Abstract
Background
Surgical site infection (SSI) is common in colorectal surgery patients and associated with morbidity and mortality. Guidelines recommend preoperative intravenous antimicrobial prophylaxis with aerobic and anaerobic coverage to reduce SSI risk. Cephalosporin based prophylaxis (CBP) regimens are recommended as first-line prophylaxis, and non-cephalosporin based are recommended as alternative prophylaxis (AP). We evaluate the efficacy of CBP versus AP in preventing surgical site infections in colorectal surgery patients.
Methods
A systematic review and meta-analysis was conducted of studies published between 2005 and 2020 in MEDLINE and Web of Science. Studies were excluded if intravenous antimicrobial prophylaxis was not administered, or if oral and intravenous prophylaxis were routinely co-administered. Heterogeneity was reported using the Q-statistic and I2-statistic. Publication bias was evaluated using a funnel plot and Egger test for small study effects. Statistical significance was defined as a two-sided p < 0.05.
Results
11 studies met inclusion criteria. AP was not associated with increased SSI risk at 30 days compared to CBP (OR 1.01, 95% CI 0.91, 1.13; OR < 1 favors AP). There was no effect size variability in subgroup analysis comparing higher-to lower-quality studies (I2 = 99%, P = 0.17). Subgroup analysis by publication year approached a significant difference in effect size between studies published prior to 2014 and later than 2014 (I2 = 99%, P = 0.06).
Conclusions
Meta-analysis of 11 studies of SSI risk in adult colorectal surgery patients suggest that SSI risk is similar for patients receiving CBP or AP, subgroup analysis of studies published since 2014 suggest increased SSI risk with AP compared to CBP.
Keywords: Colorectal surgery, Intravenous prophylaxis, Surgical site infection, Cephalosporin
Abbreviations: RCT = Randomized Control Trial, RC = Retrospective Cohort; PC = prospective Cohort, CC = case control; PD=Percent Difference, AD = Adjusted Difference; OR=Odds Ratio, RR = Relative risk
Highlights
-
•
Meta-Analysis found that surgical site infection risk is similar for patients receiving intravenous cephalosporin based or alternative prophylaxis.
-
•
Subgroup analysis of studies published since 2014 suggest increased surgical site infection risk with alternative prophylaxis compared to cephalosporin based prophylaxis.
-
•
RCT’s are needed to evaluate trends in SSI risk in cephalosporin-based prophylaxis compared to alternative prophylaxis.
1. Introduction
Surgical site infections (SSI) are defined by the Centers for Disease Control as infections affecting the superficial or deep incision space or organ space of the operative site occurring within 30 days of a surgical procedure [1]. Despite significant advances in surgical protocols aimed at reducing SSI incidence, SSIs remain significant contributors to morbidity and mortality in surgical populations [2]. Patients undergoing colorectal surgery suffer one of the highest postoperative SSI rates, with reported incidence ranging from 3 to 28% [3,4].
The 2013 American Society of Health-System Pharmacists (ASHP) Therapeutic Guidelines on Antimicrobial Prophylaxis in Surgery recommended administering preoperative intravenous antimicrobial prophylaxis with both anaerobic and aerobic coverage for adult colorectal surgery procedures. However, while the guidelines suggest cephalosporin-containing regimens as first-line, they also state that the “the optimal choice of antimicrobial agent has not been fully resolved” [5]. While prior studies have evaluated the optimal timing and frequency of intravenous antimicrobial dosing in this population, few studies have compared the effectiveness of cephalosporin-based prophylaxis regimens (CBP) to the alternative non-cephalosporin-based regimens (AP) that are recommended for SSI prevention. Furthermore, no systematic reviews or meta-analyses have been published on this topic over the past two decades, and certainly none since the widely used 2013 ASHP guidelines were widely integrated into clinical practice [6]. Thus, the equivalent efficacy of first line and alternative intravenous antimicrobial prophylaxis remains uncertain, despite the ASHP recommendations for intravenous antimicrobial prophylaxis for the colorectal surgery population. This question remains unresolved, even as the literature has moved on to new questions regarding the benefits of adjunctive oral antimicrobials and bowel preparation in SSI reduction [7].
In this systematic review and meta-analysis, the 30-day odds of acquiring SSI were compared between adult patients receiving preoperative intravenous CBP versus AP in advance of elective colorectal surgery procedures.
2. Methods
This systematic review and meta-analysis was registered by PROSPERO and conducted per PRISMA guidelines. It was deemed exempt for review by the Hamilton Integrated Research Ethics Board. A systematic literature search of studies published in English between 2005 and 2020 was performed between August 3–5, 2020 using MEDLINE and Web of Science per study protocol. The complete list of search terms, including MeSH terms and standardized language, can be found in Appendix A. Literature was compiled using Covidence (www.covidence.org). Observational cohort studies, case-control studies, and randomized controlled trials of adults undergoing clean-contaminated elective colorectal surgery procedures who received preoperative intravenous antimicrobial prophylaxis adhering to standard CDC NHSN definitions for SSI and procedure wound class reporting were included [1]. Studies were excluded if preoperative antimicrobial prophylaxis was not administered, or if oral and intravenous antimicrobial prophylaxis were routinely co-administered. Duplicate studies identified by Covidence were removed. Remaining study titles and abstracts were screened for eligibility by two reviewers with a third reviewer resolving discrepancies.
Following screening, two reviewers completed full-text appraisal and used a standardized chart to enter data from eligible studies. Study quality was assessed using Jadad Scale for randomized control trials and Newcastle-Ottawa Scale for observational studies, and an agglomerate score created for ease of analysis such that RCTs that were originally scored on the Jadad score (i.e. 5 out of 5) had their scores translated to an equivalent score (i.e. 9 out of 9) on the Newcastle-Ottawa scoring system [8,9]. Microsoft Excel was used to calculate the standardized study metameter (log odds ratio).
STATA 16 was used to perform the meta-analysis, including cumulative meta-analyses, a priori subgroup analyses, meta-regressions, and sensitivity analyses. Treatment effects were reported as SSI odds ratios with 95% confidence intervals. Between-study and between-subgroup heterogeneity was reported using Q-statistic and I2-statistics. Publication bias was evaluated using a funnel plot and Egger test for small study effects. Statistical significance was defined as a two-sided P < 0.05.
3. Results
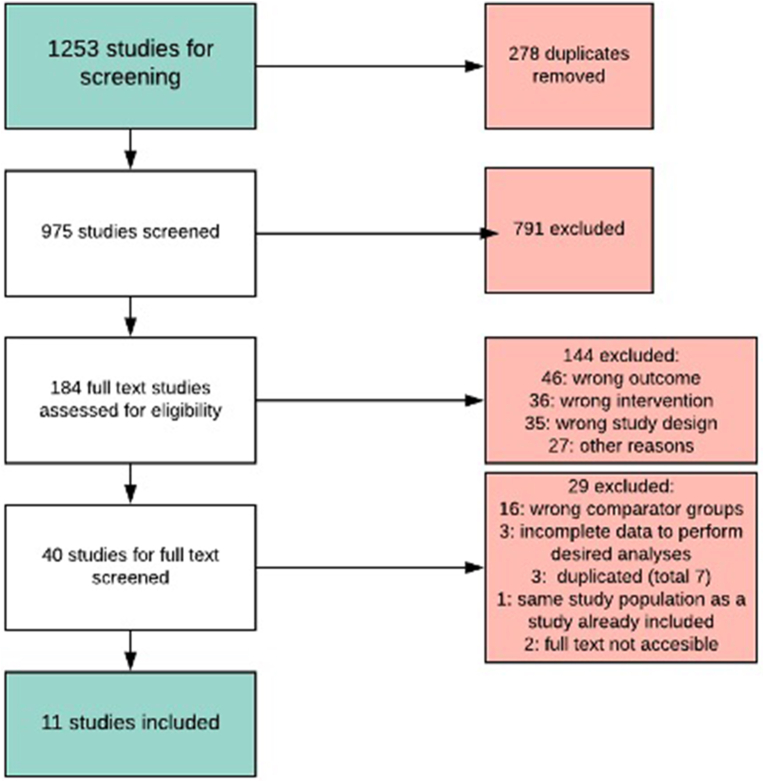
Using MEDLINE and Web of Science searches 1253 studies were identified. Of these, 278 were duplicates and 975 studies were screened for inclusion (Fig. 1). 791 of 975 were excluded because they did not meet inclusion criteria after title and abstract screening. The remaining 184 studies underwent full-text eligibility review, of which 144 were excluded; 46 (32%) due to incorrect SSI outcome, 36 (25%) due to incorrect intervention, and 35 (24%) because the study design was not amenable to data extraction for this meta-analysis. The remaining 27 (19%) were excluded for other reasons including non-English language, comparison of intravenous to oral antimicrobial prophylaxis, or lack of full text availability.
Fig. 1.
PRISMA diagram of studies included in this systematic review and meta-analysis.
The 40 remaining studies were eligible for full-text review and data abstraction, of which 29 were excluded: 16 (53%) for including a different comparator (ie. oral antimicrobial or no antimicrobial prophylaxis at all), 4 (13%) for incomplete data, and 10 (33%) for other reasons (Fig. 1). A total of 11 studies met criteria for inclusion in the meta-analysis [4,[10], [11], [12], [13], [14], [15], [16], [17], [18]]. Characteristics of all 11 studies that underwent data extraction are presented in Table 1. A total of 102,277 colorectal surgery patients comprised the combined study population. The nine observational studies included in the meta-analysis scored either 6 or 7 of 9 possible points on the Newcastle-Ottawa quality scale. This translated into three ‘good’ studies (Kuriakose, Ho, Fan) on the AHRQ scale [11,14,18]. The remaining six were fair studies per the AHRQ scale (Hawn, Baatrup, Eagye, Branch-Elliman, Branch-Elliman, Poeran) due to low scores in the outcome exposure domain, and none were considered of poor quality [4,12,13,16,17]. None of the nine observational studies reported that SSI assessment was blinded to the type of antimicrobial prophylaxis administered, and only three studies (Kuriakose, Ho, Fan) clearly reported that all subjects were evaluated for SSI following a full 30-day follow-up period [11,14,18]. Only two studies (Eagye, Fan) clearly reported that the infection was not present at the beginning of the study [4,18]. The two clinical trials included in this meta-analysis scored 5 out of 5 possible points on the Jadad scale and were considered high quality.
Table 1.
Characteristics and level of evidence for the eleven studies included in the meta-analysis.
| Study | Author | Year | Country | Design | Quality Score | Comparator | Received AP (treated) | Received BLP (control) | Total SSIs | SSIs after AP | SSIs after BLP | logOR | var (logor) | se (or) | Original study metameter | Funding source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Leng | 2014 | China | RCT | 9a | Ertapenemb | 251 | 248 | 48 | 24 | 24 | −0.006 | 0.092 | 0.304 | PD | Merck & Co. |
| 2 | Kuriakose | 2019 | USA | RC | 7 | AP | 538 | 9411 | 662 | 51 | 571 | 0.210 | 0.024 | 0.153 | OR | Blue Cross and Blue Shield Network |
| 3 | Hawn | 2013 | USA | RC | 7 | AP | 386 | 5043 | 705 | 46 | 659 | −0.046 | 0.026 | 0.163 | OR | PPO 10–296 from the VA Health Services Research and Development Service. |
| 4 | Baatrup | 2009 | Norway | CC | 6 | AP | 683 | 203 | 186 | 126 | 60 | −0.268 | 0.033 | 0.183 | RR | Not Disclosed |
| 5 | Ho | 2011 | USA | RC | 7 | AP | 376 | 219 | 130 | . | . | 0.316 | 0.077 | 0.280 | OR | Covidien, Astra Zenica, Olympus |
| 6 | Eagye | 2011 | USA | RC | 7 | Carbapenem | 1575 | 3057 | 172 | 44 | 126 | −0.175 | 0.032 | 0.178 | OR | Hartford's Hospital Center for Anti Infective Research |
| 7 | Itani | 2006 | USA | RCT | 9a | Ertapenemb | 502 | 500 | 196 | 78 | 118 | −0.225 | 0.026 | 0.162 | AD | Merck |
| 8 | Branch-Elliman | 2017 | USA | RC | 7 | AP | 188 | 7128 | 1112 | 30 | 1082 | 0.026 | 0.041 | 0.202 | RR | Veteran Affairs Health Services and Research Development |
| 9 | Branch-Elliman | 2019 | USA | RC | 6 | AP | 19417 | 54780 | 10561 | 902 | 9659 | −0.643 | 0.001 | 0.036 | OR | Veteran Affairs Health Services and Research Development |
| 10 | Poeran | 2016 | USA | RC | 6 | AP | 13615 | 47502 | 4770 | 1335 | 3435 | 0.144 | 0.001 | 0.034 | OR | NCI Cancer Center Support Grant |
| 11 | Fan | 2014 | China | PC | 7 | Carbapenem | 68 | 123 | 51 | 20 | 31 | 0.092 | 0.114 | 0.338 | OR | Not Disclosed |
Characteristics and level of evidence for the eleven studies included in the meta analysis.
RCTs that originally scored 5 out of 5 on the Jadad score had their scores translated to the highest score on the Newcastle-Ottawa scoring system for analysis.
In Leng 2014 the Ertapeneme/Metronidazole was compared to Ceftriaxone/Metronidazole. In the Itani 2006 Ertapenem was compared to Cefofetan.
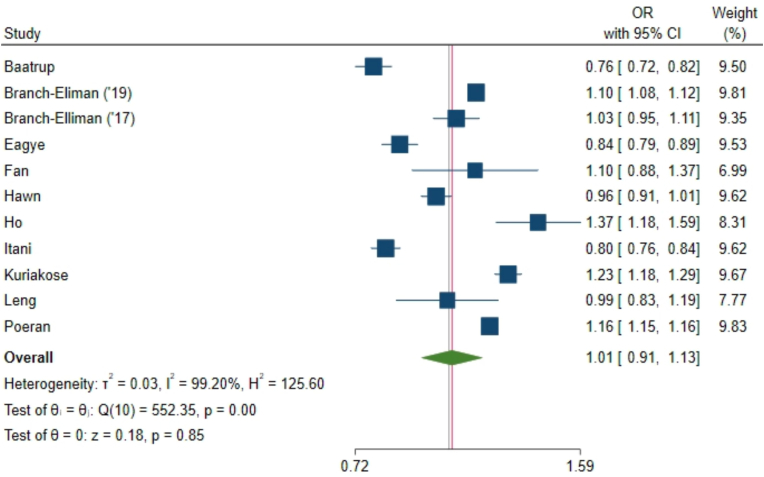
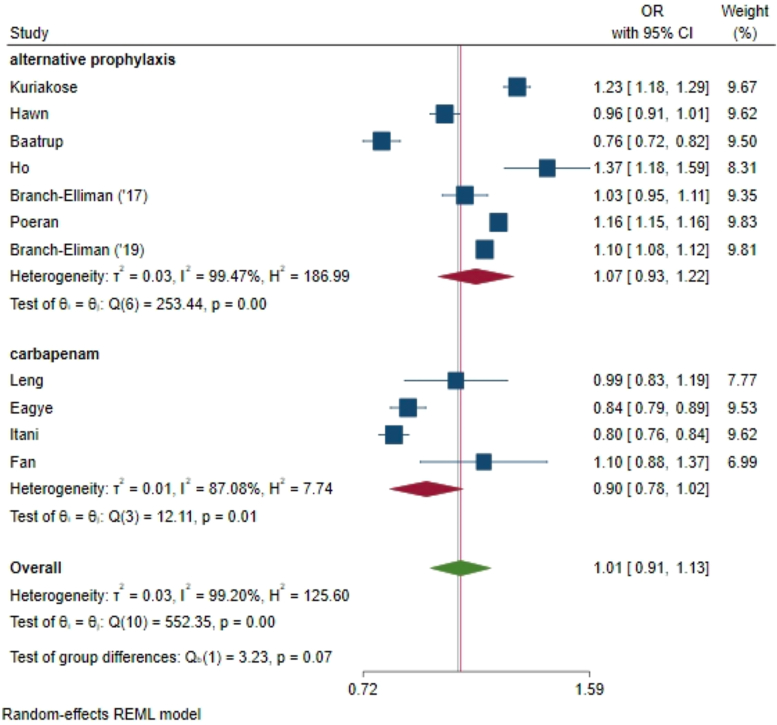
Our primary meta-analysis found no difference in the 30-day SSI risk between the AP and CBP groups (odds ratio [OR] 1.01, 95% confidence interval [CI] 0.91, 1.13; where OR < 1 favors AP, Fig. 2). There was considerable heterogeneity between studies, with an I2 value of 99% (P < 0.001), but no obvious outliers.
Fig. 2.
Meta-analysis of the odds ratio comparing AP to CBP for clean or clean-contaminated elective colorectal surgery prophylaxis for SSI risk at 30 days. Our primary meta-analysis found no difference in the 30-day SSI risk between the AP and CBP groups (odds ratio [OR] 1.01, 95% confidence interval [CI] 0.91, 1.13; where OR < 1 favors AP.
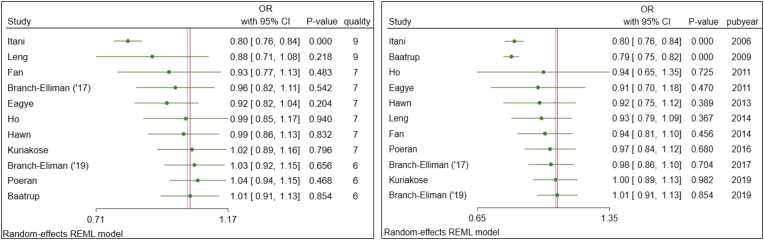
Cumulative meta-analysis of effect size by descending publication quality demonstrated a statistically non-significant trend associating higher study quality with greater effect size, as demonstrated by odds ratios further from the null (Fig. 3a). Cumulative meta-analysis of effect size by ascending publication year demonstrated a statistically non-significant trend associating later study publication with smaller effect size (Figure 3b). Cumulative meta-analysis trends were used to derive cutoffs for subsequent pre-defined subgroup analyses.
Fig. 3.
Fig. a (left). Cumulative meta-analysis of effect size by study quality demonstrated a statistically non-significant trend associating higher study quality with greater effect size, as demonstrated by odds ratios further from the null. Figure 3b (right). Cumulative meta-analysis of effect size by study publication year demonstrated a statistically non-significant trend associating later study publication with smaller effect size.
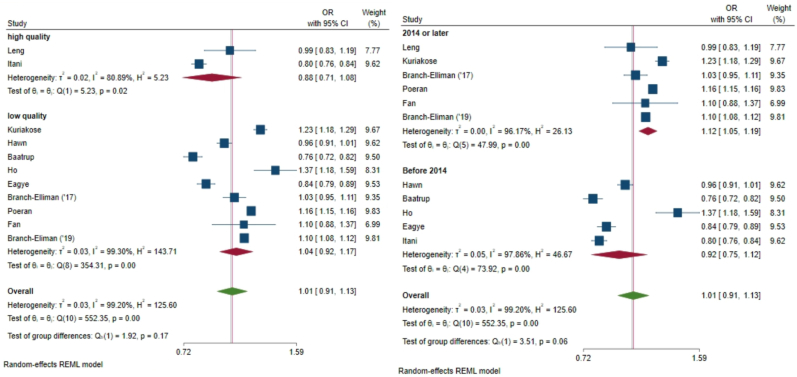
Subgroup analysis by publication year approached a significant difference in effect size between studies published prior to 2014 and those published in 2014 and later (I2 = 99%, P = 0.06) Fig. 4b). Subgroup analysis of studies published 2014 and later had a larger effect size associated with AP (OR 1.12 95% CI 1.05, 1.19), favoring CBP in reducing SSI risk. Subgroup analysis by study quality demonstrated no significant variability in effect size between higher-quality and lower-quality studies, with significant heterogeneity of effect size within the higher-quality group (I2 = 80%, P=0.02) and the lower-quality group (I2 = 99%, P<0.001) but no difference in subgroup means between the higher- and lower-quality groups (P = 0.17, Fig. 4a). Additional subgroup analyses by AP type, carbapenem versus other AP (OR 1.01, 95% CI 0.91, 1.13 Fig. 5) demonstrated a non-significant within-group and between group heterogeneity of effect size (I2 = 99.20, P = 0.07).
Fig. 4.
Fig. 4a (left) Subgroup analysis of effect size by study quality showed significant heterogeneity of effect size within the higher-quality group (I2 = 80%, P=0.02) and the lower-quality group (I2 = 99%, P<0.001) but no difference in subgroup means between the higher- and lower-quality groups. 4b (right) Subgroup analysis of effect size by publication year approached a significant difference in effect size between studies published prior to 2014 and those published in 2014 and later (I2 = 99%, P = 0.06).
Fig. 5.
Subgroup analysis of effect size by alternative antimicrobial prophylaxis type: carbapenem versus other alternative prophylaxis demonstrated non-significant within-group and between group heterogeneity of effect size.
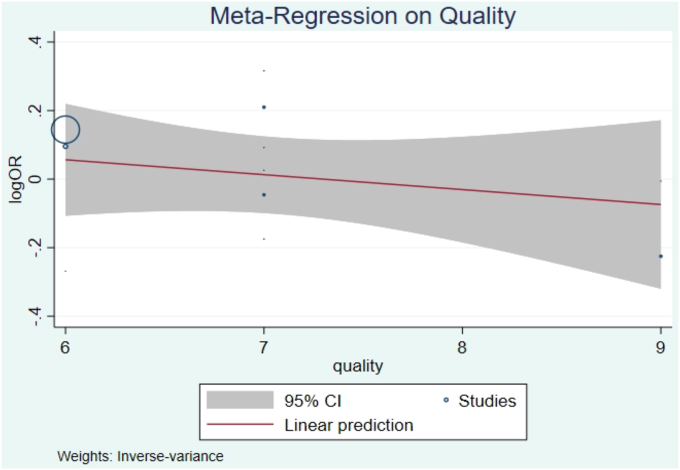
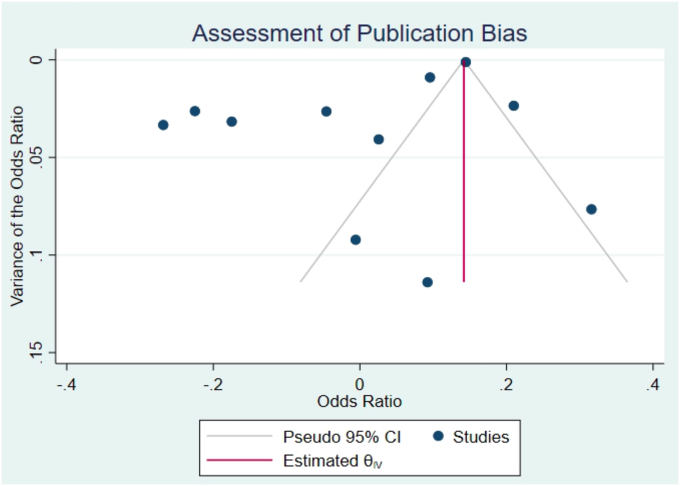
Meta-regression by study quality did not demonstrate a significant trend towards higher-quality studies having a larger effect size (logOR=-0.04, 95% CI -0.16, 0.07, P=0.45, Fig. 6). Bias analysis using funnel plot did not show significant evidence of a missing study effect, with Egger test P-value 1.0, though some small-sized population studies may be missing (seeFig. 7).
Fig. 6.
Bubble-plot showing metaregression on study quality did not demonstrate a significant trend towards higher-quality studies having a larger effect size
Fig. 7.
Funnel plot bias analysis did not show significant evidence of a missing study effect with Egger test P-value 1.0.
4. Discussion
Administration of preoperative intravenous antimicrobial prophylaxis is standard of care for elective colorectal surgery patients to reduce 30-day SSI risk, however in addition to the gold-standard CBP regimens, a variety of AP regimens are endorsed by the 2013 ASHP consensus guidelines [5]. The inclusion of multiple options reflects financial considerations, variability of regional antibiograms, and the need to provide alternatives for patients who report allergies and intolerances to specific antimicrobials. However, a growing body of cross-disciplinary evidence suggests that people reporting cephalosporin or penicillin allergies and receive AP regimens have worse clinical outcomes [19], possibly due to receiving less effective antimicrobial prophylaxis. While our meta-analysis found no difference in 30-day SSI risk between adult colorectal surgery patients receiving AP and CBP (OR 1.01, 95% CI 0.91, 1.13), included studies had significant heterogeneity of effect size.
Pre-defined subgroup analyses resulted in two important observations. First, the subgroup of studies published 2014 and later had a larger effect size associated with AP (OR 1.12 95% CI 1.05, 1.19), favoring CBP in reducing SSI risk. This finding could reflect the higher quality of more recent studies, as the Poeran study was the only lower-quality study included in this group. Changing antimicrobial resistance patterns over time could also explain the apparent differences in effectiveness of cephalosporin antimicrobial regimens over time, or differences in specific types of CBP or AP used over time. Data from later studies could also reflect a turning point in implementation of guideline-based bundled perioperative SSI reduction strategies that could influence interpretation of our results. The apparent increased risk of SSI in 2014 and later studies among patients receiving AP compared to CBP warrants further investigation to determine whether this results from changing antimicrobial resistance patterns.
Second, the subgroup analysis comparing the broad-spectrum carbapenem AP antimicrobial ertapenem to other types of AP suggested a lower risk of SSI when ertapenem was used as AP than for other AP regimens. Although the Leng study demonstrated an apparent benefit to using ertapenem AP instead of CBP in this population, this finding was not corroborated across studies that included carbapenems as an option for AP. Thus, the decision to include carbapenems for routine AP use should be balanced against antimicrobial stewardship efforts to avoid accelerating the development of antimicrobial resistance.
An important strength of this meta-analysis is the large total number of patients included, comprising over 102,177 colorectal surgeries. In addition, the included studies were of moderate- or high-quality, reducing the influence of bias from low quality studies. Furthermore, eligible studies were identified using two comprehensive databases - MEDLINE and Web of Science - to ensure all relevant studies would be captured. We limited the search period to 2005–2020 to accurately reflect current antimicrobial prophylaxis practices in colorectal surgery following widespread implementation of routine enhanced recovery after surgery (ERAS) protocols in 2005, ensuring the relevance of meta-analysis results to current practice.
Two important limitations of our study include the inherent subjectivity of quality assessment, such that included studies might have been perceived as high-quality by our reviewers despite true study biases. This risk for bias was reduced by designating repeat quality assessment by multiple reviewers using validated scoring systems. Delineating case mix (elective versus urgent) for some studies also presented a challenge. Though all study methods sections published whether they included urgent/emergent, non-elective, or non-colorectal cases, not all authors clearly reported the proportion of each case type when reporting the SSI outcome. To improve clarity in this regard, and ensure the meta-analysis included only elective cases, authors of included studies were contacted as required, and proportions were clarified as best as possible by antimicrobial prophylaxis type and SSI outcome. On the other hand, during our initial search for studies in the elective colorectal surgery population, we excluded studies conducted solely in urgent/emergent case populations. It is possible that this search strategy may have missed or excluded some studies reporting on both urgent/emergent and elective cases due to lack of clarity on study population in the title and abstract.
An additional limitation of this meta-analysis is potential variation in effectiveness between different AP and CBP regimens, which was not reported by most included studies and could have led to significant heterogeneity in SSI risk by specific prophylactic regimen, biasing the effect size towards the null hypothesis of no difference in SSI risk between AP and CBP groups. We would expect this effect to be more significant in the AP group, which has a wider variety of prophylaxis options, compared to the CBP group, which consists of fewer regimens with similar spectra of coverage. Another limitation inherent to the meta-analysis study design is difficulty adjusting for covariates that could impact SSI risk including the type of pre-operative skin preparation, the use of oral antimicrobials, surgical approach, etc. This highlights the need for further study via randomized control trials that explicitly randomize to equally distribute confounding variables, to more clearly compare the effectiveness of AP with CBP. Finally, it is important to recognize that the larger studies in this analysis could have had a larger impact on the final results. Future studies should compare standardized AP and CBP regimens to ensure the effect is not diluted by variations in prophylaxis regimen within each group, particularly in the heterogeneous AP group. Additional randomized controlled trials and better-controlled observational studies are needed to further evaluate trends towards decreased SSI risk in CBP compared to AP groups seen in more recent, higher-quality studies, so as to clarify if the CBP and AP regimens truly share equivalent efficacy in SSI prevention among elective colorectal surgery patients.
5. Conclusion
A meta-analysis of 11 studies involving 102,277 adult colorectal surgery patients demonstrated no difference in 30-day SSI rates between those receiving AP and CBP preoperative antimicrobial prophylaxis. Studies published from 2014 and later demonstrate higher SSI risk in patients receiving AP compared to those receiving CBP. Randomized controlled trials and better-controlled observational studies are needed to determine optimal preoperative antimicrobial prophylaxis in this population to minimize SSI risk.
Ethical Approval
Declared REB review exempt due to the nature of the study as a systematic review and meta-analysis (Hamilton Integrated Research Ethics Board)
Approved and registered via PROSPERO.
Funding
No funding was sought in support of this study.
Author contribution
Please specify the contribution of each author to the paper, e.g. study concept or design, data collection, data analysis or interpretation, writing the paper, others, who have contributed in other ways should be listed as contributors. Alexis N Bowder: study concept and design, data analysis and interpretation, editing and writing the paper. Christina Yen: study concept and design, data analysis and interpretation, editing and writhing the paper. Lisa M. Bebell: study concept and design, data analysis and interpretation, editing and writing the paper. Dr. Alisha R. Fernandes: proposed study concept, contributed to study design, contributed to data collection, performed data analysis, contributed to interpretation, contributed to drafting components of initial manuscript, contributed to subsequent manuscript review, did not contribute to formatting of references.
Consent
No consent required.
Registration of research studies
In accordance with the Declaration of Helsinki 2013, all research involving human participants has to be registered in a publicly accessible database. Please enter the name of the registry and the unique identifying number (UIN) of your study.
You can register any type of research at http://www.researchregistry.com to obtain your UIN if you have not already registered. This is mandatory for human studies only. Trials and certain observational research can also be registered elsewhere such as: ClinicalTrials.gov or ISRCTN or numerous other registries.
1.Name of the registry: PROSPERO
2.Unique Identifying number or registration ID: CRD42020204396
3.Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=204396
Guarantor
The Guarantor is the one or more people who accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Dr. Alisha R Fernandes.
Dr. Lisa M. Bebell.
Dr. Alexis N Bowder.
Dr. Christina Yen.
Disclaimers
None.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102401.
Contributor Information
Alexis N. Bowder, Email: abowder@mcw.edu.
Christina F. Yen, Email: cyen@bidmc.harvard.edu.
Lisa M. Bebell, Email: LBEBELL@mgh.harvard.edu.
Alisha R. Fernandes, Email: alishafernandes@hsph.harvard.edu.
Appendix A. Search Terms
This systematic literature search was designed to identify as many relevant online published studies possible comparing the risk of surgical site infection (SSI) in patients undergoing elective clean-contaminated colorectal procedures who received either cephalosporin based antimicrobial prophylaxis versus those who did not, using Medline and Web of Science database. The last search was carried out on August 3rd, 2020.The bibliographies of the studies and related reviews were included for additional references. The following search terms were used in several in the aforementioned databases:
Web of Science.
TS=((surgical OR wound OR postoperative OR “post operative”) NEAR/3 infection*)
AND.
TS=(“antibacterial” OR “antimicrobial*" OR “anti biotic*" OR “antimicrobial” OR “prophylaxis”)
AND.
TS=((colon OR colorectal OR rectal) NEAR/3 (surg* OR resection* OR ostomy*))
Medline.
(“surgical wound infection" [MeSH Terms] OR surgical site infection*[tiab] OR wound infection*[tiab])
AND.
(“Antimicrobial Prophylaxis" [Mesh] OR “Anti-Bacterial Agents" [Mesh] OR “Anti-Bacterial Agents" [Pharmacological Action] OR antibacterial [tiab] OR antimicrobial*[tiab] OR antimicrobial [tiab])
AND.
(“Colorectal Surgery" [Mesh] OR “Colorectal Neoplasms/surgery" [Mesh] OR “Colectomy" [Mesh] OR colectom*[tiab] OR “colon cancer” OR “colon neoplasm” OR “rectal cancer” OR “rectal neoplasm” OR “colorectal cancer” OR “colon/rectum adenocarcinoma” OR “adenoma” OR “unresectable polyp” OR “inflammatory bowel disease” OR “crohn's disease” OR “crohn's colitis” OR “ulcerative colitis” OR “diverticulitis” OR “diverticular stricture” OR “perforated diverticulitis” OR “hinchey” OR “Ileocecectomy” OR “right hemicolectomy” OR “partial colectomy” OR “left hemicolectomy” OR “subtotal colectomy” OR “sigmoid resection” OR “anterior resection” OR “low anterior resection” OR “abdominoperineal resection” OR “hartmann's procedure” OR “colostomy” OR “colostomy reversal OR ((surger*[tiab] OR operative [tiab]) AND (colon [tiab] OR colorectal [tiab])))
Appendix B. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Centers for disease control and prevention, Surgical Site Infection (SSI) Event. https://www.cdc.gov/nhsn/pdfs/pscManual/9pscSSIcurrent.pdf Accessed.
- 2.Berrios-Torres S.I., Umscheid C.A., Bratzler D.W. Centers for disease control and prevention guideline for the prevention of surgical site infection. JAMA Surg. 2017. 2017;152(8):784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 3.Fry D.E. The prevention of surgical site infection in elective colon surgery. Sci. Tech. Rep. 2013;2013:1–19. doi: 10.1155/2013/896297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eagye K.J., Nicolau D.P. Selection of prophylactic antimicrobial agent may affect incidence of infection in small bowel and colorectal surgery. Surg. Infect. 2011;12:451–457. doi: 10.1089/sur.2010.108. [DOI] [PubMed] [Google Scholar]
- 5.Bratzler D.W., Dellinger P., Olsen K.M. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. Feb 1 2013;70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 6.Nelson R.L., Glenny A.M., Song F. Antimicrobial prophylaxis for colorectal surgery (Review) Cochrane Database Syst. Rev. 2009;1:1–131. doi: 10.1002/14651858.CD001181.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Ohman Kerri A. Combination of oral antimicrobials and mechanical bowel preparation reduces surgical site infection in colorectal surgery. J. Am. Coll. Surg. 2017;225(4):465–471. doi: 10.1016/j.jamcollsurg.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Peterson J. Ottawa Hospital Research Institute; Ottawa: 2011. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [Google Scholar]
- 9.Moher David. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Contr. Clin. Trials. 1995;16(1):62–73. doi: 10.1016/0197-2456(94)00031-w. [DOI] [PubMed] [Google Scholar]
- 10.Leng X., Zhao Y., Qiu H. Ertapenem prophylaxis of surgical site infections in elective colorectal surgery in China: a multicentre, randomized, double-blind, active-controlled study. J. Antimicrob. Chemother. Aug 23 2014;69:3379–3386. doi: 10.1093/jac/dku302. [DOI] [PubMed] [Google Scholar]
- 11.Kuriakose J.P., Vu J., Karmakar M. β-Lactam vs non-β-lactam antimicrobials and surgical site infection in colectomy patients. J. Am. Coll. Surg. 2019;229(5):487–496. doi: 10.1016/j.jamcollsurg.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Hawn M.T., Richman J.S., Vick C.C. Timing of surgical antimicrobial prophylaxis and the risk of surgical site infection. JAMA Surg. 2013;148(7):649–657. doi: 10.1001/jamasurg.2013.134. [DOI] [PubMed] [Google Scholar]
- 13.Baatrup G., Nilsen R.M., Svensen R. Increased incidence of postoperative infections during prophylaxis with cephalothin compared to doxycycline in intestinal surgery. BMC Surg. 2009;9(17) doi: 10.1186/1471-2482-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho V.P., Barbie P.S., Stein S.L. Antimicrobial regimen and the timing of prophylaxis Are important for reducing surgical site infection after elective abdominal colorectal surgery. Surg. Infect. 2011;12(4):255–260. doi: 10.1089/sur.2010.073. [DOI] [PubMed] [Google Scholar]
- 15.Itani K.M.F., Wilson S.E., Awad S.S. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N. Engl. J. Med. 2006;355:2640–2651. doi: 10.1056/NEJMoa054408. [DOI] [PubMed] [Google Scholar]
- 16.Branch-Elliman W., Ripollone J.E., O'Brien W.J. Risk of surgical site infection, acute kidney injury, and Clostridium difficile infection following antimicrobial prophylaxis with vancomycin plus a cephalosporin versus either drug alone: a national propensity-score-adjusted retrospective cohort study. PLoS Med. 2017;14(7) doi: 10.1371/journal.pmed.1002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poeran J., Wasserman I., Zubizarreta N. Characteristics of antimicrobial prophylaxis and risk of surgical site infections in open colectomies. Dis. Colon Rectum. 2016;59(8):733–742. doi: 10.1097/DCR.0000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan Y., Ren J., Zhao Y. Fistula output microorganism-susceptible antimicrobial prophylaxis is associated with a lower risk of surgical site infection in gastrointestinal fistula patients undergoing one-stage definitive surgery. Surg. Infect. 2014;15(6):774–780. doi: 10.1089/sur.2013.143. [DOI] [PubMed] [Google Scholar]
- 19.MacFadden D.R., LaDelfa A., Leen J. Impact of reported cephalosporin allergy on inpatient outcomes: a multicenter prospective cohort study. CID. 2016;63(7):904–910. doi: 10.1093/cid/ciw462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.