Abstract
Birth cohorts are those among observational studies that provide understanding of the natural history and causality of diseases since early in life. Discussions during an International Association for Dental Research symposium in London, United Kingdom, in 2018, followed by a workshop in Bangkok, Thailand, in 2019, concluded that there are few birth cohort studies that consider oral health and that a broader discussion on similarities and differences among those studies would be valuable. This article aims to 1) bring together available long-term data of oral health birth cohort studies from the low, middle, and high-income countries worldwide and 2) describe similarities and differences among these studies. This work comprises 15 studies from all 5 continents. The most studied dental conditions and exposures are identified; findings are summarized; and methodological differences and similarities among studies are presented. Methodological strengths and weaknesses are also highlighted. Findings are summarized in 1) the negative impact of detrimental socioeconomic status on oral health changes over time, 2) the role of unfavorable patterns of dental visiting on oral health, 3) associations between general and oral health, 4) nutritional and dietary effects on oral health, and 5) intergenerational influences on oral health. Dental caries and dental visiting patterns have been recorded in all studies. Sources of fluoride exposure have been documented in most of the more recent studies. Despite some methodological differences in the way that the exposures and outcomes were measured, some findings are consistent. Predictive models have been used with caries risk tools, periodontitis occurrence, and permanent dentition orthodontic treatment need. The next steps of the group’s work are as follows: 1) establishing a consortium of oral health birth cohort studies, 2) conducting a scoping review, 3) exploring opportunities for pooled data analyses to answer pressing research questions, and 4) promoting and enabling the development of the next generation of oral health researchers.
Keywords: longitudinal studies, life span, cohort analysis, oral health outcomes, follow-up, population
Introduction
There continues to be considerable interest in the suggestion that exposures acting in early life have long-term consequences for health into adulthood, particularly for noncommunicable diseases (Lawlor et al. 2019). Central to understanding these effects are birth cohort studies, which begin at or before the birth of their participants and continue to observe the same individuals at later ages, on more than 1 occasion (Wadsworth et al. 2006).
Much of the evidence for the growing realization that early life experiences strongly influence oral disease later in life comes from birth cohort studies. A few oral health birth cohort studies (OHBCSs) began 20 to 45 y ago (Warren et al. 2006; Schooling et al. 2012; Horta et al. 2015; Poulton et al. 2015). With newer studies (Feldens et al. 2007; Chaffee et al. 2014; Do et al. 2014; Birungi et al. 2015; Seow et al. 2016; Wagner and Heinrich-Weltzien 2017; Fontana et al. 2019), they have been especially useful in understanding the multiple causes and prevention of oral diseases, providing information on the etiology and natural history of several oral conditions. They have also shown how, over time, unfavorable socioeconomic trajectories, poor oral health–related behaviors, and inadequate patterns of dental visiting have detrimental impacts on oral health (Poulton et al. 2002; Feldens et al. 2007; Thomson et al. 2010; Peres et al. 2011).
A discussion during the International Association for Dental Research (IADR) symposium held in London, United Kingdom, in July 2018 (Birth Cohort Studies of Oral Health: Main Findings and Methodological Considerations) exposed the cumulative knowledge upon this theme, and its future steps were debated. An important conclusion was that there was a need for a broader discussion on the value of OHBCSs. It was recognized that the number of such studies was relatively small and that a meeting of those most involved with these studies would be valuable. Following the IADR symposium, a 2-d workshop was convened in Bangkok, Thailand, in November 2019. Attendees were those involved with the largest and longest studies, those with advanced epidemiologic and statistical knowledge, and those involved in observational and nested intervention studies. They were from all 5 continents, but the numbers were limited to 12 due to funding constraints. This broad mix of experience allowed discussion on all aspects of OHBCSs. This collaborative group forms the basis of a consortium of OHBCSs.
This article aims to 1) bring together available long-term data of OHBCSs from the low, middle, and high-income countries worldwide and 2) describe similarities and differences among these studies.
What Is the Purpose of the Collaborative Group?
Research collaboration is seen as a unique form of partnership for the purpose of scientific research (Bukvova 2010). Science is more effective when researchers with expertise in different areas collaborate on a project with a common interest. This is the case with our OHBCS collaborative group. The objectives of the collaborative group include 1) sharing members’ experiences of working in (various) oral health studies nested in general cohort studies, exclusive OHBCSs, and interventional studies; 2) identifying the main conditions and exposures under study in those OHBCSs; 3) disseminating the collaborative group’s findings; and 4) considering methodological strengths and weaknesses.
What Studies Are in the Group?
The collaborative group comprises 15 OHBCSs: 7 that are “nested” in prospective general birth cohort studies, 4 stand-alone OHBCSs, and 4 OHBCSs combined with early-life interventional studies (Fig. 1).
Figure 1.
Oral health–related birth cohort studies.
Regarding nested studies, New Zealand’s Dunedin Multidisciplinary Health and Development Study (Poulton et al. 2015) follows a complete birth cohort comprising 1,037 babies born at Queen Mary Maternity Hospital (Dunedin’s only maternity unit at the time) between April 1, 1972, and March 31, 1973. The 1982 Pelotas Birth Cohort Study (PBCS), in Brazil, included all 5,914 children born in the city’s hospitals during the 1982 calendar year (Horta et al. 2015). It was repeated with all children who were born in 1993 (Gonçalves et al. 2018) and 2004 (Santos et al. 2014). The Hong Kong Children of 1997 project was carried out by the Department of Community Medicine of The University of Hong Kong and the Department of Health of the Hong Kong Special Administrative Region Government. Originally, 8,327 children were recruited, representing 88% of all births in Hong Kong in April and May 1997 (Schooling et al. 2012). The Prospective Cohort Study of Thai Children started in 2000 to recruit pregnant women and follow their offspring until age 24 y (Mongkolchati et al. 2010). The 2015 PBCS recruited all pregnant women and their babies from January 1 to December 31, 2015 (Hallal et al. 2018).
The stand-alone OHBCSs include the Iowa Fluoride Study, in which mothers of newborns (n = 1,882) were recruited from Iowa hospitals between March 1992 and February 1995 (Warren et al. 2006). The Queensland Birth Cohort Study recruited 1,196 healthy pregnant mothers from Logan-Beaudesert Health district in Queensland, Australia, between January 2007 and June 2008 (Seow et al. 2016). The Germany Birth Cohort Study included newborns from Jena, Thuringia, in 2009 and 2010 (Wagner and Heinrich-Weltzien 2017). The SMILE project (n = 2,181) follows a cohort of South Australian newborns and their mothers/primary caregivers from greater Adelaide, recruited between August 2013 and July 2014 (Do et al. 2014). Four birth cohort studies with interventions were included. In Brazil, the Porto Alegre Early Life Nutrition and Health Study is nested within a cluster-randomized controlled trial with 715 pregnant women who visited any of 20 Porto Alegre municipal health centers from June to December 2008 (Chaffee et al. 2014). A prospective cohort study in São Leopoldo (southern Brazil) is part of a randomized trial (n = 500) of infants at birth recruited from October 2001 to June 2002 (Feldens et al. 2007). The PROMISE-EBF study (Promoting Infant Health and Nutrition in Sub-Saharan Africa: Safety and Efficacy of Exclusive Breastfeeding Promotion in the Era of HIV) was set up as a cluster-randomized interventional behavioral study in 4 African countries: Burkina Faso in West Africa, Uganda in East Africa, Zambia in central Africa, and South Africa in southern Africa. Only the Ugandan data (collected from January 2006 to August 2008) are included here (Birungi et al. 2015). Finally, the Caries Risk Study–US (Fontana et al. 2019) is a multisite prospective study managed and coordinated by the University of Michigan. Three well-established primary care medical research networks enrolled 1,323 children. Funding for each study (with contact information) is given in the Appendix Table.
How Often Have Cohort Members Been Followed Up, and What Was the Attrition Like?
Table 1 summarizes follow-up by study. Most studies started in the early 21st century. While some have pregnancy data available (Mongkolchati et al. 2010; Birungi et al. 2015; Hallal et al. 2018), all studies collected data in childhood. Only 4 have data from late adolescence and beyond (Warren et al. 2006; Horta et al. 2015; Poulton et al. 2015; Gonçalves et al. 2018) because participants have yet to reach adulthood or are no longer being followed. The most recent assessment ages in cohorts with adult follow-up were 31 y in the 1982 PBCS, 22 y in the 1993 PBCS, and 45 y in the Dunedin study.
Table 1.
Data Collection Waves in the Cohort Studies.
| Country: Name of the Study | Enrollment | General Follow-up Assessments |
|---|---|---|
| Australia | ||
| Queensland Birth Cohort Study | Birth (2007) | 6, 12, 24, 36, 48 mo; 5, 6, 7, 12 y |
| SMILE: Study of Mothers’ and Infants’ Life Events Affecting Oral Health | Birth (2013/2014) | 3, 6, 12, 24 mo; 5 y |
| Brazil | ||
| 1982 Pelotas Birth Cohort Study | Birth (1982) | 12, 24, 48 mo; 13, 15, 18, 19, 23, 24, 30, 31 y a |
| 1993 Pelotas Birth Cohort Study | Birth (1993) | 1, 3, 6, 12 mo; 4, 6, 9, 11, 12 to 13, 15, 18, 22 y |
| 2004 Pelotas Birth Cohort Study | Birth (2004) | 3, 12, 24, 48 mo; 5, 6, 12, 13 y |
| 2015 Pelotas Birth Cohort Study | Pregnancy (2015) | Antenatal, perinatal; 3, 12, 24, 48 mo |
| Porto Alegre Early Life Nutrition and Health Study | Pregnancy (2008) | 6, 12, 38 mo; 6 y |
| Sao Leopoldo Birth Cohort Study | Birth (2001/2002) | 6 mo; 1, 4, 8,12 y |
| Hong Kong: Hong Kong Children of 1997 | Birth (1997) | 3, 9, 12 mo; annually until 2019 |
| Germany: Jena Birth Cohort Study | Birth (2009/2010) | 9 mo; 3, 5, 8 y |
| New Zealand: The Dunedin Multidisciplinary Health and Development Study | Birth (1972/1973) | 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, 38, 45 y |
| Thailand: The Prospective Cohort Study of Thai Children–Khon Kaen Site | Pregnancy (2000) | 21 d; 3, 6, 12, 18, 24, 30 mo |
| Uganda: PROMISE-EBF studyb | Pregnancy (2006) | 3, 6, 12, 24 wk; 2 and 5 y |
| USA | ||
| Caries Risk Study–US | 9/15 mo (2012) | Every 4 mo up to age 4 y, then starting at age 6.5 y again every 4 mo |
| IFS: The Iowa Fluoride Study | Birth (1992) | 5, 9, 11, 13, 15, 17, 19, 23 y and 3- to 6-mo intervals throughout the study period |
Mean ages.
The PROMISE-EBF study was set up as a cluster study in 4 African countries (Burkina Faso, Uganda, Zambia, and South Africa).
Figure 2 provides an overview of oral health assessments and attrition rates by study. Unsurprisingly, most follow-up assessments concentrated on the index age groups of the World Health Organization (2013). The attrition rate by the last follow-up ranges from 10% in the 2015 PBCS to 80% in the Germany study. Fieldwork was ongoing in 4 of the 15 studies (Do et al. 2014; Seow et al. 2016; Wagner and Heinrich-Weltzien 2017; Fontana et al. 2019) as of this writing in June 2020.
Figure 2.
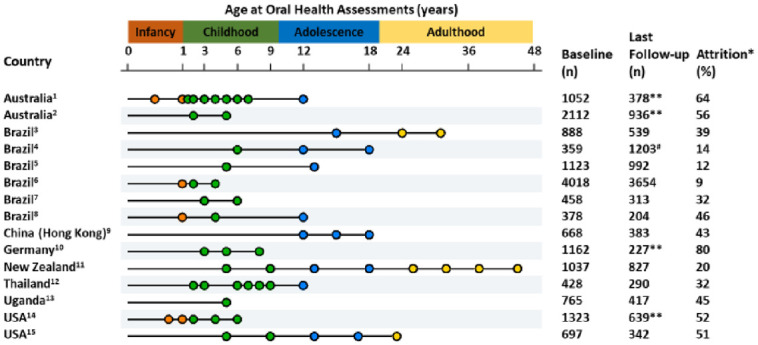
Data collection waves for oral health information in the cohort studies. Age range in figure not to scale (infancy expanded; adulthood condensed). *Attrition refers to losses to follow-up between the first and last oral health assessment, not necessarily attrition since birth. **Ongoing fieldwork. #Sample was inflated in the last follow-up. 1Queensland Birth Cohort Study. Dental examinations also performed at 2.5, 3.5, 4.5 5.5, and 6.5 y. Attrition rate between baseline and last completed follow-up (7 y) = 64.1%. 2Study of Mothers’ and Infants’ Life Events Affecting Oral Health (SMILE). Attrition rate between baseline and last completed follow-up (2 y) = 50.8%. 31982 Pelotas Birth Cohort Study. 41993 Pelotas Birth Cohort Study. 52004 Pelotas Birth Cohort Study. 62015 Pelotas Birth Cohort Study. 7Porto Alegre Early Life Nutrition and Health Study. 8Sao Leopoldo Ten Steps Healthy Feeding and Nutrition Study. 9Hong Kong Children of 1997. 10Jena Birth Cohort Study. Children from the prevention group have been examined every 3 to 6 mo. 11The Dunedin Multidisciplinary Health and Development Study. 12The Prospective Cohort Study of Thai Children–Khon Kaen Site. 13The oral health cohort study is a subsample of the Ugandan cohort of the PROMISE-EBF study. Mothers’ dental examinations at 28 wk of gestation and at 5 y of age of their children. 14Caries Risk Study–US. Attrition rate between baseline and last completed cohort follow-up (4 y) = 25.8%. Cohort for age 6.5 y is ongoing at the time of this report. The collection waves described in the figure include clinical assessments. There are additional data collections every 4 mo for in-person visits from age 1 to 4 y and 6.5 to 9.5 y that do not involve an in-person visit. 15The Iowa Fluoride Study. Age 9 y is the mean age of examined children.
What Has Been Measured?
General information—such as birth weight and height, socioeconomic indicators, maternal and paternal education—has been collected in most (if not all) of the cohort studies. Several have also collected data on conditions such as hypertension, obesity, and anxiety with biological samples. Behavioral characteristics that have been investigated include feeding patterns, sugar consumption, smoking, alcohol intake, and physical activity, as detailed elsewhere (Warren et al. 2006; Feldens et al. 2007; Mongkolchati et al. 2010; Tylleskär et al. 2011; Schooling et al. 2012; Chaffee et al. 2014; Do et al. 2014; Santos et al. 2014; Horta et al. 2015; Poulton et al. 2015; Seow et al. 2016; Wagner and Heinrich-Weltzien 2017; Gonçalves et al. 2018; Hallal et al. 2018; Fontana et al. 2019). Of the 15 studies, 10 have collected data on toothache in at least 1 follow-up (Chaffee et al. 2014; Do et al. 2014; Santos et al. 2014; Birungi et al. 2015; Horta et al. 2015; Seow et al. 2016; Wagner and Heinrich-Weltzien 2017; Gonçalves et al. 2018; Hallal et al. 2018; Fontana et al. 2019).
Oral Diseases and Conditions
All studies have data on dental caries experience, with the number of assessments depending on study duration (Table 2; note that where tooth- and surface-level data have been recorded, only DMFS/dmfs is indicated in the table). Of the 15 cohorts, 11 have detailed data on early childhood caries and/or severe early childhood caries. Dental plaque and hypoplasia/opacities were the second-most investigated dental condition (12 of 15 studies), followed by malocclusion (11 of 15 studies). The collection of periodontal data was less consistent. Very few studies have investigated mutans streptococci levels or maxillofacial development. Only 1 study used the PUFA index (pulpal involvement, ulceration, fistula, and abscess; Monse et al. 2010). Variation in the indices and indicators used reflects the progress of the criteria over time and the epidemiologic profile of the most common oral conditions. For instance, the DMFS index replaced the DMFT index over time, and new indicators have been introduced, such as the International Caries Detection and Assessment System and PUFA indices. Differences in the way in which variables are measured are challenges inherent to long-term cohort studies.
Table 2.
Oral Health and Oral Health–Related Information by Site.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral health data | |||||||||||||||
| DMFT/dmft | × | × | × | × | × | ||||||||||
| DMFS/dmfs | × | × | × | × | × | × | × | × | × | × | |||||
| Malocclusion | × | × | × | × | × | × | × | × | × | × | × | × | |||
| ECC/severe ECC | × | × | × | × | × | × | × | × | × | × | × | ||||
| Dental trauma | × | × | × | × | × | × | × | × | × | ||||||
| Dental erosion | × | × | × | × | × | ||||||||||
| Oral lesions | × | × | × | × | × | × | × | ||||||||
| Gingival bleeding | × | × | × | × | × | × | × | × | |||||||
| Calculus | × | × | × | × | × | ||||||||||
| Periodontal pockets | × | × | × | × | |||||||||||
| Enamel hypoplasia/opacities | × | × | × | × | × | × | × | × | × | × | × | × | |||
| Dental plaque | × | × | × | × | × | × | × | × | × | × | × | × | |||
| Dental fluorosis | × | × | × | × | × | × | × | ||||||||
| Mutans streptococci | × | × | |||||||||||||
| Maxillofacial development | × | ||||||||||||||
| PUFA index | × | ||||||||||||||
| Oral health–related components | |||||||||||||||
| Ever been to the dentist | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| First dental visit | × | × | × | × | × | × | × | × | × | × | |||||
| Last dental visit | × | × | × | × | × | × | × | × | × | × | × | ||||
| Place of dental visit | × | × | × | × | × | × | × | ||||||||
| Type of treatment | × | × | × | × | × | × | × | ||||||||
| Type of dental service | × | × | × | × | × | × | × | ||||||||
| Type of dental treatment | × | × | × | × | × | × | × | ||||||||
| Toothbrushing frequency | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Age when started toothbrushing | × | × | × | × | × | × | × | × | × | × | |||||
| Who brushes | × | × | × | × | × | × | × | × | × | × | × | × | |||
| Type of toothpaste | × | × | × | × | × | × | × | × | × | × | × | × | |||
| Age when started toothpaste use | × | × | × | × | × | × | × | × | |||||||
| Use of dental floss | × | × | × | × | × | × | × | × | × | ||||||
| Professional fluoride treatment (gel, varnish) | × | × | × | × | × | × | × | × | × | × | |||||
| Use of mouth rinse | × | × | × | × | × | × | |||||||||
| Access to fluoridated water | × | × | × | × | × | × | × | × | × | × | × | × | |||
| Other sources of fluoride (dietary supplements, foods, and drinks) | × | × | × | × | × | × | × | × | × | ||||||
| Oral health–related quality of life | × | × | × | × | × | × | × | × | × | ||||||
ECC, early childhood caries; PUFA, pulpal involvement, ulceration, fistula, and abscess.
Queensland Birth Cohort Childhood Caries Study.
SMILE: Study of Mothers’ and Infants’ Life Events Affecting Oral Health.
1982 Pelotas Birth Cohort Study.
1993 Pelotas Birth Cohort Study.
2004 Pelotas Birth Cohort Study.
2015 Pelotas Birth Cohort Study.
Porto Alegre Early Life Nutrition and Health Study.
Sao Leopoldo Ten Steps Healthy Feeding and Nutrition Study.
Hong Kong Children of 1997.
Jena Birth Cohort Study.
The Dunedin Multidisciplinary Health and Development Study.
The Prospective Cohort Study of Thai Children–Khon Kaen Site.
The Oral Health Cohort Study is a subsample of the Ugandan cohort of the PROMISE-EBF study.
Caries Risk Study–US.
IFS: The Iowa Fluoride Study.
Oral Health–Related Components
All studies have recorded data on dental visiting patterns, albeit with differences across ages. Similarly, data on oral hygiene behaviors (e.g., toothbrushing habits) have been collected largely consistently. Fluoride exposure sources have been documented in most of the more recent studies. Oral health–related quality of life data are available in 8 of the 15 studies.
What Has the Collaborative Group Found to Date?
Table 3 shows the main findings of the studies, grouped into 5 main topics: 1) the negative impact of detrimental socioeconomic status (SES) on oral health changes over time, 2) the role of unfavorable patterns of dental visiting on oral health, 3) associations between general and oral health, 4) nutritional and dietary effects on oral health, and 5) intergenerational influences on oral health. Despite some methodological differences in the way that the exposures and outcomes were measured, some findings are consistent.
Table 3.
Findings from Cohort Studies.
| Module: Exposure Outcome | Findings | Study |
|---|---|---|
| SES: socioeconomic changes | ||
| Dental caries, plaque, gingivitis, periodontitis | Low childhood socioeconomic circumstances have long-lasting negative influences on adult periodontitis and caries level | 10 |
| Periodontitis | Low-income trajectories from birth to age 30 y affect periodontitis level at 31 y | 3 |
| Unsound teeth | Poverty in the life course affects number of unsound teeth among adults | 3 |
| Early sugar consumption | Clear SES gradient and early sugar introduction | 2, 13 |
| Excess sugar intake (>5%) | SES gradients related to excess energy consumption >5% from sugar | 2 |
| Dental visiting: routine visits | ||
| DMFS, self-rated oral health | Long-term unfavorable pattern of dental visiting linked to poor adult oral health | 10 |
| Preschool children dental visit | Positive association between favorable maternal dental visits and routine visits in preschool children | 5 |
| General health: obesity | ||
| Dental caries | Central obesity associated with increase dental caries in adolescents | 8 |
| Periodontitis | Central and diet-induced obesity linked with periodontitis in adulthood | 3 |
| Traumatic dental injuries | Early-life overweight and obesity as risk factors for traumatic dental injuries in preschool children | 6 |
| General health: heart diseases | ||
| Periodontitis | Markers of cardiovascular diseases are not associated with periodontitis | 3, 10 |
| Nutritional and dietary: breastfeeding | ||
| Dental caries | Prolonged breastfeeding associated with early childhood caries | 5, 6, 7, 9 |
| Breastfeeding between 6 and 11 mo is linked to lower dmfs | 11 | |
| Malocclusion | Exclusive breastfeeding drops the risk for malocclusion in deciduous teeth | 4, 9 |
| Sugar intake | Breastfeeding between 6 and 11 mo reduces energy intake due to sugar | 2 |
| Nutritional and dietary: infant feeding | ||
| Dental caries | Infant feeding recommendations do not reduce early childhood caries | 6, 12 |
| Dietary advice at home reduced caries experience in infants | 1, 7 | |
| Nutritional and dietary: sugar consumption | ||
| Dental caries | Regular intake of assorted sugar sources increases caries experience | 4, 6, 7, 14 |
| Intergenerational: maternal dental characteristics | ||
| Dental caries | Caregiving behaviors mediate the association between maternal dental anxiety and unfavorable care patterns on the increase in child’s dmfs | 5 |
| Quality of life–OHRQoL | Maternal oral conditions predict child’s dental caries and OHRQoL in adulthood | 10 |
OHRQoL, oral health–related quality of life; SES, socioeconomic status.
Queensland Birth Cohort Childhood Caries Study.
SMILE: Study of Mothers’ and Infants’ Life Events Affecting Oral Health.
1982 Pelotas Birth Cohort Study.
1993 Pelotas Birth Cohort Study.
2004 Pelotas Birth Cohort Study.
2015 Pelotas Birth Cohort Study.
Porto Alegre Early Life Nutrition and Health Study.
Sao Leopoldo Ten Steps Healthy Feeding and Nutrition Study.
Hong Kong Children of 1997.
Jena Birth Cohort Study.
The Dunedin Multidisciplinary Health and Development Study.
The Prospective Cohort Study of Thai Children–Khon Kaen Site.
The Oral Health Cohort Study is a subsample of the Ugandan cohort of the PROMISE-EBF study.
Caries Risk Study–US.
IFS: The Iowa Fluoride Study.
The dynamic of socioeconomic changes over the life course on oral health conditions has been suggested, as has the importance of socioeconomic disadvantage at specific critical ages (Poulton et al. 2002; Peres et al. 2011; Ha et al. 2017; Schuch et al. 2018; Devenish et al. 2019; Fontana et al. 2019). The Dunedin cohort study uniquely revealed a long-term unfavorable pattern of dental visiting as being associated with poor oral health (Thomson et al. 2010), and routine visits for children were positively associated with regular maternal dental visits in preschool children in Pelotas (Camargo et al. 2012). Several studies elucidated the complex relationship between general and oral health from our collaborative team. Obesity, for example, has been confirmed as having a direct effect on periodontitis (Nascimento, Peres, Mittinty, Mejia, et al. 2017; Nascimento, Peres, Mittinty, Peres, et al. 2017) and has been associated with greater experience of dental caries (Li et al. 2017) and traumatic dental injury (Borges et al. 2017). However, studies from the 2 longest cohort studies (Shearer et al. 2017; Leite et al. 2020) showed that periodontitis is not associated with surrogate markers of cardiovascular disease, challenging the notion that periodontitis is a risk factor for cardiovascular disease. Research from our collaborative team is unique in having prospectively and simultaneously investigated nutritional and dietary patterns and their effects on dental caries (Marshall et al. 2003; Feldens et al. 2007; Feldens et al. 2010; Chaffee et al. 2013; Chaffee et al. 2014; Birungi et al. 2015; Seow et al. 2016; Nirunsittirat et al. 2016; Peres et al. 2016; Peres et al. 2017; Wagner and Heinrich-Weltzien 2017), sugar intake in childhood (Devenish et al. 2019), and malocclusion (Peres, Cascaes, et al. 2015; Wagner and Heinrich-Weltzien 2017). Intergenerational studies suggest that socioeconomic and behavioral factors, much more than biological mechanisms, explain observed intrafamily concordance in oral health (Shearer et al. 2011a, 2011b; Goettems et al. 2018).
Additionally, predictive diseases models have been developed, and the natural histories of oral conditions have been described. US work showed that caries-predictive risk factors—such as diet pattern and oral hygiene—differ according to Medicaid status and race/ethnicity (Fontana et al. 2019). The Pelotas study has shown that accuracy of predicting periodontitis occurrence depends on how cases are defined (Leite et al. 2017) and that deciduous-dentition malocclusion is a predictor of permanent-dentition orthodontic treatment need (Peres et al. 2015). Only prospective cohort studies can shed light on the natural history of oral conditions, particularly for adults. In this respect, the Dunedin study has shown that caries increment is constant through life (Broadbent et al. 2008) and that incremental tooth loss commences relatively early in adulthood (Thomson et al. 2000). Periodontitis starts early in adulthood, and its progression accelerates with age, particularly among smokers (Thomson et al. 2013). Sustained effective plaque control is crucial, and poor oral hygiene and smoking have a synergistic effect on periodontal disease experience (Broadbent et al. 2011). Periodontal attachment loss rates accelerate from the third to the fourth decade of life among susceptible people (Zeng et al. 2014). New understanding of the natural history and antecedents of dental anxiety was also elucidated (Thomson et al. 2009). The Iowa study showed that >30% of noncavitated pit-and-fissure lesions progressed to frank decay or filled surfaces over 4 y, while very few noncavitated smooth surface lesions did so (Warren et al. 2006). High birth weight and rapid growth during the first year of life were associated with developmental defects of enamel (DDEs; Wong et al. 2015). DDEs are determinants of primary dentition caries (Seow et al. 2016), which in turn has been associated with DDEs in permanent successor teeth (Broadbent et al. 2005). Children who were shorter at birth and stunted at 6 mo of age had fewer emerged teeth by the age of 12 mo than their counterparts (Bastos et al. 2007). Heavier children at birth and those with a slower growth rate from birth to 3 mo are more likely to have a complete permanent dentition by age 12 y (Wong et al. 2019).
The relationship between breastfeeding and dental caries was the most common topic investigated among the studies of this collaborative group. Table 4 depicts how breastfeeding duration was differently associated with primary dental caries across studies. Further pooled and sensitivity analysis, made possible through the collaborative group, may help identify the potential effect modifiers responsible for these differences by study setting.
Table 4.
Site-Specific Associations between Measures of Breastfeeding and Dental Caries in Primary Dentition.
| Study | Exposure | Outcome/Age | Findings (95% CI) |
|---|---|---|---|
| SMILE study (Do et al. 2014) | BF >12 moa | ECC prevalence/2 to 3 y | PR: 1.42 (0.85 to 2.38) |
| 2004 Pelotas Birth Cohort Study (Peres et al. 2017) | BF ≥24 mo | 1. Caries prevalence 2. S-ECC/5 y |
1. RR: 1.9 (1.5 to 2.4) 2. RR: 2.4 (1.7 to 3.3) |
| Porto Alegre Early Life Nutrition and Health Study (Chaffee et al. 2014) | 1. BF 6 to 11 mob
2. BF 12 to 23 mob 3. BF ≥24 mob |
S-ECC/38 mo | 1. PR: 1.77 (1.12 to 2.85) 2. PR: 1.82 (0.85 to 3.20) 3. PR: 2.10 (1.50 to 3.25) |
| Sao Leopoldo Ten Steps Healthy Feeding and Nutrition Study (Feldens et al. 2010) | 1. BF 3 to 6/d 12 mo 2. BF ≥7/d 12 mo |
S-ECC/4 y | 1. RR: 2.04 (1.22 to 3.39) 2. RR: 1.97 (1.45 to 2.68) |
| Jena Birth Cohort Study (Wagner and Heinrich-Weltzien 2017) | BFc ≥12 mo | Caries prevalence/5 y | OR: 6.20 (3.25 to 11.75) |
| The Prospective Cohort Study of Thai Children–Khon Kaen Site (Nirunsittirat et al. 2016) | Full BFd 6 to 11 mo | 1. Caries prevalence/3 to 4 y 2. dmfs (count variable) |
1. RRe: 0.45 (0.22 to 0.90) 2. RR: 0.77 (0.63 to 0.93) |
| PROMISE-EBF study (Birungi et al. 2015) | Exclusive BF/6 mo | ECC prevalence/5 y | IRR: 0.62 (0.43 to 0.91) / 0.60 (0.41 to 0.88)f |
| Caries Risk Study–US (Fontana et al. 2019) | BFg 12 mo | Decay = ICDAS ≥3/1 to 4 y | OR: 2.04 (1.22 to 3.41) |
| IFS: The Iowa Fluoride Study (Hong et al. 2014) | 1. BF <6 vs ≥6 mo | 2. Primary second molar (cavitated enamel/dentin and/or filled)/5 y | OR: 15.58 (P < 0.05) |
For the findings, the measure of association was controlled for confounders.
BF, breastfeeding; ECC, early childhood caries; ICDAS, International Caries Detection and Assessment System; IRR, incidence relative ratio; OR, odds ratio; PR, prevalence ratio; RR, risk ratio; S-ECC, severe early childhood caries.
Reference category: BF, 6 to 12 mo.
Reference category: BF, <6 mo.
BF in combination with bottle-feeding.
Feeding breast milk but not formula, regardless of other liquids and foods. Reference category: BF <6 mo.
Two regression models.
Child goes to sleep daily while breastfeeding or drinking something other than water from bottle/sippy cup.
What Are the Studies’ Main Strengths and Weaknesses, and How Can Collaboration Help?
Birth cohort studies provide insight into the earliest determinants of oral health. Well after childhood, they continue to reveal how oral health responds to changing conditions over time, especially as risk factors, vulnerabilities, and major health concerns evolve throughout adolescence, adulthood, and later life. Opportunities to intervene may be identified, and the effect of varying the timing, strength, and type of intervention can be modeled with data from such studies.
As prospective cohort studies, birth cohorts are well suited to detect risk and protective factors, as well inform disease prediction models, with a high degree of certainty on the temporal sequence of predictors, outcomes, and confounders of interest. An additional and generally held advantage of birth cohorts is excellent population representativeness. With birth being a universal human experience that is often connected to the health care system and government record keeping, it is possible to enroll cohorts that are representative of surrounding communities. This enables descriptive natural history studies and oral disease burden estimates with high external validity.
Drawing evidence from multiple OHBCSs as a coordinated, collaborative undertaking has several advantages. Notably, the consistency of findings can be assessed across studies that may differ in their methodological specifics. Should a finding persist across studies—despite differences in study populations, measurements, and statistical approaches—there is greater confidence of underlying consistency in findings.
When studies with broadly similar designs combine data systematically, patterns not evident in any single study may be revealed. Synthesis may involve data pooling at the individual participant level or as a meta-analytic summary of findings. One advantage of combined analyses is greater statistical power. Another is the ability to examine research questions with greater global representation and in different contexts and periods. Whether a quantitative or narrative synthesis, a global view can explore interactions between oral health determinants and various sociocultural influences, economic conditions, and health systems (Richter et al. 2012). Notwithstanding the ecologic fallacy, looking across studies also affords a wider range of exposures (e.g., levels of per capita sugar consumption) otherwise not observable within any individual population (which is more homogeneous in behaviors and experiences).
Beyond these methodological advantages, a collaborative undertaking has the potential to enhance the overall quality and utility of birth cohort research. Collaboration creates opportunities for standardization in study design and data collection to facilitate future data syntheses. It can foster innovation and creativity and raise the profile of such research to attract investigators and potential funders. In addition to bolstering current research, support and guidance from the collaboration can help establish the next research study and the next generation of oral health birth cohort researchers.
Extensive heterogeneity in variable measurement, case definitions, and other aspects of the study and analytic protocols is the most notable challenge in synthesizing findings from existing studies. Measurement and variable specification differences contribute additional variance, if not systematic bias, to summary estimates. Furthermore, selection bias can result if data syntheses include only studies consistent in certain design features. Finally, differences in underlying disease burden affect the interpretation of numeric summary measures, such as the relative risk; for example, a relative risk of 2.0 corresponds to widely divergent absolute differences when reference group caries prevalence is 5% than when it is 40%, and it is unrealizable if the reference group prevalence exceeds 50%.
Combining study data also presents logistical challenges. Pooling data may require formal data-sharing agreements to protect intellectual property, as well as protocols for data management, archiving, and analysis. Even if limited identifiable participant information is involved, data sharing could require additional ethical review and possibly permission from participants, which may be unobtainable if studies are no longer active.
Among the limitations of OHBCSs themselves, many existing studies have not continued beyond childhood, precluding their study of the diseases of adulthood. Moreover, relatively high attrition rates in some studies erode the sample sizes and, consequently, may affect the generalizability of the findings. At least 2 strategies have been adopted to minimize this impact. Data imputation may help to reduce bias created due to missing data, facilitate data handling and analysis, and increase precision (Sterne et al. 2009). Concerted efforts to rescue participants lost during previous follow-up waves are often needed to maintain the cohort and ensure a sufficient sample.
What Are the Next Steps?
The next steps include conducting a scoping review of birth cohort studies of oral health, consolidating the consortium’s makeup and purpose, exploring opportunities for pooled data analyses to answer pressing research questions, and facilitating the development of the next generation of OHBCS researchers.
Scoping Review
The purpose of a scoping review is to determine the scope, extent, and nature of the published scientific literature on a topic. It involves a stocktake of the available evidence in a given field to identify 1) the available evidence types, 2) the key concepts and definitions, 3) the dominant methodological approaches used, 4) the important relationships, and 5) the nature and importance of existing knowledge gaps (Munn et al. 2018).
To date, there has been no scoping review of OHBCSs. Accordingly, a crucial part of our program will be to conduct one, to inform and guide future work in the field.
Consortium
A consortium is an alliance of ≥2 entities to enable their participation in a common activity or pooling their resources for a common goal. A consortium of OHBCSs would have several benefits. First, it would facilitate greater standardization and/or commonality of methods across studies. This would enable data pooling to make comparing findings easier. Second, it would allow more recently established teams to learn from the experiences of more long-standing studies. Third, the resultant closer collaboration and harmonization of methods would raise studies’ profiles and lend more authority to their findings. Fourth, the consortium would be positioned to identify possibilities for and facilitate new birth cohort studies in low- and middle-income countries.
Pooling Data to Enhance Statistical Power
Some research questions require more statistical power than can be afforded by any single study (Richter et al. 2012). Combining data sets would enable examination of more subtle associations and interactions, particularly for gene-by-environment interactions or microbiome studies.
As well as greater statistical power, making contextual comparisons would allow for investigation of a broader range of research questions than that with a single study. A consortium using pooled data could study the effects of the same exposure in different settings; for example, how consistent across different populations is the strength of the association of birth trauma with molar-incisor hypomineralization? It would also be possible to compare the antecedents of a particular outcome in different settings; for example, are the risk factors for severe early childhood caries similar across different populations, or are there particular practices/exposures that come into play?
Training of the Next Generation of Researchers
New researchers are an essential part of the machinery of knowledge generation, playing an important role in collecting data and formulating research questions under the guidance of more experienced ones. Birth cohort studies provide an excellent opportunity for training and development.
It is also important to bear in mind that, even though a particular OHBCS may not at first have been visualized as a longer-term life course study, circumstances can change, particularly as the study’s worth becomes more apparent as each assessment wave adds value to the earlier data. For example, the renowned Dunedin (Poulton et al. 2015) and 1982 Pelotas (Horta et al. 2015) studies were not initially planned to be the decades-long (and counting) life course studies that they have become; however, the emerging findings attracted attention and funding from international agencies and researchers, and the studies are now well into their fourth or fifth decade and providing unprecedented information on the natural history of oral conditions. Any such study will involve turnover in research staff: just as the cohort itself ages, so do the researchers, and an essential self-preservation strategy for any such study is to identify, develop, and retain scientists. Moreover, those scientists should come from as broad a range of disciplines as possible. Clearly, any OHBCS will need to have some dentally qualified staff (so that clinical oral data can be collected and interpreted appropriately), but others involved should have training and experience in fields such as biostatistics, epidemiology, social psychology, social anthropology, and so on.
Author Contributions
K.G. Peres, M.A. Peres, A.J. Rugg-Gunn, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; W.M. Thomson, B.W. Chaffee, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; N. Birungi, L.G. Do, C.A. Feldens, M. Fontana, T.A. Marshall, W. Pitiphat, W.K. Seow, Y. Wagner, H.M. Wong, contributed to data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520942208 for Oral Health Birth Cohort Studies: Achievements, Challenges, and Potential by K.G. Peres, W.M. Thomson, B.W. Chaffee, M.A. Peres, N. Birungi, L.G. Do, C.A. Feldens, M. Fontana, T.A. Marshall, W. Pitiphat, W.K. Seow, Y. Wagner, H.M. Wong and A.J. Rugg-Gunn in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
The Borrow Foundation supported the 2019 IADR Symposium on Birth Cohort Studies (London, UK, July 2018) and the 2-day workshop of the Oral Health–Related Birth Cohort Study Collaboration (Bangkok, Thailand, November 2019).
The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: K.G. Peres  https://orcid.org/0000-0002-1730-2123
https://orcid.org/0000-0002-1730-2123
W.M. Thomson  https://orcid.org/0000-0003-0588-6843
https://orcid.org/0000-0003-0588-6843
M.A. Peres  https://orcid.org/0000-0002-8329-2808
https://orcid.org/0000-0002-8329-2808
N. Birungi  https://orcid.org/0000-0002-9071-196X
https://orcid.org/0000-0002-9071-196X
C.A. Feldens  https://orcid.org/0000-0002-9783-9309
https://orcid.org/0000-0002-9783-9309
M. Fontana  https://orcid.org/0000-0003-2357-7534
https://orcid.org/0000-0003-2357-7534
H.M. Wong  https://orcid.org/0000-0003-3411-6442
https://orcid.org/0000-0003-3411-6442
References
- Bastos JL, Peres MA, Peres KG, Barros AJ. 2007. Infant growth, development and tooth emergence patterns: a longitudinal study from birth to 6 years of age. Arch Oral Biol. 52(6):598–606. [DOI] [PubMed] [Google Scholar]
- Birungi N, Fadnes LT, Okullo I, Kasangaki A, Nankabirwa V, Ndeezi G, Tumwine JK, Tylleskär T, Lie SA, Åstrøm AN. 2015. Effect of breastfeeding promotion on early childhood caries and breastfeeding duration among 5-year-old children in eastern Uganda: a cluster randomised trial. PloS One. 10(5):e0125352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges TS, Chaffee BW, Kramer PF, Feldens EG, Vítolo MR, Feldens CA. 2017. Relationship between overweight/obesity in the first year of age and traumatic dental injuries in early childhood: findings from a birth cohort study. Dent Traumatol. 33(6):465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent JM, Thomson WM, Boyens JV, Poulton R. 2011. Dental plaque and oral health during the first 32 years of life. J Am Dent Assoc. 142(4):415–426. [DOI] [PubMed] [Google Scholar]
- Broadbent JM, Thomson WM, Poulton R. 2008. Trajectory patterns of dental caries experience in the permanent dentition to the fourth decade of life. J Dent Res. 87(1):69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent JM, Thomson WM, Williams SM. 2005. Does caries in primary teeth predict enamel defects in permanent teeth? A longitudinal study. J Dent Res. 84(3):260–264. [DOI] [PubMed] [Google Scholar]
- Bukvova H. 2010. Studying research collaboration: a literature review. Sprouts: Working Papers on Information Systems. 10(3):326. [Google Scholar]
- Camargo MBJ, Barros AJD, Frazao P, Matijasevivh A, Santos IS, Peres MA, Peres KG. 2012. Predictors of dental visits for routine check-ups and for the resolution of problems among preschool children. Rev Saude Publica. 46(1): 87–97. [DOI] [PubMed] [Google Scholar]
- Chaffee BW, Feldens CA, Vítolo MR. 2013. Cluster-randomised trial of infant nutrition training for caries prevention. J Dent Res. 92(7):29S–36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee BW, Vítolo MR, Feldens CA. 2014. The porto alegre early life nutrition and health study. Rev Bras Epidemiol. 17(4):1015–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish G, Ytterstad E, Begley A, Do L, Scott J. 2019. Intake, sources, and determinants of free sugars intake in Australian children aged 12–14 months. Matern Child Nutr. 15(2):e12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do LG, Scott JA, Thomson WM, Stamm JW, Rugg-Gunn AJ, Levy SM, Wong C, Devenish G, Ha DH, Spencer AJ. 2014. Common risk factor approach to address socioeconomic inequality in the oral health of preschool children—a prospective cohort study. BMC Public Health. 14:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldens CA, Giugliani ER, Vigo Á, Vítolo MR. 2010. Early feeding practices and severe early childhood caries in four-year-old children from southern Brazil: a birth cohort study. Caries Res. 44(5):445–452. [DOI] [PubMed] [Google Scholar]
- Feldens CA, Vítolo MR, Drachler Mde L. 2007. A randomised trial of the effectiveness of home visits in preventing early childhood caries. Community Dent Oral Epidemiol. 35(3):215–223. [DOI] [PubMed] [Google Scholar]
- Fontana M, Eckert GJ, Keels MA, Jackson R, Katz BP, Kemper AR, Levy BT, Levy SM, Yanca E, Kelly S, et al. 2019. Predicting caries in medical settings—risk factors in diverse infant groups. J Dent Res. 98(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettems ML, Nascimento GG, Peres MA, Santos IS, Matijasevich A, Barros AJD, Peres KG, Demarco FF. 2018. Influence of maternal characteristics and caregiving behaviours on children’s caries experience: an intergenerational approach. Community Dent Oral Epidemiol. 46(5):435–441. [DOI] [PubMed] [Google Scholar]
- Gonçalves H, Wehrmeister FC, Assunção MCF, Tovo-Rodrigues L, Oliveira IO, Murray J, Anselmi L, Barros FC, Victora CG, Menezes AMB. 2018. Cohort profile update: the 1993 Pelotas (Brazil). Birth cohort follow-up at 22 years. Int J Epidemiol. 47(5):1389–1390e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha DH, Do LG, Spencer AJ, Thomson WM, Golley RK, Rugg-Gunn AJ, Levy SM, Scott JA. 2017. Factors influencing early feeding of foods and drinks containing free sugars—a Birth Cohort Study. Int J Environ Res Public Health. 14(10):E1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallal PC, Bertoldi AD, Domingues MR, da Silveira MF, Demarco FF, da Silva ICM, Barros FC, Victora CG, Bassani DG. 2018. Cohort profile: the 2015 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol. 47(4):1048–1048h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L, Levy SM, Warren JJ, Broffitt B. 2014. Infant breast-feeding and childhood caries: a nine-year study. Pediatr Dent. 36(4):342–347. [PMC free article] [PubMed] [Google Scholar]
- Horta BL, Gigante DP, Gonçalves H, dos Santos Motta J, Loret de Mola C, Oliveira IO, Barros FC, Victora CG. 2015. Cohort profile update: the 1982 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol. 44(2):441, 441a–441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Lewcock M, Rena-Jones L, Rollings C, Yip V, Smith D, Pearson RM, Johnson L, Millard LAC, Patel N, et al. 2019. The second generation of the Avon Longitudinal Study of Parents and Children (ALSPAC-G2): a cohort profile. Wellcome Open Res. 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite FRM, Nascimento GG, Peres KG, Demarco FF, Horta BL, Peres MA. 2020. Collider bias in the association of periodontitis and carotid intima-media thickness. Community Dent Oral Epidemiol [epub ahead of print 3 Mar 2020]. doi: 10.1111/cdoe.12525 [DOI] [PubMed] [Google Scholar]
- Leite FRM, Peres KG, Do LG, Demarco FF, Peres MA. 2017. Prediction of periodontitis occurrence: influence of classification and sociodemographic and general health information. J Periodontol. 88(8):731–743. [DOI] [PubMed] [Google Scholar]
- Li LW, Wong HM, McGrath CP. 2017. Longitudinal association between obesity and dental caries in adolescents. J Pediatr. 189:149–154.e5. [DOI] [PubMed] [Google Scholar]
- Mongkolchati A, Thinkhamrop B, Mo-Suwan L, Chittchang U, Choprapawon C. 2010. Prevalence and incidence of child stunting from birth to two years of life in Thai children: based on the Prospective Cohort Study of Thai Children (PCTC). J Med Assoc Thai. 93(12):1368–1378. [PubMed] [Google Scholar]
- Monse B, Heinrich-Weltzien R, Benzian H, Holmgren C, van Palenstein Helderman W. 2010. PUFA—an index of clinical consequences of untreated dental caries. Community Dent Oral Epidemiol. 38(1):77–82. [DOI] [PubMed] [Google Scholar]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. 2018. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 18(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento GG, Peres KG, Mittinty MN, Mejia GC, Silva DA, Gonzalez-Chica D, Peres MA. 2017. Obesity and periodontal outcomes: a population-based cohort study in Brazil. J Periodontol. 88(1):50–58. [DOI] [PubMed] [Google Scholar]
- Nascimento GG, Peres MA, Mittinty MN, Peres KG, Do LG, Horta BL, Gigante DP, Corrêa MB, Demarco FF. 2017. Diet-induced overweight and obesity and periodontitis risk: an application of the parametric G-formula in the 1982 Pelotas Birth Cohort. Am J Epidemiol. 185(6):442–451. [DOI] [PubMed] [Google Scholar]
- Nirunsittirat A, Pitiphat W, McKinney CM, DeRouen TA, Chansamak N, Angwaravong O. 2016. Breastfeeding duration and childhood caries: a cohort study. Caries Res. 50(5):498–507. [DOI] [PubMed] [Google Scholar]
- Peres KG, Cascaes AM, Peres MA, Demarco FF, Santos IS, Matijasevich A, Barros AJ. 2015. Exclusive breastfeeding and risk of dental malocclusion. Pediatrics. 136(1):e60–e67. [DOI] [PubMed] [Google Scholar]
- Peres KG, Nascimento GG, Peres MA, Mittinty MN, Demarco FF, Santos IS, Matijasevich A, Barros AJD. 2017. Impact of prolonged breastfeeding on dental caries: a population-based birth cohort study. Pediatrics. 140(1):e20162943. [DOI] [PubMed] [Google Scholar]
- Peres MA, Peres KG, Thomson WM, Broadbent JM, Gigante DP, Horta BL. 2011. The influence of family income trajectories from birth to adulthood on adult oral health: findings from the 1982 Pelotas Birth Cohort. Am J Public Health. 101(4):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres KG, Peres MA, Thomson WM, Broadbent J, Hallal PC, Menezes AB. 2015. Deciduous-dentition malocclusion predicts orthodontic treatment needs later: findings from a population-based birth cohort study. Am J Orthod Dentofacial Orthop. 147(4):492–498. [DOI] [PubMed] [Google Scholar]
- Peres MA, Sheiham A, Liu P, Demarco FF, Silva AE, Assunção MC, Menezes AM, Barros FC, Peres KG. 2016. Sugar consumption and changes in dental caries from childhood to adolescence. J Dent Res. 95(4):388–394. [DOI] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, Moffitt TE. 2002. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 360(9346):1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Moffitt TE, Silva PA. 2015. The Dunedin Multidisciplinary Health and Development Study: overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 50(5):679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter LM, Victora CG, Hallal PC, Adair LS, Bhargava SK, Fall CH, Lee N, Martorell R, Norris SA, Sachdev HS, et al. 2012. Cohort profile: the consortium of health-orientated research in transitioning societies. Int J Epidemiol. 41(3):621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos IS, Barros AJ, Matijasevich A, Zanini R, Chrestani Cesar MA, Camargo-Figuera FA, Oliveira IO, Barros FC, Victora CG. 2014. Cohort profile update: 2004 Pelotas (Brazil) Birth Cohort Study. Body composition, mental health and genetic assessment at the 6 years follow-up. Int J Epidemiol. 43(5):1437–1437f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooling CM, Hui LL, Ho LM, Lam TH, Leung GM. 2012. Cohort profile: “children of 1997. ” A Hong Kong Chinese birth cohort. Int J Epidemiol. 41(3):611–620. [DOI] [PubMed] [Google Scholar]
- Schuch HS, Peres KG, Demarco FF, Horta BL, Gigante DP, Peres MA, Do LG. 2018. Effect of life-course family income trajectories on periodontitis: birth cohort study. J Clin Periodontol. 45(4):394–403. [DOI] [PubMed] [Google Scholar]
- Seow WK, Leishman SJ, Palmer JE, Walsh LJ, Pukallus M, Barnett AG. 2016. A longitudinal observational study of developmental defects of enamel from birth to 6 years of age. JDR Clin Trans Res. 1(3):285–291. [DOI] [PubMed] [Google Scholar]
- Shearer DM, Thomson WM, Broadbent JM, Mann J, Poulton R. 2017. Periodontitis is not associated with metabolic risk during the fourth decade of life. J Clin Periodontol. 44(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer DM, Thomson WM, Broadbent JM, Poulton R. 2011. a. Does maternal oral health predict child oral health-related quality of life in adulthood? Health Qual Life Outcomes. 9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer DM, Thomson WM, Broadbent JM, Poulton R. 2011. b. Maternal oral health predicts their children’s caries experience in adulthood. J Dent Res. 90(5):672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. 2009. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WM, Broadbent JM, Locker D, Poulton R. 2009. Trajectories of dental anxiety in a birth cohort. Community Dent Oral Epidemiol. 37(3):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WM, Poulton R, Kruger E, Boyd DM. 2000. Socioeconomic and behavioural risk factors for tooth loss from age 18 to 26 among participants in the Dunedin Multidisciplinary Health and Development Study. Caries Res. 34(5):361–366. [DOI] [PubMed] [Google Scholar]
- Thomson WM, Shearer DM, Broadbent JM, Foster Page LA, Poulton R. 2013. The natural history of periodontal attachment loss during the third and fourth decades of life. J Clin Periodontol. 40(7):672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WM, Williams SM, Broadbent JM, Poulton R, Locker D. 2010. Long-term dental visiting patterns and adult oral health. J Dent Res. 89(3):307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylleskär T, Jackson D, Meda N, Engebretsen IM, Chopra M, Diallo AH, Doherty T, Ekström EC, Fadnes LT, Goga A, et al. 2011. Exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa (PROMISE-EBF): a cluster-randomised trial. Lancet. 378(9789):420–427. [DOI] [PubMed] [Google Scholar]
- Wadsworth M, Kuh D, Richards M, Hardy R. 2006. Cohort profile: The 1946 National Birth Cohort (MRC National Survey of Health p.441-441e.h and Development). Int J Epidemiol. 35(1):49–54. [DOI] [PubMed] [Google Scholar]
- Wagner Y, Heinrich-Weltzien R. 2017. Evaluation of a regional German interdisciplinary oral health programme for children from birth to 5 years of age. Clin Oral Investig. 21(1):225–235. [DOI] [PubMed] [Google Scholar]
- Warren JJ, Levy SM, Broffitt B, Kanellis MJ. 2006. Longitudinal study of non-cavitated carious lesion progression in the primary dentition. J Public Health Dent. 66 (2):83–87. [DOI] [PubMed] [Google Scholar]
- Wong HM, Peng SM, King NM, McGrath C. 2015. Infant growth and the occurrence of developmental defects of enamel in 12-year-olds. Caries Res. 49(6):575–582. [DOI] [PubMed] [Google Scholar]
- Wong HM, Peng SM, McGrath CPJ. 2019. Association of infant growth with emergence of permanent dentition among 12 year-aged southern Chinese school children. BMC Oral Health. 19(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2013. Oral health survey: basic methods. Report of a WHO Consultation. 5th ed. Geneva (Switzerland): World Health Organization. [Google Scholar]
- Zeng J, Williams SM, Fletcher DJ, Cameron CM, Broadbent JM, Shearer DM, Thomson WM. 2014. Re-examining the periodontal effects of smoking and periodontitis in the Dunedin study with an enhanced analytical approach. J Periodontol. 85(10):1390–1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520942208 for Oral Health Birth Cohort Studies: Achievements, Challenges, and Potential by K.G. Peres, W.M. Thomson, B.W. Chaffee, M.A. Peres, N. Birungi, L.G. Do, C.A. Feldens, M. Fontana, T.A. Marshall, W. Pitiphat, W.K. Seow, Y. Wagner, H.M. Wong and A.J. Rugg-Gunn in Journal of Dental Research