Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Cheminova A/S submitted a request to the competent national authority in Spain to modify the existing maximum residue levels (MRLs) for the active substance acrinathrin in peaches and sweet peppers. The data submitted in support of the request were found sufficient to derive MRL proposals for both crops. Adequate analytical methods for enforcement are available to control the residues of acrinathrin and its enantiomer in the plant matrices under consideration at the validated limit of quantification (LOQ) of 0.02 mg/kg. Based on the risk assessment results, EFSA concluded that the short‐ and long‐term intake of residues resulting from the use of acrinathrin according to the reported agricultural practices is unlikely to present a risk to consumer health.
Keywords: acrinathrin, peaches, nectarines, sweet peppers, pesticide, MRL, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, Cheminova A/S submitted an application to the competent national authority in Spain (evaluating Member State, EMS) to modify the existing maximum residue levels (MRLs) for the active substance acrinathrin in peaches and sweet peppers. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 1 June 2017. To accommodate for the intended uses of acrinathrin in southern Europe (SEU), the EMS proposed to raise the existing MRLs in both crops from the limit of quantification (LOQ) of 0.02 to 0.1 mg/kg.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified data requirements, which were requested from the EMS. On 12 April 2021 the EMS submitted a revised evaluation report, which replaced the previously submitted evaluation report.
Based on the conclusions derived by EFSA in the framework of Regulation (EC) No 1107/2009 (EU pesticides peer review) and in the review of the existing EU MRLs of acrinathrin according to Article 12 of Regulation (EU) No 396/2005 (MRL review), the assessment of a recent MRL application and the additional data provided by the EMS in the framework of this application, the following conclusions are derived.
The metabolism of acrinathrin following foliar application was investigated during the EU pesticides peer review and in the MRL review in fruit crops and leafy crops. In the recent assessment of an MRL application on lettuce, a new metabolism study on lettuce was provided. In the framework of the current assessment, the applicant submitted a new metabolism study on tomatoes. Amongst all available metabolism studies, only the metabolism study on grapes (which detects the enantiomeric pairs of isomers but not acrinathrin enantiomer), oranges (separation of acrinathrin (and enantiomer) from other enantiomeric pairs of isomers) and the new metabolism studies in lettuce and tomatoes (which detect the enantiomeric pairs of isomers as well as acrinathrin enantiomer) could be considered valid to address the metabolism in leafy and fruit crops. The results from new, overdosed metabolism studies indicate negligible formation of acrinathrin isomers in tomatoes and a complete absence of acrinathrin enantiomer at the preharvest intervals (PHIs) investigated.
Standard hydrolysis studies investigating the effect of processing on the nature of acrinathrin are not available and are not required, considering the low contribution of residues in the crops under assessment to the dietary exposure of acrinathrin and the residues below 0.1 mg/kg in raw agricultural commodity (RAC).
According to the EU pesticides peer review, for rotational crops there is no need to set a specific residue definition due to the low residue levels identified in the metabolism studies.
Based on the metabolic pattern identified in grape metabolism study, the toxicological significance of the metabolites and acrinathrin isomers, the capabilities of analytical enforcement methods, the residue definitions for fruit crops were proposed by the EU pesticides peer review as ‘acrinathrin and its enantiomer’ for enforcement and ‘acrinathrin and all isomers’ for the risk assessment. The risk assessment residue definition has been proposed, pending the investigation of the toxicological profile of the 15 acrinathrin potential isomers.
According to the previous EFSA assessment, the proposed residue definitions can be extended also to leafy crop group. On the basis of studies in three fruit crops, EFSA concludes that the acrinathrin isomers (including its enantiomer) will not occur at relevant levels in the crops under consideration when treated according to the intended Good Agricultural Practice (GAP). The lettuce and tomatoes metabolism studies also indicate that there might be no need to consider all acrinathrin isomers in the risk assessment residue definition. However, since isomerisation depends on the crop and the PHI, this conclusion is applicable only for the crops under consideration. Moreover, metabolism studies are available only for two crop groups (fruit crops and leafy crops), and therefore a final conclusion on the need for revision of the risk assessment residue definition cannot be taken.
For the crops assessed in this application, EFSA concludes that metabolism of acrinathrin in primary crops has been addressed and that the previously derived residue definitions are applicable.
Sufficiently validated analytical methods are available to quantify residues at or above 0.02 mg/kg (LOQ) for routine analysis in the crops assessed in this application according to the enforcement residue definition. A lower LOQ of 0.01 mg/kg was achieved with an analytical method assessed in a previous EFSA opinion. The methods are not stereoselective and quantify the sum of acrinathrin and its enantiomer, thus complying with the enforcement residue definition.
The available residue trials are sufficient to derive an MRL proposal of 0.08 mg/kg both in peaches and sweet peppers in support of intended SEU uses. The EMS derived an MRL proposal of 0.1 mg/kg, by rounding up the value of 0.08 mg/kg.
Specific studies investigating the magnitude of acrinathrin residues in processed commodities have not been submitted and are currently not required, since significant residues are not expected in the RAC and the total theoretical maximum daily intake (TMDI) is below the trigger value of 10% of the ADI.
Based on the available information on the nature of residues in rotational crops, EFSA concludes that significant translocation of residues is not expected if crops are grown in rotation with sweet peppers, being treated according to the intended GAP.
Residues of acrinathrin in commodities of animal origin were not assessed since the crops under consideration in this MRL application are normally not fed to livestock.
The toxicological profile of acrinathrin was assessed in the framework of the EU pesticides peer review under Regulation (EC) No 1107/2009 and the data were sufficient to derive an acceptable daily intake (ADI) of 0.01 mg/kg body weight (bw) per day and an acute reference dose (ARfD) of 0.01 mg/kg bw. The applicant in the framework of the current assessment provided additional information, which allowed to conclude that isomers included in the risk assessment residue definition are not genotoxic and three of these acrinathrin isomers are expected to be less acutely neurotoxic than acrinathrin but for the other 12 isomers, including the enantiomer of acrinathrin, no conclusion could be made regarding their general toxicity. However, since the metabolism of acrinathrin is clearly depicted in two crop groups only and since the potential formation of isomers depends on the crop/PHI and the general toxicity of certain isomers has not been addressed, a worst‐case conversion factor of 1.1 derived from grape metabolism study and proposed by the MRL review was applied to the crops under consideration in the consumer exposure assessment.
The consumer risk assessment was performed with revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo). The short‐term exposure assessment was performed only for peaches and sweet peppers and did not exceed the ARfD, accounting for a maximum of 52% of the ARfD and 33% of the ARfD, respectively. For the calculation of the chronic exposure, EFSA used the median residue values (STMR) as derived from the residue trials submitted and the STMRs available from previously issued EFSA opinions. The estimated long‐term dietary intake accounted for a maximum of 2% of the ADI (PT general diet). The contribution of residues expected in peaches and sweet peppers to the overall long‐term exposure accounted for a maximum of 0.12% of the ADI for peppers and 0.08% of the ADI for peaches.
Although general uncertainties related to the toxicological profile of different isomers remain, the data gap set by the EU pesticides peer review regarding the toxicological profile of acrinathrin isomers is not relevant for the crops under consideration. Thus, EFSA concludes that residues in crops from the intended and existing uses are unlikely to pose a risk to consumer's health.
EFSA proposes to amend the existing MRLs as reported in the summary table below.
Full details of all end points and the consumer risk assessment can be found in Appendices B–D.
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Acrinathrin and its enantiomerF | ||||
| 0140030 | Peaches | 0.02* | 0.08 | The submitted data are sufficient to derive MRL proposal for the intended SEU uses. Although general uncertainties related to the toxicological profile of different isomers remain, for the uses under consideration this data gap is not fully justified. Risk for consumers unlikely. |
| 0231020 | Sweet peppers/bell peppers | 0.02* | 0.08 | |
MRL: maximum residue level; SEU: southern Europe; GAP: Good Agricultural Practice.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Fat soluble.
Assessment
The European Food Safety Authority (EFSA) received an application to modify the existing maximum residue levels (MRL) for the active substance acrinathrin in peaches and sweet peppers. The detailed description of the intended uses of acrinathrin, which is the basis for the current MRL application, is reported in Appendix A.
Acrinathrin is the ISO common name for (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate (IUPAC).
The four asymmetric centres present in acrinathrin lead to the possibility of 16 isomers, which compose eight pairs of enantiomers; acrinathrin is one isomer of an enantiomeric pair.
The chemical structures of the active substance, its isomers and main metabolites are reported in Appendix E.
Acrinathrin was evaluated in the framework of Regulation (EC) No 1107/20091 with France designated as rapporteur Member State (RMS) for the representative uses as an insecticide and acaricide on wine grapes, table grapes and ornamentals. The draft assessment report (DAR) prepared by the RMS has been peer reviewed by EFSA (EFSA, 2013). Acrinathrin was approved2 for the use as insecticide and acaricide on 1 January 2012 with a specific provision related to application rate not exceeding 22.5 g/ha per application.
The EU MRLs for acrinathrin are established in Annex II of Regulation (EC) No 396/20053. The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) has been performed (EFSA, 2015) and the proposed modifications have been implemented in the MRL legislation only for the commodities where the GAPs were compliant with the current approval restrictions of acrinathrin. The MRL proposal from the recent reasoned opinion on lettuce has not been implemented yet in regulation.4
In accordance with Article 6 of Regulation (EC) No 396/2005, Cheminova A/S submitted an application to the competent national authority in Spain (evaluating Member State, EMS) to modify the existing MRLs for the active substance acrinathrin in peaches and sweet peppers. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to EFSA on 1 June 2017. To accommodate for the intended uses of acrinathrin, the EMS proposed to raise the existing MRLs from the limit of quantification (LOQ) at 0.02 to 0.1 mg/kg for both crops.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified data requirements, which were requested from the EMS. On 12 April 2021, the EMS submitted a revised evaluation report, which replaced the previously submitted evaluation report.
EFSA based its assessment on the evaluation report submitted by the EMS (Spain, 2017), the draft assessment report (DAR) and its addenda (France, 2007, 2010, 2012, 2013) prepared under Regulation (EC) No 1107/2009, the Commission review report on acrinathrin (European Commission, 2017a), the conclusions on the peer review of the pesticide risk assessment of the active substance acrinathrin (EFSA, 2013) as well as the conclusions from previous EFSA opinions on acrinathrin (EFSA, 2015, 2020), including the reasoned opinion on the MRL review according to Article 12 of Regulation No 396/2005.
For this application, the data requirements established in Regulation (EU) No 544/20115 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 1997a,b,c,d,e,f,g, 2000, 2010a,b, 2017b; OECD, 2011). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20116.
A selected list of end points of the studies assessed by EFSA in the framework of this MRL application including the end points of relevant studies assessed previously, are presented in Appendix B.
The evaluation report submitted by the EMS (Spain, 2017) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Mammalian toxicology
The toxicity of acrinathrin has been investigated in the framework of the EU pesticides peer review which derived an acceptable daily intake (ADI) value of 0.01 mg/kg body weight (bw) per day and an acute reference dose (ARfD) of 0.01 mg/kg bw (EFSA, 2013). However, during the EU pesticides peer review, the data gap for further identification/quantification of the potential 16 acrinathrin isomers and further assessment of the toxicological profile of the 15 isomers of acrinathrin was established (EFSA, 2013). The EU pesticides peer review concluded that, depending on further identification/quantification of the 16 possible isomers to which the consumers could be exposed to, and further assessment of the toxicity of the 15 isomers which are not the active substance, the applicability of the toxicological reference values for acrinathrin (being only one single isomer) to other isomers might need to be reconsidered (EFSA, 2013).
In the framework of the current assessment, the applicant provided information on the identity of 16 isomers as outlined in Appendix E. Additionally, the applicant provided toxicity assessment of 15 isomers included in the proposed residue definition for the risk assessment (Spain, 2017).
Following the assessment of available studies, the EMS and EFSA concluded that the 15 isomers of acrinathrin (including its enantiomer) are not genotoxic as outlined in Appendix B. Three of these isomers – enantiomer of RU45120 (1S, cis, Z, αS), RU39592 (1R, trans , Z, αS) and RU45120 (1R, cis, Z, αR) – are expected to be less acutely neurotoxic than acrinathrin but for the other 12 isomers no conclusion could be made regarding their general toxicity (high, equal, less acutely neurotoxic than acrinathrin). The data also did not allow to conclude on the general toxicity of acrinathrin enantiomer (R‐acrinathrin or RU 48238).
The metabolite 3‐phenoxybenzoic acid (PBA or 3‐PBAcid), which was identified in the metabolism study of tomato (above 10% total radioactive residue (TRR), but at low actual levels) is a common metabolite to many of the pyrethroids in general. During the EU pesticides peer review of lambda‐cyhalothrin, it was concluded that the toxicological properties of this compound are not sufficiently addressed. Pending on the toxicological profile of the aforementioned metabolite,7 it needs to be considered if a similar risk assessment approach as for the triazole derivative metabolites (TDMs) will have to be chosen in future (EFSA, 2014).
2. Residues in plants
2.1. Nature of residues and methods of analysis in plants
2.1.1. Nature of residues in primary crops
The metabolism of acrinathrin on fruit crops (grapes, apples, cucumbers) and on leafy crops (cabbages) has been investigated in the framework of the EU pesticides peer review (EFSA, 2013) and on oranges in the MRL review (EFSA, 2015). During the EU pesticides peer review and the MRL review it was concluded that, since the levels of each of the isomers or enantiomeric pairs of isomers present had not been quantified in apples, cabbages, cucumbers, these studies were considered insufficient to determine the nature of acrinathrin residues in primary crops.
In grapes (2 × 22.5 g/ha (both radiolabels); 2 × 0.225 kg/ha (one label)), acrinathrin constituted the most important component of the residue in berries (38% TRR; 0.0037–0.031 mg eq/kg) and in leaves (15.4–18.8% TRR; 0.136–0.139 mg eq/kg). The isomer fraction accounted for a maximum of 9% TRR in berries (0.008 mg eq/kg) and 5.4% TRR (0.046 mg eq/kg) in leaves 28 DAT. At the 10N application rate, acrinathrin was also the most important component of the residue in berries (54% TRR; 0.33 mg eq/kg), whereas the isomer fraction accounted for maximum of 4% TRR (0.023 mg eq/kg) in berries and of 4.6% TRR (0.31 mg eq/kg) in leaves 28 DAT. Up to 11 minor metabolites were present in berries at < 0.01 mg eq/kg and < 10% TRR. All were unidentified except one which was tentatively identified as 3‐phenoxybenzyl alcohol (3‐PBAlc). Some photoisomerisation and epimerisation were observed in the studies but acrinathrin isomers were present at low levels in berries at harvest (5–9% TRR; 0.004–0.008 mg eq/kg).
The relatively small amounts of metabolites (other than isomers) detected are not expected to contribute significantly to the toxicological burden (EFSA, 2015). Based on the metabolism study in grapes, which was the only study available for the EU pesticides peer review analysing all pairs of isomers, it was concluded that isomerisation could occur as part of the normal metabolic process and the isomer profile is dependent on the preharvest interval (PHI) (EFSA, 2013). This study, however, did not use chiral HPLC method and was therefore not able to distinguish between acrinathrin and its own enantiomer. The EU pesticides peer review concluded that although the amount of isomers other than acrinathrin and its enantiomer is expected to be low for the representative use in grapes, it is unknown if the acrinathrin enantiomer will be present and to what extent, and if its toxicity will be comparable to that of acrinathrin.
In oranges (2 × 0.09 kg/ha), the radioactivity in fruits at harvest was mainly found in the peel (89–92% TRR) with the remainder in the rinse solution, pulp and juice. The residue was mainly acrinathrin (66–85% of TRR in the peel; 0.095–0.205 mg eq/kg). Radioactivity levels in pulp and juice were too low to allow identification and quantification of metabolites. It is noted that the isomers represent less than 10% of the TRR in peel and, whilst the analytical methods used do not allow clear identification of individual isomers, the data indicate that isomerisation was limited (EFSA, 2015).
A new metabolism study representing fruit crops (tomatoes, 3 × 60 g/ha) has been submitted in support of the present MRL application and evaluated under the current assessment (Spain, 2017). Furthermore, a new study representing leafy crops (lettuce, 2 × 60 g/ha) was recently evaluated in the framework of a previous MRL application on lettuce (EFSA, 2020).
In lettuce, acrinathrin was the most prominent component detected and accounted for 37.2–64.5% of TRR (0.103–1.197 mg eq/kg). Significant photoisomerisation of acrinathrin did not occur; acrinathrin enantiomer was not detected. Other isomers of acrinathrin were not present in lettuce at relevant levels. Other identified metabolites were in the range of 0.4–8.0% of TRR (0.003–0.064 mg eq/kg). The low levels of isomers found in the new metabolism study in lettuce suggested that in lettuce the main component of the residues is parent acrinathrin only. Other isomers, for which the data gap regarding their toxicological profile was set in the peer review and the MRL review, were not present at significant amounts (EFSA, 2020).
Although metabolism studies in lettuce and grapes indicate low extent of isomerisation, it was also noted that isomerisation varies between crops and depends on the PHI (EFSA, 2013, 2015).
In tomato fruit at PHI of 3–14 days, the total TRR accounted for 0.036–0.039 mg eq/kg for the cyclopropyl label study and 0.011–0.028 mg eq/kg for the phenoxy label study. Acrinathrin was the main component of the TRR, accounting for 24.5–61.4% (0.007–0.022 mg eq/kg) and majority of it was found in fruit rinses. Metabolite 3 phenoxy‐benzoic acid (3‐PBacid or PBA) was identified at levels of 6.7–10.9% TRR (0.001–0.003 mg eq/kg). Other metabolites were detected at levels ranging from 0.1% to 7.1% TRR (< 0.001–0.003 mg eq/kg). Three complementary HPLC methods were utilised to allow identification of metabolites and photoisomers of acrinathrin: reverse‐phase analysis was used to separate acrinathrin from its metabolic transformation products directly in the rinses and combined extracts from fruit; normal‐phase analysis was used to separate enantiomeric pairs of acrinathrin from each other and, if necessary, chiral analysis was used to separate enantiomers from each other (Spain, 2017).
The unidentified compounds ranged from 22.4% to 52.5% TRR. In the cyclopropyl label study, their amounts increased with longer PHI, but none of them individually exceeded trigger values of 10% TRR or 0.01 mg/kg. In the phenoxy label study the levels of unidentified compounds were generally the same at all PHIs and three components individually accounted for 10.2–12.2% TRR with very low actual amounts (≤ 0.003 mg eq/kg).
The partition of selected rinses and fruit extracts with hexane was used to separate acrinathrin and its isomers from metabolites. Significant photoisomerisation was not evidenced; acrinathrin accounted for 91.6–100% in surface rinses and extracts from tomato. The enantiomer of acrinathrin (RU 48238) was not present in any of the fractions analysed. In fruit rinses from samples taken at 3 and 7 days after last application (DALA) no isomerisation was observed (acrinathrin 100%); in fruit extracts from samples taken 3 DALA, acrinathrin represented 97–100%. The isomerisation to a minor extent (7.4–8.4%) was observed in leaf rinse, however, only samples taken at 14 DALA were analysed.
The metabolism in tomatoes indicates that acrinathrin is the main residue in fruit and that the isomerisation occurs at negligible levels, if at all. Neither the acrinathrin enantiomer (included in the enforcement residue definition), nor the 15 remaining potential isomers of acrinathrin (included in the provisional risk assessment residue definition), were present in tomato fruits at levels requiring further consideration. The data from grapes and oranges metabolism study are also relevant for the intended uses on fruit crops and indicates that the isomers’ exposure is low. Nevertheless, the EU pesticides peer review could not conclude whether the acrinathrin enantiomer will be present or not in grapes at higher application rates and if its toxicity will be comparable to that of acrinathrin (EFSA, 2013).
On the basis of studies in three fruit crops, EFSA concludes that acrinathrin isomers are not expected to occur at relevant levels in the crops under consideration when treated according to the intended GAP. The lettuce and tomato metabolism studies indicate that there might be no need to consider all acrinathrin isomers in the risk assessment residue definition. However, since isomerisation depends on the crop and the PHI, this conclusion is applicable only for the fruit crops under consideration and, in leafy crops. Moreover, metabolism studies are available only for two crop groups (fruit crops and leafy crops), and therefore a final conclusion on the need for revision of existing risk assessment residue definition cannot be taken.
2.1.2. Nature of residues in rotational crops
Sweet pepper can be grown in a crop rotation and therefore the nature of acrinathrin residues in rotational crops would need to be investigated. According to the soil degradation studies evaluated in the framework of the EU pesticides peer review (EFSA, 2013), the DT90 value of acrinathrin and its enantiomer ranged from 0.5 to 887 days. The trigger value of 100 days was exceeded and therefore further studies investigating the nature of residues in rotational crops were required. The DT90 value of the relevant soil metabolite (DPA‐A) (identified at 10.5% applied radioactivity (AR)) is below the trigger value of 100 days.
The metabolism of acrinathrin in rotational crops (wheat, spinaches, carrots and kohlrabi) has been evaluated during the peer review (EFSA, 2013) with 14C‐labelled acrinathrin applied on a bare soil at application rate of 113–158 g/ha. The residues were not further identified due to the very low amounts detected. Therefore, a specific residue definition for rotational crops was not deemed necessary.
The same conclusions are applicable also for the intended use on the sweet peppers.
2.1.3. Nature of residues in processed commodities
The effects of processing on the nature of acrinathrin residues under processing conditions have not been investigated.
EFSA agrees with the arguments of the applicant and the EMS that there is no need to conduct a standard hydrolysis study since residues of acrinathrin in the crops under consideration are below 0.1 mg/kg and the theoretical maximum daily intake (TMDI) is expected to be below 10% of the acceptable daily intake (ADI) according to current guidance (European Commission, 1997f).
However, in case higher residues are expected in raw agricultural commodities from future intended uses, a need for a hydrolysis study would need to be considered.
2.1.4. Methods of analysis in plants
Acrinathrin is an enantiomerically pure synthetic pyrethroid having three stereogenic centres and a double bond in a defined configuration. Having three stereogenic centres and a double bond, a total of 16 possible isomers – which comprise eight pairs of enantiomers – exist. It is important to note that except for acrinathrin and its own enantiomer, reference standards for only one compound of each enantiomeric pair were synthetized (EFSA, 2020).
Analytical methods for the determination of acrinathrin residues were assessed during the MRL review (EFSA, 2015). Sufficiently validated methods are available for the determination of residues of acrinathrin and its enantiomer for routine analysis in the crops under consideration (high water content matrices) at an LOQ of 0.02 mg/kg. The enforcement methods are not stereoselective and quantify residues of acrinathrin together with its enantiomer.
In addition, a new analytical method was provided in the context of the previous MRL application on lettuce (EFSA, 2020). It was concluded that in crop matrices with high acid content (strawberry), high water content (sweet pepper, tomatoes), high oil content (soybeans) and high starch content (wheat grain) residues of acrinathrin can be enforced at a lower LOQ of 0.01 mg/kg. Since chiral analysis was not employed, also for this analytical method the acrinathrin peak could include its enantiomer.
EFSA concludes that sufficiently validated analytical enforcement methods are available for the determination of acrinathrin residues in the crops under consideration.
2.1.5. Storage stability of residues in plants
The storage stability of acrinathrin in plants stored under frozen conditions prior to analysis was investigated in the framework of the EU pesticides peer review demonstrating storage stability for acrinathrin in high water content commodities up to 24 months (EFSA, 2013). The storage stability of acrinathrin in the crops under consideration is thus addressed.
2.1.6. Proposed residue definitions
Based on the metabolic pattern identified in metabolism studies with grapes and oranges, the toxicological significance of metabolites and acrinathrin isomers and the capabilities of enforcement analytical methods, the following residue definitions were proposed by the EU pesticides peer review and confirmed by the MRL review (EFSA, 2013, 2015):
residue definition for risk assessment: acrinathrin and all isomers (as long as the toxicity of the individual isomers including enantiomers has not been addressed);
residue definition for enforcement: acrinathrin and its enantiomer.
The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical with the above‐mentioned residue definition. These residue definitions were limited to the uses on fruit crops.
In the assessment of a recent MRL application, a new metabolism study in lettuce was submitted and EFSA concluded that the proposed residue definitions can be extended also to leafy crop group (EFSA, 2020). The new tomato study submitted under the present MRL application, addresses some uncertainties identified by the EU pesticides peer review in the grape metabolism study, related to identification and quantification of acrinathrin isomers and confirms the proposed residue definitions.
It is highlighted that the inclusion of all potential isomers in the residue definition for risk assessment could eventually be reviewed due to the very low amounts of isomers found in metabolism studies with tomatoes and lettuce. However, considering the absence of metabolism studies on other crop categories, the revision of the existing risk assessment residue definition for fruit crops is not proposed in the framework of the current assessment.
2.2. Magnitude of residues in plants
2.2.1. Magnitude of residues in primary crops
In support of the MRL application, the applicant submitted residue trials on peaches, apricots and sweet peppers.
Residue trial samples were analysed by a non‐chiral method and therefore the peak associated with acrinathrin would also include its potential enantiomer, and thus refers to the sum of both enantiomers according to the residue definition for monitoring. The method used to analyse the residue trial samples was sufficiently validated and fit for purpose. The samples prior to analysis were stored for a period not affecting the integrity of samples (Spain, 2017).
In order to derive residue data according to the risk assessment definition, the applicant and the EMS proposed to apply the conversion factor (CF) of 1.1 from enforcement to risk assessment as derived by the EU pesticides peer review from the metabolism study on grapes (EFSA, 2013). When using the data from the tomato metabolism study, the derived CF would be lower (1.02). EFSA agrees with the EMS to apply a more critical CF of 1.1. The factor is applicable both to peaches/apricots and sweet peppers residue data.
Peaches
In support of the intended use on peaches and nectarines,8 the applicant submitted four GAP‐compliant residue trials on peaches and four GAP‐compliant residue trials on apricots, which were performed in various southern Europe (SEU) countries in 2015. To address the EFSA's data requirement that residue trials were carried out over only one season, the applicant provided data from overdosed peaches/nectarines trials from 1995, 2000 and 2007 growing seasons. These trials were scaled to the intended nominal application rate of the GAP, and the scaled data were then compared with the trials from 2015, indicating that results were of the same range. The arguments provided by the applicant are thus considered sufficient to address the data requirement of EFSA. The scaled data, however, were not used to derive the MRL proposal since a sufficient number of GAP‐compliant residue trials is available.
The applicant proposes to extrapolate residue data from apricots to peaches. Such an extrapolation is supported according to EU guidance documents (European Commission, 2017b). The combined residue data on peaches and apricots was used to derive an MRL proposal of 0.08 mg/kg in support of the intended SEU use of acrinathrin on peaches. The EMS derived a higher MRL proposal of 0.1 mg/kg, by rounding up the value of 0.08 mg/kg.
Sweet peppers
In support of the intended use on sweet peppers in the SEU, the applicant submitted eight GAP‐compliant residue trials on peppers, which were performed in various SEU countries in 2015. No justification was provided by the applicant on the fact that trials were carried out over one growing season. However, considering a wide distribution of trials among Member States and the fact that the supporting residue data provided for peaches (see above) demonstrated the same range of residues in a crop regardless of season, EFSA accepts this deviation from the trial requirements.
The data are sufficient to derive an MRL proposal of 0.08 mg/kg in peppers in support of the intended SEU use of acrinathrin. The EMS derived a higher MRL proposal of 0.1 mg/kg, by rounding up the value of 0.08 mg/kg.
2.2.2. Magnitude of residues in rotational crops
The possible transfer of acrinathrin residues to crops that are grown in crop rotation has been assessed in the EU pesticides peer review (EFSA, 2013). The available studies demonstrated that significant residues (above 0.01 mg/kg) are not expected in succeeding crops when planted in soil previously treated at 113–158 g a.s./ha.
Since the total application rate on sweet peppers (0.045 kg as/ha) is lower than the application rate tested in the rotational crop studies, it is concluded that no residues are expected in following crops grown in rotation with sweet peppers, provided that the active substance is applied according to the proposed GAP.
2.2.3. Magnitude of residues in processed commodities
Specific processing studies investigating the effect of processing on the magnitude of acrinathrin residues in processed peaches and sweet peppers were not submitted and are not required as significant residues are not expected in raw agricultural commodity (RAC) and the total theoretical maximum daily intake (TMDI) is below the trigger value of 10% of the ADI (European Commission, 1997f).
2.2.4. Proposed MRLs
The available data are considered sufficient to derive MRL proposals as well as risk assessment values for the commodities under evaluation. In Section 4 EFSA assessed whether residues on these crops resulting from the intended uses are likely to pose a consumer health risk.
3. Residues in livestock
The investigation of the nature and magnitude in the livestock is not necessary as peaches and sweet peppers are not fed to livestock.
4. Consumer risk assessment
EFSA performed a dietary risk assessment using revision 3.1 of the EFSA PRIMo (EFSA, 2018, 2019). This exposure assessment model contains food consumption data for different subgroups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (FAO, 2016).
The toxicological reference values for acrinathrin used in the risk assessment were derived in the framework of the EU pesticides peer review where an ADI of 0.01 mg/kg bw per day and an ARfD of 0.01 mg/kg bw were set (European Commission, 2017a,b). During the EU pesticides peer review, the data gap regarding further assessment of the toxicological profile of the 15 isomers of acrinathrin was established and further confirmed by the MRL review (EFSA, 2013, 2015). The MRL review proposed to consider the toxicological reference values set for acrinathrin tentatively valid also for the other isomers. The applicant in the framework of the current assessment has provided additional information, which allows to conclude that isomers included in the risk assessment residue definition are not genotoxic and three of these acrinathrin isomers are expected to be less acutely neurotoxic than the parent but for the other 12 isomers, including the enantiomer of acrinathrin, no conclusion could be made regarding their general toxicity compared to the parent.
As reported in the nature of residues in primary crops section, the isomers in fruit crops were present at negligible levels; the enantiomer of acrinathrin was not identified. Thus, EFSA agrees with the EMS, that the exposure to acrinathrin isomers from the intake of treated peaches/nectarines and sweet peppers will be negligible. Moreover, since only few of the existing uses – on grapes and some lettuces – were maintained after the MRL review, the overall consumer exposure to acrinathrin residues is low with a large safety margin.
However, since the potential formation of isomers depends on the crop/PHI and the toxicity of all the isomers has not been addressed, a conversion factor of 1.1 derived from the grape metabolism study (EFSA, 2013) as proposed by the MRL review (EFSA, 2015) is applied to all crops considered in the consumer exposure assessment.
Short‐term (acute) dietary risk assessment
The highest residue (HR) value as derived from the submitted residue trials and multiplied by the conversion factor of 1.1 was used as an input value. The complete list of input values can be found in Appendix D.1. The short‐term exposure did not exceed the ARfD for the crops assessed in this application, accounting for a maximum of 52% of the ARfD for peaches and 33% of the ARfD for sweet peppers. EFSA concludes that the use of acrinathrin according to the reported agricultural practice is unlikely to present a short‐term risk to consumer health.
Long‐term (chronic) dietary risk assessment
The long‐term exposure assessment was performed using the median residue levels (STMR) in peaches and sweet peppers as derived from the submitted residue trials, multiplied by the conversion factor for risk assessment of 1.1. For the remaining commodities covered by the MRL regulation, the median residue levels derived in the MRL review and in the previous MRL application on lettuce were selected, multiplied by the conversion factor for risk assessment of 1.1. Those crops on which authorised uses were not supported after the MRL review (i.e. the commodities where the GAPs were not compliant with the restriction of use of acrinathrin) or for which no GAP was reported during the MRL review, were not included in the exposure calculation. The complete list of input values can be found in Appendix D.1.
The estimated long‐term dietary intake accounted for a maximum of 2% of the ADI (PT general diet). The contribution of residues expected in peaches and sweet peppers to the overall long‐term exposure accounted for a maximum of 0.12% of the ADI for peppers and 0.08% of the ADI for peaches.
Although general uncertainties related to the toxicological profile of different isomers remain, the data gap set by the EU pesticides peer review regarding the toxicological profile of acrinathrin isomers is not relevant for the crops under consideration. Thus, EFSA concludes that residues in crops from the intended and existing uses are unlikely to pose a risk to consumer's health.
For further details on the exposure calculations, a screenshot of the report sheet of the PRIMo is presented in Appendix C.
5. Conclusion and Recommendations
The data submitted in support of this MRL application were found to be sufficient to derive an MRL proposal for peaches and sweet peppers.
EFSA concluded that the proposed use of acrinathrin on the crops under consideration will not result in a consumer exposure exceeding the toxicological reference values and therefore is unlikely to pose a risk to consumers’ health.
The MRL recommendations are summarised in Appendix B.5.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CF
conversion factor for enforcement to risk assessment residue definition
- CXL
Codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- DT90
period required for 90% dissipation (define method of estimation)
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- EW
emulsion, oil in water
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GC‐MS
gas chromatography with mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- InChiKey
International Chemical Identifier Key
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- NEU
northern Europe
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
Appendix A – Summary of intended GAP triggering the amendment of existing EU MRLs
1.
| Crop and/or situation | NEU, SEU, MS or country | F G or Ia | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. (g/kg) | Method kind | Range of growth stages & seasonc | Number min‐max | Interval between application (days) min‐max | g a.s./hL min–max | Water (L/ha) min–max | Rate min–max | Unit | ||||||
| Peaches, nectarine | SEU (ES) | F | Red mites, thrips | EW | 75 g/L | Foliar–spray | BBCH 67–87 | 1–2 | 10 | 2.25–3.375 | 650–1,000 | 22.5 | g/ha | 7 | Against mobile forms 30–45 mL product/hL (max 0.3 L/ha) |
| Sweet peppers | SEU (ES) | F | Red mites, thrips | EW | 75 g/L | Foliar–spray | From BBCH 10 | 1–2 | 10 | 3.0–4.5 | 500–700 | 22.5 | g/ha | 3 | Against mobile forms. At first signs of pest 40–60 mL product/hL (max 0.3 L/ha) |
MRL: maximum residue level; GAP: Good Agricultural Practice; NEU: northern European Union; SEU: southern European Union; MS: Member State; a.s.: active substance; EW: emulsion, oil in water.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
Appendix B – List of end points
B.1. Mammalian toxicity

B.2. Residues in plants
B.2.1. Nature of residues and methods of analysis in plants
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Grape vine | Foliar, Fa 2 × 0.0225 kg a.s./ha | 0, 13, 27, 41 | Radiolabelled active substance: [14C‐gemdimethyl]‐ and [14C‐benzyl]‐acrinathrin (EFSA, 2013) | |
| Foliar, Fa 2 × 0.225 kg a.s./ha | 0, 13, 27, 41 | Radiolabelled active substance: [14C‐gem‐dimethyl]‐acrinathrin (EFSA, 2013) | |||
| Apples | 1 × 45‐91 μg per fruit, 52‐145 μg per leafb | 0, 7, 14, 21, 28, 42, 56c 0, 28d | Radiolabelled active substance: [14C‐gemdimethyl]‐ and [14C‐benzyl]‐acrinathrin Supportive, not fully valid (EFSA, 2013) Application by brush to individual fruits and leaves. | ||
| Oranges | Foliar, Fa 2 × 0.09 kg a.s./ha | 21 | Radiolabelled active substance: [14C‐gemdimethyl]‐, [14C‐benzyl]‐ and [14C‐hexafluoroisopropyl]‐ acrinathrin. Identification of individual isomers not undertaken (EFSA, 2015) | ||
| Cucumbers | Foliar, Fa 2 × 0.078 kg a.s./ha | 0, 14, 42 | Radiolabelled active substance: [14C‐hexafluoroisopropyl]‐, [14C‐benzyl]‐ and [14C‐gemdimethyl]‐acrinathrin. Supportive, not fully valid (EFSA, 2013) | ||
| Tomatoes | Foliar, Fa 3 × 0.060 kg a.s./ha | 3, 7, 14 | Radiolabelled active substance: [cyclopropyl‐1‐14C]‐ and [phenoxy‐U‐14C]‐acrinathrin (Spain, 2017) | ||
| Leafy crops | Cabbages | 2 × 587–679 μg per plantb | 0, 28, 56c 0, 14, 28d | Radiolabelled active substance: [14C‐gemdimethyl]‐ and [14C‐benzyl]‐acrinathrin. Supportive, not fully valid (EFSA, 2013) Application by brush to heart and four innermost leaves | |
| Lettuce | Foliar, Fa 2 × 0.060 kg a.s./ha | 3, 7, 14 | Radiolabelled active substance: [cyclopropyl‐1‐14C]‐ and phenoxy‐U‐14C]‐acrinathrin (EFSA, 2020) |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Root/tuber crops | Carrot, kohlrabi | 0.113–0.158 kg a.s./ha | 29, 70 | Radiolabelled active substance: [14C‐gemdimethyl]‐ and [14C‐benzyl]‐acrinathrin (EFSA, 2013) Treatment to bare soil. | |
| Leafy crops | Spinaches | ||||
| Cereal (small grain) | Wheat |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Not triggered | ||
| Baking/brewing/boiling (60 min, 100°C, pH 5) | Not triggered | ||
| Sterilisation (20 min, 120°C, pH 6) | Not triggered | ||
| Other processing conditions | – |
Outdoor/field application (F) or glasshouse/protected/indoor application (G).
Total applied radioactivity (TAR) for each sample.
Application 8 weeks before normal harvest.
Application 4 weeks before normal harvest.

B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Cucumbers, green beans | –18°C | 24 | Months | Acrinathrin | EFSA (2013) | |
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Regiona | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) | CFd |
|---|---|---|---|---|---|---|---|
| Enforcement residue definition: acrinathrin and its enantiomer Risk assessment residue definition: acrinathrin and all isomers | |||||||
| Peaches | NEU | Mo: Peaches: 3 × 0.02; 0.05 Apricots: 0.02; 3 × 0.01 RA: – | Residue trials on apricots and peaches compliant with the GAP on peaches. Extrapolation from combined data set possible. | 0.08 | Mo: 0.05 RA: – | Mo: 0.02 RA: – | 1.1 |
| Sweet peppers | NEU | Mo: 2 × < 0.01; 2 × 0.01; 2 × 0.02; 0.03; 0.05 RA: – | Residue trials on sweet peppers compliant with the GAP. | 0.08 | Mo: 0.05 RA: – | Mo: 0.02 RA: – | 1.1 |
MRL: maximum residue level; GAP: Good Agricultural Practice; Mo: monitoring; RA: risk assessment.
NEU: Outdoor trials conducted in northern Europe; SEU: Outdoor trials conducted in southern Europe; EU: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment; derived from the grape metabolism study (EFSA, 2013).
B.1.2.2. Residues in rotational crops

B.1.2.3. Processing factors
No processing studies were submitted in the framework of the present MRL application.
B.2. Residues in livestock
Not relevant
B.3. Consumer risk assessment


B.4. Recommended MRLs
| Codea | Commodity | Existing U MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: AcrinathrinF | ||||
| 0140030 | Peaches | 0.02* | 0.08 | The submitted data are sufficient to derive MRL proposal for the intended SEU uses. Although general uncertainties related to the toxicological profile of different isomers remain, for the uses under consideration this data gap is not fully justified. Risk for consumers unlikely. |
| 0231020 | Sweet peppers/bell peppers | 0.02* | 0.08 | |
MRL: maximum residue level; SEU: southern Europe; GAP: Good Agricultural Practice.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Fat soluble.
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.

Appendix D – Input values for the exposure calculations
D.1. Consumer risk assessment
| Commodity | Existing/Proposed MRL (mg/kg) | Source | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Commenta | |||
| Risk assessment residue definition: acrinathrin and all isomers (EFSA, 2013, 2015) | ||||||
| Peaches | 0.08 | Intended use | 0.022 | STMR‐RAC*CF (1.1) | 0.055 | HR‐RAC*CF (1.1) |
| Table grapes | 0.05 | EFSA (2015) | 0.055 | STMR‐RAC*CF (1.1) | 0.055 | HR‐RAC*CF |
| Wine grapes | 0.1 | EFSA (2015) | 0.055 | STMR‐RAC*CF (1.1) | 0.066 | HR‐RAC*CF |
| Sweet peppers/bell peppers | 0.08 | Intended use | 0.022 | STMR‐RAC*CF (1.1) | 0.055 | HR‐RAC*CF (1.1) |
| Lamb's lettuce/corn salads | 0.06 | EFSA (2015) | 0.022 | STMR‐RAC*CF (1.1) | 0.033 | HR‐RAC*CF |
| Lettuces | 0.1 | EFSA (2020) | 0.011 | STMR‐RAC*CF (1.1) | 0.055 | HR‐RAC*CF |
| Escaroles/broad‐leaved endives | 0.06 | EFSA (2015) | 0.022 | STMR‐RAC*CF (1.1) | 0.033 | HR‐RAC*CF |
| Cress and other sprouts and shoots | 0.06 | EFSA (2015) | 0.022 | STMR‐RAC*CF (1.1) | 0.033 | HR‐RAC*CF |
| Roman rocket/rucola | 0.06 | EFSA (2015) | 0.022 | STMR‐RAC*CF (1.1) | 0.033 | HR‐RAC*CF |
| Red mustards | 0.06 | EFSA (2015) | 0.022 | STMR‐RAC*CF (1.1) | 0.033 | HR‐RAC*CF |
STMR‐RAC: supervised trials median residue in raw agricultural commodity; HR‐RAC: highest residue in raw agricultural commodity; PeF: Peeling factor.
Input values for the commodities which are not under consideration for the acute risk assessment are reported in grey.
Appendix E – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac | Code/Name | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|---|---|---|
| N d | (S)‐cyano(3‐phenoxyphenyl)methyl (1S,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐RNOTXBHXSA‐N |

|
Dd RU45199 | (R)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1c[C@@H]([C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐BIXLXFTDSA‐N |

|
| Od Acrinathrin RU38702 | (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐WEQBUNFVSA‐N |

|
Cd RU 48238 | (R)‐cyano(3‐phenoxyphenyl)methyl (1S,3R)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐QTGMIWFCSA‐N |

|
| P d RU45120 ( 1S , cis, Z, αS) | (S)‐cyano(3‐phenoxyphenyl)methyl (1S,3R)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐BJRXWDRESA‐N |
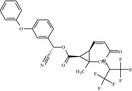
|
Bd R‐Acrinathrin RU 45120 (1R, cis, Z, αR) | (R)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐UTFPFDPTSA‐N |

|
| Qd Z‐trans‐Acrinathrin RU39592 | (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐CEGVRPJUSA‐N |

|
A d | (R)‐cyano(3‐phenoxyphenyl)methyl (1S,3S)‐3‐{(1Z)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1YLFSVIMMRPNPFK‐LVHZCJPFSA‐N |

|
| J e | (S)‐cyano(3‐phenoxyphenyl)methyl (1S,3S)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐WWLKQQBESA‐N |

|
He RU45198 | (R)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐CEDMXCAPSA‐N |

|
| Ke E‐Acrinathrin RU39319 | (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐HIMIUADYSA‐N |

|
F e | (R)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐KPJVQRDYSA‐N |

|
| L e | (S)‐cyano(3‐phenoxyphenyl)methyl (1S,3R)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐IJSXBTRHSA‐N |

|
Ge RU45168 | (R)‐cyano(3‐phenoxyphenyl)methyl (1S,3R)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐OCFKXNCMSA‐N |

|
| Me E‐trans‐Acrinatrhin RU39506 | (S)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@@H]([C@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐SFKSTPLFSA‐N |

|
Ee | (R)‐cyano(3‐phenoxyphenyl)methyl (1S,3S)‐3‐{(1E)‐3‐[(1,1,1,3,3,3‐hexafluoropropan‐2‐yl)oxy]‐3‐oxoprop‐1‐en‐1‐yl}‐2,2‐dimethylcyclopropane‐1‐carboxylate CC1(C)[C@H]([C@@H]1/C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C(=O)O[C@@H](C#N)c1cccc(Oc2ccccc2)c1 YLFSVIMMRPNPFK‐SZSGGGEMSA‐N |

|
| 3‐PBA 3‐PBAcid | 3‐phenoxybenzoic acid O=C(O)c1cc(Oc2ccccc2)ccc1 NXTDJHZGHOFSQG‐UHFFFAOYSA‐N |

|
|||
| DPA‐A | (2Z)‐3‐(3‐{[2‐amino‐2‐oxo‐1‐(3‐phenoxyphenyl)ethoxy]carbonyl}‐2,2‐dimethylcyclopropyl)prop‐2‐enoic acid OC(=O)/C=C\C1C(C(=O)OC(c2cccc(Oc3ccccc3)c2)C(N)=O)C1(C)C OGXOFXISOFSZPL‐QXMHVHEDSA‐N |

|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019).
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019).
Z‐isomer.
E‐isomer.
Suggested citation: EFSA (European Food Safety Authority) , Bellisai G, Bernasconi G, Brancato A, Carrasco Cabrera L, Ferreira L, Giner G, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Ruocco S, Santos M, Scarlato AP, Theobald A, Vagenende B and Verani A, 2021. Reasoned opinion on the modification of the existing maximum residue levels for acrinathrin in peaches and sweet peppers. EFSA Journal 2021;19(7):6681, 29 pp. 10.2903/j.efsa.2021.6681
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00471
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to thank: Stathis Anagnos, Laszlo Bura, Andrea Mioč, Marta Szot, Aikaterini Vlachou for the support provided to this scientific output.
Adopted: 8 June 2021
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 974/2011 of 29 September 2011 approving the active substance acrinathrin, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011 and Commission Decision 2008/934/EC. OJ L 255, 1.10.2011, p. 1–5.
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
For an overview of all MRL Regulations on this active substance, please consult: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/active-substances/?event=search.as
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
It is noted that on 18 February 2021, EFSA received from the European Commission the request for an Opinion of the Scientific Panel on Plant Protection Products and their Residues (PPR Panel) on the toxicity profile of PBA and PBA(OH) (metabolites common to several pyrethroid substances) and for EFSA to consider the impact on the consumer risk assessment. The Scientific Opinion is currently under preparation and will be finalised by 30 September 2022.
It is noted that nectarines are classified in Annex I Part B to Regulation (EC) No 396/2005 as a product (commodity code 0140030‐002) to which the same MRL applies as to peaches.
References
- EFSA (European Food Safety Authority), 2013. Conclusion on the peer review of the pesticide risk assessment of the active substance acrinathrin. EFSA Journal 2013;11(12):3469, 82 pp. 10.2903/j.efsa.2013.3469 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Conclusion on the peer review of the pesticides risk assessment of the active substance lambda‐cyhalothrin. EFSA Journal 2014;12(5):3677, 170 pp. 10.2903/j.efsa.2014.3677 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Review of the existing maximum residue levels for acrinathrin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2015;13(7):4203, 44 pp. 10.2903/j.efsa.2015.4203 Available online: www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Reasoned opinion on the modification of the existing MRL(s) for acrinathrin in lettuce. EFSA Journal 2020;18(7):6218, 24 pp. 10.2903/j.efsa.2020.6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019;EN‐1605, 15 pp. 10.2903/sp.efsa.2019.en-1605 [DOI] [Google Scholar]
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/VI/95‐rev.3, 22 July 1997.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirements for Annex II (part A, section 4) and Annex III (part A, section 5) of Directive 91/414. SANCO/3029/99‐rev. 4. 11 July 2000.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010-rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2017a. Final Review report for the active substance acrinathrin finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 15 July 2011 in view of the approval of acrinathrin to the list of active substances approved under Regulation (EC) No 1107/2009. SANCO/11357/2011 Rev. 7 24 January 2017.
- European Commission , 2017b. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.3, 13 June 2017.
- FAO (Food and Agriculture Organization of the United Nations), 2016. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 3rd Edition. FAO Plant Production and Protection Paper 225, 298 pp.
- France , 2007. Draft Assessment Report (DAR) on the active substance acrinathrin prepared by the rapporteur Member State France in the framework of Directive 91/414/EEC, July 2007. Available online: www.efsa.europa.eu
- France , 2010. Final Addendum to the Additional Report on acrinathrin, compiled by EFSA, September 2010. Available online: www.efsa.europa.eu
- France , 2012. Addendum to the revised Draft Assessment Report (DAR) on the active substance acrinathrin prepared by the rapporteur Member State France in the framework of Regulation (EC) No 1107/2009, May 2012. Available online: www.efsa.europa.eu
- France , 2013. Final Addendum to the revised Draft Assessment Report (DAR) on acrinathrin, compiled by EFSA, September 2013. Available online: www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org
- Spain , 2017. Evaluation report on the setting of MRLs for acrinathrin in peach and pepper. Updated 30 March, 2021, 99 pp.


