Abstract
Cardiorenal syndromes constellate primary dysfunction of either heart or kidney whereby one organ dysfunction leads to the dysfunction of another. The role of several microRNAs (miRNAs) has been implicated in number of diseases, including hypertension, heart failure, and kidney diseases. Wide range of miRNAs has been identified as ideal candidate biomarkers due to their stable expression. Current study was aimed to identify crucial miRNAs and their target genes associated with cardiorenal syndrome and to explore their interaction analysis. Three differentially expressed microRNAs (DEMs), namely, hsa-miR-4476, hsa-miR-345-3p, and hsa-miR-371a-5p, were obtained from GSE89699 and GSE87885 microRNA data sets, using R/GEO2R tools. Furthermore, literature mining resulted in the retrieval of 15 miRNAs from scientific research and review articles. The miRNAs-gene networks were constructed using miRNet (a Web platform of miRNA-centric network visual analytics). CytoHubba (Cytoscape plugin) was adopted to identify the modules and the top-ranked nodes in the network based on Degree centrality, Closeness centrality, Betweenness centrality, and Stress centrality. The overlapped miRNAs were further used in pathway enrichment analysis. We found that hsa-miR-21-5p was common in 8 pathways out of the top 10. Based on the degree, 5 miRNAs, namely, hsa-mir-122-5p, hsa-mir-222-3p, hsa-mir-21-5p, hsa-mir-146a-5p, and hsa-mir-29b-3p, are considered as key influencing nodes in a network. We suggest that the identified miRNAs and their target genes may have pathological relevance in cardiorenal syndrome (CRS) and may emerge as potential diagnostic biomarkers.
Keywords: CRS, DEMs, literature mining, miRNA-mRNA network, module analysis, pathways
Introduction
The performance of the heart and the renal function is strongly interconnected with each other. It is well known that acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ. 1 The presence of impaired renal function during heart disease and vice versa is collectively known as cardiorenal syndrome (CRS). 1 The CRS is categorized in 5 different subcategories (types 1-5) on the basis of potential underlying pathophysiological mechanisms. 2 Renal and cardiovascular complications cover a wide range of diseases such as chronic kidney disease (CKD), coronary artery disease (CAD), congestive heart failure, and arrhythmias. 3 MicroRNAs (miRNAs), an endogenous small (18-24 nucleotides) noncoding RNA molecules, have been found associated with several pathological conditions. 4 It is well known that miRNAs regulate expression of genes at posttranscriptional level by degrading or blocking the translation mechanism of target gene/messenger RNA (mRNA). 5 The miRNAs are involved in regulation of many cellular processes, including proliferation, differentiation, and programmed cell death. 6 Stable forms of miRNAs have been traced in human body fluids like blood and urine. 7 The miRNAs have been recently discovered with a wider role in renal cancer, diabetic nephropathy, and hypertensive renal injury. 8 Moreover, it has been found that a single miRNA may be responsible for altering complex genetic networks by affecting different genes simultaneously. 9
The therapeutic management of CRS is considered as a huge challenge. Drugs supposed to treat the cardiovascular diseases may lead to impairment in the functioning of kidneys and vice versa. 10 The miRNAs are involved in the development and progression of different diseases and therefore may be used as biomarkers. Several miRNAs with various expression degrees have been reported in both acute and chronic state of primary organ failure in CRS.11,12 The miR-21 has been found common in all CRS types. 13 Interestingly, miR-21 has a higher level of expression in both heart and kidneys and its elevated levels have been found associated with poor outcome in most of the primary organ failures. 12 These findings provide a notion that suppression of miR-21 may show a ray of hope for therapeutics and treatment of CRS. 12
This study was aimed to identify crucial miRNAs and their target genes associated with CRS. GSE89699 and GSE87885 data sets were chosen for the analysis of differentially expressed microRNAs (DEMs). Interaction analysis of miRNA and their target genes may pave for the finding of novel pathological mechanisms and biomarkers for CRS.
Materials and Methods
Data retrieval and preprocessing of data
The GSE89699 and GSE87885 miRNA expression profiles were retrieved from the functional genomics data repository, Gene Expression Omnibus, and NCBI (www.ncbi.nlm.nih.gov/geo/). The miRNA data set GSE89699 consists of data from 8 samples, 7 diseased and 1 normal sample, whereas GSE87885 consists of data from 5 samples, 2 diseased and 3 normal samples. 14 The miRNA expression profile in GSE89699 was detected using the GPL18402 platform (Unrestricted_Human_miRNA_V19.0_Microarray, Design Id: 046064; Agilent, Karnataka, India), whereas miRNA expression profile in GSE87885 was detected using the GPL22555 platform (μParaflo human miRNA array; LC Sciences, Qingdao, China). The GSE89699 and GSE87885 were normalized and preprocessed through R/GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/) tool. R/GEO2R is a Web-based analytical tool with in-built Linear Models for Microarray Data (Limma) R package and GEO query. 15
Literature mining
The PubMed search engine was used for literature mining of key miRNAs related to CRS. The keywords for literature mining included microRNA expression, miRNA expression, cardiorenal syndrome, renocardio syndrome, cardiovascular and chronic/acute kidney disease, heart and kidney disease, renal and myocardial/heart/congestive failure, acute kidney injury and coronary artery disease, CRS biomarkers, CRS Type 1-5, CKD and CVD, AKI and MI, CAD and CRF, diagnosis and prognosis. In addition, published research and review articles were manually searched to add more data on miRNAs related to CRS. A detailed description of workflow is given in Figure 1.
Figure 1.
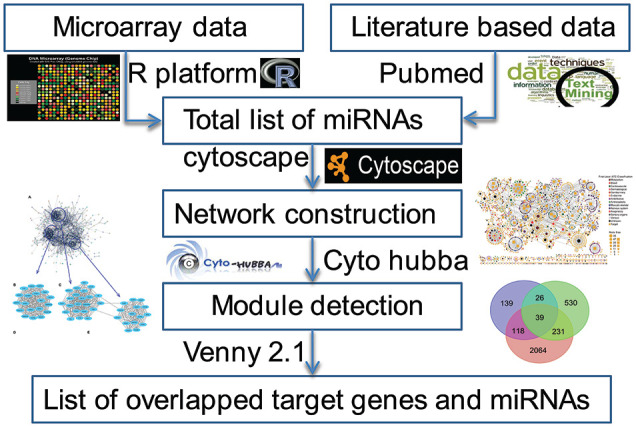
Methodology which has been adopted to retrieve miRNAs from different sources. miRNA indicates microRNA.
MiRNA gene network construction
Generated list of miRNAs was submitted to miRNet tool (an miRNA-centric network visual analytics platform) for construction of miRNAs-mRNA network. 16 The miRNet is a Google Cloud Computing Engine with 64G RAM and 8 CPU cores (n2-highmem-8). Four well-annotated databases (miRTarBase v8.0, TarBase v8.0, miRanda, and miRecords) were used to retrieve the data of target genes searched against CRS-related miRNAs. Visualization and analysis of miRNAs-mRNA network were carried out by using the Cytoscape software (version 3.7.1). 17
Module detection and pathways enrichment analysis
Cytoscape with cytoHubba plugin was used to identify highly interconnected regions in the miRNAs-mRNA network. It is a facilitated platform for the analysis and visualization of molecular interaction networks. 18 The cytoHubba (version 0.1) was used to identify the important functional modules and the higher ranked genes/proteins in the network based on Degree centrality, Closeness centrality, Betweenness centrality, and Stress centrality. Degree centrality assigns an importance score based simply on the number of links held by each node. Degree tells us that how many direct, “one hop” connections each node has to other nodes in the network. It is the simplest measure of node connectivity. Sometimes it is useful to look at in-degree (number of inbound links) and out-degree (number of outbound links) as distinct measures, eg, when looking at transactional data or account activity. Closeness centrality scores each node based on their “closeness” to all other nodes in the network. Closeness measure calculates the shortest paths between all nodes and then assigns each node a score based on its sum of shortest paths. It is used for finding the factors that are best placed to influence the entire network most quickly. Betweenness centrality measures the number of times a node lies on the shortest path between other nodes. Betweenness tells us which nodes are “bridges” between nodes in a network. It does this by identifying all the shortest paths and then counting how many times each node falls on one. It is useful for analyzing communication dynamics. The stress of a node in a biological network, for instance, a protein-signaling network, can indicate the relevance of a protein as functionally capable of holding together communicating nodes. The higher is the value, the higher is the relevance of the protein in connecting regulatory molecules. Due to the nature of this stress centrality, it is possible that the stress simply indicates a molecule heavily involved in cellular processes but not relevant to maintain the communication between other proteins. The cytoHubba provides a user interface in a very simple way to analyze a network with 11 scoring methods. Top-ranked nodes of a particular scoring method (Degree centrality, Closeness centrality, Betweenness centrality, and Stress centrality) were retrieved from the cytoHubba tab in the Cytoscape control panel. Furthermore, DIANA-mirPath, a Web-based server, was used for the analysis of overlapped miRNAs and for generating heatmaps. 19
Results
Identification of DEMs
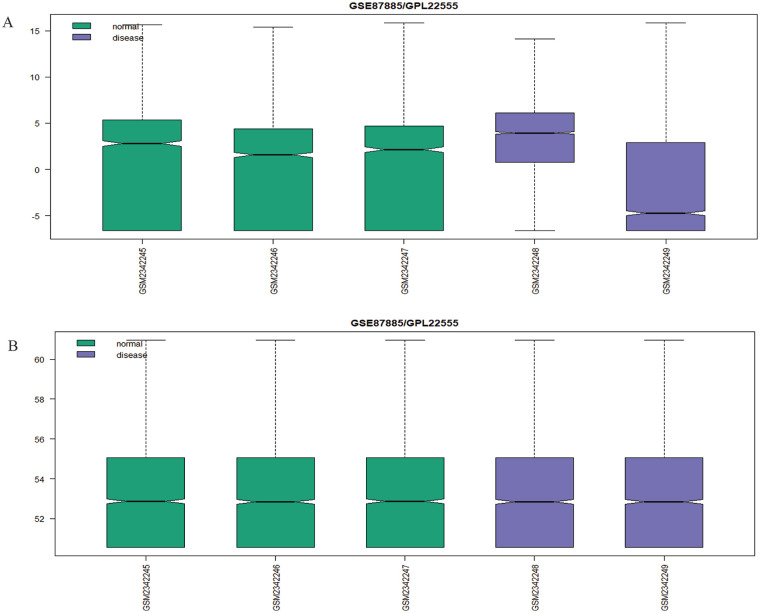
The GSE89699 and GSE87885 data sets were first preprocessed and normalized, and then differentially expressed microRNAs (DEMs) were extracted. The P value < .05 and |log fold change| > 0.5 were taken as cutoff values for statistically significant differentially expressed genes (DEGs) or miRNAs. The data sets obtained before and after normalization are depicted in boxplots (Figure 2). The GSE87885 revealed 171 total DEMs in which 93 were upregulated and 78 were downregulated miRNAs, whereas GSE89699 revealed 81 down-regulated miRNAs. Overlapped DEMs among these 2 data sets were explored by Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/). Results showed that 3 miRNAs were common in both GSE data sets. Finally, we have 18 miRNAs listed in Table 1. Fifteen miRNAs among these were retrieved from literature.
Figure 2.
Boxplot indicating the expression values in the GSE series data sets. If the data sets are not normalized, it shows false results (the value of log fold change varies) of the probe expression due to noise, duplicity, and redundancy so that normalization of data sets is required. (A) Prenormalization of GSE87885 where green color indicates normal samples (GSM2342245, GSM2342246 and GSM2342247) and violet color for disease samples (GSM2342248 and GSM2342249). (B) Normalized data sets.
Table 1.
List of different miRNAs, their target gene counts, and mature sequences which have been retrieved by literature mining and microarray data sets.
| miRBase ID | Retrieval method | Target genes count | Mature sequence | References |
|---|---|---|---|---|
| hsa-miR-21-5p | Literature based | 612 | UAGCUUAUCAGACUGAUGUUGA | [7, 12] |
| hsa-miR-22-3p | Literature based | 163 | AGUUCUUCAGUGGCAAGCUUUA | [12] |
| hsa-miR-126-5p | Literature based | 119 | CAUUAUUACUUUUGGUACGCG | [14, 15] |
| hsa-miR-223-5p | Literature based | 284 | CGUGUAUUUGACAAGCUGAGUU | [15] |
| hsa-miR-29b-3p | Literature based | 261 | UAGCACCAUUUGAAAUCAGUGUU | [16] |
| hsa-miR-155-3p | Literature based | 68 | CUCCUACAUAUUAGCAUUAACA | [17] |
| hsa-miR-212-5p | Literature based | 159 | ACCUUGGCUCUAGACUGCUUACU | [18] |
| hsa-miR-143-3p | Literature based | 228 | UGAGAUGAAGCACUGUAGCUC | [19] |
| hsa-miR-192-5p | Literature based | 994 | CUGACCUAUGAAUUGACAGCC | [20] |
| hsa-miR-122-5p | Literature based | 610 | UGGAGUGUGACAAUGGUGUUUG | [21] |
| hsa-miR-146a-5p | Literature based | 202 | UGAGAACUGAAUUCCAUGGGUU | [22] |
| hsa-miR-24-3p | Literature based | 855 | UGGCUCAGUUCAGCAGGAACAG | [23] |
| hsa-miR-23a-3p | Literature based | 249 | AUCACAUUGCCAGGGAUUUCC | [24] |
| hsa-miR-145-5p | Literature based | 238 | GUCCAGUUUUCCCAGGAAUCCCU | [25] |
| hsa-miR-222-3p | Literature based | 394 | AGCUACAUCUGGCUACUGGGU | [26, 27] |
| hsa-miR-4476 | Microarray based | 199 | CAGGAAGGAUUUAGGGACAGGC | |
| hsa-miR-345-3p | Microarray based | 81 | GCCCUGAACGAGGGGUCUGGAG | |
| hsa-miR-371a-5p | Microarray based | 345 | ACUCAAACUGUGGGGGCACU |
Abbreviation: miRNA, microRNA.
Construction of miRNA-target gene network
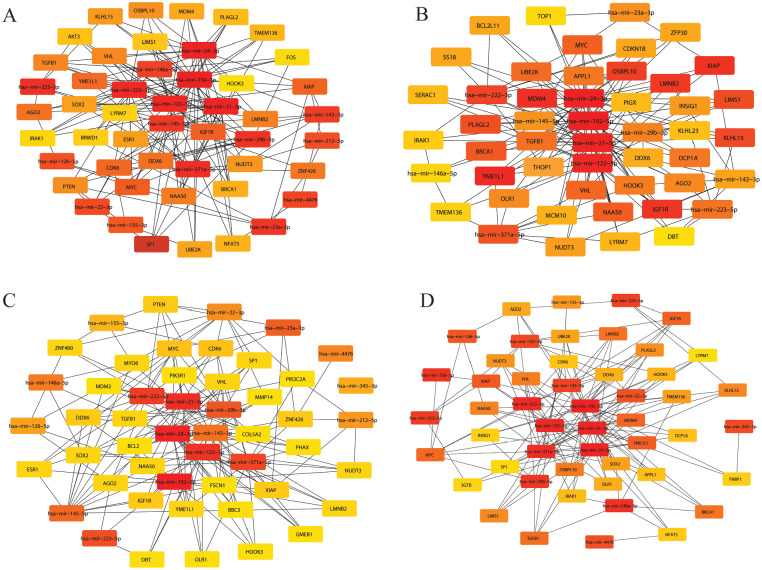
The list of 15 miRNAs was submitted to miRNet tool for construction of miRNAs-mRNA network. Results showed that the network contains 2800 nodes and 2900 edges. In network analysis, red color diamond represents the miRNAs and blue color circles are their target genes (Figure 3). Corresponding target genes for each miRNAs are presented in Table 1.
Figure 3.
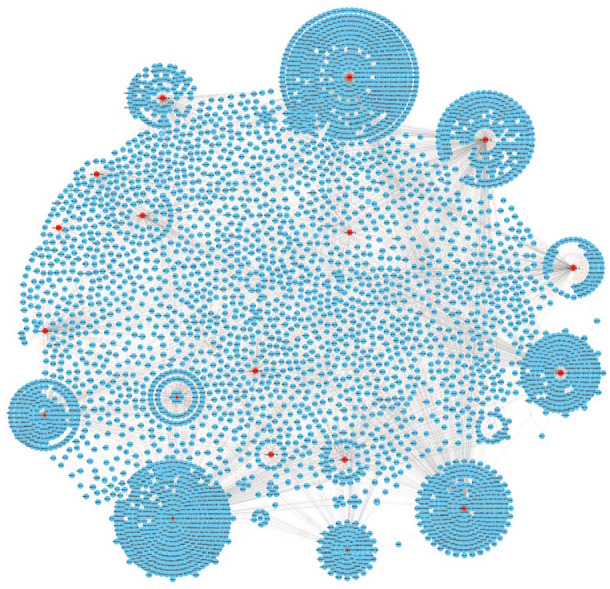
The miRNA-target gene interaction network containing 4540 nodes and 6060 edges. The red diamond (18 miRNAs) indicates our key miRNAs, and the blue circles are the interacting partners. miRNA indicates microRNA.
Module selection and pathways analysis
The cytoHubba was used to identify the valued modules and top-ranked nodes in the whole network presented in Figure 4. Module detection process was carried out based on Degree centrality, Closeness centrality, Betweenness centrality, and Stress centrality. Top scoring 50 nodes from network were extracted. After comparing the modules using Venny tools, we found 24 overlapped nodes, namely, hsa-mir-122-5p, hsa-mir-222-3p, hsa-mir-21-5p, hsa-mir-146a-5p, hsa-mir-29b-3p, hsa-mir-24-3p, hsa-mir-143-3p, hsa-mir-145-5p, hsa-mir-223-5p, hsa-mir-371a-5p, hsa-mir-192-5p, hsa-mir-23a-3p, VHL, TGFB1, XIAP, LMNB2, NAA50, NUDT3, YME1L1, IGF1R, DDX6, AGO2, MYC, and HOOK3. Of 18 miRNAs, 12 miRNAs overlapped in 4 clustering methods along with their 12 target genes.
Figure 4.
Significant modules extracted from the main network based on (A) Betweenness centrality, which includes 50 nodes and 154 edges; (B) Closeness centrality which includes 50 nodes and 149 edges; (C) Degree centrality which includes 50 nodes and 156 edges; and (D) Stress centrality which includes 50 nodes and 146 edges. Strong red to light yellow color in modules indicates the rank from top to bottom.
Discussion
The miRNAs are involved in multiple biological processes, including cellular differentiation and proliferation, cell metabolism, hemostasis, apoptosis and inflammation, and in pathophysiology of many other diseases. Numerous studies have reflected miRNAs as promising diagnostic and prognostic biomarkers of many diseases. The serum miR-122-5p can be used as a novel, noninvasive prognostic biomarker in renal cell carcinomas patients. 20 The miR-222 was first discovered in human umbilical vein endothelial cells (HUVECs) and plays an important role to maintain many cardiac physiological functions. Deregulation of miR-222 has been associated with several cardiovascular diseases. 21 Thus, targeting miR-222 may emerge as a significant therapeutic option for cardiovascular diseases. One of the most abundant types of miRNAs in the human tissues is miR-21. It is associated with different biological functions. It helps to protect the kidney in response to inflammation and stress. Recently, a study showed that up-regulation of miR-21 protects the kidney from delayed ischemic preconditioning effects (IPCs). 22 The miR-21 has been reported upregulated in kidney tissues of patients of CKD and kidney disease animal models. 23 The hsa-miR-155-5p and hsa-miR-146a-5p are positively related to proteinuria and inversely related to glomerular filtration rate (GFR). It was proposed that in IgA nephropathy patients, hsa-miR-155-5p and hsa-miR-146a-5p regulate the expression of pro-inflammatory molecules. 24
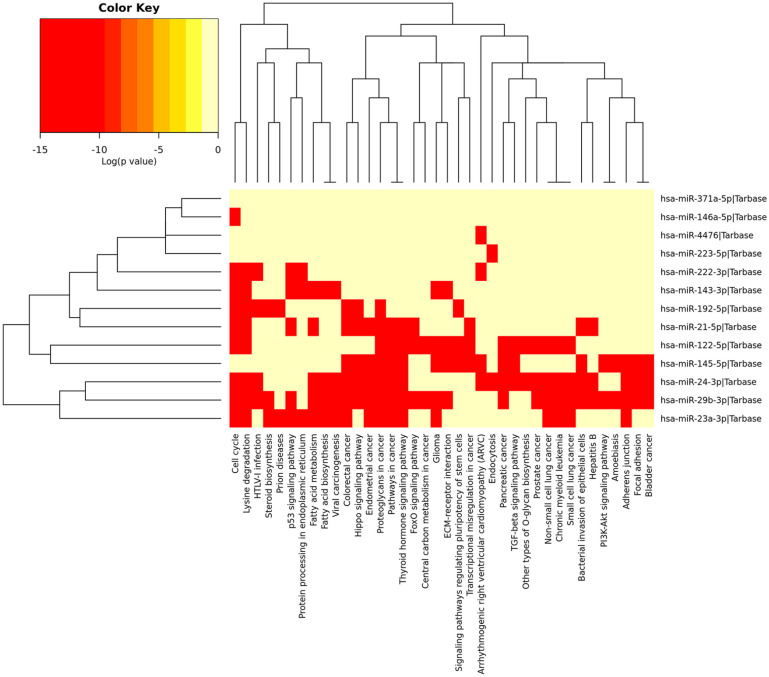
In cardiovascular disease, arterial calcification is considered as notable pathological risk factor connected with miR-29b-3p which targets matrix metalloproteinase-2. Clinical evidences are reflecting that reduction in the miR-29b-3p expression is associated with fibrosis in kidney, skin, liver, heart, and lung, whereas an increase in the expression of miR-29b-3p helps to prevent tissue fibrosis. The hsa-miR-24-3p is recognized as a master regulator for smooth muscle cell multiplication. 25 Proteome profiling revealed that hsa-miR-24-3p regulates the heme oxygenase-1 that in turn affects the activity of different cellular stress–related factors. 26 The ex vivo and in vitro models have shown that hsa-miR-24-3p inhibits vasculature development in engineered heart tissues. 27 The miR-143 is categorized as a cardiovascular-specific miRNA due to its significant expression in aorta and heart. The miR-143-3p regulates the expression of ACE2 gene involved in blood pressure regulation. 28 The hsa-miR-145-5p is involved in the regulation and development of vascular smooth muscle cells (VSMCs). 29 It has been observed that the VSMCs deficient in hsa-miR-143-5p and hsa-miR-145-5p are unable to respond to vasocontractile stimuli irrespective of increased synthetic activity. 30 In carcinoma with below par differentiation, the miR-4476 was found to be upregulated. The study carried by Ulbing et al 31 found decreased level of miR-223in patients with CKD. However, the level of miR-223 increased after renal graft. It was found that epinephrine can induce miR 371a-5p, which results in improved BAG3 protein expression. 32 The miR-192-5p found in kidney is one of most common types of miRNAs targeting mRNA for β1 subunit of Na+/K+-ATPase (ATP1B1). 33 The miR-23a helps in cardiomyocyte apoptosis regulation which is a vital pathogenesis factor in heart failure. It regulates cardiomyocyte apoptosis by targeting the vasculogenesis and manganese superoxide dismutase (MnSOD) of CAD through epidermal growth factor (EGF) receptor. 34 The extracellular miR-23a can probably act as a novel biomarker for CAD. An increase in miR-23a level can help to predict presence and intensity of coronary lesions in CAD patients. The DIANA mirPathv3 database study represented that most of the overlapped miRNAs were enriched in the top 10 pathways (Table 2). The pathway enrichment showed that most of the overlapped miRNAs were involved in the p53 signaling pathway, thyroid hormone signaling pathway, hippo signaling pathway, and pathways of multiple cancers (Figure 5).
Table 2.
Immersion of the key miRNAs in different pathways based on the minimum count of 5.
| Sr. no. | Pathways | P value | Count | miRNAs |
|---|---|---|---|---|
| 1 | Proteoglycans in cancer (hsa05205) | 1e-325 | 7 | hsa-miR-21-5p, hsa-miR-122-5p, hsa-miR-29b-3p, hsa-miR-24-3p, hsa-miR-145-5p, hsa-miR-192-5p, hsa-miR-23a-3p |
| 2 | Top of Form Top of Form Fatty acid metabolism (hsa01212) |
5.08E-13 | 5 | hsa-miR-29b-3p, hsa-miR-24-3p, hsa-miR-143-3p, hsa-miR-23a-3p, hsa-miR-21-5p |
| 3 | Lysine degradation (hsa00310) | 7.81E-13 | 8 | hsa-miR-21-5p, hsa-miR-222-3p, hsa-miR-122-5p, hsa-miR-29b-3p, hsa-miR-24-3p, hsa-miR-143-3p, hsa-miR-192-5p, hsa-miR-23a-3p |
| 4 | Pathways in cancer (hsa05200) | 1.21E-11 | 6 | hsa-miR-122-5p, hsa-miR-29b-3p, hsa-miR-24-3p, hsa-miR-145-5p, hsa-miR-23a-3p, hsa-miR-21-5p |
| 5 | Colorectal cancer (hsa05210) | 5.03E-10 | 6 | hsa-miR-29b-3p, hsa-miR-24-3p, hsa-miR-145-5p, hsa-miR-192-5p, hsa-miR-23a-3p, hsa-miR-21-5p |
| 6 | Cell cycle (hsa04110) | 2.84E-08 | 9 | hsa-miR-222-3p, hsa-miR-146a-5p, hsa-miR-122-5p, hsa-miR-29b-3p, hsa-miR-24-3p, hsa-miR-143-3p, hsa-miR-192-5p, hsa-miR-23a-3p, hsa-miR-21-5p |
| 7 | Hippo signaling pathway (hsa04390) | 1.07E-06 | 5 | hsa-miR-29b-3p, hsa-miR-24-3p, hsa-miR-145-5p, hsa-miR-192-5p, hsa-miR-21-5p |
| 8 | Thyroid hormone signaling pathway (hsa04919) | 0.000687 | 6 | hsa-miR-122-5p, hsa-miR-29b-3p, hsa-miR-24-3p, hsa-miR-145-5p, hsa-miR-23a-3p, hsa-miR-21-5p |
| 9 | p53 signaling pathway (hsa04115) | 2.74E-06 | 5 | hsa-miR-222-3p, hsa-miR-29b-3p, hsa-miR-143-3p, hsa-miR-23a-3p, hsa-miR-21-5p |
| 10 | Glioma (hsa05214) | 0.000134 | 5 | hsa-miR-29b-3p, hsa-miR-143-3p, hsa-miR-145-5p, hsa-miR-23a-3p, hsa-miR-122-5p |
Abbreviation: miRNA, microRNA.
Figure 5.
Heatmap of overlapped miRNAs reflecting their role in different enriched functional pathways. Many miRNAs such as hsa-miR-24-3p and hsa-miR-29b-3p were found involved in the progression of different types of cancers. miRNA indicates microRNA.
Conclusions
As reflected by various studies, gene/mRNA may be targeted by multiple miRNAs to contribute in many cellular functions. MiRNA can affect the different biological mechanisms through several pathways by interacting with diverse mRNAs. The mirPath-v3 database study revealed that most of the overlapped miRNAs were enriched in the p53/thyroid hormone/hippo signaling pathway and in multiple cancers. The analysis of 4 clustering methods of the most significant modules showed that the miRNA/mRNAs in these modules were mainly associated with cancer. Results showed that many pathways were regulated by hsa-mir-21-5p. This study identified 24 overlapped nodes, namely, hsa-mir-122-5p, hsa-mir-222-3p, hsa-mir-21-5p, hsa-mir-146a-5p, hsa-mir-29b-3p, hsa-mir-24-3p, hsa-mir-143-3p, hsa-mir-145-5p, hsa-mir-223-5p, hsa-mir-371a-5p, hsa-mir-192-5p, hsa-mir-23a-3p, VHL, TGFB1, XIAP, LMNB2, NAA50, NUDT3, YME1L1, IGF1R, DDX6, AGO2, MYC, and HOOK3. We suggest that the identified miRNA and target genes may have pathological relevance in CRS and may emerge as potential diagnostic biomarkers. Findings of the current study provide an important view that can be considered in CRS diagnosis and cure.
Footnotes
Author Contributions: MMA, ST, and RI conceptualized the work; MMA and AA did data curation; MMA, RA, and ST prepared the figures; MMA, RA, ST, AA, AF, NI, NT, SA, MZM, AS, and RI wrote the manuscript; and all the authors read and approved the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MMA, ST, AF, and NI were financially supported by Indian Council of Medical Research, Government of India under Senior Research Fellow (FTS No. 525507). MZM and AA were financially supported by Department of Health and Research, Ministry of Health and Family Welfare, Government of India under young scientist (FTS No. 3146887). NT was financially supported by Council of Scientific & Industrial Research (CSIR) under Junior Research Fellow.
ORCID iDs: Romana Ishrat  https://orcid.org/0000-0001-9744-9047
https://orcid.org/0000-0001-9744-9047
Aftab Alam  https://orcid.org/0000-0002-6989-9398
https://orcid.org/0000-0002-6989-9398
Armiya Sultan  https://orcid.org/0000-0001-5373-8824
https://orcid.org/0000-0001-5373-8824
References
- 1. Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139:e840-e878. [DOI] [PubMed] [Google Scholar]
- 2. McCullough PA. Cardiorenal syndromes. World J Cardiol. 2011;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Lullo L, Bellasi A, Barbera V, et al. Pathophysiology of the cardio-renal syndromes types 1–5: an uptodate. Indian Heart J. 2017;69:255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ardekani AM, Naeini MM. The role of microRNAs in human diseases. Avicenna J Med Biotechnol. 2010;2:161-179. [PMC free article] [PubMed] [Google Scholar]
- 5. O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dai C, Zhang Y, Xu Z, Jin M. MicroRNA-122-5p inhibits cell proliferation, migration and invasion by targeting CCNG1 in pancreatic ductal adenocarcinoma. Cancer Cell Int. 2020;20:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LaPierre MP, Stoffel M. MicroRNAs as stress regulators in pancreatic beta cells and diabetes. Mol Metab. 2017;6:1010-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, Kowdley KV. MicroRNAs in common human diseases. Geno Proteomic Bioinform. 2012;10:246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mukhadi S, Hull R, Mbita Z, Dlamini Z. The role of MicroRNAs in kidney disease. Non-Coding RNA. 2015;1:192-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. House AA, Haapio M, Lassus J, Bellomo R, Ronco C. Pharmacological management of cardiorenal syndromes. Int J Nephrol. 2011;2011:630809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Liang Y, Zhao W, et al. Circulating miRNA-21 as a diagnostic biomarker in elderly patients with type 2 cardiorenal syndrome. Sci Rep. 2020;10:4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C-K, Bär C, Thum T. miR-21, mediator, and potential therapeutic target in the cardiorenal syndrome. Front Pharmacol. 2020;11:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pogribny IP. MicroRNAs as biomarkers for clinical studies. Exp Biol Med (Maywood). 2018;243:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clough E, Barrett T. The gene expression omnibus database. In: Mathé E, Davis S. (eds) Statistical genomics, vol. 1418. New York, NY: Springer; 2016:93-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wan G, Ji L, Xia W, Cheng L, Zhang Y. Bioinformatics identification of potential candidate blood indicators for doxorubicin‑induced heart failure. Exp Ther Med. 2018;16:2534-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan Y, Siklenka K, Arora SK, Ribeiro P, Kimmins S, Xia J. miRNet—dissecting miRNA-target interactions and functional associations through network-based visual analysis. Nucleic Acids Res. 2016;44:W135-W141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. You G, Zu B, Wang B, Fu Q, Li F. Identification of miRNA–mRNA–TFs regulatory network and crucial pathways involved in tetralogy of fallot. Front Genet. 2020;11:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chin C-H, Chen S-H, Wu H-H, Ho C-W, Ko M-T, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vlachos IS, Zagganas K, Paraskevopoulou MD, et al. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43:W460-W466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heinemann FG, Tolkach Y, Deng M, et al. Serum miR-122-5p and miR-206 expression: non-invasive prognostic biomarkers for renal cell carcinoma. Clin Epigenetics. 2018;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ding S, Huang H, Xu Y, Zhu H, Zhong C. MiR-222 in cardiovascular diseases: physiology and pathology. Biomed Res Int. 2017;2017:4962426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu X, Kriegel AJ, Liu Y, et al. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int. 2012;82:1167-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun IO, Lerman LO. Urinary microRNA in kidney disease: utility and roles. Am J Physiol-Ren Physiol. 2019;316:F785-F793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang G, Kwan Lai FM, Chow KM, Li PK, Szeto CC. Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis Markers. 2011;30:171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin X, Zhan JK, Wang YJ, et al. Function, role, and clinical application of MicroRNAs in vascular aging. Biomed Res Int. 2016;2016:6021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci. 2006;103:18255-18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mellis D, Caporali A. MicroRNA-based therapeutics in cardiovascular disease: screening and delivery to the target. Biochem Soc Trans. 2018;46:11-21. [DOI] [PubMed] [Google Scholar]
- 28. Chen L-J, Xu R, Yu H-M, Chang Q, Zhong JC. The ACE2/apelin signaling, microRNAs, and hypertension. Int J Hypertens. 2015;2015:896861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li L, Mao D, Li C, Li M. miR-145-5p Inhibits vascular smooth muscle cells (VSMCs) proliferation and migration by dysregulating the transforming growth factor-b signaling cascade. Med Sci Monit. 2018;24:4894-4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boettger T, Beetz N, Kostin S, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ulbing M, Kirsch AH, Leber B, et al. MicroRNAs 223-3p and 93-5p in patients with chronic kidney disease before and after renal transplantation. Bone. 2017;95:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mizushima W, Sadoshima J. BAG3 plays a central role in proteostasis in the heart. J Clin Invest. 2017;127:2900-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baker MA, Wang F, Liu Y, et al. MiR-192-5p in the kidney protects against the development of hypertension. Hypertension. 2019;73:399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Long B, Gan T-Y, Zhang R-C, Zhang YH. miR-23a regulates cardiomyocyte apoptosis by targeting manganese superoxide dismutase. Mol Cells. 2017;40:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]