Abstract
There have been no studies as to whether parthanatos, a poly (adenosine diphosphate-ribose) polymerase-1 (PARP-1)-dependent and apoptosis-inducing factor (AIF)-mediated caspase-independent programmed cell death, is present in pulmonary hypertension (PH). Basic studies have, however, been conducted on several of the key molecules in parthanatos, such as PARP-1, AIF, and macrophage migration inhibitory factor (MIF). For this study, we collected blood samples from 88 incident male patients with PH and 50 healthy controls at the Shanghai Pulmonary Hospital. We measured the key factors of parthanatos (PARP-1, PAR, AIF, and MIF) by enzyme-linked immunosorbent assay and performed a logistic regression, Cox proportional hazards analysis, and Kaplan–Meier test to assess the prognostic value of the key molecules in diagnosing and predicting survival. The patients who ultimately died had a significantly poorer clinical status during the study than those who survived. The PARP-1, PAR, AIF, and MIF levels were significantly higher in the patients than in the controls (all p < .0001), and the PARP-1, PAR, and AIF levels were higher in the nonsurvivors than in the survivors (all p < .0001). PARP-1 and AIF levels served as independent predictors of disease onset and mortality in these patients (all p < .005). Patients with PARP-1 levels <11.24 ng/mL or AIF levels <1.459 pg/mL had significantly better survival than those with higher PARP-1 or AIF levels (p < .0001). Circulating levels of PARP-1 and AIF were independent predictors for PH onset and mortality, which indicated that parthanatos might be associated with the pathogenesis of PH.
Keywords: pulmonary hypertension, parthanatos, poly (adenosine diphosphateribose) polymerase-1, apoptosis inducing factor
Introduction
Pulmonary hypertension (PH) is a growing public healthcare problem that seriously threatens human physical and mental health (Galie et al., 2016; Hansmann et al., 2019). PH is mainly characterized by increased pulmonary vascular resistance (PVR) and narrowing of vascular lumen due to an imbalance in the proliferation and death of vascular and inflammatory cells (Thenappan et al., 2018). With the onset of pulmonary vascular remodeling, the right ventricular structure will be remodeled, thereby making right ventricular function an important prognostic factor (Wang et al., 2019). A variety of pathophysiological conditions such as hypoxia, oxidation stress, and inflammation, in addition to mitochondrial metabolic, genetic, and epigenetic factors can lead to changes in pulmonary vascular and myocardial cell phenotypes (Thenappan et al., 2018). The changes in cell phenotype constitute a complex phenomenon that involves the activation of numerous secondary pathways (Singh et al., 2019). Numerous studies have analyzed the abnormal proliferation and apoptotic mechanisms of vascular and myocardial cells; however, there have been no reports on the mechanisms behind the non-apoptotic programmed cell death known as parthanatos in vascular and myocardial cells during PH (Ma et al., 2020).
Parthanatos is a form of regulated cell death initiated by poly (adenosine diphosphate-ribose) (PAR) polymerase 1 (PARP-1) hyperactivation and precipitated by the consequent bioenergetic catastrophe coupled with apoptosis-inducing factor (AIF)-dependent and macrophage migration inhibitory factor (MIF)-dependent DNA degradation (Wang et al., 2016). Parthanatos appears to occur not only as a consequence of severe/prolonged alkylating DNA damage but also in response to oxidative stress, hypoxia, hypoglycemia, and inflammatory cues (David et al., 2009; Lipton et al., 1993). Previous studies have suggested that monocrotaline and Sugen/hypoxia (SuHx) induce PH in rats by increasing the apoptosis of the right ventricular myocardium via numerous pathways (Neto-Neves et al., 2017). The main mechanisms of parthanatos are overactivation of PARP-1/PAR in the nucleus and transfer to the mitochondria, as well as mitochondrial AIF nuclear translocation and the transfer of macrophage MIF by AIF to the nucleus, resulting in DNA cleavage thereby creating large fragments (Tarayrah-Ibraheim et al., 2021; Wang et al., 2011, 2016; Zhou et al., 2021). PARP-1, PAR, AIF, and MIF are therefore key factors in parthanatos. Previous studies have indicated that PARP-1 and MIF induce overproliferation and apoptosis in PH (Le Hiress et al., 2018, p. 1); however, there have been no studies on the effects of these key factors on the onset and mortality of PH.
The present study was therefore designed to investigate the expression of the key factors of parthanatos in PH and to analyze the potential association with the onset of PH and its outcomes.
Method
Study Sample
From August 2015 to September 2020, the study enrolled 88 patients with PH and 50 healthy controls aged 18 or older from the Department of Cardiopulmonary Circulation of Shanghai Pulmonary Hospital, China. The patients included those with idiopathic pulmonary arterial hypertension (IPAH; 55 patients), chronic obstructive pulmonary disease-related pulmonary hypertension (COPD-PH; 21 patients), and chronic thromboembolic pulmonary hypertension (CTEPH; 12 patients). The PH diagnosis was based on the European Society of Cardiology/European Respiratory Society criteria (Galie et al., 2016) and was determined according to the latest guidelines on PH. The exclusion criteria were as follows: anorexigen-associated PH, connective tissue disease, congenital heart disease, portal hypertension, HIV infection, other chronic respiratory diseases, acute or chronic diseases that might affect the hormone metabolism, and undergoing treatment with hormones or drugs that significantly inhibit hormone production. The study complied with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Shanghai Pulmonary Hospital (Study#K19-070Y). The research procedure was approved by the local ethics committee, and written informed consent from all participants was obtained.
Clinical Assessment
Demographic information, including age, body mass index (BMI), 6-min walk distance (6MWD), N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, and World Health Organization functional class (WHO-FC) were obtained at admission. The hemodynamic parameters were collected by right heart catheterization (RHC) at baseline. An 8F catheter sheath was placed in the right or left internal jugular vein, and a Swan-Ganz thermodilution catheter was inserted into the sheath (Edwards Life Sciences Company, Irving City, CA, USA). The patients’ mean pulmonary arterial pressure (mPAP), mean right atrial pressure (mRAP), mean pulmonary artery wedge pressure (mPAWP), PVR, and cardiac index (CI) were then evaluated.
Samples were collected in ethylenediamine tetraacetic acid (EDTA) anticoagulant under fasting conditions for preliminary evaluation. The plasma was immediately separated and frozen at −20 °C and stored at −80 °C until all samples were ready for the experiment. The plasma concentrations of PARP-1, PAR, AIF, and MIF were determined by enzyme-linked immunosorbent assay in the biochemical laboratory of Shanghai Pulmonary Hospital.
Statistical Analysis
The continuous variables conforming to normal distribution are expressed as means ± standard deviation. We employed an independent sample T test to compare the groups. The measurement data that did not conform to a normal distribution are expressed as medians and interquartile distances. We employed a Mann–Whitney U test to compare the groups (Yuan et al., 2020). The classification variables are expressed by the composition ratio. We employed a chi-squared test to compare the groups and Pearson’s or Spearman’s correlation test in the single factor correlation analysis. We employed single factor and multiple logistic and Cox regression to analyze the independent predictors for the onset and mortality of PH. We analyzed the receiver-operating characteristic (ROC) curves for the independent parameters. We measured the areas under the curves, cut-off values, sensitivity, and specificity of the independent parameters using an ROC analysis and estimated the survival using the Kaplan–Meier method and analyzed it using the log-rank test after grouping the patients using the cut-off value for each independent predictor. A p < .05 was considered a statistically significant difference. For the statistical analysis, we employed SPSS (Statistical Package for Social Science, Chicago, IL) version 22.0 and GraphPad Prism (San Diego, ca, USA) version 9.0 software.
Results
Characteristics of the Study Sample
A total of 88 patients with PH who met the inclusion criteria and 50 healthy controls were recruited for the present study. The mean follow-up duration was 34.7 ± 15.8 months, during which 15 (13%) patients died. None of the patients were lost to follow-up, resulting in a 100% follow-up rate. The mean ages were 57 ± 19 years for the nonsurvivors and 57 ± 16 years for the survivors. Table 1 presents the clinical features and hemodynamic data at baseline.
Table 1.
Baseline Characteristics of Controls and Patients With PH.
| Control |
PH patients |
p
value |
||
|---|---|---|---|---|
| Nonsurvivor | Survivor | |||
| (n = 50) | (n = 15) | (n = 73) | ||
| Age, y | 54 ± 12 | 57 ± 19 | 57 ± 16 | .134 |
| HR, bpm | 78.3 ± 10.6 | 87.1 ± 15.9 | 79.8 ± 13.4 | .064 |
| Diagnosis | .008 | |||
| IPAH, n | 7 | 48 | ||
| COPD-PH, n | 8 | 13 | ||
| CTEPH, n | 0 | 12 | ||
| WHO-FC, n | .136 | |||
| Ⅰ-Ⅱ/Ⅲ/Ⅳ | 2005/6/4 | 28/40/5 | ||
| 6MWD, m | 291.2 ± 151.5 | 366.2 ± 98.7 | .017 | |
| NT-proBNP, pg/mL | 2225.5 (209.0, 2948.5) | 359 (136.5, 1032.5) | .005 | |
| Hemodynamics | ||||
| mRAP, mmHg | 7.4 ± 5.5 | 3.9 ± 2.2 | .026 | |
| mPAP, mmHg | 58.9 ± 18.5 | 45.3 ± 13.2 | .001 | |
| mPAWP, mmHg | 11.2 ± 5.4 | 7.3 ± 4.9 | .007 | |
| PVR, Wood units | 14.7 ± 4.4 | 8.5 ± 4.3 | <.001 | |
| CI, L/min/m2 | 2.3 ± 0.8 | 3.1 ± 0.7 | <.001 | |
| Specific medications | .672 | |||
| PDE-5 inhibitors, % | 7 (46.7) | 21 (28.8) | ||
| ERAs, % | 1 (6.7) | 10 (13.7) | ||
| Prostacyclin analogs, % | 0 (0.0) | 1 (1.3) | ||
| Combination, % | 1 (6.7) | 5 (6.8) | ||
| Nonspecific medication, % | 6 (40.0) | 36 (49.3) | ||
Note. Data are presented as n (%); mean ± SD, and interquartile range. 6MWD = 6-minute walk distance, CI = cardiac index, ERA = endothelial receptor antagonist, HR = heart rate, mPAP = mean pulmonary arterial pressure, mPAWP = mean pulmonary capillary wedge pressure, mRAP = mean right atrial pressure, PDE-5 = phosphodiesterase type 5, PVR = pulmonary vascular resistance, WHO-FC = World Health Organization Functional Class.
The nonsurvivors had significantly lower 6MWDs and higher NT-proBNP levels than the survivors (p = .017 and p = .005, respectively; Table 1). The nonsurvivors had significantly higher mRAPs, mPAPs, mPAWPs, and PVRs, and lower CIs at baseline than the survivors (p = .026, p = .001, p = .007, p < .001, and p < .001, respectively; Table 1).
The targeted PH drugs included inhaled iloprost, intravenous iloprost, oral beraprost, oral bosentan, oral ambrisentan, oral sildenafil, oral vardenafil, and oral tadalafil (Table 1). There were no significant differences in the intake of these drugs between the nonsurvivors and survivors (Table 1).
Expression of PARP-1, PAR, AIF, and MIF in the Controls and Patients
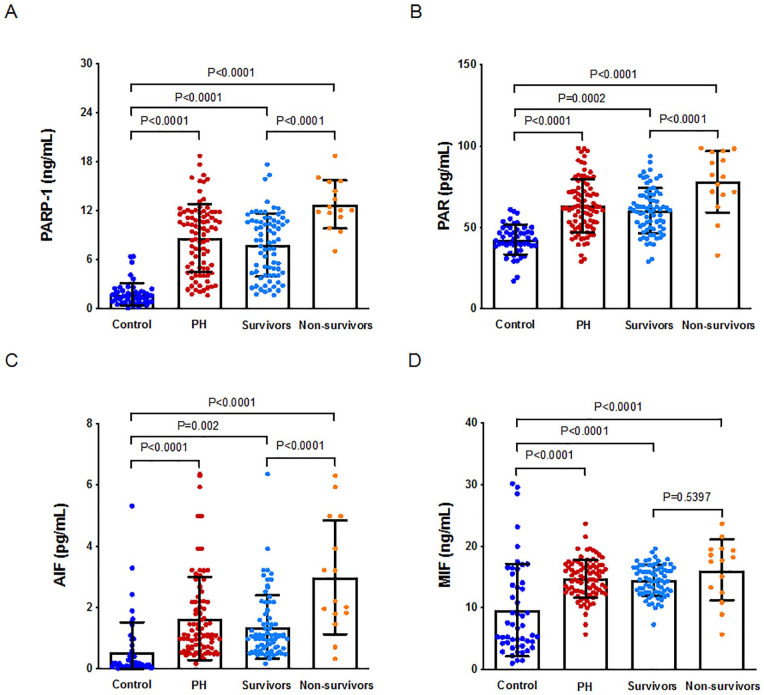
The levels were similarly upregulated in all patients (p < .0001, p = .0002, p < .0001, and p = .0002 for PARP-1, PAR, AIF, and MIF, respectively; Figure 1). The plasma PARP-1, PAR, and AIF levels were significantly lower in the survivors than in the nonsurvivors (p < .0001, p < .0001, and p < .0001, respectively; Figure 1), but there were no significant differences in MIF levels between the survivors and nonsurvivors (p < .5397; Figure 1).
Figure 1.
Expression of PARP-1, PAR, AIF and MIF in controls and patients with PH.
A. Expression of PARP-1 in controls and patients with PH. B. Expression of PAR in controls and patients with PH. C. Expression of AIF in controls and patients with PH. D. Expression of MIF in controls and patients with PH. Note. PH = pulmonary hypertension, PARP-1 = poly (adenosine diphosphate-ribose) polymerase-1, PAR = poly adenosine diphosphate-ribose, AIF = apoptosis inducing factor, MIF = migration inhibition factor.
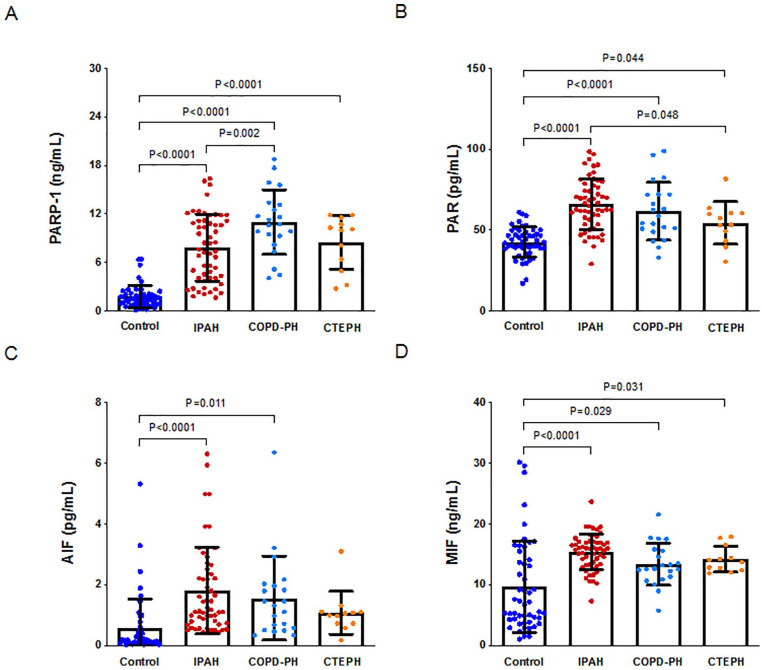
PARP-1 levels were significantly upregulated in the patients with IPAH, COPD-PH, and CTEPH compared with the controls (p < .0001, p < .0001, and p < .0001, respectively; Figure 2A). The increased PAR levels were consistent with the PARP-1 levels (p < .0001, p < .0001, and p = .044 for IPAH, COPDPH, and CTEPH, respectively; Figure 2B) and with the MIF levels (p < .0001, p = .029, and p = .031, respectively; Figure 2D). AIF levels were significantly upregulated in the patients with IPAH and COPD-PH compared with the controls (p < .0001 and p = .011; Figure 2C) but not in the patients with CTEPH (p > .05; Figure 2C).
Figure 2.
Expression of PARP-1, PAR, AIF and MIF in controls and different subgroups with PH.
A. PARP-1 levels in controls and different subgroups with PH. B. PAR levels in controls and different subgroups with PH. C. AIF levels in controls and different subgroups with PH. D. MIF levels in controls and different subgroups with PH. Note. IPAH = idiopathic pulmonary arterial hypertension, COPD-PH = chronic obstructive pulmonary disease-related pulmonary hypertension, CTEPH = chronic thromboembolic pulmonary hypertension.
PARP-1 levels were lower in the patients with IPAH than in the patients with COPD-PH (p = .002; Figure 2A). PAR levels were higher in the patients with IPAH than in the patients with CTEPH (p = .048; Figure 2B). However, there were no significant differences in plasma AIF and MIF levels among the various PH subgroups.
Correlations Between Plasma Levels of the PARP-1, PAR, AIF, and MIF and Clinical Parameters
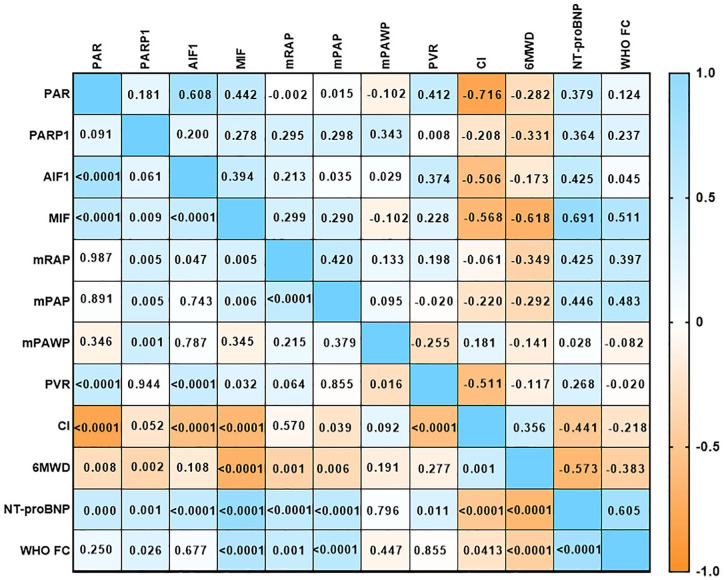
We found mild to moderate negative correlations between the plasma PARP-1, PAR, and MIF levels and 6MWD in all patients (p = .002, p = .008, and p < .0001, respectively; Figure 3). We also observed moderate positive correlations between PARP-1, PAR, AIF, and MIF and NT-proBNP levels in the patients (p = .001, p = .0001, p < .0001, and p < .0001, respectively; Figure 3) and a moderate positive correlation between plasma MIF levels and WHO-FC in the patients (p = .005; Figure 3). There were no significant correlations in the plasma AIF levels for 6MWD and WHO-FC in these patients (Figure 3).
Figure 3.
Correlations between plasma levels of the PARP-1, PAR, AIF, and MIF and clinical parameters in patients with PH.
Note. PH = pulmonary hypertension, PARP-1 = poly (adenosine diphosphate-ribose) polymerase-1, PAR = poly adenosine diphosphate-ribose, AIF = apoptosis inducing factor, MIF = migration inhibition factor.
There were mild to moderate positive and negative correlations between the PAR, AIF, MIF and PVR or and CI levels (but not PARP-1) in the patients (p = .0001, p < .0001, p = .032, and p = .944; p = .008, p < .0001, p < .0001, and p = .052, respectively; Figure 3). There were also mild positive correlations between the plasma PARP-1, AIF, and MIF levels and mRAP in these patients (p = .001, p < .0001, and p = .005, respectively; Figure 3). There were similar mild positive correlations between the plasma PARP-1 and MIF levels and mPAP (p = .005 and p = .006, respectively; Figure 3). There were no significant correlations between the PAR or AIF levels and mPAP in the patients (Figure 3).
Factors Influencing Onset and Mortality of Pulmonary Hypertension
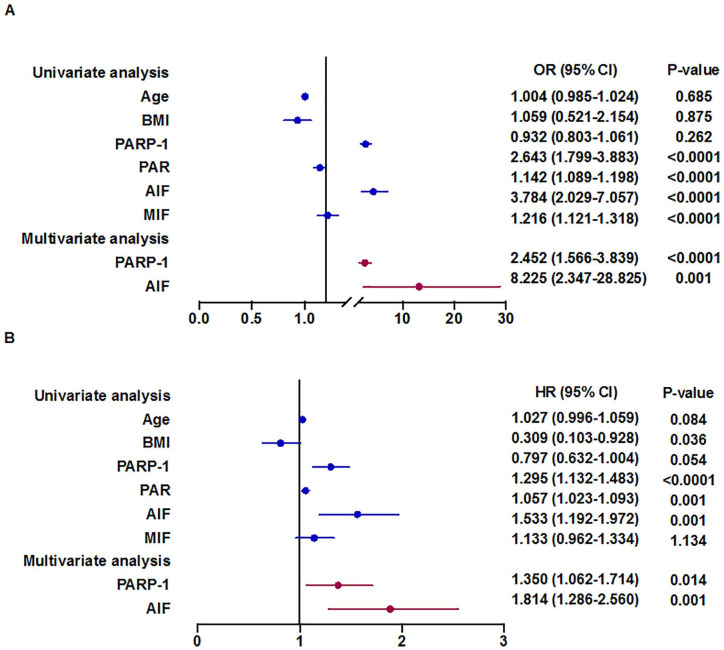
In the univariate logistic regression analysis, PARP-1, PAR, AIF, and MIF levels were significantly related to the onset of PH in all patients (p < .0001, p < .0001, p < .0001, and p < .0001, respectively; Figure 4). The multivariate forward stepwise logistic regression analysis revealed that PARP-1 and AIF levels were independent predictors of the onset of PH in all patients after adjusting for age and BMI (p < .0001 and p = .001, respectively; Figure 4). In the univariate Cox proportional hazards analysis, PARP-1, PAR, and AIF levels were significantly related to mortality for all patients (p < .0001, p = .001, and p = .001, respectively; Figure 4). The multivariate forward stepwise Cox proportional hazards analysis revealed that PARP-1 and AIF levels were independent predictors of survival for all patients after adjusting for age and BMI (p < .014 and p = .001, respectively; Figure 4). PARP-1 and AIF therefore play a significant role in predicting the onset and outcome of PH in this cohort.
Figure 4.
Factors influencing onset and mortality of PH.
A. Univariate and multivariate cox proportional hazards analysis relating OR to selected PARP-1, PAR, AIF and MIF levels. B. Univariate and multivariate cox proportional hazards analysis relating survival to selected PARP-1, PAR, AIF and MIF levels. Note. Multivariate analysis adjusted by age, sex, and BMI. BMI = body mass index, PARP-1 = poly (adenosine diphosphate-ribose) polymerase-1, PAR = poly adenosine diphosphate-ribose, AIF = apoptosis inducing factor, MIF = migration inhibition factor.
Receiver-Operating Characteristic Analysis
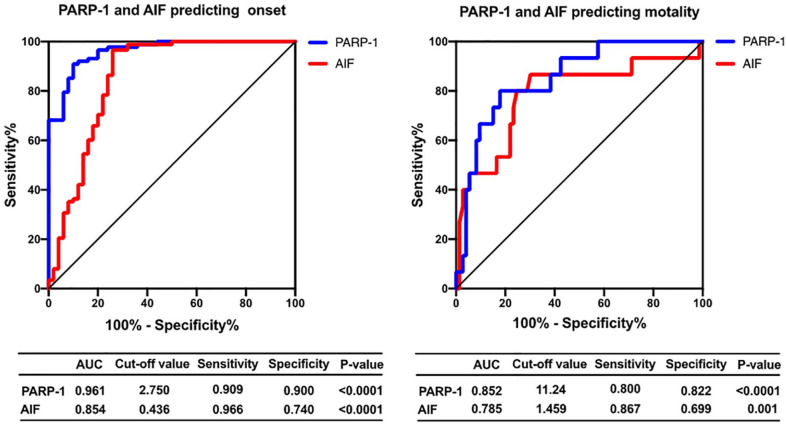
We performed ROC analyses to assess the sensitivity and specificity of PARP-1 and AIF as predictors for the onset and mortality of PH (Figure 5). Specifically, PARP-1 showed an area under the curve of 0.961 as a predictor of the onset of PH (p < .0001; Figure 5). The ROC-optimal PARP-1 cut-off value was 11.24 ng/mL, with a sensitivity and specificity of 90.9% and 90.0%, respectively. AIF could also be a predictor of the onset of PH, with an initial cut-off value of 0.436 for predicting the onset and a sensitivity and specificity of 96.6% and 74.0%, respectively (area under the curve, 0.854; p < .0001; Figure 5). In a further analysis, PARP-1 showed an area under the curve of 0.852 as a predictor of mortality (p < .0001; Figure 5). The ROC-optimal PARP-1 cut-off value was 11.24 ng/mL, with a sensitivity and specificity of 80.0% and 86.7%, respectively. AIF could also be a predictor of mortality, with an initial cut-off value of 1.459 for predicting mortality and a sensitivity and specificity of 86.7% and 69.9%, respectively (area under the curve, 0.785; p = .001; Figure 5).
Figure 5.
Receiver operating characteristics of PARP-1 and AIF in PH.
Note. PH = pulmonary hypertension, PARP-1 = poly (adenosine diphosphate-ribose) polymerase-1, AIF = apoptosis inducing factor.
Kaplan–Meier Survival Analysis
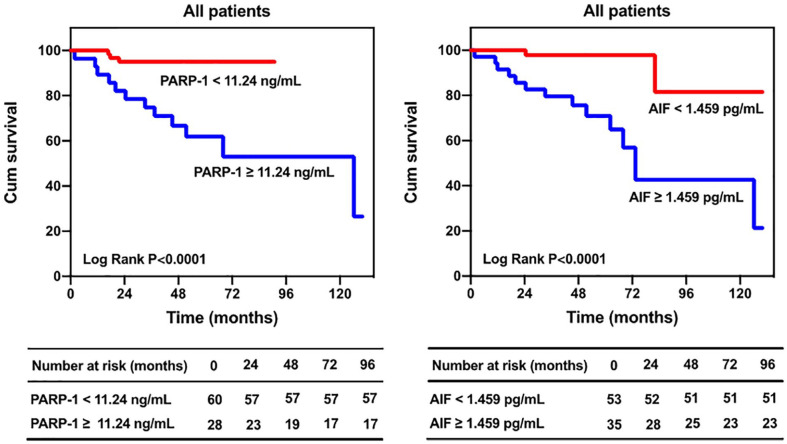
We grouped the patients by cut-off value. The Kaplan–Meier analysis indicated that the prognosis was significantly better for the patients with PARP-1 levels <11.24 ng/mL than for the patients with PARP-1 levels ≥11.24 ng/mL (p < .0001; Figure 6), and the prognosis was significantly better for the patients with AIF levels <1.459 pg/ mL than for the patients with AIF levels ≥1.459 pg/mL (p < .0001; Figure 6).
Figure 6.
Kaplan–Meier estimates of survival in patients with PH. Note. PH = pulmonary hypertension.
Discussion
The present study found that circulating levels of PARP-1, PAR, AIF, and MIF in the nonsurvivors were higher than in the survivors. There were significant correlations between plasma levels of PARP-1, PAR, AIF, MIF and mPAP, mRAP, PVR, and CI. Higher circulating levels of PARP-1 and AIF might influence the onset and mortality of PH, which indicates that parthanatos could be a powerful predictor of increased cardiopulmonary vascular risk.
The nonsurvivors in the present study had more severe PH, reduced 6MWDs, higher NT-proBNP levels, and more prominent hemodynamic parameters, suggesting that the nonsurvivors might have greater pulmonary arterial remodeling and vascular calcification, which contribute to pulmonary arterial complications and mortality independent of the classical cardiopulmonary vascular risk factors for PH. Our data indicated that PARP-1 levels were higher in the patients, especially in the nonsurvivors, results that are consistent with a previous study (Meloche et al., 2014) that reported that PARP-1 overactivation caused by DNA damage plays an important role in the development of PH. Another study demonstrated that PARP-1 played an essential role in arteriosclerotic calcification (Li et al., 2020), which suggests that PARP-1 might contribute to vascular cell and cardiomyocyte calcification in PH. The increased PARP-1 levels in the nonsurvivors, which were associated with higher mPAP, PVR, and CI in this cohort, therefore indicates that PARP-1 might be associated with cardiopulmonary vascular calcification and remodeling in PH.
Plasma AIF levels were also upregulated in the patients and were more expressed in the nonsurvivors. AIF was similarly correlated with NT-proBNP, PVR, mRAP, and CI levels. The data also demonstrated that AIF might play a role in the development of pulmonary arterial and right ventricular remodeling in PH, which could partly be explained by the fact that AIF is an important component of the apoptosis pathway under hyperoxia (Kondrikov et al., 2015; Madungwe et al., 2018). PARP-1 and AIF-dependent parthanatos might also play an important role in the right ventricular remodeling of PH. AIF levels were not significantly upregulated in patients with CTEPH. There were two possible reasons for this result. One is the pathogenesis of CTEPH maybe different from IPAH and COPD-PH. Another reason maybe that the sample size of the CTEPH group was the relatively small, which leads to no significant difference. However, it can also present an upward trend in CTEPH group compared with control group. Perhaps increasing the sample size in our further study will also have significant differences in CTEPH patients.
As a pleiotropic upstream proinflammatory mediator, MIF is a promising molecular target for PH therapy because MIF contributes to perivascular inflammation and pulmonary artery remodeling (Jalce & Guignabert, 2020). In addition, the increase in MIF levels in the pulmonary artery of PH will induce the proliferation of pulmonary vascular smooth muscle cells, leading to pulmonary artery remodeling (Li et al., 2017). Our data also showed that MIF levels were increased in the patients, with a similar correlation between MIF levels and clinical parameters, especially WHO-FC, mPAP, and CI. The increase in PARP-1, PAR, AIF, and MIF levels might be related to cell proliferation or apoptosis; however, when the factors work in conjunction, the new type of cell death (parthanatos) can occur. At present, there have been no reports on parthanatos in PH, but there have been various indications that parthanatos might be present in the onset and development of PH.
Among the noninvasive variables measured in the present study, plasma PARP-1 and AIF levels can independently predict the onset and mortality of PH in the regression analysis. Patients with higher plasma PARP-1 and AIF levels have a higher risk of PH onset and mortality. However, we did not find that PAR and MIF were independent predictors for the onset and mortality of PH in this cohort, which might be due to the limited sample size of our study. Whether this result is related to the different mechanisms of PAR and MIF in the process of parthanatos requires further study.
There are a number of limitations to the present study. First, the number of male patients included for analysis was relatively small. Other factors, such as sex, ethnicity, and genetic predisposition, which could influence the metabolic and exercise capacity, were not considered in the present study. Further study is needed to explore the mechanisms of parthanatos in contributing to the pathogenesis of PH.
Conclusion
Plasma PARP-1 and AIF levels are positively correlated with the severity of PH, and high plasma PARP-1 and AIF levels were independently associated with an increased risk for the diagnosis and mortality of PH. Future studies with larger sample sizes are needed to confirm our findings.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Program of National Natural Science Foundation of China (81870042, 81700045 and 81900050), Program of Shanghai Pulmonary Hospital (FKLY20005), National Science and Technology Information System of the People’s Republic of China (2018YFC1313603), and Program of Natural Science Foundation of Shanghai (21ZR1453800, 18ZR1431500), Program of Shanghai Science and Technology Committee (201409004100).
ORCID iD: Ping Yuan  https://orcid.org/0000-0001-5096-4850
https://orcid.org/0000-0001-5096-4850
References
- David K. K., Andrabi S. A., Dawson T. M., Dawson V. L. (2009). Parthanatos, a messenger of death. Frontiers in Bioscience (Landmark Edition), 14, 1116–1128. 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie N., Humbert M., Vachiery J. L., Gibbs S., Lang I., Torbicki A., & Group, E. S. C. S. D. (2016). 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS): Endorsed by: Association for european paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). European Heart Journal, 37(1), 67–119. 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- Hansmann G., Koestenberger M., Alastalo T. P., Apitz C., Austin E. D., Bonnet D., Zartner P. (2019). 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. Journal of Heart and Lung Transplantation, 38(9), 879–901. 10.1016/j.healun.2019.06.022. [DOI] [PubMed] [Google Scholar]
- Jalce G., Guignabert C. (2020). Multiple roles of macrophage migration inhibitory factor in pulmonary hypertension. American Journal of Physiology: Lung Cellular and Molecular Physiology, 318(1), L1–L9. 10.1152/ajplung.00234.2019. [DOI] [PubMed] [Google Scholar]
- Kondrikov D., Fulton D., Dong Z., Su Y. (2015). Heat shock protein 70 prevents hyperoxia-induced disruption of lung endothelial barrier via caspase-dependent and AIF-dependent pathways. PLoS One, 10(6), e0129343. 10.1371/journal.pone.0129343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hiress M., Akagah B., Bernadat G., Tu L., Thuillet R., Huertas A., Guignabert C. (2018). Design, synthesis, and biological activity of new N-(Phenylmethyl)-benzoxazol-2-thiones as Macrophage Migration Inhibitory Factor (MIF) antagonists: Efficacies in experimental pulmonary hypertension. Journal of Medicinal Chemistry, 61(7), 2725-2736. 10.1021/acs.jmedchem.7b01312. [DOI] [PubMed] [Google Scholar]
- Li H., Wang Y., Chen L., Han L., Li L., He H., Wang W. (2017). The role of MIF, cyclinD1 and ERK in the development of pulmonary hypertension in broilers. Avian Pathology, 46(2), 202–208. 10.1080/03079457.2016.1245409. [DOI] [PubMed] [Google Scholar]
- Li P., Wang Y., Liu X., Liu B., Wang Z. Y., Xie F., Zhang M. X. (2020). Loss of PARP-1 attenuates diabetic arteriosclerotic calcification via Stat1/Runx2 axis. Cell Death & Disease, 11(1), 22. 10.1038/s41419-019-2215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Stamler J. S. (1993). A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature, 364(6438), 626–632. 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Ma H., Zheng L., Qin H., Wang W. (2020). Myocardial infarction-associated transcript knockdown inhibits cell proliferation, migration, and invasion through miR-490-3p/intercellular adhesion molecule 1 axis in oxidized low-density lipoprotein-induced vascular smooth muscle cells. Journal of Cardiovascular Pharmacology, 76(5), 617–626. 10.1097/FJC.0000000000000901. [DOI] [PubMed] [Google Scholar]
- Madungwe N. B., Feng Y., Lie M., Tombo N., Liu L., Kaya F., Bopassa J. C. (2018). Mitochondrial inner membrane protein (mitofilin) knockdown induces cell death by apoptosis via an AIF-PARP-dependent mechanism and cell cycle arrest. American Journal of Physiology-Cell Physiology, 315(1), C28–C43. 10.1152/ajpcell.00230.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche J., Pflieger A., Vaillancourt M., Paulin R., Potus F., Zervopoulos S., Bonnet S. (2014). Role for DNA damage signaling in pulmonary arterial hypertension. Circulation, 129(7), 786–797. 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- Neto-Neves E. M., Frump A. L., Vayl A., Kline J. A., Lahm T. (2017). Isolated heart model demonstrates evidence of contractile and diastolic dysfunction in right ventricles from rats with sugen/hypoxia-induced pulmonary hypertension. Physiological Reports, 5(19). 10.14814/phy2.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I., Rahaghi F. N., Naeije R., Oliveira R. K. F., Systrom D. M., Waxman A. B. (2019). Right ventricular-arterial uncoupling during exercise in heart failure with preserved ejection fraction: Role of pulmonary vascular dysfunction. Chest, 156(5), 933–943. 10.1016/j.chest.2019.04.109. [DOI] [PubMed] [Google Scholar]
- Tarayrah-Ibraheim L., Maurice E. C., Hadary G., Hur S. B., Kolpakova A., Braun T., Peleg Y., Yacobi-Sharon K., Arama E. (2021). DNase II mediates a parthanatos-like developmental cell death pathway in Drosophila primordial germ cells. Nature Communications, 12(1), 2285. 10.1038/s41467-021-22622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenappan T., Ormiston M. L., Ryan J. J., Archer S. L. (2018). Pulmonary arterial hypertension: Pathogenesis and clinical management. British Medical Journal, 360, j5492. 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang Q., Tian Z., Guo X., Lai J., Li M., Fang Q. (2019). Right ventricular function is associated with quality of life in patients with systemic lupus erythematosus associated pulmonary arterial hypertension. Heart, Lung & Circulation, 28(11), 1655–1663. 10.1016/j.hlc.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Wang Y., An R., Umanah G. K., Park H., Nambiar K., Eacker S. M., Dawson T. M. (2016). A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science, 354(6308). 10.1126/science.aad6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kim N. S., Haince J. F., Kang H. C., David K. K., Andrabi S. A., Dawson T. M. (2011). Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Science Signal, 4(167), ra20. 10.1126/scisignal.2000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Wang Z., Wang L., Zhao Q., Gong S., Sun Y., Yuan P. (2020). Increased levels of Runt-related transcription factor 2 are associated with poor survival of patients with idiopathic pulmonary arterial hypertension. American Journal of Mens Health, 14(4), 1557988320945458. 10.1177/1557988320945458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liu L., Tao S., Yao Y., Wang Y., Wei Q., Shao A., Deng Y. (2021).Parthanatos and its associated components: Promising therapeutic targets for cancer. Pharmacological Research, 163, 105299. 10.1016/j.phrs.2020.105299. [DOI] [PubMed] [Google Scholar]