Abstract
Macrophage plays a critical part in host defense, tissue repair, and anti-inflammation; Macrophage reprogramming is responsible for disease development or regression. We aimed to clarify the effect of sinomenine-4-hydroxy-palmitate (C16), on macrophage reprogramming and anti-inflammatory in endotoxemia model. According to a structure modification of SIN (Sinomenine), C16 was found. Then, based on the endotoxin model, the mice liver and kidney toxicity was evaluated and serum cytokines level of IL-6 (Interleukin-6), TNF-α (Tumor necrosis factor-α), and IL-1β (Interleukin-1β) were measured by ELISA (Enzyme linked immunosorbent assay). Then, we confirmed the effect of C16 on macrophages reprogramming, we used the flow cytometry to test the effect of C16 on macrophages apoptosis in vitro. Then, iNOS (Inducible nitric oxide synthase), M1-type related cytokines, such as IL-1β, TNF-α, and M2-type related cytokines, such as Arg-1 (Arginase-1), CD206, Fizz1, and Ym1 was detected, which expressed in ANA-1 and primary peritoneal macrophages. To further explore the molecular mechanism of C16 in reprogramming of macrophages from M1 toward M2 phenotype, the expression of STAT1 (signal transducer and activator of Transcription 1), STAT3, ERK1/2 (extracellular signal regulated kinase1/2), AKT, p38, and its corresponding phosphorylation were determined by western blot. Our results demonstrated that C16 improved the survival rate of LPS- (lipopolysaccharide) challenged mice and decreased the inflammatory cytokines expression; After C16 treatment, the expression of M1 phenotype correlation factors decreased significantly, while the expression of M2 phenotype correlation factors increased significantly at different levels compared with normal group. It indicated that C16 reprogram macrophages phenotype from M1 toward M2 following LPS stimulus. Furthermore, the results also showed that C16 showed anti-inflammatory effect by inhibiting LPS-induced p38, AKT and STAT1 phosphorylation and contributing ERK1/2 activation. C16 promoted macrophage reprogramming toward M2-like phenotype via p-p38/p-AKT or STAT1 signals pathway and C16 might be a valid candidate for inflammatory disease.
Keywords: anti-inflammatory, endotoxemia, macrophage reprogram, signaling pathway, sinomenine-4-hydroxy-palmitate
Introduction
Macrophages play a critical role in maintenance the tissue homeostasis, especially, anti-microbial infection, and participate in the innate and adaptive immunity of inflammatory diseases. 1 Furthermore, on the one hand macrophages involved in the amplification of inflammation during injury; on the other hand macrophages down-regulate the inflammatory response to avoid excess tissue damage through macrophages reprogramming following their predominating pro-or anti-inflammatory microenvironment, especially cytokines. 2 Two major macrophages subpopulation switch different functions which represent extreme of a continuum in a universe of activation states, including classically activated/inflammatory (M1) and alternatively activated/regenerative (M2) macrophages, have long been recognized. 3 Macrophages may switch among these phenotypes in response to some signals in their dynamic local microenvironment, 4 namely “reprogramming”; which permits adaptation to a myriadly likely variations in dysfunctional immune response or damaged tissue, including acute or chronic inflammatory.
Endotoxemia is a serious medical disease, which is characterized by an inappropriate systemic inflammatory response due to a harmful or destructive host response to infection, leading to multiple organ failure, and death. 5 It remains the main reason of death in hospital although early active antibiotic treatments to control bacterial infection. 6 It is necessary to understand the dysregulation of the host response in endotoxemia, which will provide a new opportunity for therapeutic intervention. 7
Sinomenine-4-hydroxy-palmitate (C16), is a novel derivative of sinomenine (SIN) 8 ; which is a natural alkaloid derived from Chinese medicinal plant Sinomenium acutum. C16 was designed and synthesized in our lab, and their bioactivities were evaluated using endotoxemia mice. Our results clearly demonstrated that C16 obviously increased the survival rate of endotoxemia mice and decreased the inflammatory cytokines expression; which was dependent on macrophage reprogramming toward M2-like phenotype from a proinflammatory M1-like phenotype; Furthermore, C16 promoted macrophage reprogramming toward M2-like phenotype via p-p38/p-AKT signals pathway.
Materials and methods
Cells and mice
Male BALB/c mice (6–8 weeks old, 18–22 g) were obtained from the animal center of Yangzhou University and raised in the Animal Center of Jiangsu University according to the guidelines for the care and use of experimental animals (NIH Publication No. 85-23, revised 1996). Housing details include the type of facility was specific pathogen free; non-toxic plastic mouse box and stainless steel wire cage cover were used in the experiment and the water fountain was made of plastic bottles with metal drinking pipes attached to the stopper; the bedding material was wood shavings or sawdust from broadleaf trees; the cage companions were divided into four groups with five mice in each group. Husbandry conditions include the light/dark cycle was12/12 h; mice were fed a whole nutrient pellet diet containing a certain proportion of crude fiber. All mice were placed at room temperature of about 23°C ± 2°C, and kept the temperature constant; they were provided with sufficient food and water, several days before modeling, they could be fed with food such as melon seeds and eggs to enhance their immunity. The experimental schemes were authorized by the Committee for Ethical Affairs of the Jiangsu University (Zhenjiang, China) and the methods were implemented according to the approved guidelines. Peritoneal macrophages were isolated from BALB/c mice. ANA-1 macrophages cell line were purchased from American Type Culture Collection (Rockville, MD). Both of them were cultured with RPMI (Roswell Park Memorial Institute)-1640 containing 10% FBS (Fetal bovine serum) (Gibco, Life Technologies).
Endotoxemia model
Referring to the methods in the literature9,10 and the previous experimental results in our group, the model of inducing endotoxin blood will be induced, and appropriate improvements will be made according to the actual situation. BALB/c mice were divided into four groups (n = 5): Control, lipopolysaccharide (LPS), LPS + C16, and LPS + SIN. According to the reports in the literature11,12 and the previous work of the research group, the model of endotoxemia in vivo was induced by injecting 10 mg/kg LPS into the abdominal cavity of mice according to the body weight of mice. The state of mice was observed and the model was successful. In order to compare the anti-inflammatory effect of sinomenine before and after modification, high (5 mg/kg) and low (2.5 mg/kg) dose groups of sinomenine and sinomenine 4-hydroxypalmitate were designed to observe the vital signs and activities of mice within 24 h. There was no significant change in activity during the first 4 h after LPS treatment. The mice in the LPS group gradually died after about 12 h. The results showed that the effect of C16 was stronger than that of SIN, and the anti-inflammatory effect of high-dose group was stronger than that of low-dose group. All the mice were administered 5 mg/kg sinomenine (SIN) (Sigma-Aldrich, St. Louis, MO), 5 mg/kg C16, or PBS by i.p. injection before LPS challenge. And then endotoxemia was induced by i.p. injection of 0.2 mL of normal saline (NS) containing 10 mg/kg LPS (Sigma-Aldrich, St. Louis, MO). NC group was injected the same volume of PBS. Survival rate was monitored for 24 h.
Quantitative RT-PCR (RT-qPCR)
iNOS, Arg-1, IL-1β, IL-6, CD206, Fizz1 expression levels were measured by RT-qPCR. Total RNA was isolated from ANA-1 macrophages or peritoneal macrophages or tissues using Trizol regent (Invitrogen Life Technologies, USA) and reverse transcribed to cDNA using Moloney murine leukemia virus reverse transcribed system (Invitrogen Life Technologies, USA). Real-time PCR was carried out using iQ SYBR Green Supermix (Bio-Rad, USA) and a 7500 Real-Time PCR System (Applied Biosystem USA) with GAPDH or 18 s as internal control. The amplification conditions was 1 cycle of 95°C for 2 min followed by 40 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s. The primers used were showed in Table 1.
Table 1.
The primers used in present work.
| Genes | Sequence (5′–3′) | Size (bp) |
|---|---|---|
| iNOS | Fw: AACTTGTTTGCAGGCGTCAG | 127 |
| Rv: CACATTGCTCAGGGGATGGA | ||
| Arg-1 | Fw: ACATTGGCTTGCGAGACGTA | 109 |
| Rv: ATCACCTTGCCAATCCCCAG | ||
| IL-1β | Fw: CAAATCTCGCAGCAGCACAT | 261 |
| Rv: ACGAGGCTTTTTTGTTGTTCAT | ||
| IL-6 | Fw: GGCCTTCCCTACTTCACAAG | 126 |
| Rv: ATTTCCACGATTTCCCAGAG | ||
| GAPDH | Fw: GGCATTGCTCTCAATGACAA | 200 |
| Rv: TGTGAGGGAGATGCTCAGTC |
Apoptosis assay and cytokine assays
ANA-1/peritoneal macrophages were pretreated with 5 µmol/L C16, 5 µmol/L SIN or the same volume of DMSO (Dimethyl sulfoxide) for 24 h, respectively and then added 500 ng/mL LPS for another 12 h. The treated cells were gathered for apoptosis assay. Briefly, the cells were washed twice with cold PBS buffer and then resuspended in 1 × Annexin binding buffer. The 100 µL solution were transferred to a 5 mL culture tube. Five microliter of Annexin V conjugated to FITC and 1 µL PI (100 mg/mL) for 15 min at RT (37°C). Then 400 µL of 1 × Annexin binding buffer were added to each tube. The cells were evaluated by flow cytometry. Supernatant was collected for cytokine assays. IL-1β and TNF-a cytokines were detected by ELISA kits (LianKe Bio.Co., Ltd, Nanjing) according to the manufacture’s protocols; OD value was measured at 450 nm with a microplate analyzer (Labsystems Dragon, Finland). NO was detected using Griess Reagent System (Promega, USA).
Western blot
The specimens were collected, loading buffer was added, ultrasonic cracking was performed, centrifuged at 12,000 g at 4°C for 15 min, and the supernatant was taken and stored in a boiling water bath for 10 min at −20°C. Protein isolated from ANA-1 cell line was electrophoresed by 12% SDS-PADE gels before being transferred to PVDF transfer membranes for 90 min (PerkinElmer, USA). Membranes were blocked with 5% (w/v) non-fat dry milk/TBST for 1 h at room temperature and incubated for 24 h with primary antibodies of Arg-1 (1:200, Rabbit polyclonal, YT0311), p-AKT (1:500, Rabbit polyclonal, YP0006), p-ERK1/2 (1:1000, Mouse monoclonal, YM1464), total ERK1/2 (1:1000, Mouse monoclonal, YM3677), STAT1(Rabbit polyclonal, YT4439), p-STAT1 (Rabbit polyclonal, YP0249), STAT3 (Rabbit polyclonal, YT4443) and p- STAT3(1:100, Rabbit polyclonal, YP0250) were obtained from Immonoway; iNOS (1:1000, Rabbit monoclonal, ab178945), GAPDH (1:5000, Mouse monoclonal, ab8245) was purchased from Abcam (Abcam, Shanghai, China), followed by horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. Detection was performed with electrochemiluminesce (ECL) and relevant blots quantified by densitometry using the accompanying computerized image analysis program (Amercontrol Biosciences, USA).
Statistical analysis
All statistical analysis was performed by Graphpad Prism 5 software. Data were expressed as the mean ± standard deviation (SD) of thee independent experiments. Comparisons between groups were performed using the paired t-test or one-way ANOVA analysis. p value of <0.05 was considered statistically significant.
Results
C16 more effectively protected mice from endotoxemia
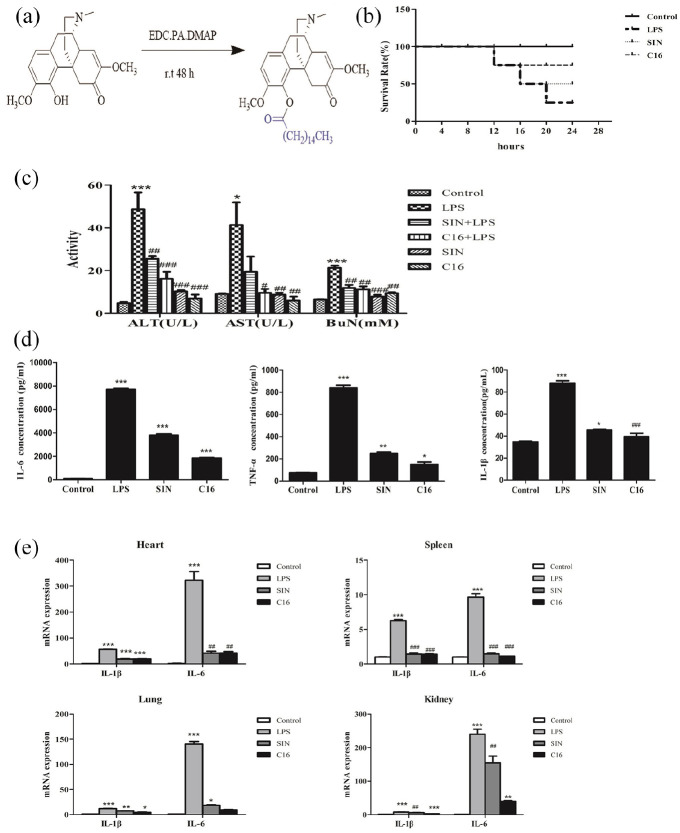
In our lab, based on the principle of prodrug combination, the structure of sinomenine A ring was modified, With DMAP as catalyst and EDC as shrinkage agent and four hydroxyl substituents were substituted to connect with palmitic acid, a novel derivative of SIN, C16 (Figure 1a) was found. Some solids were selected for mass spectrum and hydrogen spectrum identification, and the structure of the compound was confirmed. And C16 more effectively alleviated the LPS-induced mortality in mice comparing with the same concentration of SIN (Figure 1b). Additionally, the ALT, AST, and BuN in SIN and C16 pre-treated groups were obviously decreased (24.48 ± 2.11 U/L, 19.32 ± 3.21 U/L, and 13.13 ± 1.87 mM in SIN group; 17.42 ± 2.35 U/L, 9.43 ± 2.27 U/L, and 12.41 ± 1.32 mM in C16 group) comparing with the LPS group (48.48 ± 5.21 U/L, 42.92 ± 6.81 U/L, and 21.97 ± 1.57 mM) (p < 0.01) (Figure 1c). And the activities of ALT and AST in C16 treated group was more effectively inhibited comparing with SIN treated group. Furthermore, C16 dramatically decreased the level of IL-1β, IL-6, and TNF-α in the serum (39.54 ± 3.01 pg/mL, 1850.00 ± 70.71 pg/mL, 150.23 ± 22.26 pg/mL, respectively) comparing with LPS group (88.76 ± 12.13 pg/mL, 7865.00 ± 154.32 pg/mL, 873.32 ± 43.26 pg/mL, respectively) (p < 0.01). Comparing with SIN group (45.32 ± 3.33 pg/mL, 3875.00 ± 97.23 pg/mL, 243.27 ± 24.36 pg/mL, respectively), C16 was more effectively down-regulation the level of IL-1β, IL-6, and TNF-α (p < 0.05) (Figure 1d). Additionally, RT-qPCR data of IL-1β and IL-6 in heart, spleen, kidney, and lung were furthering confirmed above conclusion (Figure 1e). Histopathological assays didn’t show any obvious changes (the data was not shown).
Figure 1.
C16 more effectively protected mice from endotoxemia. (a) Synthesis of 4-palmitoyl-sinomenine. C16/SIN were intraperitoneally administered 1 h before LPS (10 mg/kg) stimulation. (b) Survival ratio within 24 h was observed and calculated n = 5. Serum and organ tissues were collected. (c) ALT, AST activity, and BuN content were measured by kits. (d) Serum cytokines level of IL-6, TNF-α, and IL-1β were detected by ELISA. (e) Total RNA were extracted from heart, spleen, lung and kidney tissues and the inflammatory cytokines expression in mRNA level were assessed by RT-qPCR.
*P < 0.05. **P < 0.01. ***P < 0.001 versus control group.
#P < 0.05. ##P < 0.01. ###P < 0.001 versus LPS group.
C16 reprogram macrophages phenotype from M1 toward M2 following LPS stimulus
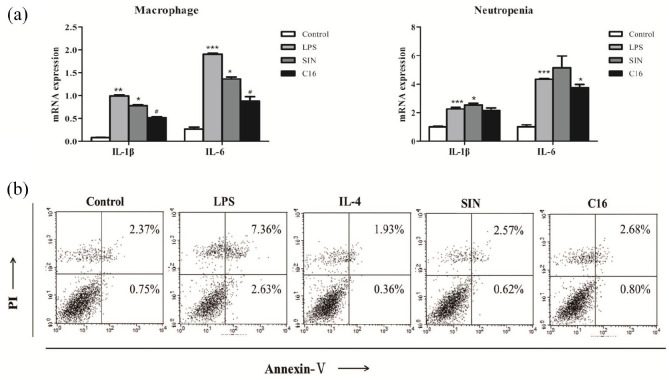
As Figure 2a showed that C16 group, IL-1 and IL-6 expression of macrophages not neutrophils following LPS stimulus. Furthermore, C16 could ameliorate LPS induced macrophages apoptosis (3.48 ± 1.87% vs 9.99 ± 2.52%, p < 0.05, Figure 2b); which indicated that C16 preferentially modulated macrophages and independent on inducing macrophages apoptosis.
Figure 2.
C16 preferentially modulated macrophages and independent on inducing macrophages apoptosis cells were incubated with 5 uM C16/SIN for 24 h, and then 500 ng/mL LPS challenged for another 6 h. Neutropils were separated from mice. (a) The mRNA levels of IL-1β and IL-6 were assayed by RT-qPCR. (b) Cells were stained with Annexin V/PI and measured by flow cytometry.
*P < 0.05. **P < 0.01. ***P < 0.001 versus control group.
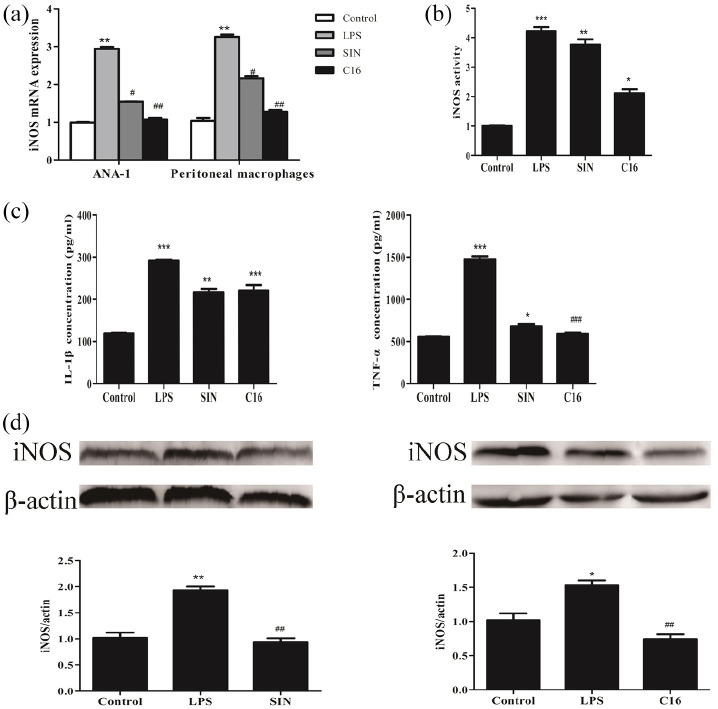
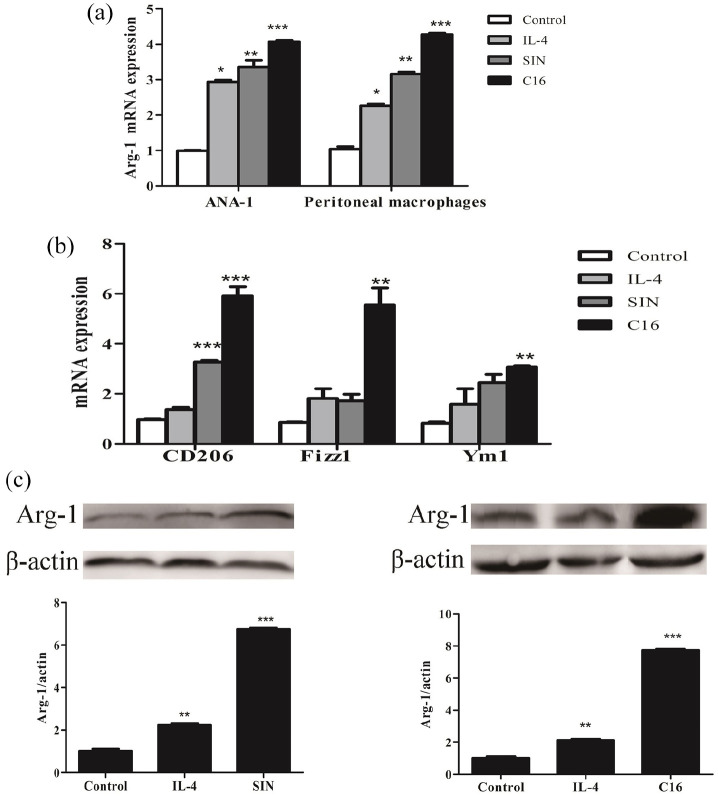
Then the C16 could protect mice from endotoxemia via promoting macrophages reprogramming. Therefore, macrophages were dealt with 500 ng/mL LPS. As shown in Figure 3, C16 can significantly decrease mRNA and protein levels of iNOS; inhibit iNOS activity; down-regulate cytokine IL-1β and TNF-α in the supernatant on peritoneal macrophages/ANA-1 macrophages treated by LPS. Conversely, C16 could furthering enhance mRNA expression of Fizz1, Ym1, CD206 and Arg-1 as well as Arg-1 protein in macrophages co-cultured with IL-4 (Figure 4). Which indicated that C16 reprogram macrophages phenotype from M1 toward M2 following LPS stimulus.
Figure 3.
Reprogram macrophages phenotype from M1 toward M2 following LPS stimulus ANA-1/peritoneal macrophages were incubated with C16/SIN or the same volume of DMSO for 24 h and then 500 ng/mL LPS challenged for another 6 or 12 h. Cells and supernatnat were collected, respectively. (a) Total RNA were isolated, iNOS expression was assayed by RT-qPCR. (b and c) iNOS activity, IL-1β and TNF-α cytokines in supernatnat were meadured by kits.(d) The expression of iNOS (131 KD) in protein level were measured by western blot.
*P < 0.05. **P < 0.01. ***P < 0.001 versus control group.
#P < 0.05. ##P < 0.01. ###P < 0.001 versus control group.
Figure 4.
C16 could furthering enhance mRNA expression of Fizz1, Ym1, CD206, and Arg-1 as well as Arg-1 protein in macrophages. (a and b) The level of Arg-1, CD206, Fizz1, and Ym1 relative expression were detected by RT-qPCR. (c) Arg-1 (35 KD) protein expression was performed by western blot.
*P < 0.05. **P < 0.01. ***P < 0.001 versus control group.
C16 promoted macrophages reprogramming from M1 toward M2 dependent on P38/AKT or STAT1 signals following LPS stimulus
In order to further explore the molecular mechanism of C16 on macrophages reprogramming from M1 toward M2 phenotype, As Figure 5a shown. C16 significantly inhibited LPS-induced p38, AKT and STAT1 phosphorylation. However, STAT3 phosphorylation didn’t have obviously changes. Conversely, C16 contributed ERK1/2 activation; which indicated that P38/AKT or STAT1 might be involved in C16 promoted macrophages reprogramming from M1 toward M2.
Figure 5.
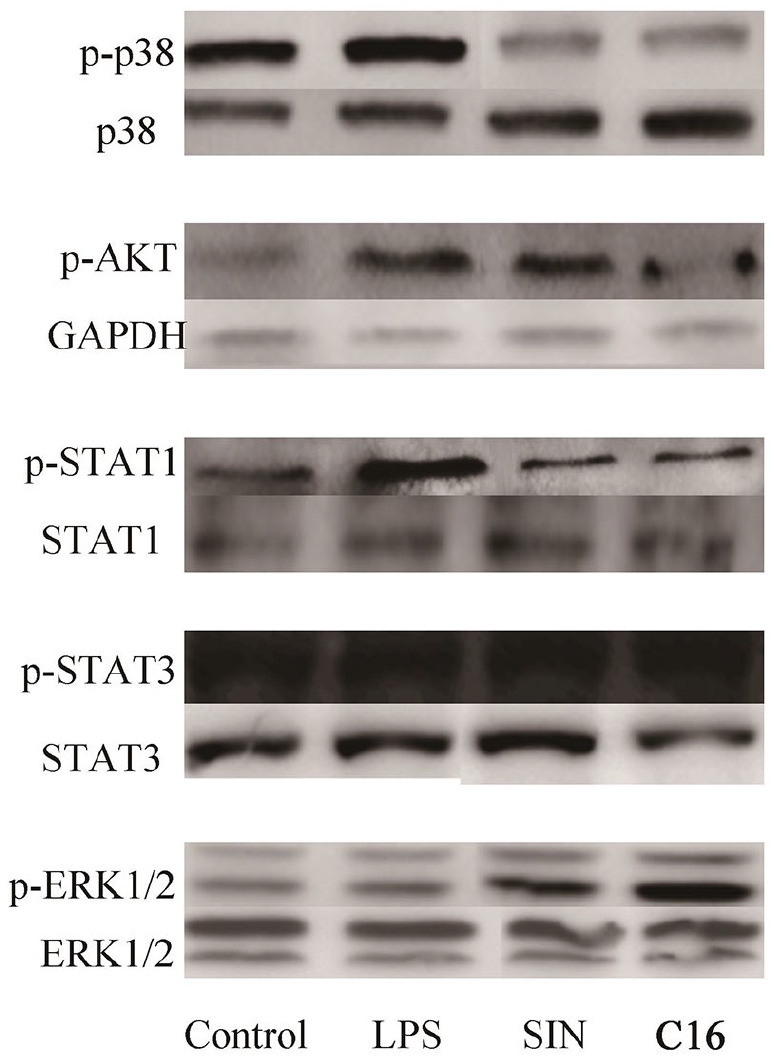
C16 promoted macrophages reprogramming from M1 toward M2 dependent on P38/AKT or STAT1 signals following LPS stimulus. The expression of STAT1, STAT3, ERK1/2, AKT, p38, and its corresponding phosphorylation were determined by western blot. All the data were from three independent experiments and the similar data were obtained. The representative bands were shown.
The molecular weight markers are GAPDH (36KD), p-STAT1 (87KD), STAT1 (87KD), p-STAT3 (85KD), STAT3 (88KD), p-ERK1/2 (42-44KD), ERK1/2 (42-44KD), p-AKT (55KD), p38 (38KD), p-p38 (38KD).
Discussion
Macrophages are heterogenous polyclonal cells, which can transform phenotype/ energy through microenvironment signals. Functional phenotypes of macrophages participated in different diseases. Macrophages can be be polarized into different subtypes in different micro-environments, in which type M1 has “proinflammatory” function and secretes inflammatory cytokines such as IL-Iβ, IL-6, and TNF-α; and type M2 has “anti-inflammatory” function and participates in tissue repair, with high expression of CD206 and Ym1.13,14 In recent years, macrophage reprogramming and its molecular mechanism have attracted a growing number of researchers’ attention. Regulating macrophage reprogramming has been recognized as a potential application in the treatment of inflammatory diseases.
SIN, a natural compound, has been widely used as an immunosuppressive drug for the treatment of autoimmune diseases, such as RA, anti-cancers,15,16 inhibition of chronic cardiac allograft rejection, 17 and anti-inflammatory.18,19 However, it also has certain adverse effects for example, inefficacy for some inflammatory disease, 20 short half-life, thermal instability, easy to decompose, etc. sinomenine and the ester derivatives of fatty acids have good anti-neuritis effects. On the one hand, sinomenine and fatty acids are expected to improve the absorption, transport and metabolism of drugs by changing the lipid balance constant of these compounds. On the other hand, sinomenine and fatty acids cooperate with anti-inflammatory activity based on NF-kB and PPARs respectively. Therefore, more attention has been paid to improve its bioactivities by modifying its structure.21,22 In the article, a new derivative of SIN, C16, showed significant immunosuppressive activity comparing with its parent natural compound.
Endotoxemia lead to high death rate since the mortal disordered inflammatory responses, but there is no specific drug, if a good anti-endotoxemia compound can be found, we believe that the research is of practical significance. The LPS-induced mouse model is a relatively mature inflammatory model in vivo, which is commonly used for the activity screening of anti-inflammatory drugs, to evaluate the anti-inflammatory activity of C16: to explore the anti-inflammatory mechanism of C16 based on regulating the polarization of macrophages, it was found that C16 significantly improved the survival of mice and inhibited inflammatory cytokines in LPS-induced endotoxemia. In vitro, C16 could obviously inhibited M1-related markers expression, conversely, promoted M2-associated factors such as Fizz1, Ym1, and CD206 dampened the pro-inflammatory factors; The results showed that C16 could inhibit the polarization of macrophages to M1 type and promote the polarization of macrophages to M2 type. However, when investigating the regulation effect of C16 on the polarization of macrophages, the concentration gradient of the action of the compound was not set, only preliminary experiments were done, which will be improved in the future research. In order to study the molecular mechanism of C16 regulating macrophage polarization, the expression of related signals in MAPK, JAK/STAT, and PI3K/AKT pathways was measured by Western Blot. Studies have found that the molecular mechanism of C16 regulating macrophage polarization depends on P38/AKT or STAT1 signaling pathways. However, due to time, only some protein phosphorylation in each pathway was detected, which was not comprehensive and the relationship between its upstream and downstream could not be determined. Additionally, C16 showed no toxic effect either on macrophages or liver and kidney suggesting. Howeve, there are few evaluation indicators, and its toxic and side effects cannot be completely excluded. In the future research, we can continue to investigate its internal metabolism, test its half-life and test its chronic toxic side effect for better development utilization. Therefore, targeting M1-polarized macrophage might be a promising strategy for the treatment of endotoxemia. From point of view, C16 might be an effective candidate for inflammatory disease, such as endotoxemia.
Conclusion
To sum up, C16 significantly improved mice survival and inhibited inflammatory cytokines in LPS-induced endotoxemia via reprogram macrophages phenotype from M1 toward M2.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Hospital level project of Taizhou People’s Hospital [grant number ZL201808]; and the Zhenjiang Science and Technology project[grant number SH2019076].
Ethics approval: Ethical approval for this study was obtained from* THE COMMITTE FOR ETHICAL AFFAIRS OF THE JIANGSU UNIVERSITY(BUT THERE’S NO APPROVAL NUMBER/ID)*.
Animal welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
ORCID iD: Ping Ni  https://orcid.org/0000-0003-4430-1078
https://orcid.org/0000-0003-4430-1078
References
- 1. Ka MB, Daumas A, Textoris J, et al. (2014) Phenotypic diversity and emerging new tools to study macrophage activation in bacterial infectious diseases. Frontiers in Immunology 5: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qin HW, Holdbrooks AT, Liu YD, et al. (2012) SOCS3 deficiency promotes M1 macrophage polarization and inflammation. The Journal of Immunology 189: 3439–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoeksema MA, Stöger JL, de Winther MPJ. (2012) Molecular pathways regulating macrophage polarization: Implications for atherosclerosis. Current Atherosclerosis Reports 14: 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sica A, Mantovani A. (2012) Macrophage plasticity and polarization: In vivo veritas. The Journal of Clinical Investigation 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hotchkiss RS, Karl IE. (2003) The pathophysiology and treatment of sepsis. New England Journal of Medicine 348: 138–150. [DOI] [PubMed] [Google Scholar]
- 6. Dahdah A, Gautier G, Attout T, et al. (2014) Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. The Journal of Clinical Investigation 124: 4577–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riedemann NC, Guo RF, Ward PA. (2003) Novel strategies for the treatment of sepsis. Nature Medicine 9: 517–524. [DOI] [PubMed] [Google Scholar]
- 8. Jiang Y, Gao M, Wang WM, et al. (2015) Sinomenine hydrochloride protects against polymicrobial sepsis via autophagy. International Journal of Molecular Sciences 16: 2559–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deguang S, Xin Z, Haiwen Z, et al. (2015) Antimicrobial peptide cathelicidin-BF prevents intestinal barrier dysfunction in a mouse model of endotoxemia. International Immunopharmacology 25: 141–147. [DOI] [PubMed] [Google Scholar]
- 10. Abdelmageed ME, Elawady MS, Suddek GM, et al. (2016) Apocynin ameliorates endotoxin-induced acute lung injury in rats. International Immunopharmacology 30: 163–170. [DOI] [PubMed] [Google Scholar]
- 11. Boyd JH, Kan B, Roberts H, et al. (2008) S100A8 and S100A9 mediate endotoxin- induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circularion Research 102: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 12. Peng T, Zhang T, Lu X, et al. (2009) JNKI/c-fos inhibits cardiomyocyte TNF-α expression via a negative crosstalk with ERK and p38 MAPK in endotoxaemia. Cardiovascular Research 81: 733–741. [DOI] [PubMed] [Google Scholar]
- 13. Feng L, Song P, Zhou H, et al. (2014) Pentamethoxyflavanone regulates macrophage polarization and ameliorates sepsis in mice. Biochemical Pharnacology 89: 109–118. [DOI] [PubMed] [Google Scholar]
- 14. Zhang L, Wang Y, Zhang L, et al. (2012) Macrophage polarization and its mechanism. Chinese Journal 28: 2661–2665. [Google Scholar]
- 15. Wang J, Yang ZR, Dong WG, et al. (2013) Cooperative inhibitory effect of sinomenine combined with 5-fluorouracil on esophageal carcinoma. World Journal of Gastroenterology 19: 8292–8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu XL, Zeng J, Chen YL, et al. (2013) Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: Involvement of cell cycle arrest and apoptosis induction. International Journal of Oncology 42: 229–238. [DOI] [PubMed] [Google Scholar]
- 17. Mark W, Schneeberger S, Seiler R, et al. (2003) Sinomenine blocks tissue remodeling in rat model of chronic cardiac allograft rejection. Transplantation 75: 940–945. [DOI] [PubMed] [Google Scholar]
- 18. Gao T, Hao J, Wiesenfeld-Hallin Z, et al. (2013) Analgesic effect of sinomenine in rodents after inflammation and nerve injury. European Journal of Pharmacology 721: 5–11. [DOI] [PubMed] [Google Scholar]
- 19. Oh YC, Kang OH, Kim SB, et al. (2012) Anti-inflammatory effect of sinomenine by inhibition of pro-inflammatory mediators in PMA plus A23187 -stimulated HMC-1 Cells. European Review for Medical and Pharmacological Sciences 16: 1184–1191. [PubMed] [Google Scholar]
- 20. Xu M, Liu L, Qi C, et al. (2008) Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Medica 74: 1423–1429. [DOI] [PubMed] [Google Scholar]
- 21. Teng P, Liu HL, Zhang L, et al. (2012) Synthesis and biological evaluation of novel sinomenine derivatives as anti-inflammatory agents. European Journal of Medicinal Chemistry 50: 63–74. [DOI] [PubMed] [Google Scholar]
- 22. Yan LC, Bi EG, Lou YT, et al. (2010) Novel sinomenine derivative 1032 improves immune suppression in experimental autoimmune encephalomyelitis. Biochemical and Biophysical Research Communications 391: 1093–1098. [DOI] [PubMed] [Google Scholar]