Abstract
Dentists prescribe a large portion of all oral antibiotics, and these are associated with a risk of adverse drug reactions (ADRs). The aim of this study was to quantify the risk of ADRs associated with oral antibiotics commonly prescribed by dentists. NHS Digital Prescribing data and Yellow Card Drug Analysis data for 2010 to 2017 were abstracted to quantify dental antibiotic prescribing in England, and the rate and types of ADRs associated with them. During the period of study, the mean number of actively practicing dentists in England was 23,624. Amoxicillin accounted for 64.8% of dental antibiotic prescribing and had the lowest reported rate of fatal ADRs (0.1/million prescriptions) and overall ADRs (21.5/million prescriptions). Indeed, amoxicillin was respectively 6 and 3 times less likely to cause an ADR than the other penicillins, penicillin V and amoxicillin + clavulanic acid, and appears to be very safe in patients with no history of penicillin allergy. In contrast, clindamycin, which is often used in patients with penicillin allergy, had the highest rate of fatal (2.9/million prescriptions) and overall (337.3/million prescriptions) ADRs, with Clostridiodes (formerly Clostridium) difficile infections pivotal to its ADR profile. Other amoxicillin alternatives, clarithromycin and metronidazole, while significantly worse than amoxicillin, were 3 and nearly 5 times less likely to cause an ADR than clindamycin. Ranked from least to most likely to cause an ADR, antibiotics most commonly prescribed were as follows: amoxicillin < cephalosporins < erythromycin < tetracyclines < azithromycin < metronidazole < amoxicillin + clavulanic acid < clarithromycin < penicillin V < clindamycin. This study confirmed the high level of safety associated with use of amoxicillin by dentists and the significantly worse rates of fatal and nonfatal ADRs associated with other penicillins and alternatives to amoxicillin for those who are penicillin allergic. In particular, clindamycin had the highest rate of fatal and nonfatal ADRs of any of the antibiotics commonly prescribed by dentists.
Keywords: dentistry, adverse drug reaction, allergy, Clostridiodes difficile, Clostridium difficile, infection
Introduction
Dental prescribing accounts for approximately 10% of all antibiotic prescriptions dispensed in primary care (Hicks et al. 2015; Durkin et al. 2017). Although antibiotics are invaluable therapeutic and prophylactic agents, like any medication, they are associated with a risk of adverse drug reactions (ADRs). It is important, therefore, that dentists are aware of the potential risks associated with the antibiotics that they prescribe. The aim of this study was to evaluate the antibiotic-prescribing practices of dentists in England over the period of 2010 to 2017 and to quantify the ADR risk associated with the antibiotics that they commonly prescribe.
Methods
Prescription Cost Analysis data held by NHS Digital (https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis) were abstracted to detect all oral antibiotics prescribed in primary care by general medical practitioners, nurse practitioners, and other health care providers, including dentists, in England from 2010 to 2017. In addition, we were able to separately extract data on all oral antibiotics prescribed by general dental practitioners in England over the same period. Hospital and private prescribing of antibiotics was not included. Private dental prescribing in the United Kingdom is uncommon and is primarily for those ineligible for NHS prescriptions (e.g., foreign nationals).
National Yellow Card Interactive Drug Analysis Profile data from the Medicines and Healthcare Products Regulatory Agency (MHRA; http://yellowcard.mhra.gov.uk/iDAP/) were interrogated for those antibiotics most frequently prescribed by dentists in primary care and data extracted for all prescriptions of oral antibiotics or for prescriptions where the route of administration was not stated but reactions to drugs administered parenterally, topically, or by other routes were excluded. The ADR rate/million prescriptions issued in primary care was calculated for those antibiotics commonly prescribed by dentists over the period of study. The Yellow Card system has been in place for >50 years and allows patients and health care professionals to report ADRs to the MHRA, either online or by postage-free mail-in. Although ADRs are subdivided into those that are nonserious, serious, and fatal, the distinction between serious and nonserious is left for the person reporting to determine. All ADR reports are categorized with the Medical Dictionary for Regulatory Activities (MedDRA; International Council for Harmonisation 2019). The MedDRA hierarchy of terms has 5 levels. There are >70,000 individual descriptive “lowest-level terms,” and these are matched to terms used in the ADR report (e.g., feeling queasy). These are grouped into “preferred terms” (e.g., nausea) and then through 3 further levels of hierarchy: “high-level terms” (e.g., nausea and vomiting symptoms), “high-level group terms” (e.g., gastrointestinal signs and symptoms), and finally “system organ class” (e.g., gastrointestinal disorders). There are 27 “system organ class” categories. However, no or very few antibiotic ADRs are reported for approximately one-half of the “system organ class” categories. To avoid reporting negative “system organ class” data, we excluded any categories that did not appear among the top 5 reported for at least 1 of the antibiotics being studied. This reduced the number of categories to 14. ADRs that fell under any other “system organ class” categories were grouped under a 15th category: “other.” Even though data were reported by “system organ class,” we were able to delve deeper into the hierarchical structure where necessary (down to “preferred term” level) to gain better insight into the precise cause of ADRs.
Because of the small numbers of prescriptions for individual cephalosporin or tetracycline preparations, all cephalosporin class or tetracycline class prescriptions were grouped. For the purposes of this investigation, the azalide azithromycin was included under the antibiotic class of macrolides, which included erythromycin and clarithromycin. Aminoglycosides were excluded from this investigation, since they accounted for only 0.002% of overall oral antibiotic prescribing and zero dental prescribing. Fosfomycin was not included due to limited use, and linezolid and tedizolid were not included since these agents were prescribed only in secondary care. Fluoroquinolone data were included in the overall analysis of oral antibiotic use, although there was no dental prescribing of fluoroquinolones. Further details regarding oral fluoroquinolone use were published elsewhere (Baddour et al. 2019). Oral antibiotics (isoniazid, rifampin, ethambutol, para-aminosalicylic acid, pyrazinamide, clofazimine, bedaquiline, rifabutin, rifapentine, dapsone, ethionamide, cycloserine, prothionamide, and delamanid) used primarily to treat mycobacterial infections, including tuberculosis, were excluded.
Because all data reported herein were obtained from national data resources and completely anonymized, ethics approval was not required.
Results
Dental Antibiotic-Prescribing Trends
Between 2010 and 2017, there were 28,825,698 oral antibiotic prescriptions issued by dentists in England, and the mean number of actively practicing dentists was 23,624. On average, this amounted to 3,603,212 prescriptions per annum (67 prescriptions per 1,000 of the English population). Amoxicillin was the most frequently prescribed (18,667,126 prescriptions) and accounted for 64.8% of all dental oral antibiotic prescriptions. Metronidazole was the next-most frequently prescribed antibiotic (8,082,568), accounting for 28.0% of all dental antibiotic prescribing. These were followed by erythromycin (1,255,878, 4.4% of dental antibiotics), penicillin V (phenoxymethylpenicillin; 270,782, 0.9%), clindamycin (167,426, 0.6%), amoxicillin + clavulanic acid (co-amoxiclave) (132,134, 0.5%), cephalosporins (121,234, 0.4%), tetracyclines (81,983, 0.3%), clarithromycin (33,519, 0.1%), and azithromycin (12,503, <0.1%).
Incidence of ADRs
The ADR rates were slightly lower for oral antibiotics prescribed by dentists than for those prescribed in primary care overall. This was true for all ADRs (50.9 vs. 57.9/million prescriptions), fatal reactions (0.5 vs. 0.7/million prescriptions), and severe reactions (30.5 vs. 36.8/million prescriptions) (Table 1). However, the differences were not clinically significant.
Table 1.
Adverse Drug Reaction Data: 2010 to 2017.
| Adverse Drug Reactions/Million Prescriptions | ||||
|---|---|---|---|---|
| Drug Name | Nonserious | Serious | Fatal | Total |
| Amoxicillin | 9.4 | 11.9 | 0.1 | 21.5 |
| Amoxicillin + clavulanic acid | 20.2 | 49.5 | 1.5 | 71.2 |
| Penicillin V | 75.0 | 61.7 | 0.4 | 137.0 |
| Cephalosporins | 9.0 | 17.9 | 0.5 | 27.4 |
| Tetracyclines | 16.8 | 32.6 | 0.9 | 50.2 |
| Azithromycin | 12.1 | 45.8 | 0.8 | 58.7 |
| Clarithromycin | 31.2 | 65.5 | 1.3 | 98.0 |
| Erythromycin | 19.8 | 26.7 | 0.7 | 47.2 |
| Clindamycin | 101.2 | 233.2 | 2.9 | 337.3 |
| Metronidazole | 18.5 | 51.4 | 0.7 | 70.6 |
| All antibiotics prescriptions by dentists | 19.9 | 30.5 | 0.5 | 50.9 |
| All antibiotics | 20.4 | 36.8 | 0.7 | 57.9 |
Among oral antibiotics commonly prescribed by dentists, clindamycin had the highest fatal (2.9/million prescriptions), serious (233.2/million prescriptions), and overall (337.3/million prescriptions) ADR rates. This was more than twice that of any other commonly used dental antibiotic (Table 1, Fig.) and >15 times higher than amoxicillin, the most widely used antibiotic, which had the lowest fatal (0.1/million prescriptions), serious (11.9/million prescriptions), and overall (21.5/million prescriptions) ADR rates.
Figure.
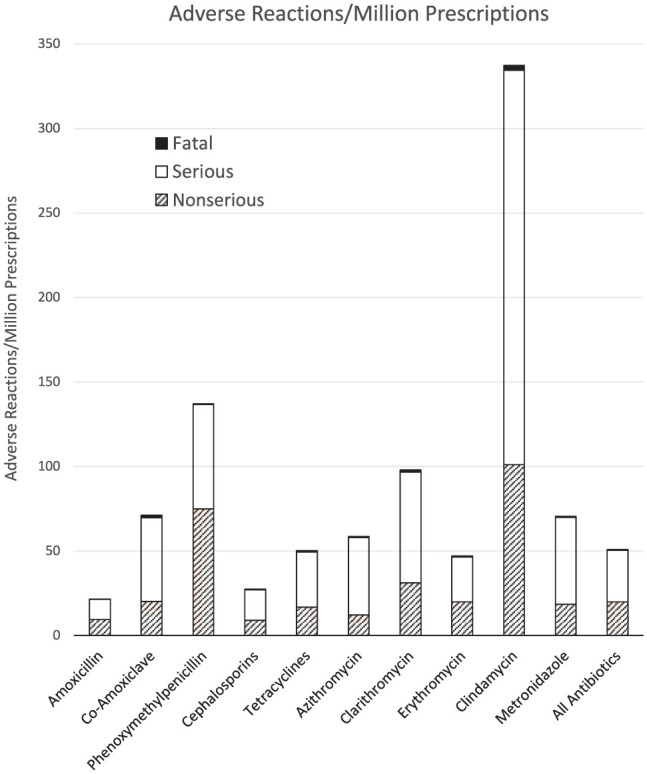
The number of adverse drug reactions per million prescriptions of each oral antibiotic type.
Of the penicillins, penicillin V had an overall (137.0/million prescriptions), serious (61.7/million prescriptions), and fatal (0.4/million prescriptions) ADR rate more than 4 times that of amoxicillin. Amoxicillin + clavulanic acid had an overall (71.2/million prescriptions) and serious (51.4/million prescriptions) ADR rate >3 times that of amoxicillin. Perhaps most concerning, however, amoxicillin + clavulanic acid had the highest fatal ADR rate (1.5/million prescriptions) among the penicillins, which was second only to clindamycin.
Cephalosporins and tetracyclines had relatively low overall ADR rates. Indeed, the cephalosporin overall ADR rates were similar to those of amoxicillin, and the tetracycline overall ADR rates were close to the average for all antibiotics (Table 1, Fig.). Among the macrolide antibiotics (erythromycin, azithromycin, and clarithromycin), erythromycin had the lowest overall ADR rate; azithromycin overall ADR rates were only marginally higher, while overall ADR rates for clarithromycin were almost twice those for erythromycin (overall, 98.0/million prescriptions; serious, 65.5/million prescriptions; fatal, 1.3/million prescriptions); clarithromycin had the third-highest fatal ADR rate among those oral antibiotics commonly prescribed by dentists.
Metronidazole was the second-most commonly prescribed antibiotic by dentists in England and was associated with overall (70.6/million prescriptions), serious (51.4/million prescriptions), and fatal (0.7/million prescriptions) ADR rates that were greater than the average and >3 times that of amoxicillin but nearly a fifth of those of clindamycin.
Types of ADR
Clindamycin had the highest fatal ADR rate (2.9/million prescriptions). All fatal reactions were related to infection or gastrointestinal problems, mostly related to Clostridiodes difficile infection (Table 2). These also accounted for a significant proportion of all clindamycin-related ADRs. Skin reactions, however, accounted for a majority of nonfatal clindamycin ADRs, and of them, the majority were allergic rashes or pruritus.
Table 2.
Adverse Drug Reactions/Million Prescriptions between 2010 and 2017 by Organ System Affected.
| Adverse Drug Reactions/Million Prescriptions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| System | Amox | Amox + Clav | Pen V | Ceph | Tetra | Azith | Clarith | Eryth | Clinda | Metro |
| Cardiac | 0.25 | 2.39 | 0.26 | 0.56 | 1.07 | 6.3 | 7.64 | 2.55 | 4.4 | 1.98 |
| Eye | 0.65 | 1.84 | 1.8 | 4.47 | 4.52 | 4.28 | 3.39 | 2.05 | 2.93 | 8.12 |
| Gastrointestinal | 5.85 | 32.43 | 25.28 | 12.39 | 26.91 | 26.7 | 52.99 | 37.22 | 155.46 | 42.91 |
| General and administration site | 4.48 | 17.25 | 18.33 | 7.83 | 13.39 | 21.91 | 33.11 | 13.69 | 68.93 | 31.19 |
| Hepatobiliary | 0.64 | 15.36 | 0.31 | 0.75 | 2.26 | 2.52 | 2.82 | 1.18 | 10.27 | 1.98 |
| Immune system | 2.64 | 5.94 | 28.62 | 3.17 | 1.59 | 1.76 | 3.97 | 5.1 | 20.53 | 3.74 |
| Infections and infestations | 1.51 | 6.85 | 2.78 | 3.63 | 3.45 | 18.39 | 6.78 | 1.87 | 33.73 | 4.66 |
| Injury, poisoning, and procedural complications | 0.56 | 2.94 | 0.72 | 3.26 | 1.89 | 3.02 | 5.4 | 1.37 | 14.67 | 4.16 |
| Investigations | 1.41 | 11.38 | 0.98 | 2.14 | 3.54 | 11.84 | 12.82 | 3.24 | 26.4 | 6.21 |
| Nervous system | 2.1 | 11.14 | 5.82 | 6.8 | 12.54 | 16.88 | 42.59 | 6.1 | 44 | 49.4 |
| Psychiatric | 1.49 | 5.69 | 2.47 | 3.35 | 6.5 | 7.56 | 35.58 | 1.68 | 17.6 | 20.89 |
| Renal and urinary | 0.45 | 2.88 | 0.82 | 1.03 | 1.34 | 1.51 | 5.17 | 1.18 | 1.47 | 6.56 |
| Respiratory, thoracic, and mediastinal | 1.74 | 6 | 9.88 | 2.7 | 4.79 | 7.81 | 12.24 | 4.05 | 19.07 | 8.47 |
| Skin and subcutaneous | 16.42 | 33.9 | 93.44 | 14.91 | 19.53 | 11.59 | 30.46 | 19.23 | 217.06 | 27.74 |
| Other | 2.44 | 12.83 | 5 | 6.16 | 12.4 | 18.89 | 27.66 | 9.42 | 64.52 | 26.11 |
| Total | 42.63 | 168.82 | 196.51 | 73.15 | 115.72 | 160.96 | 282.62 | 109.93 | 701.04 | 244.12 |
| Fatal Adverse Drug Reactions/Million Prescriptions | ||||||||||
| Cardiac | 0.01 | 0.18 | 0.00 | 0.00 | 0.09 | 0.50 | 0.40 | 0.25 | 0.00 | 0.00 |
| Eye | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Gastrointestinal | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.06 | 0.00 | 1.47 | 0.00 |
| General and administration site | 0.04 | 0.37 | 0.15 | 0.09 | 0.15 | 0.25 | 0.23 | 0.00 | 0.00 | 0.21 |
| Hepatobiliary | 0.03 | 0.18 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.06 | 0.00 | 0.35 |
| Immune system | 0.00 | 0.12 | 0.15 | 0.09 | 0.00 | 0.00 | 0.06 | 0.12 | 0.00 | 0.07 |
| Infections and infestations | 0.03 | 0.18 | 0.05 | 0.09 | 0.09 | 0.00 | 0.06 | 0.12 | 1.47 | 0.07 |
| Injury, poisoning, and procedural complications | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 |
| Investigations | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 |
| Nervous system | 0.00 | 0.06 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Psychiatric | 0.00 | 0.06 | 0.00 | 0.00 | 0.12 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 |
| Renal and urinary | 0.01 | 0.06 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Respiratory, thoracic, and mediastinal | 0.00 | 0.12 | 0.00 | 0.00 | 0.03 | 0.00 | 0.06 | 0.06 | 0.00 | 0.00 |
| Skin and subcutaneous | 0.02 | 0.06 | 0.00 | 0.00 | 0.03 | 0.00 | 0.11 | 0.06 | 0.00 | 0.00 |
| Other | 0.00 | 0.13 | 0.00 | 0.02 | 0.03 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 |
| Total | 0.14 | 1.53 | 0.36 | 0.47 | 0.82 | 0.76 | 1.26 | 0.68 | 2.93 | 0.71 |
Individuals may have adverse reactions affecting >1 organ system at any 1 time. Hence, total number of organ systems affected (total reactions or total fatal reactions) for each antibiotic may exceed the total number of reactions reported in Table 1.
Amox, amoxicillin; Amox + Clav, amoxicillin + clavulanic acid; Azith, azithromycin; Ceph, cephalosporins; Clarith, clarithromycin; Clinda, clindamycin; Eryth, erythromycin; Metro, metronidazole; Pen V, penicillin V; Tetra, tetracyclines.
Gastrointestinal disturbances accounted for a significant proportion of the ADRs recorded for all antibiotics examined, although clindamycin had the highest rate of gastrointestinal disturbance reactions reported, followed by clarithromycin, metronidazole, and erythromycin. The most frequent gastrointestinal disturbances reported for all antibiotics were nausea and diarrhea.
Skin reactions, mainly allergic in nature, were also a feature of all antibiotics studied. The rate of skin reactions was considerably higher for clindamycin than for any other antibiotic, although none of these were fatal. Penicillin V had the next-highest rate of skin reactions, followed by amoxicillin + clavulanic acid, clarithromycin, and metronidazole. Fatal skin reactions, however, were seen only with amoxicillin, amoxicillin + clavulanic acid, tetracyclines, erythromycin, and clarithromycin.
Immune system ADRs were reported most frequently with penicillin V. These were all due to allergic reactions, and more than one-fifth were reported as anaphylactic or anaphylactoid reactions. These included 0.15 deaths/million prescriptions. Immune system ADRs also occurred with amoxicillin and amoxicillin + clavulanic acid but to a lesser degree than with penicillin V. All of these reactions were allergic in nature, and a significant proportion were anaphylactic or anaphylactoid. However, no fatal anaphylactic or anaphylactoid reactions were recorded with oral amoxicillin and only 0.12 fatal reactions/million prescriptions with amoxicillin + clavulanic acid. Cephalosporins and erythromycin were also associated with a considerable number of allergic reactions, including a small number of fatal anaphylactic reactions.
Nervous system ADRs accounted for a significant proportion of ADRs seen with metronidazole, clindamycin, and the macrolide antibiotics clarithromycin and azithromycin. Nervous system ADRs were not a significant feature of the penicillins. With metronidazole, nervous system ADRs presented mainly as headaches, dizziness, altered taste, or paraesthesia. Clindamycin was associated with headaches and neurologic disorders, particularly altered taste. For macrolides, nervous system ADRs presented mainly as dizziness, altered taste, somnolence, or paraesthesia.
Psychiatric disorder ADRs were also a feature with clarithromycin and metronidazole but were not seen with penicillins. For clarithromycin and metronidazole, psychiatric disorder ADRs largely presented as anxiety disorders, confusion, hallucinations, or sleep disturbance. For metronidazole, some psychiatric ADRs also presented as depressive mood disorders.
Amoxicillin + clavulanic acid was the only antibiotic associated with hepatobiliary ADRs, which presented almost entirely with features of cholestatic jaundice, a rare but well-recognized reaction.
Cardiac arrhythmia ADRs were the most common cause of death for the macrolides erythromycin and clarithromycin and accounted for two-thirds of deaths associated with azithromycin. Macrolides are known to prolong the QT interval, which can predispose to the development of torsades de pointes (Hancox et al. 2013).
Discussion
Although all antibiotics are associated with ADRs, our study quantifies which antibiotics are safer for patients. Penicillins, the antibiotic class most commonly prescribed by dentists, are well known for their capacity to induce hypersensitivity reactions, usually manifesting as skin rashes, which can include serious and fatal skin reactions, such as Stevens-Johnson syndrome or toxic epidermal necrolysis, and more rarely anaphylaxis. In the current investigation, skin and immune reactions accounted for a considerable proportion of ADRs reported for penicillins. However, gastrointestinal disturbances, which were less frequently reported by health care providers, were also prominent. Nevertheless, fatal, serious, and overall ADR rates for amoxicillin were lower than for any other antibiotic. Indeed, amoxicillin appeared to be the safest, particularly for those with no history of penicillin allergy. It is important to point out that during the period of the study (2010 to 2017), national guidance recommended against all use of antibiotic prophylaxis in patients at risk of infective endocarditis (IE) in the United Kingdom (National Institute for Health and Care Excellence 2008). Unlike in the United States, there has never been guidance recommending antibiotic prophylaxis for those with prosthetic joints or organ transplants in the United Kingdom. Nor is antibiotic prophylaxis recommended for those at risk of medication- or radiotherapy-related osteonecrosis of the jaw or for patients with underlying medical conditions, such as diabetes mellitus or immunosuppression (https://bnf.nice.org.uk/guidance/prescribing-in-dental-practice.html). Thus, it is likely that the majority of antibiotic prescribing identified in this study was for treating dental infections. Nonetheless, in an earlier study, using data from a period when antibiotic prophylaxis was still recommended to prevent IE, we demonstrated that a single 3-g dose of amoxicillin was also safe and had an extremely low incidence of ADRs (Thornhill et al. 2015). The results of the current investigation suggest that amoxicillin is a safe antibiotic for both treatment and prophylaxis and may be safer than penicillin V and amoxicillin + clavulanic acid. Perhaps not surprising, the risk of a skin or immune reaction was roughly twice as high with amoxicillin + clavulanic acid than with amoxicillin alone. Penicillin V harbored a risk of skin or immune reaction that was respectively 6 and 11 times higher than that of amoxicillin. The reason for this is unclear but may relate to the subtly different chemical structures of amoxicillin and penicillin V.
Although skin and immune reactions are most commonly associated with penicillins, it is noteworthy that nonpenicillin-based antibiotics also can cause skin and immune reactions. This is exemplified by macrolide antibiotics (erythromycin, clarithromycin, and azithromycin), metronidazole, and clindamycin. Indeed, for clindamycin, the immune system ADR rate was second only to penicillin V and the rate of skin and subcutaneous reactions more than twice that of any other antibiotic. These results serve as a reminder that metronidazole and clindamycin are not necessarily a safer alternative to penicillin.
Ideally, although often difficult to achieve in primary care dental practice, amoxicillin should not be replaced with alternatives without confirmatory evidence of penicillin allergy (Shenoy et al. 2019). Ninety percent of patients who report a history of penicillin allergy are not confirmed to be penicillin allergic by skin testing combined with an oral amoxicillin challenge (Shenoy et al. 2019). If further evaluation is not done for those reporting penicillin sensitivity, then this could result in exposure to antibiotics with potentially worse ADR profiles, in particular clindamycin, an increased risk of causing antibiotic resistance, suboptimal antibiotic therapy, and higher health care costs (Macy and Contreras 2014; Drug and Therapeutics Bulletin 2017; Har and Solensky 2017; Trubiano et al. 2017; Centers for Disease Control and Prevention 2019; Shenoy et al. 2019). Better screening of patients with self-reported penicillin allergy could significantly reduce the number of individuals unnecessarily denied penicillins and improve antimicrobial stewardship and patient safety (Macy et al. 2009; Macy and Ngor 2013; Macy 2014; Macy and Contreras 2014; National Institute for Health and Care Excellence 2014; Gonzalez-Estrada and Radojicic 2015; Drug and Therapeutics Bulletin 2017; Har and Solensky 2017; Trubiano et al. 2017; Centers for Disease Control and Prevention 2019; Shenoy et al. 2019).
Gastrointestinal disturbance, not unexpectedly, occurs frequently with oral antibiotics, but disturbances differ considerably in severity, from mild nausea to severe C. difficile infection. The more severe C. difficile infection cases, which can be complicated by mortality, were reported in either the “gastrointestinal” or “infections and infestations” organ systems categories. In both categories, amoxicillin had the lowest rate of ADRs of all oral antibiotics used by dentists, including other penicillins.
Historically, the macrolides, particularly erythromycin, have been characterized by gastrointestinal disturbance. However, our study revealed that the newer macrolides, which have superior pharmacokinetic properties, had higher ADR rates within the Yellow Card database. Specifically, clarithromycin was more likely than erythromycin to cause gastrointestinal upset; azithromycin also had one of the highest rates of ADR. It is possible, however, that this reflects a greater likelihood for clinicians to report ADRs associated with newer drugs (see Limitations section).
Clindamycin was clearly the worst of the oral antibiotics prescribed by dentists for risk of ADRs related to the “gastrointestinal” (155.5/million prescriptions) or “infections” (343.7/million prescriptions) category, including fatal ADRs (1.5/million prescriptions each). Although clindamycin was likely prescribed to treat dental infections, the data are consistent with the results of an earlier investigation that examined the risk of ADRs following a single oral dose of clindamycin for dental IE prophylaxis. This investigation also demonstrated a much higher risk of gastrointestinal- and infection-related ADRs, including fatal ADRs, with a single 600-mg oral dose of clindamycin, than with other antibiotics used for IE prophylaxis (Thornhill et al. 2015). Our findings are also consistent with the results of a systematic review of the risk of C. difficile infection following the use of different antibiotics in the community setting: the risk of C. difficile infection was highest with clindamycin, followed by macrolides; penicillins and tetracyclines had little to no risk (Brown et al. 2013). Given that clindamycin is recommended as an alternative to amoxicillin for antibiotic prophylaxis globally, this underscores the importance of performing penicillin skin testing and, if negative, subsequent oral amoxicillin challenge to confirm that amoxicillin can be used as antibiotic prophylaxis with avoidance of clindamycin.
Metronidazole was the second-most frequently prescribed antibiotic by dentists in the United Kingdom. Moreover, dental prescribing accounted for 57% of all metronidazole prescribing in primary care. In England, metronidazole has been the primary alternative to amoxicillin for treating dental infections in those who are penicillin allergic and accounts for 28% of all dental oral antibiotic prescribing. In contrast, clindamycin accounted for only 0.6% of prescribing. Metronidazole has also been the preferred alternative to amoxicillin in many parts of Europe (Palmer et al. 2000; Al-Haroni and Skaug 2007), Africa (Fadare et al. 2017), the Middle East (Dar-Odeh et al. 2008), the Indian subcontinent (Tanwir et al. 2015; Konde et al. 2016), and Australasia (Ford et al. 2017; Teoh et al. 2018). In the United States, however, metronidazole has accounted for <0.9% of dental antibiotic prescribing, and clindamycin is regarded as the primary alternative to amoxicillin, accounting for 15% of dental antibiotic prescribing (Durkin et al. 2017).
There are scant published data regarding the risk and types of ADRs associated with oral metronidazole other than the well-known “Antabuse” reaction that can occur in patients who ingest alcohol while taking metronidazole. Metronidazole was the third-most likely oral antibiotic to cause an ADR. Of these, gastrointestinal ADRs, including nausea and vomiting, were common. However, nervous system ADRs, including dizziness, altered taste, and somnolence, were more frequent than with any other dental antibiotic. Psychiatric disturbance, including confusion, depression, altered perception, and sleep disturbance, were also more common than with any other antibiotic except clarithromycin. Skin reactions were not uncommon.
The newer macrolides, azithromycin and clarithromycin, generally had a higher frequency of ADRs than erythromycin. In particular, they were associated with higher rates of nervous system ADRs, including altered taste, dizziness, and somnolence, and psychiatric ADRs, including anxiety symptoms, sleep disturbance, confusion, and altered perception. Azithromycin and clarithromycin also had a higher rate of cardiac ADRs, mainly rhythm disturbances, than any other antibiotics prescribed by dentists, and a significant proportion of these (10% to 20%) were associated with a fatal outcome.
Limitations
This study has several limitations. The Yellow Card reporting scheme is dependent on clinicians and patients reporting ADRs. This is likely to result in underreporting, particularly of nonserious ADRs. It may also result in higher reporting rates for newer drugs and underreporting for older drugs, where the ADR profile is well recognized and associated mechanisms responsible for ADRs better understood. Reporting rates are not, therefore, the same as incidence rates and are likely to underestimate the true incidence. A further limitation is that categorization of ADR severity is not defined and is decided by the individual reporting an ADR. Although there should be no error in the reporting of fatal ADRs, the distinction between serious and nonserious ADRs is arbitrary. The MedDRA (International Council for Harmonisation 2019) is helpful in categorizing the diverse terminology used by individuals reporting ADRs into the different organ systems affected. However, given the 5 terminological hierarchies in the MedDRA, encompassing >70,000 individual descriptors, it is impossible to express the vast array of data available with the full depth of detail contained within the Interactive Drug Analysis Profile data included for each antibiotic by the MHRA.
An advantage of the data evaluated in this study is that they were abstracted from a large data set that represented all available ADR data for oral antibiotics prescribed in primary care in England during the period of 2010 to 2017.
Conclusions
The current investigation demonstrated that amoxicillin, the most commonly prescribed antibiotic in dental practice, is remarkably safe, particularly in those with no history of penicillin allergy. Other penicillins, including amoxicillin + clavulanic acid and penicillin V, had significantly worse ADR profiles than amoxicillin. Of the antibiotics commonly used as alternatives to penicillins, particularly in those with a history of penicillin allergy, clindamycin has the worst ADR profile of any of the oral antibiotics prescribed by dentists. Overall, clindamycin was 15 times more likely to cause an ADR than amoxicillin and nearly 30 times more likely to cause a fatal ADR. This underscores the importance of assessing patients with a purported history of penicillin allergy with penicillin skin testing and amoxicillin oral challenge. Clarithromycin and metronidazole had the next-worst ADR profiles among the penicillin alternatives but were respectively still 3 times and nearly 5 times less likely than clindamycin to cause an ADR. Ranked from least to most likely to cause an ADR, antibiotics most commonly prescribed by dentists were as follows: amoxicillin < cephalosporins < erythromycin < tetracyclines < azithromycin < metronidazole < amoxicillin + clavulanic acid < clarithromycin < penicillin V < clindamycin.
Author Contributions
M.H. Thornhill, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; M.J. Dayer, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; M.J. Durkin, P.B. Lockhart, contributed to data interpretation, critically revised the manuscript; L.M. Baddour, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: M.H. Thornhill  https://orcid.org/0000-0003-0681-4083
https://orcid.org/0000-0003-0681-4083
M.J. Durkin  https://orcid.org/0000-0003-4652-7089
https://orcid.org/0000-0003-4652-7089
References
- Al-Haroni M, Skaug N. 2007. Incidence of antibiotic prescribing in dental practice in Norway and its contribution to national consumption. J Antimicrob Chemother. 59(6):1161–1166. [DOI] [PubMed] [Google Scholar]
- Baddour LM, Dayer MJ, Thornhill MH. 2019. Fluoroquinolone use and associated adverse drug events in England. J Infect. 78(3):251–253. [DOI] [PubMed] [Google Scholar]
- Brown KA, Khanafer N, Daneman N, Fisman DN. 2013. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 57(5):2326–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2019. Is it really a penicillin allergy? [accessed 2019 Mar 5]. https://www.cdc.gov/antibiotic-use/community/pdfs/penicillin-factsheet.pdf.
- Dar-Odeh NS, Abu-Hammad OA, Khraisat AS, El Maaytah MA, Shehabi A. 2008. An analysis of therapeutic, adult antibiotic prescriptions issued by dental practitioners in Jordan. Chemotherapy. 54(1):17–22. [DOI] [PubMed] [Google Scholar]
- Drug and Therapeutics Bulletin. 2017. Penicillin allergy-getting the label right. BMJ. 358:j3402. [DOI] [PubMed] [Google Scholar]
- Durkin MJ, Hsueh K, Sallah YH, Feng Q, Jafarzadeh SR, Munshi KD, Lockhart PB, Thornhill MH, Henderson RR, Fraser VJ, et al. 2017. An evaluation of dental antibiotic prescribing practices in the United States. J Am Dent Assoc. 148(12):878–886.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadare JO, Oshikoya KA, Obimakinde OS, Sijuade AO, Afolayan JM, Adeleke AA, Godman B, Ojumu DO. 2017. Patterns of drugs prescribed for dental outpatients in Nigeria: findings and implications. Acta Odontol Scand. 75(7):496–506. [DOI] [PubMed] [Google Scholar]
- Ford PJ, Saladine C, Zhang K, Hollingworth SA. 2017. Prescribing patterns of dental practitioners in Australia from 2001 to 2012. Antimicrobials. Aust Dent J. 62(1):52–57. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Estrada A, Radojicic C. 2015. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 82(5):295–300. [DOI] [PubMed] [Google Scholar]
- Hancox JC, Hasnain M, Vieweg WV, Crouse EL, Baranchuk A. 2013. Azithromycin, cardiovascular risks, QTc interval prolongation, torsade de pointes, and regulatory issues: a narrative review based on the study of case reports. Ther Adv Infect Dis. 1(5):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Har D, Solensky R. 2017. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 37(4):643–662. [DOI] [PubMed] [Google Scholar]
- Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH, Jr, Schrag SJ. 2015. Us outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 60(9):1308–1316. [DOI] [PubMed] [Google Scholar]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. 2019. Medical dictionary for regulatory activities; [accessed 2019 Feb 24]. https://www.meddra.org.
- Konde S, Jairam LS, Peethambar P, Noojady SR, Kumar NC. 2016. Antibiotic overusage and resistance: a cross-sectional survey among pediatric dentists. J Indian Soc Pedod Prev Dent. 34(2):145–151. [DOI] [PubMed] [Google Scholar]
- Macy E. 2014. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep. 14(11):476. [DOI] [PubMed] [Google Scholar]
- Macy E, Contreras R. 2014. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 133(3):790–796. [DOI] [PubMed] [Google Scholar]
- Macy E, Ngor EW. 2013. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 1(3):258–263. [DOI] [PubMed] [Google Scholar]
- Macy E, Schatz M, Lin C, Poon KY. 2009. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 13(2):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. 2008. Clinical guideline [cg64]. Prophylaxis against infective endocarditis. http://www.nice.org.uk/guidance/cg64. [PubMed]
- National Institute for Health and Care Excellence. 2014. Clinical guideline [cg183]. Drug allergy: diagnosis and management; [accessed 2019 Mar 5]. https://www.nice.org.uk/guidance/CG183. [PubMed] [Google Scholar]
- Palmer NO, Martin MV, Pealing R, Ireland RS. 2000. An analysis of antibiotic prescriptions from general dental practitioners in England. J Antimicrob Chemother. 46(6):1033–1035. [DOI] [PubMed] [Google Scholar]
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. 2019. Evaluation and management of penicillin allergy: a review. JAMA. 321(2):188–199. [DOI] [PubMed] [Google Scholar]
- Tanwir F, Marrone G, Tariq A, Lundborg CS. 2015. Diagnosis and prescribing pattern of antibiotics and painkillers among dentists. Oral Health Prev Dent. 13(1):75–83. [DOI] [PubMed] [Google Scholar]
- Teoh L, Stewart K, Marino RJ, McCullough MJ. 2018. Current prescribing trends of antibiotics by dentists in Australia from 2013 to 2016. Part 1. Aust Dent J. 63(3):329–337. [DOI] [PubMed] [Google Scholar]
- Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. 2015. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother. 70(8):2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trubiano JA, Adkinson NF, Phillips EJ. 2017. Penicillin allergy is not necessarily forever. JAMA. 318(1):82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


