Abstract
Both sensation seeking and affiliation with deviant peer groups are risk factors for delinquency in adolescence. In this study, we use a sample of adolescent twins (n = 549), 13 to 20 years old (M age = 15.8 years), in order to test the interactive effects of peer deviance and sensation seeking on delinquency in a genetically informative design. Consistent with a socialization effect, affiliation with deviant peers was associated with higher delinquency even after controlling for selection effects using a co-twin-control comparison. At the same time, there was evidence for person-environment correlation; adolescents with genetic dispositions toward higher sensation seeking were more likely to report having deviant peer groups. Genetic influences on sensation seeking substantially overlapped with genetic influences on adolescent delinquency. Finally, the environmentally mediated effect of peer deviance on adolescent delinquency was moderated by individual differences in sensation seeking. Adolescents reporting high levels of sensation seeking were more susceptible to deviant peers, a Person × Environment interaction. These results are consistent with both selection and socialization processes in adolescent peer relationships, and they highlight the role of sensation seeking as an intermediary phenotype for genetic risk for delinquency.
Keywords: sensation seeking, peers, adolescence, delinquency, behavioral genetics
Adolescence is a peak period of risk for engaging in antisocial behavior, that is, acts that violate rules, social norms, and/or the rights of others (Jessor & Jessor, 1977; Moffitt, 1993). Research on antisocial behavior from a developmental psychopathology perspective investigates how normative developmental processes in adolescence intersect with individual vulnerabilities and social contexts to shape why teenagers are generally more prone to antisocial behavior than are children or adults, and why certain teenagers are at particular risk compared with others (Frick & Viding, 2009; Krueger, Markon, Patrick, & Iacono, 2005). In the current study, we adopt a developmental psychopathology approach to consider the intersections among genes, sensation seeking, and peer deviance in the etiology of adolescent delinquency. We test for gene-environment correlation, in which adolescents with greater genetic liability toward sensation-seeking and antisocial behavior are more likely to affiliate with deviant peers, and Person × Environment interaction, in which adolescents higher in sensation seeking (a genetically influenced trait) are more susceptible to the environmental effects of peer deviance on antisocial behavior.
Peer Selection and Socialization
Teenagers whose friends engage in delinquent behaviors are more likely than teenagers without such friends to engage in delinquency themselves. Some part of peer group similarity for delinquency is due to selection processes, as friends are not chosen at random. Adolescents may select friends partly on the basis of delinquency itself or on the basis of correlated behaviors (e.g., smoking or drinking; Caspi, 1995; Ennett & Bauman, 1994; Burk, van der Vorst, Kerr, & Stattin, 2012) and traits (e.g., sensation seeking; Mann, Kretsch, Tackett, Harden, & Tucker-Drob, 2015). Additionally, an adolescent’s potential pool of friends (e.g., other teens in the same school or neighborhood) are stratified by demographic variables (e.g., low socioeconomic status) that may be risks for delinquent behavior, inducing similarity among peer groups (social homogeny) in the absence of active and evocative selection processes (McPherson, Smith-Lovin, & Cook, 2001). Nevertheless, research using longitudinal and quasi-experimental methods capable of controlling for selection factors, confirms that deviant peer groups do, in fact, exert influence on adolescent delinquency, above and beyond selection processes and social homogeny (Dishion & Tipsord, 2011).
One method for estimating peer influences on delinquency is a co-twin-comparison design. When comparing two identical twins, who have overlapping but not entirely identical peer groups, does the twin with more deviant peers also engage in more delinquent behavior? Although any cross-sectional design is, of course, not able to establish direction of causation, a within-twin-pair comparison controls for selection and social homogeny processes that are driven by genetic and environmental factors that are shared by identical twins raised in the same home. That is, a within-identical-twin-pair association between peer deviance and delinquency cannot be confounded by genetically based propensities to seek out certain types of friends, because identical twins are genetically identical; nor can this within-twin-pair association be attributed to social homogeny due to school or neighborhood environments, socioeconomic status, or race/ethnicity—all of which are identical within both identical and fraternal twin pairs raised together.
Consistent with a socialization effect of peers, previous twin research on peer influence has found evidence for a within-twin-pair association between peer deviance and delinquency and related behaviors (Cruz, Emery, & Turkheimer, 2012; Harden, Hill, Turkheimer, & Emery, 2008; Kretsch, Mendle, & Harden, 2016). At the same time, twin research has also documented the importance of gene-environment correlations (rGE): More genetically similar individuals (e.g., identical twins as compared with fraternal twins) experience more similar peer environments, indicating that adolescents are selecting and being selected into peer groups partially on the basis of their genetically influenced traits (Agrawal et al., 2010; Cleveland, Wiebe, & Rowe, 2005; Fowler, Settle, & Christakis, 2011; Harden et al., 2008; reviewed in Brendgen, 2012). Interestingly, there is preliminary evidence that rGE for peer relationships emerge in adolescence, and twin research with prepubertal children has failed to find substantial genetic effects on peer characteristics (Brendgen, 2012).
Person × Context Interactions
A twin-comparison-approach can also be used to estimate whether individual characteristics confer greater sensitivity (or resilience) to the environmental effects of peer influence, a Person × Environment interaction. Identifying individual differences that moderate the strength of peer influence processes is a research goal that is “essential [for] the development of more precise, targeted intervention efforts” (Brechwald & Prinstein, 2011, p. 174). Because characteristics that make adolescents more vulnerable to peer influence may themselves be genetically influenced, such Person × Environment interactions may serve as mechanisms of Gene × Environment interaction.
Gene × Environment interactions involving the peer environment are among the most consistently replicated effects in behavioral genetics literature. Multiple twin studies have found that genetic influences on delinquency and related behaviors (e.g., drinking) are magnified among teenagers with deviant peers (Boardman, Saint Onge, Haberstick, Timberlake, & Hewitt, 2008; Button et al., 2007, 2009; Fowler et al., 2007; Guo, Elder, Cai, & Hamilton, 2009; Harden et al., 2008; Hicks, South, Dirago, Iacono, & McGue, 2009). Similar results have been obtained in candidate gene studies, with variants in the BDNF and CHRM2 genes found to interact with peer characteristics to predict adolescent antisocial behavior (Kretschmer, Dijkstra, Ormel, Verhulst, & Veenstra, 2013; Kretschmer, Vitaro, & Barker, 2014; Latendresse et al., 2011).
Most recently, Salvatore and colleagues (2015) constructed a polygenic risk index based on results from a genome-wide association study of externalizing disorders (i.e., substance use disorders, antisocial personality disorder) in adults, and then examined interactions between peer deviance and this polygenic score in the prediction of antisocial behavior in adolescents. As hypothesized, they found that the polygenetic risk score was more strongly associated with adolescent antisocial behavior among adolescents who reported high levels of peer deviance. A polygenic risk score approach is similar to a twin study, in that it estimates the effects of many genetic variants (rather than single variants, as in a candidate gene study), but involves entirely different assumptions, as genetic risk is measured directly from DNA rather than inferred from resemblance between biological relatives. The convergence of results across candidate gene, polygene, and twin methods is a testament to the robustness of Gene × Peer effects. However, it is not currently clear what specific genetically influenced psychological traits interact with peer context to give rise to these Gene × Environment interactions. The current study focuses on sensation seeking, a personality trait that may act as a mediator of genetic effects on delinquency.
Sensation Seeking as an Endophenotype
Although previous research has found consistent evidence for both rGE and G × E in the association between peer deviance and delinquency, there has been comparatively little research into the specific genetically influenced psychological traits that serve as mechanisms of these effects. A largely separate line of research suggests that sensation seeking may be an endophenotype (Gottesman & Gould, 2003) that mediates genetic influences on delinquency. An endophenotype is an intermediary construct, usually biological or psychological, that helps bridge the explanatory gap between individual differences in genetic make-up and individual differences in complex behavior. Sensation seeking is a facet of disinhibited personality characterized by a preference for novel, rewarding, and/or thrilling experiences and sensations (Zuckerman, 1971). Phenotypically, average levels of sensation seeking increase dramatically from late childhood (about age 10) to mid-adolescence (about age 16; Harden & Tucker-Drob, 2011), an age-trend that parallels the rise of delinquency (Harden, Quinn, & Tucker-Drob, 2012). Moreover, genetically influenced differences in the rate of change in sensation seeking substantially account for individual differences in the escalation of delinquency (Harden et al., 2012).
Convergent evidence for the utility of sensation seeking as an index of genetic risk comes from a genetic association study by Aliev and colleagues (2015). They focused on a candidate set of 215 measured genetic variants, each of which had been previously found to be associated with alcohol dependence or externalizing disorder phenotypes in adults. They tested the associations between each variant and antisocial behavior outcomes, substance use outcomes, and personality traits (including extraversion, conscientiousness, impulsivity, and sensation seeking). Interestingly, they found the greatest number of significant genetic associations with sensation seeking, with enrichment analyses indicating that sensation seeking was more consistently associated with the candidate genetic variants than any other phenotype.
Sensation seeking is also correlated with affiliation with more deviant peer groups (Hampson, Andrews, & Barckley, 2008; Mann et al., 2015; Yanovitzky, 2005) and has been shown to moderate the phenotypic association between peer deviance and adolescent delinquency (Mann et al., 2015). Putting these lines of evidence together, as illustrated in Figure 1, we hypothesize that (1) sensation seeking mediates genetic influences on delinquency; (2) sensation seeking mediates gene-environment correlations with peer deviance; and (3) sensation seeking moderates the effects of peer influence on delinquency. Although individual components of this model have been tested in—and supported by—previous research, the relations between sensation seeking, peer deviance, and antisocial behavior have not yet been comprehensively tested in a genetically informative study that simultaneously considers both person-environment correlations and interactions.
Figure 1.
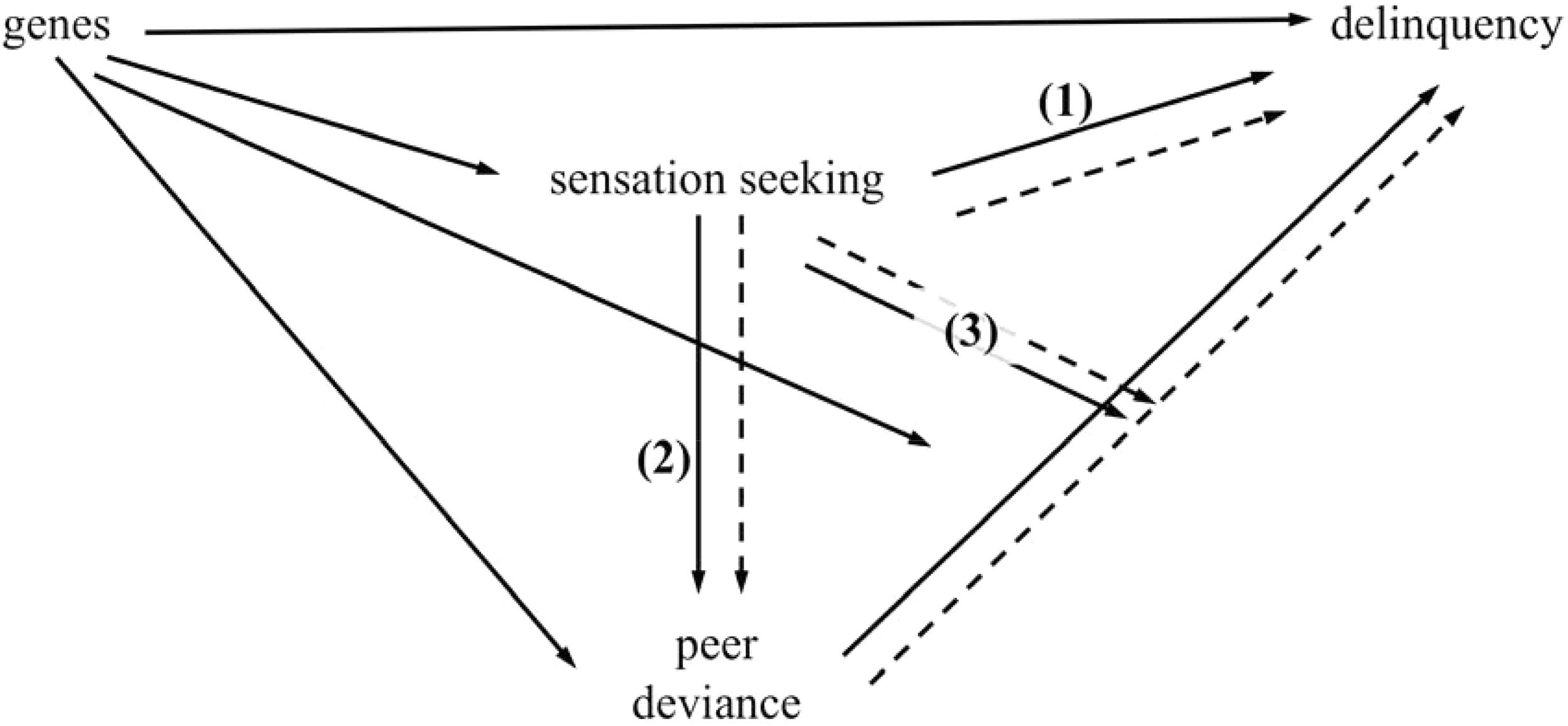
Theoretical pathways between genes, sensation seeking, peer deviance, and delinquency. Solid lines represent genetic pathways, and dashed lines represent environmental pathways. Numbers correspond with hypotheses tested in the current study. (1) Sensation seeking mediates genetic influences on delinquency. (2) Sensation seeking mediates gene-environment correlations with peer deviance. (3) Sensation seeking moderates the environmental effects of peer influence on delinquency.
Goals of the Current Study
We test three hypotheses. First, motivated by previous research indicating that sensation seeking is an endophenotype for delinquent behavior (Harden et al., 2012), we examine the extent to which genetic influences on sensation seeking account for genetic influences on delinquent behavior. Second, we examine the extent to which genetic influences on sensation seeking account for genetic influences on affiliation with deviant peers. We hypothesize that the genetic correlations between sensation seeking, peer deviance, and delinquency will be moderate to large in magnitude. Third, we examine the extent to which sensation seeking moderates the environmental association between peer deviance and delinquency, using a model that controls for rGE while using a co-twin-control to estimate peer influence. We hypothesize the environmental effect of peer deviance on delinquency will be magnified among adolescents high in sensation seeking. We test our hypotheses using multivariate behavioral genetic twin-comparison models.
Method
Participants
Households with identical and fraternal twins were identified using public school rosters, and families were invited by phone call or mailing to participate in an in-laboratory study as part of the Texas Twin Project (Harden, Tucker-Drob, & Tackett, 2013). Verbal and written consent was obtained from parents, and assent was obtained from adolescents prior to participation. Participants were adolescents (n = 549), ages 13–20 years (M age = 15.8 years, SD = 1.4 years), who were either enrolled in high school during the previous school year or expected to enroll in high school in the next fall semester. The sample was 52% male and 48% female. The racial composition of the sample was 59.7% non-Hispanic Caucasian, 20.3% Hispanic/Latino, 10.4% African American, 1.0% Native American, 1.5% East Asian, 2.2% South-east Asian, and 4.8% mixed-race/other. Approximately 7% of parents did not complete high school, 7% completed no more than high school, 3% completed a vocational or technical degree, 19% attended college but did not obtain a degree, 6% completed an associate degree, and 58% a bachelor degree or higher.
A different trained research assistant independently assessed each twin. Honest reporting of sensitive information (e.g., delinquent behavior) was encouraged by allowing participants to enter information directly into the computer and by notifying them that the study data was protected from disclosure by a federal certificate of confidentiality.
Zygosity Classification
Zygosity information was missing for 5 twin pairs; therefore, behavioral genetic analyses were performed on a subsample of 539 adolescent twins. Same-sex pairs were classified on the basis of self, parent, and experimenter report of twin pairs’ physical similarity and likelihood/frequency of being mistaken for each other. Specifically, items were completed by the twins’ parents, two research assistants following the twins’ lab visit, and both twins themselves. Responses to items were analyzed using latent class analysis (LCA), which assigns participants to groups (in this case, monozygotic, MZ, and dizygotic, DZ, twins). Compared with determining zygosity using molecular genetics techniques, LCA of questionnaire data has a misclassification rate of less than 1% (Heath et al., 2003). The LCA solution had an entropy statistic of 0.999, indicating very little uncertainty in classifying pairs. (Opposite sex twin pairs were not included in LCA because they are necessarily DZ.). The sample included 255 sets of twin and 27 sets of triplets from 9 families, with each triplet family contributing three pairwise combinations; there were 97 MZ pairs (45 female-female, 52 male-male), and 185 DZ pairs (60 female-female, 39 male-male, 86 female-male).
Measures
Sensation seeking.
Sensation seeking was measured using the Brief Sensation Seeking Scale (BSSS; Hoyle, Stephenson, Palmgreen, Lorch, & Donohew, 2002; Stephenson, Hoyle, Palmgreen, & Slater, 2003). Adolescents rated 8 items on a scale ranging from 1 (strongly disagree) to 5 (strongly agree). Sample items include, “I like to do frightening things,” “I prefer friends who are excitingly unpredictable,” and “I would like to explore strange places.” Descriptive statistics (means, standard deviations, and internal consistencies) for sensation seeking and other study constructs are summarized in Table 1.
Table 1.
Descriptive Statistics and Zero-Order Correlations
| N = 547 | α | M (SD) | Age | SS | PD | DEL |
| Age | — | 15.82 (1.45) | 1.0 | .09* | .08 | .35** |
| SS | .72 | 3.20 (.69) | 1.0 | .31** | .45** | |
| PD | .86 | 1.73 (.33) | 1.0 | .50** | ||
| DEL | .87 | 6.66 (7.26) | 1.0 | |||
| Effects of Biological Sex on Study Variables | ||||||
| t | df | p | d | Male M (SD) | Female M (SD) | |
| Age | −.97 | 547 | .33 | −.08 | 15.76 (1.35) | 15.88 (1.54) |
| SS | 1.85 | 538 | .06 | .16 | 3.25 (.70) | 3.14 (.68) |
| PD | 4.56 | 521 | <.01 | .40 | 1.79 (.33) | 1.67 (.32) |
| DEL | 3.78 | 515 | <.01 | .33 | 7.80 (7.88) | 5.49 (6.38) |
Note. Means (M) and standard deviations (SD) reported for untransformed variables. Correlation coefficients and test statistics reported for transformed variables. N = total number of participants; α= Cronbach’s alpha; SS = sensation seeking; PD = peer deviance; DEL = delinquent behavior; t = two-sample test statistic; df = degrees of freedom; p = two-tailed probability; d = standardized difference between means (Cohen’s d).
p < .05.
p < .01.
Peer deviance.
Peer deviance was assessed with a self-report questionnaire adapted from Thornberry, Lizotte, Krohn, Farnworth, and Jang (1994), which asked how many friends engage in delinquent behaviors, such as skipping school and destroying property, and prosocial behaviors, like participating in school activities and getting along with teachers. All 22 items were rated on a scale ranging from 1 (none of them) to 4 (all of them). Prosocial items were reverse scored before summing item scores to form a composite scale.
Delinquency.
A self-report measure adapted from Survey, Huizinga, Esbensen, and Weiher (1991) was employed to assess individual differences in adolescents’ delinquent behavior. The severity of delinquent behaviors ranged from minor offenses to relatively severe crimes, including “skipped class or school without an excuse” and “made obscene telephone calls (calling someone and saying dirty things),” or “sold drugs such as heroin, cocaine, LSD, ecstasy, or prescription pills” and “been involved in a group fight or gang fight.” Thirty-six items were assessed on a 3-point scale (1 = never, 2 = once, 3 = more than once) and summed to form a composite scale.
Results
Prior to fitting univariate and multivariate twin models, internal consistencies (Cronbach’s alpha), descriptive statistics (means and standard deviations), and phenotypic associations (t tests and zero-order Pearson correlations) were calculated using R version 3.1.2 (R Core Team, 2013; see Table 1). Behavioral genetic analyses were conducted within a structural equation modeling (SEM) framework using Mplus version 7.1 (Muthén & Muthén, 1998–2010). For phenotypic analyses, participants with missing data were excluded pairwise. For behavioral genetic analyses, the full dataset was analyzed with missing data estimated using full information maximum likelihood (Enders & Bandalos, 2001). The delinquency scale was log-transformed to correct for positive skew. Standard errors and fit statistics were adjusted for nonindependence of data (Asparouhov & Muthén, 2006), which was necessary in behavioral genetic models because pairwise combinations of triplets were nested within the same household. MZ and DZ twin-pair correlations and SEM parameters control for the main effects of Caucasian, Hispanic, and African American race; age; sex; and Age × Sex interaction (McGue & Bouchard, 1984). All models were estimated using maximum likelihood with robust standard errors, and model fit was evaluated using model χ2, root mean squared error of approximation (RMSEA) and comparative fit index (CFI). Nested models were compared using Satorra-Bentler scaled chi-squared difference tests (Satorra & Bentler, 2001). Non-nested models were compared using Akaike Information Criteria (AIC).
Univariate Twin Models
Twin models were fit as SEMs that decompose total observed variance in a construct into three components: additive genetic variance (A), shared environmental variance (C), and nonshared environmental variance, plus measurement error (E). Heritability is the proportion of total variance in a phenotype attributable to additive genetic differences between individuals, that is, the A variance. Heritability is inferred from the extent to which MZ twins correlate higher on that phenotype than do DZ twins. Shared environmental variance includes factors that occur at the family level that make sibling similar to one another, like socioeconomic status, culture, and religion. Shared environmental factors are inferred from the extent to which twin correlations are higher than can be explained by genetics alone. Nonshared environmental variance, on the other hand, refers to factors uniquely experienced by siblings that make them different from one another (e.g., peer groups). Nonshared environmental factors are inferred from the extent to which identical twins raised together do not perfectly resemble one another on the phenotype.
With respect to model specification, MZ and DZ cross-twin correlations between C factors and between E factors are fixed to 1.0 and 0, respectively. For MZ twins, the cross-twin correlation between A factors are fixed to 1.0, reflecting the fact that MZ twins share approximately 100% of their segregating genes. For DZ twins, the cross-twin correlation between A factors is fixed to .50 because they share approximately 50% of their segregating genes.
Univariate ACE models were fit to each of the focal study variables (sensation seeking, peer deviance, and delinquency) and parameter estimates and model fit statistics can be found in Table 2. The MZ twin-pair correlation (rMZ = .449) for sensation seeking was more than double the DZ twin-pair correlation (rDZ = .176), indicating possible dominance genetic effects.1 However, although a model that included dominance genetic effects in place of shared environmental effects for sensation seeking showed better fit (AIC = 1,538.295) than an ACE model (AIC = 1,538.873), an ADE model did not fit significantly better than a more parsimonious AE model that only included additive genetic and nonshared environmental effects (χ2 diff. = .569, p = .440 for nested model comparison). The preferred model (AE), which showed good fit to the data (model χ2 = 4.896, df = 7, p = .662, RMSEA = .000, CFI = 1.00), was therefore selected and carried forward to multivariate analyses.
Table 2.
Twin-Pair Correlations and Results From Univariate Twin Models
| Twin correlations |
|||||||||||
| Variable | rMZ | rDZ | |||||||||
| SS | .45 (.32, .58) | .18 (.03, .33) | |||||||||
| PD | .56 (.42, .71) | .33 (.19, .47) | |||||||||
| DEL | .59 (.43, .74) | .37 (.19, .47) | |||||||||
| Standardized parameter estimates from ACE models |
|||||||||||
| Variable | A | C | E | ||||||||
| SS | .43 (.31, .55) | .00 (.00, .00) | .56 (.45, .69) | ||||||||
| PD | .30 (.01, .60) | .00 (.00, .00) | .70 (.40, .99) | ||||||||
| DEL | .42 (.03, .81) | .14 (.00, .44) | .44 (.29, .59) | ||||||||
| Standardized parameter estimates from ADE models |
|||||||||||
| Variable | A | D | E | ||||||||
| SS | .22 (.00, .82) | .24 (.00, .88) | .54 (.42, .66) | ||||||||
| PD | .30 (.01, .60) | — | .70 (.40, .99) | ||||||||
| DEL | .58 (.45, .70) | — | .42 (.30, .55) | ||||||||
| Model comparison results | |||||||||||
| Variable | Model | AIC | RMSEA | CFI | χ2 | df | c | Nested comparison | Δχ2 | p | Preferred model |
| SS | ACE | 1,538.9 | .00 | 1.00 | 4.27 | 6 | 1.20 | ||||
| ADE | 1,538.3 | .00 | 1.00 | 4.42 | 6 | 1.03 | |||||
| AE | 1,536.9 | .00 | 1.00 | 4.99 | 7 | 1.03 | ADE vs. AE | .57 | .44 | AE | |
| PD | ACE | 1,480.1 | .05 | .938 | 7.47 | 5 | 1.39 | ||||
| AE | 1,478.1 | .05 | .926 | 8.96 | 6 | 1.16 | ACE vs. AE | .00 | .96 | AE | |
| DEL | ACE | 1,457.2 | .00 | 1.00 | 4.21 | 6 | .88 | ||||
| AE | 1,455.9 | .00 | 1.00 | 5.01 | 7 | .89 | ACE vs. AE | .78 | .37 | AE | |
Note. Sibling contrast effects were modeled for peer deviance. 95% confidence intervals are reported in parentheses. SS = sensation seeking; PD = peer deviance; DEL = delinquency; rMZ = monozygotic twin-pair correlation; rDZ = dizygotic twin-pair correlation; A = additive genetics; C = shared environment; E = nonshared environment; D = dominance genetics; AIC = Akaike information criteria; df = degrees of freedom; c = scaling correction factor for model chi-squared. Differences between χ2 values obtained using maximum likelihood estimation with robust standard errors are not themselves χ2 distributed. Therefore, model χ2 values with scaling correction factors were used to compute Satorra-Bentler χ2 tests for nested model comparisons.
A similar procedure was used for selecting univariate twin models of peer deviance and delinquency (see Table 2). The ACE model of adolescent delinquency showed good fit to the data (model χ2 = 4.213, df = 6, p = .647, RMSEA = .000, CFI = 1.000). Although nested model comparisons indicate that omitting estimates of shared environmental influences did not result in significant misfit to the data, past behavior genetic research has emphasized the importance of shared environmental influences on a range of externalizing and antisocial behaviors, particularly rule-breaking behaviors (Burt, Krueger, McGue, & Iacono, 2001; Burt, McGue, & Iacono, 2010; Burt, McGue, Iacono, & Krueger, 2006; Burt, McGue, Krueger, & Iacono, 2007). Therefore, estimates of shared environmental influences on delinquency were included in multivariate analyses.
An initial ACE model of peer deviance showed relatively poor fit to the data (model χ2 = 10.939, df = 6, p = .093, RMSEA = .076, CFI = .876). Therefore, we fit a second ACE model that also estimated sibling effects (Carey, 1986), which improved model fit (model χ2 = 7.465, df = 5, p = .188, RMSEA = .059, CFI = .938). Sibling effects are parameterized in terms of two regression coefficients (Twin 1’s phenotype on Twin 2’s phenotype, and Twin 2’s phenotype on Twin 1’s phenotype) that are constrained to be equal in magnitude. Conceptually, these regressions posit that twins are similar to one another, not just because of shared genes and shared environmental influences, but also because they directly influence each other. Given that teenage twins likely share friends, this twin-to-twin influence is particularly plausible for peer deviance. Mathematically, sibling effects imply that the variances of the phenotypes will differ in MZ versus DZ twins, as was observed in this sample (MZ σ2 = 1.260, DZ σ2 = 0.797). Similar to sensation seeking, predictive fit indices and nested model comparisons indicated that AE models were preferred over ACE models. Consequently, for peer deviance, an AE model with sibling effects was carried forward to multivariate analyses.
Multivariate Twin Model: Gene-Environment Correlation and Interaction
First, to provide a baseline model for comparison and to assess whether peer deviance has a main environmental effect on delinquent behavior after genetic and shared environmental factors are controlled (i.e., a within-MZ twin-pair association), we fit a multivariate twin model. This model showed adequate fit to the data (model χ2 = 56.182, df = 37, p = .023, RMSEA = .061, CFI = .935, AIC = 4290.001). The association between peer deviance and delinquency is divided into two parts. The genetic cross-path (bA1) between peer deviance and delinquency tests whether adolescents who are genetically predisposed to select more deviant peers also show higher delinquency. In contrast, the environmental cross-path between peer deviance and delinquency (bE1) tests whether identical twins who differ in peer deviance also differ in their levels of delinquency. This within-twin comparison controls for potential genetic and environmental confounds that are shared by twins raised in the same family. This model also simultaneously accounts for genetic and environmental correlations with sensation seeking. The genetic (bA1 = .533, 95% CI = .306, .761, p < .001) and the environmental (bE1 = .144, 95% CI = .054, .234, p = .002) cross-paths from peer deviance to delinquency were positive and significant. This suggests that adolescents who are genetically predisposed to select more deviant peers also show higher levels of delinquency. Moreover, after controlling for these genetic selection effects, the environmental effect of peer deviance on adolescent delinquency remains.
The multivariate twin model described above was then expanded to include interaction terms, such that the components of variance in peer deviance and adolescent delinquency, as well as the genetic and environmental cross-paths between peer deviance and delinquency (bA1 and bE1), were allowed to interact with individual differences in sensation seeking. This model is designed to test for G × E interaction in the presence of rGE (Rathouz, Van Hulle, Rodgers, Waldman, & Lahey, 2008) and is depicted in Figure 2. Note that the primary pathways of interest (i.e., genetic and environmental cross paths between peer deviance and adolescent delinquency) now include two freely estimated parameters: a main effect (bA1 and bE1) and an interaction with sensation seeking (bA1’ and bE1’).
Figure 2.

Twin model of sensation seeking, peer deviance, and delinquent behavior. Person-environment correlations and person-environment correlations are depicted for only one twin per pair. A = additive genetics; C = shared environment; E = nonshared environment; SS = sensation seeking. Sibling effects were modeled for peer deviance, but omitted from presentation.
This interaction model fit significantly better than the previous model (χ2 difference = 41.473, df = 7, p < .001, AIC = 4,246.050), which did not allow genetic and environmental variance in peer deviance and adolescent delinquency to interact with individual differences in sensation seeking. Parameter estimates and confidence intervals from the interaction model are reported in Table 3.
Table 3.
Unstandardized Parameter Estimates From Model of Person-Environment Interaction and Correlation
| Regression parameters | b | 95% CI |
| Variance in sensation seeking | ||
| Main genetic effect (ASS) | .632 | [.522, .742] |
| Main nonshared environmental effect (ESS) | .769 | [.683, .855] |
| Variance in peer deviance | ||
| Main genetic effect (APD) | .794 | [.695, .894] |
| Gene × Sensation seeking interaction (APD’) | −.182 | [−.299, −.064] |
| Main nonshared environmental effect (EPD) | .582 | [.489, .675] |
| Gene × Nonshared environmental interaction (EPD’) | .562 | [.468, .656] |
| Peer deviance → Delinquency | ||
| Main genetic path (bA1) | .357 | [.206, .509] |
| Gene × Sensation seeking interaction (bA1’) | −.098 | [−.194, −.002] |
| Main nonshared environmental path (bE1) | .191 | [.083, .299] |
| Nonshared environment × Sensation seeking interaction (bE1’) | .214 | [.120, .308] |
| Variance in delinquency unique of peer deviance | ||
| Main genetic effect (ADEL) | .642 | [.506, .779] |
| Gene × Sensation seeking interaction (ADEL’) | −.020 | [−.126, .087] |
| Main shared environmental effect (CDEL) | .000 | [−.001, .001] |
| Shared environmental × Sensation seeking interaction (CDEL’) | .000 | [.000, .000] |
| Main nonshared environmental effect (EDEL) | .623 | [.532, .714] |
| Gene × Nonshared environmental interaction (EDEL’) | .033 | [−.069, .135] |
| Correlation coefficients | r | 95% CI |
| Sensation seeking and peer deviance | ||
| Additive genetic (rA1) | .415 | [.256, .575] |
| Nonshared environmental (rE1) | .068 | [−.084, .219] |
| Sensation seeking and delinquency | ||
| Additive genetic (rA2) | .534 | [.286, .783] |
| Nonshared environmental (rE2) | .181 | [−.003, .366] |
Note. b = beta coefficients; r = correlation coefficients; 95% CI = 95% confidence intervals.
The genetic (APD’ = −.182, p = .002) and environmental (EPD’ = .562, p < .001) components of peer deviance were moderated by individual differences in sensation seeking. The E component of peer deviance represents the extent to which identical twins differ in their experience of peer deviance, whereas the A component represents the extent to which identical twins experience more similar peer environments than fraternal twins. Therefore, the positive moderation of the E path by sensation seeking, in conjunction with the negative moderation of the A path, indicates that highly sensation seeking twins experience more differentiated and idiosyncratic peer environments.
Additionally, individual differences in sensation seeking significantly moderated the environmental (bE1’ = .214, p < .001) and genetic (bA1’ = −.098, p = .046) paths between peer deviance and adolescent delinquency, such that the environmental and genetic effects of peer deviance on delinquency increased and decreased, respectively, among high sensation-seeking adolescents. Moderation of the environmental (E) path between peer deviance and adolescent delinquency is consistent with our main hypothesis that peer influence will be magnified among adolescents high in sensation seeking.
This E moderation effect is illustrated in Figure 3, which shows individual-level data on delinquency from MZ twins. Each twin was classified according to whether he or she reported higher or lower peer deviance than his or her identical twin (“higher PD than co-twin” [plotted using circles] or “lower PD than co-twin” [plotted using triangles]). In addition, each twin was classified as either “high sensation seeking” or “low sensation seeking” based on whether he or she was above or below the sample mean for sensation seeking. As shown in Figure 3, regardless of peer deviance, adolescents who reported “low sensation seeking” were clearly less delinquent than adolescents who reported “high sensation seeking.” Moreover, the difference between the “higher PD than co-twin” and “lower PD than co-twin” groups is the difference in delinquency between identical twins who are discordant for peer deviance. This within-MZ-twin-pair difference represents the E pathway from peer deviance to delinquency in the structural equation model. Consistent with the results from the interaction model summarized in Table 3, identical twins who experience differing levels of peer deviance show corresponding differences in delinquent behavior only at high levels of sensation seeking and not at low levels of sensation seeking.
Figure 3.
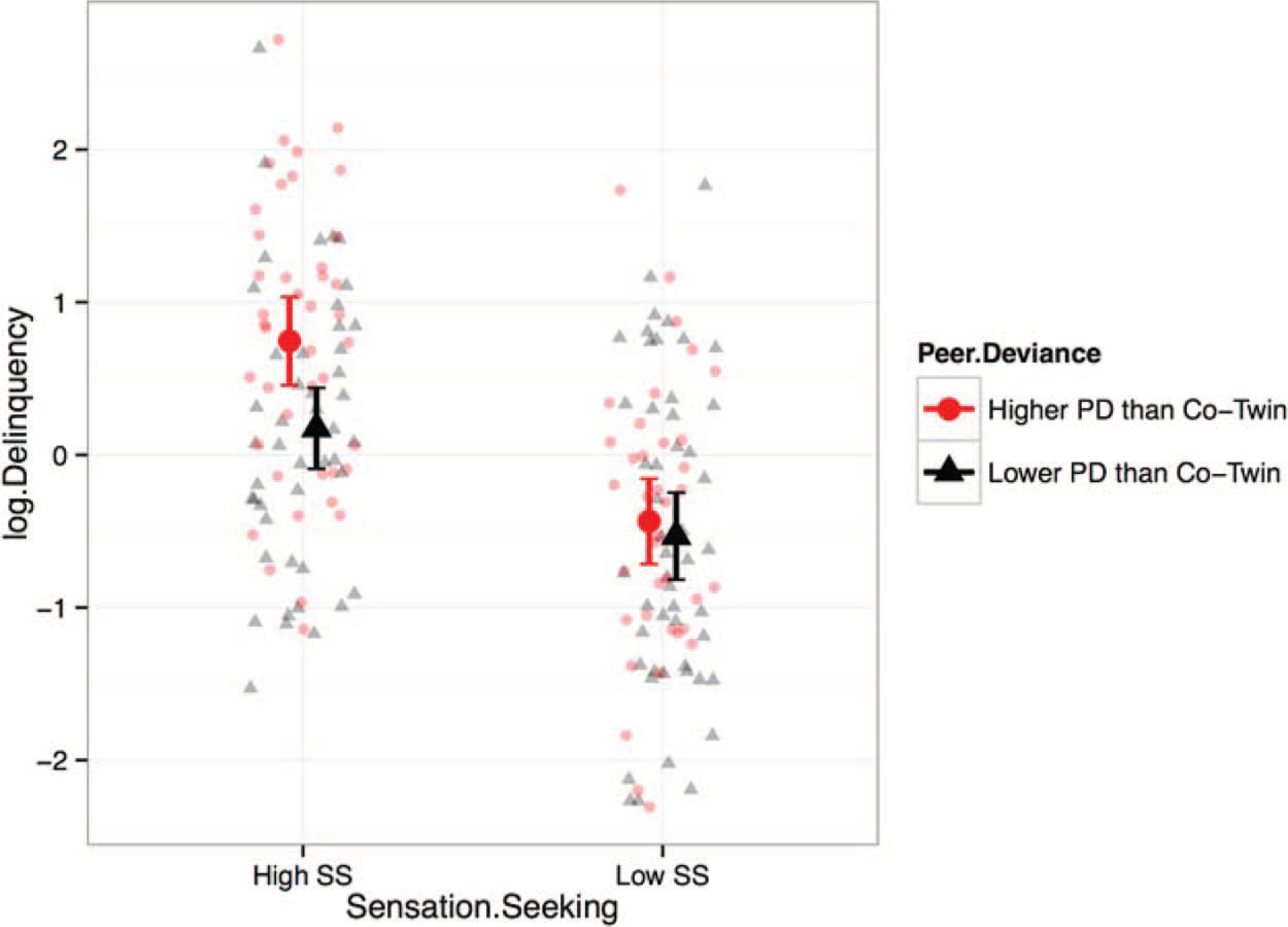
Individual-level data on delinquency by sensation seeking and within-MZ-twin-pair differences in peer deviance. Small dots and triangles represent individual-level data. Large dots and triangles represent means. Error bars represent ± 2 SEs around the mean. Only data from MZ twins are presented. “higher PD than co-twin” (plotted using circles) or “lower PD than co-twin” (plotted using triangles) were classified based on whether the individual reported higher or lower peer deviance than his or her identical co-twin. “High sensation seeking” or “low sensation seeking” was classified based on whether the individual reported sensation seeking above or below the sample mean. See the online article for the color version of this figure.
We are hesitant to interpret the interaction between the A path from peer deviance of delinquency, because we did not have an a priori hypothesis regarding this moderation effect and because the significance of the parameter was very close to p = .05. Nevertheless, we can consider the implications of the negative genetic interaction in conjunction with the positive (and larger) nonshared environmental interaction. Compared with adolescents who are low in sensation seeking, the total association between peer deviance and sensation seeking is stronger in adolescents who are high in sensation seeking. Moreover, the relative balance of genetic versus nonshared environmental processes shifts. Among adolescents who are low in sensation seeking, the relationship is almost entirely accounted for by genetic selection, whereas among adolescents who are high in sensation seeking the association between peer deviance and delinquency is less reflective of genetic selection and more reflective of an environmental pathway.
To summarize results, we found that the genes that influence sensation seeking overlap with genetic influences on affiliation with deviant peers (rGE). In a co-twin control design, identical twins who differ in peer deviance also differ in delinquency, indicating an environmental effect of peers on delinquency. Finally, as hypothesized, this environmentally mediated peer effect was moderated by individual differences in sensation seeking, a Person × Environment interaction.
Discussion
This article reports results of the first genetically informative study of sensation seeking, peer deviance, and antisocial behavior to consider simultaneously both person-environment correlations and interactions. By estimating genetic correlations, we tested the extent to which genetic influences on sensation seeking account for genetic influences on (a) delinquent behavior and (b) peer deviance. By allowing cross-paths between peer deviance and adolescent delinquency to interact with sensation seeking, we tested (c) whether the environmental association between peer deviance and adolescent delinquency (i.e., the environmental effect of peer influence) is moderated by individual differences in sensation seeking.
Our findings build on previous behavioral genetic research on peers and externalizing behaviors, which has found evidence for both selection and socialization processes (Boardman, Saint Onge, Haberstick, Timberlake, & Hewitt, 2008; Button et al., 2007, 2009; Cruz et al., 2012; Fowler et al., 2007; Harden et al., 2008). More genetically similar individuals (e.g., identical twins) tend to experience more similar peer environments than do less similar individuals (e.g., fraternal twins), indicating that genetically influenced traits lead adolescents to systematically select and attract peer groups that behave similar to themselves. We find evidence that suggests, even after controlling for this gene-environment correlation, identical twins who differ in their peer environments show differing levels of involvement in delinquent behavior, a result that is consistent with a socializing effect of peers. Our findings also build on past behavior genetic and molecular genetics research indicating Gene × Peer interactions on delinquency and closely related behaviors, such as substance use (Guo et al., 2009; Harden et al., 2008; Hicks et al., 2009). Results of the current study are consistent with conceptualizing sensation seeking as a genetically influenced trait that underlies individual differences in vulnerability to peer influence. Previous work in both quantitative genetics and molecular genetics has found evidence that genetic variants influencing antisocial behavior are mediated by sensation seeking (Aliev et al., 2015; Harden et al., 2012; Salvatore et al., 2015), but no previous study that we are aware of has examined sensation seeking as a mechanism of both gene-environment correlation and Person × Environment interaction with respect to peer deviance and delinquency. Thus, the current study synthesizes multiple sets of findings from previous work to provide evidence that (a) sensation seeking accounts for a considerable proportion of the genetic influences on delinquent behavior, (b) peer deviance is associated with delinquent behavior even when common genetic and shared environmental influences are controlled, and (c) the environmentally mediated effect of peer deviance on adolescent delinquency is moderated by sensation seeking.
Limitations
A limitation of the current study is that the constructs were measured exclusively using twin reports of their own behavior; conclusions drawn would be bolstered by additional evidence from teachers, parents, administrators, or legal authorities. In the current project, we were able to detect significant moderation effects; though, the current sample of adolescents (547 individuals comprising 282 twin pairs) is small-to-moderate for testing biometric interactions. Therefore, it is important not to draw definitive conclusions about the moderating effects of sensation seeking on the genetic and environmental associations between peer deviance and adolescent delinquency. This is particularly the case for the moderation of the genetic association between peer deviance and adolescent delinquency, which just met conventional standards for statistical significance, had correspondingly wide confidence intervals, and was not a hypothesized or predicted effect. Importantly, the precision and reliability of the current results may be further clarified with replications in larger samples. Prior to replication, the moderation effects documented in the current study (both genetic and environmental) should be considered tentative.
Avenues for Future Research
Two critical questions for ongoing research on adolescent delinquency (and for all complex, heritable phenotypes) are (a) what are the specific genetic variants that constitute the heritable variation, and (b) what are the intermediary biological pathways that translate genetic differences into phenotypic differences. On the one hand, discovering a specific genetic variant yields new insights regarding the underlying biology of a phenotype. For example, the discovery that a polymorphism in CACNA1C is reliably associated with schizophrenia in genome-wide association studies has focused attention on understanding the role of calcium channel signaling in the pathophysiology of serious psychiatric diseases (Curtis et al., 2011; Nyegaard et al., 2010). Then again, understanding the underlying neurobiology of a phenotype can point to candidate gene sets. For example, on the basis of research showing that long-term learning depends on proteins in excitatory neuronal synapses, Hill et al. (2014) tested associations between general intelligence and a candidate set of genes coding for these proteins. Discovering specific genes and delineating the pathways from genotype to adolescent delinquency and its precursor traits, such as sensation seeking, are mutually informative enterprises that will ultimately help to propel our understanding of how biological factors intersect with social contexts to shape adolescent delinquency and related externalizing behaviors.
Our results suggest new avenues for research in humans and in animal models to address these questions. For example, Roman high- and low-avoidance (RHA-I vs. RLA-I) ratlines are inbred animals that differ in sensation-seeking and novelty-seeking behavior (Giorgi, Piras, & Corda, 2007). In a study using brain tissue to examine differences in gene expression, 38 genes were found to be either upregulated or downregulated in the RLA-I rats versus RHA-I rats, including PRL (which codes for prolactin) and CRHBP (which codes for corticotropin releasing hormone binding protein; Sabariego et al., 2011). The finding that much of the genetic influence on delinquency is mediated via genetic influences on sensation seeking may indicate that these genes and the hormonal systems that they regulate may be promising targets for genetic association studies of delinquent behavior in humans.
Additionally, future research using cognitive neuroscience might test the hypothesis that the neural correlates of sensation seeking mediate genetic influences on delinquency. Research in developmental cognitive neuroscience has begun to illuminate the neurobiological correlates of developmental changes in sensation seeking. For example, adolescents show stronger activation of the ventral striatum in response to rewards, such as money, than do either children or adults (Galvan, 2010). Ventral striatum activity, in turn, is positively correlated with risk-taking on laboratory tasks, preference for immediate rewards, and self-reported delinquency and substance use (Galvan, 2010; Geier, 2013). In addition to rewards, the ventral striatum (along with the amygdala) has also been shown to respond to threatening cues in some adolescents, a response that is associated with increasing testosterone levels (Spielberg et al., 2015). This pattern of neural activation might underlie thrill-seeking tendencies, in which physically dangerous activities are experienced as rewarding. Synthesizing these findings from developmental cognitive neuroscience with the behavioral genetic findings reported here suggest the interesting hypothesis that ventral striatal function may be a key neurobiological mediator between genotype and delinquency phenotype (Harden & Mann, 2015).
Although we interpret the Sensation seeking × Peer deviance interaction through the lens of previous research on Gene × Peer deviance interactions, it is important to note that the phenotypic measurement of sensation seeking (rather than just its genetically influenced component) was entered as the moderator in our interaction models. The moderating effects of sensation seeking, therefore, include both the environmental and genetic variance in sensation seeking. Research on more general domains of personality (i.e., Big Five) have found that genetic influences on personality are more stable in adolescence than environmental influences (Briley & Tucker-Drob, 2014). Moreover, theoretical work in person-environment transactions has hypothesized that genetically influenced variation in personality will be most consequential for shaping subsequent behavioral development, precisely because the genetic influenced variation is more likely to be stable and recurring over development (Briley & Tucker-Drob, 2015; Tucker-Drob & Harden, 2012). On the other hand, research in both humans and animals has suggested that one source of “environmental” influence on impulsive personality traits is substance use (Ashenhurst, Harden, Corbin, & Fromme, 2015; Irimia et al., 2015; Quinn, Stappenbeck, & Fromme, 2011)—which is, of course, correlated with the nonsubstance-related delinquent behaviors we examine in the current project. One can imagine positive feedback loops in which early exposure to substances sensitizes the development of sensation seeking, leading to greater sensitivity to peer deviance and the escalation of socially problematic behavior. However, tracing these sorts of feedback loops, while also discriminating genetic selection from environmental causation, will require longitudinal data.
Finally, as we observe in our current sample, a socially problematic expression of sensation seeking is to engage in delinquent behavior. However, prosocial expressions of sensation seeking are also possible. For example, highly sensation seeking teens might also be more likely to become socially dominant leaders, as has previously been found for high-testosterone males with prosocial friends (Rowe, Maughan, Worthman, Costello, & Angold, 2004). Among adults who were all employed full-time, high sensation seeking was associated with better supervisor ratings of job performance, higher entrepreneurship, and higher involvement with and enjoyment of work (Jackson, 2011). However, whether these results generalize to teenagers, for whom many avenues of prosocial risk-taking (e.g., entrepreneurship, military service) are proscribed, is unclear. How to best conceptualize and measure potentially prosocial manifestations of sensation seeking in adolescents is, therefore, an interesting avenue for future research.
Measuring Peer Influence
Peer influence can be operationalized in a variety of ways. One limitation of the current study is that we rely on adolescents’ reports of their peers’ behaviors. Previous research has shown that teenagers tend to both overestimate their peers’ involvement in socially deviant activities (Prinstein & Wang, 2005) and to overestimate their peers’ similarity to themselves (Bauman & Ennett, 1996). Additionally, participants may have differed in how broadly or narrowly they construed the prompt about their “friends,” either considering only intimate relationships or counting even casual acquaintances. Nevertheless, the current results converge with results from previous twin research on peer influence that used sociometric nominations to define peer groups and that used peers’ reports on their own behavior (Cruz et al., 2012; Harden et al., 2008). In addition, our measure of peer deviance includes items asking about peer involvement in deviant behaviors (e.g., “used force to get money or things,” “sold drugs”) and (reverse-coded) items asking about prosocial behaviors (e.g., “has been involved in school activities/athletics,” “gets along well with teachers at school”). Thus, very low scores on the peer deviance measure represents affiliation with peers who are engaged in a variety of normative and constructive activities.
No cross-sectional study can definitively ascertain direction of causation. We have interpreted the correlation between peer deviance and delinquency in terms of the socializing effects of peers on the (twin) target, but socializing influences are reciprocal and dynamic. An additional (and not mutually exclusive) interpretation of our data is that highly sensation-seeking adolescents are particularly influential in shaping the behaviors of their friends. It is true that adolescents who engage in minor delinquency and substance use are more popular (Allen, Porter, McFarland, Marsh, & McElhaney, 2005), consistent with the idea that pseudoadult behavior is a “coveted social asset” (Moffitt, 1993). However, in laboratory studies where susceptibility to peer influence in operationalized in terms of changing one’s opinion in negotiation with a close friend, socially influential teenagers actually showed less engagement in delinquent behavior than did teenagers who were highly susceptible to peer influence (Allen, Porter, & McFarland, 2006). Ultimately, disentangling the impact of sensation seeking on one’s susceptibility to peer influence versus the strength of one’s influence on others will require longitudinal research that pays close attention to the developmental contexts, in addition to the broader social contexts, in which individuals are embedded.
Considering Other Subtypes of Antisocial Behavior
Our measure of delinquent behavior inquired about involvement in mostly nonaggressive, rule- breaking acts (e.g., “purposely damaged or destroyed property that did not belong to you,” “painted graffiti or signs on someone else’s property or in a public place,” etc.). Moreover, in our school-based, community sample of teenagers, we expect that most individuals who are currently engaging in delinquent acts will desist from antisocial behavior as they transition to adulthood. Importantly, the etiology of adolescent-limited, rule-breaking antisocial behavior has been found to differ from other, more serious and persistent, subtypes of ASB defined by childhood-onset behavior problems, aggression, and callous-unemotionality (Burt, 2012; Harden et al., 2015; Viding, Jones, Paul, Moffitt, & Plomin, 2008). It is unclear whether the results observed in this study (high genetic correlations with sensation seeking and environmentally mediated effects of peer deviance) will generalize to other subtypes of antisocial behavior. This remains an interesting question for future research.
Conclusions
In sum, the current study combines insights from two separate lines of research, the first of which has examined sensation seeking as psychological mediator of genetic influences on delinquent adolescent behavior, and the second of which has examined Gene × Peer context interactions on delinquency. We find evidence that genetic influences on sensation seeking predispose adolescents to affiliate with deviant peers and engage in delinquent behavior themselves. But, even after controlling for this gene-environment correlation using a co-twin-comparison, peer deviance remains associated with adolescent delinquency. Further, we find evidence that sensation seeking moderates the environmental effect of peer deviance on delinquency. Compared with low sensation-seeking adolescents, high sensation-seekers are more vulnerable to the social influence of their peers. These results are consistent with both selection and socialization processes contributing to delinquent behavior in adolescence, particularly mediated through individual differences in sensation seeking.
General Scientific Summary.
Sensation seeking and deviant peer groups are risk factors for delinquent behavior. The current study suggests that adolescents with genetic dispositions toward higher sensation seeking are more likely to affiliate with deviant peers. At the same time, adolescents high in sensation seeking are also more susceptible to the influence of deviant peers.
Acknowledgments
Population Research Center at the University of Texas at Austin, which is supported by National Institutes of Health (NIH) center Grant R24-HD042849, provided a seed grant for the establishment of the Texas Twin Project. Ongoing work on the Texas Twin Project is supported by NIH Grant R21-AA020588 to K. Paige Harden and Elliot M. Tucker-Drob.
Footnotes
At any particular locus (i.e., a specific location on a chromosome), there are two copies of a particular version of a gene, called alleles. One allele is inherited from one’s mother and the other from one’s father. If an individual inherits different alleles from each parent, then they are heterozygous (Aa) on that particular gene. If an individual inherits the same alleles from each parent, then they are either homozygous recessive (aa) or homozygous dominant (AA) on that gene. For phenotypes influenced by additive genes, the presence of one allele does not alter the effects of the other allele at the same locus. For phenotypes influenced by dominant genes, one allele—that is, the dominant one—masks the effects of the second allele—that is, the recessive one. Thus, for additive genes, individuals who are heterozygous (Aa) express a phenotype that is intermediary between individuals who are homozygous recessive (aa) and homozygous dominant (AA). For dominant genes, however, individuals who are heterozygous (Aa) express the same phenotype as those who are homozygous dominant (AA); the alternate phenotype is only expressed when two recessive alleles are inherited—that is, homozygous recessive (aa). In terms of twin-pair correlations, compared with what is predicted under an exclusively additive model, dominance genetic effects decrease the phenotypic similarity of dizygotic twins (who are not always matched on the other allele), but not monozygotic twins (who are guaranteed to be matched on the other allele).
Contributor Information
Frank D. Mann, University of Texas at Austin
Megan W. Patterson, University of Texas at Austin
Andrew D. Grotzinger, University of Texas at Austin
Natalie Kretsch, Texas A&M University.
Jennifer L. Tackett, Northwestern University
Elliot M. Tucker-Drob, University of Texas at Austin
K. Paige Harden, University of Texas at Austin.
References
- Agrawal A, Balasubramanian S, Smith EK, Madden PA, Bucholz KK, Heath AC, & Lynskey MT (2010). Peer substance involvement modifies genetic influences on regular substance involvement in young women. Addiction, 105, 1844–1853. 10.1111/j.1360-0443.2010.02993.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev F, Wetherill L, Bierut L, Bucholz KK, Edenberg H, Foroud T, & Dick DM (2015). Genes associated with alcohol outcomes show enrichment of effects with broad externalizing and impulsivity phenotypes in an independent sample. Journal of Studies on Alcohol and Drugs, 76, 38–46. 10.15288/jsad.2015.76.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Porter MR, & McFarland FC (2006). Leaders and followers in adolescent close friendships: Susceptibility to peer influence as a predictor of risky behavior, friendship instability, and depression. Development and Psychopathology, 18, 155–172. 10.1017/S0954579406060093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Porter MR, McFarland FC, Marsh P, & McElhaney KB (2005). The two faces of adolescents’ success with peers: Adolescent popularity, social adaptation, and deviant behavior. Child Development, 76, 747–760. 10.1111/j.1467-8624.2005.00875.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashenhurst JR, Harden KP, Corbin WR, & Fromme K (2015). Trajectories of binge drinking and personality change across emerging adulthood. Psychology of Addictive Behaviors, 29, 978–991. 10.1037/adb0000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T, & Muthén B (2006, August). Multilevel modeling of complex survey data. In Proceedings of the Joint Statistical Meeting in Seattle (pp. 2718–2726). [Google Scholar]
- Bauman KE, & Ennett ST (1996). On the importance of peer influence for adolescent drug use: Commonly neglected considerations. Addiction, 91, 185–198. 10.1111/j.1360-0443.1996.tb03175.x [DOI] [PubMed] [Google Scholar]
- Boardman JD, Saint Onge JM, Haberstick BC, Timberlake DS, & Hewitt JK (2008). Do schools moderate the genetic determinants of smoking? Behavior Genetics, 38, 234–246. 10.1007/s10519-008-9197-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechwald WA, & Prinstein MJ (2011). Beyond homophily: A decade of advances in understanding peer influence processes. Journal of Research on Adolescence, 21, 166–179. 10.1111/j.1532-7795.2010.00721.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendgen M (2012). Genetics and peer relations: A review. Journal of Research on Adolescence, 22, 419–437. 10.1111/j.1532-7795.2012.00798.x [DOI] [Google Scholar]
- Briley DA, & Tucker-Drob EM (2014). Genetic and environmental continuity in personality development: A meta-analysis. Psychological Bulletin, 140, 1303–1331. 10.1037/a0037091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley DA & Tucker-Drob EM (2015). Comparing the developmental genetics of cognition and personality over the lifespan. Journal of Personality. Advance online publication 10.1111/jopy.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk WJ, van der Vorst H, Kerr M, & Stattin H (2012). Alcohol use and friendship dynamics: Selection and socialization in early-, middle-, and late-adolescent peer networks. Journal of Studies on Alcohol and Drugs, 73, 89–98. 10.15288/jsad.2012.73.89 [DOI] [PubMed] [Google Scholar]
- Burt SA (2012). How do we optimally conceptualize the heterogeneity within antisocial behavior? An argument for aggressive versus nonaggressive behavioral dimensions. Clinical Psychology Review, 32, 263–279. 10.1016/j.cpr.2012.02.006 [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, & Iacono WG (2001). Sources of covariation among attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder: The importance of shared environment. Journal of Abnormal Psychology, 110, 516–525. 10.1037/0021-843X.110.4.516 [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, & Iacono WG (2010). Environmental contributions to the stability of antisocial behavior over time: Are they shared or non-shared? Journal of Abnormal Child Psychology, 38, 327–337. 10.1007/s10802-009-9367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Iacono WG, & Krueger RF (2006). Differential parent-child relationships and adolescent externalizing symptoms: Cross-lagged analyses within a monozygotic twin differences design. Developmental Psychology, 42, 1289–1298. 10.1037/0012-1649.42.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, & Iacono WG (2007). Environmental contributions to adolescent delinquency: A fresh look at the shared environment. Journal of Abnormal Child Psychology, 35, 787–800. 10.1007/s10802-007-9135-2 [DOI] [PubMed] [Google Scholar]
- Button TM, Corley RP, Rhee SH, Hewitt JK, Young SE, & Stallings MC (2007). Delinquent peer affiliation and conduct problems: A twin study. Journal of Abnormal Psychology, 116, 554–564. 10.1037/0021-843X.116.3.554 [DOI] [PubMed] [Google Scholar]
- Button TM, Stallings MC, Rhee SH, Corley RP, Boardman JD, & Hewitt JK (2009). Perceived peer delinquency and the genetic predisposition for substance dependence vulnerability. Drug and Alcohol Dependence, 100, 1–8. 10.1016/j.drugalcdep.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey G (1986). Sibling imitation and contrast effects. Behavior Genetics, 16, 319–341. 10.1007/BF01071314 [DOI] [PubMed] [Google Scholar]
- Caspi A (1995). Puberty and the gender organization of schools: How biology and social context shape the adolescent experience. In Crockett LJ & Crouter AC (Eds.), Pathways through adolescence: Individual development in relation to social contexts (pp. 57–74). New York, NY: Erlbaum. [Google Scholar]
- Cleveland HH, Wiebe RP, & Rowe DC (2005). Sources of exposure to smoking and drinking friends among adolescents: A behavioral-genetic evaluation. The Journal of Genetic Psychology: Research and Theory on Human Development, 166, 153–169. [PubMed] [Google Scholar]
- Cruz JE, Emery RE, & Turkheimer E (2012). Peer network drinking predicts increased alcohol use from adolescence to early adulthood after controlling for genetic and shared environmental selection. Developmental Psychology, 48, 1390–1402. 10.1037/a0027515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D, Vine AE, McQuillin A, Bass NJ, Pereira A, Kandaswamy R, … Gurling HM (2011). Case-case genome-wide association analysis shows markers differentially associated with schizophrenia and bipolar disorder and implicates calcium channel genes. Psychiatric Genetics, 21, 1–4. 10.1097/YPG.0b013e3283413382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, & Tipsord JM (2011). Peer contagion in child and adolescent social and emotional development. Annual Review of Psychology, 62, 189–214. 10.1146/annurev.psych.093008.100412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8, 430–457. 10.1207/S15328007SEM0803_5 [DOI] [PubMed] [Google Scholar]
- Ennett ST, & Bauman KE (1994). The contribution of influence and selection to adolescent peer group homogeneity: The case of adolescent cigarette smoking. Journal of Personality and Social Psychology, 67, 653–663. 10.1037/0022-3514.67.4.653 [DOI] [PubMed] [Google Scholar]
- Fowler JH, Settle JE, & Christakis NA (2011). Correlated genotypes in friendship networks. Proceedings of the National Academy of Sciences of the United States of America, 108, 1993–1997. 10.1073/pnas.1011687108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T, Shelton K, Lifford K, Rice F, McBride A, Nikolov I, … van den Bree MB (2007). Genetic and environmental influences on the relationship between peer alcohol use and own alcohol use in adolescents. Addiction, 102, 894–903. 10.1111/j.1360-0443.2007.01824.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, & Viding E (2009). Antisocial behavior from a developmental psychopathology perspective. Development and Psychopathology, 21, 1111–1131. 10.1017/S0954579409990071 [DOI] [PubMed] [Google Scholar]
- Galvan A (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience, 4, 6. 10.3389/neuro.09.006.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF (2013). Adolescent cognitive control and reward processing: Implications for risk taking and substance use. Hormones and Behavior, 64, 333–342. 10.1016/j.yhbeh.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Giorgi O, Piras G, & Corda MG (2007). The psychogenetically selected Roman high- and low-avoidance rat lines: A model to study the individual vulnerability to drug addiction. Neuroscience and Biobehavioral Reviews, 31, 148–163. 10.1016/j.neubiorev.2006.07.008 [DOI] [PubMed] [Google Scholar]
- Gottesman II, & Gould TD (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. The American Journal of Psychiatry, 160, 636–645. 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- Guo G, Elder GH, Cai T, & Hamilton N (2009). Gene– environment interactions: Peers’ alcohol use moderates genetic contribution to adolescent drinking behavior. Social Science Research, 38, 213–224. 10.1016/j.ssresearch.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson SE, Andrews JA, & Barckley M (2008). Childhood predictors of adolescent marijuana use: Early sensation-seeking, deviant peer affiliation, and social images. Addictive Behaviors, 33, 1140–1147. 10.1016/j.addbeh.2008.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Hill JE, Turkheimer E, & Emery RE (2008). Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behavior Genetics, 38, 339–347. 10.1007/s10519-008-9202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, & Mann FD (2015). Biological risk for the development of problem behavior in adolescence: Integrating insights from behavioral genetics and neuroscience. Child Development Perspectives, 9, 211–216. 10.1111/cdep.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Quinn PD, & Tucker-Drob EM (2012). Genetically influenced change in sensation seeking drives the rise of delinquent behavior during adolescence. Developmental Science, 15, 150–163. 10.1111/j.1467-7687.2011.01115.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, & Tucker-Drob EM (2011). Individual differences in the development of sensation seeking and impulsivity during adolescence: Further evidence for a dual systems model. Developmental Psychology, 47, 739–746. 10.1037/a0023279 [DOI] [PubMed] [Google Scholar]
- Harden KP, Tucker-Drob EM, & Tackett JL (2013). The Texas Twin project. Twin Research and Human Genetics, 16, 385–390. 10.1017/thg.2012.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Nyholt DR, Neuman R, Madden PA, Bucholz KK, Todd RD,... Martin NG (2003). Zygosity diagnosis in the absence of genotypic data: An approach using latent class analysis. Twin Research, 6, 22–26. 10.1375/136905203762687861 [DOI] [PubMed] [Google Scholar]
- Hicks BM, South SC, Dirago AC, Iacono WG, & McGue M (2009). Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry, 66, 640–648. 10.1001/archgenpsychiatry.2008.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Davies G, van de Lagemaat LN, Christoforou A, Marioni RE, Fernandes CPD, … Deary IJ (2014). Human cognitive ability is influenced by genetic variation in components of postsynaptic signalling complexes assembled by NMDA receptors and MAGUK proteins. Translational Psychiatry, 4, e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, & Donohew RL (2002). Reliability and validity of a brief measure of sensation seeking. Personality and Individual Differences, 32, 401–414. 10.1016/S0191-8869(01)00032-0 [DOI] [Google Scholar]
- Irimia C, Wiskerke J, Natividad LA, Polis IY, de Vries TJ, Pattij T, & Parsons LH (2015). Increased impulsivity in rats as a result of repeated cycles of alcohol intoxication and abstinence. Addiction Biology, 20, 263–274. 10.1111/adb.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CJ (2011). How sensation seeking provides a common basis for functional and dysfunctional outcomes. Journal of Research in Personality, 45, 29–36. 10.1016/j.jrp.2010.11.005 [DOI] [Google Scholar]
- Jessor R, & Jessor SL (1977). Problem behavior and psychosocial development: A longitudinal study of youth. New York, NY: Academic Press. [Google Scholar]
- Kretsch N, Mendle J, & Harden KP (2016). A twin study of objective and subjective pubertal timing and peer influence on risk-taking. Journal of Research on Adolescence, 26, 46–59. 10.1111/jora.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer T., Dijkstra JK., Ormel J., Verhulst FC., & Veenstra R. (2013). Dopamine receptor D4 gene moderates the effect of positive and negative peer experiences on later delinquency: The Tracking Adolescents’ Individual Lives Survey study. Development and Psychopathology, 25, 1107–1117. 10.1017/S0954579413000400 [DOI] [PubMed] [Google Scholar]
- Kretschmer T, Vitaro F, & Barker ED (2014). The association between peer and own aggression is moderated by the BDNF Val-met polymorphism. Journal of Research on Adolescence, 24, 177–185. 10.1111/jora.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, & Iacono WG (2005). Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology, 114, 537–550. 10.1037/0021-843X.114.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latendresse SJ, Bates JE, Goodnight JA, Lansford JE, Budde JP, Goate A, … Dick DM (2011). Differential susceptibility to adolescent externalizing trajectories: Examining the interplay between CHRM2 and peer group antisocial behavior. Child Development, 82, 1797–1814. 10.1111/j.1467-8624.2011.01640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann FD, Kretsch N, Tackett JL, Harden KP, & Tucker-Drob EM (2015). Person × Environment interactions on adolescent delinquency: Sensation seeking, peer deviance and parental monitoring. Personality and Individual Differences, 76, 129–134. 10.1016/j.paid.2014.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, & Bouchard TJ Jr. (1984). Adjustment of twin data for the effects of age and sex. Behavior Genetics, 14, 325–343. 10.1007/BF01080045 [DOI] [PubMed] [Google Scholar]
- McPherson M, Smith-Lovin L, & Cook JM (2001). Birds of a feather: Homophily in social networks. Annual Review of Sociology, 27, 415–444. 10.1146/annurev.soc.27.1.415 [DOI] [Google Scholar]
- Moffitt TE (1993). Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review, 100, 674–701. 10.1037/0033-295X.100.4.674 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998 –2010). Mplus user’s guide (6th ed.). Los Angeles, CA: Author. [Google Scholar]
- Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sørensen KM, … Børglum AD (2010). CACNA1C (rs1006737) is associated with schizophrenia. Molecular Psychiatry, 15, 119–121. 10.1038/mp.2009.69 [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, & Wang SS (2005). False consensus and adolescent peer contagion: Examining discrepancies between perceptions and actual reported levels of friends’ deviant and health risk behaviors. Journal of Abnormal Child Psychology, 33, 293–306. 10.1007/s10802-005-3566-4 [DOI] [PubMed] [Google Scholar]
- Quinn PD, Stappenbeck CA, & Fromme K (2011). Collegiate heavy drinking prospectively predicts change in sensation seeking and impulsivity. Journal of Abnormal Psychology, 120, 543–556. 10.1037/a0023159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathouz PJ, Van Hulle CA, Rodgers JL, Waldman ID, & Lahey BB (2008). Specification, testing, and interpretation of gene-by-measured-environment interaction models in the presence of gene-environment correlation. Behavior Genetics, 38, 301–315. 10.1007/s10519-008-9193-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing [Computer software]. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/ [Google Scholar]
- Rowe R, Maughan B, Worthman CM, Costello EJ, & Angold A (2004). Testosterone, antisocial behavior, and social dominance in boys: Pubertal development and biosocial interaction. Biological Psychiatry, 55, 546–552. 10.1016/j.biopsych.2003.10.010 [DOI] [PubMed] [Google Scholar]
- Sabariego M, Gómez MJ, Morón I, Torres C, Fernández-Teruel A, Tobeña A, … Esteban FJ (2011). Differential gene expression between inbred Roman high- (RHA-I) and low- (RLA-I) avoidance rats. Neuroscience Letters, 504, 265–270. 10.1016/j.neulet.2011.09.044 [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Aliev F, Bucholz K, Agrawal A, Hesselbrock V, Hesselbrock M, … Dick DM (2015). Polygenic risk for externalizing disorders: Gene-by-development and gene-by-environment effects in adolescents and young adults. Clinical Psychological Science, 3, 189–201. 10.1177/2167702614534211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A, & Bentler PM (2001). A scaled difference chi-square test statistic for moment structure analysis. Psychometrika, 66, 507–514. 10.1007/BF02296192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Forbes EE, Ladouceur CD, Worthman CM, Olino TM, Ryan ND, & Dahl RE (2015). Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Social Cognitive and Affective Neuroscience, 10, 408–415. 10.1093/scan/nsu062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson MT, Hoyle RH, Palmgreen P, & Slater MD (2003). Brief measures of sensation seeking for screening and large-scale surveys. Drug and Alcohol Dependence, 72, 279–286. 10.1016/j.drugalcdep.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Survey DY, Huizinga D, Esbensen FA, & Weiher AW (1991). Are there multiple paths to delinquency? The Journal of Criminal Law & Criminology, 82, 83–118. 10.2307/1143790 [DOI] [Google Scholar]
- Thornberry TP, Lizotte AJ, Krohn MD, Farnworth M, & Jang SJ (1994). Delinquent peers, beliefs, and delinquent behavior: A longitudinal test of interactional theory. Criminology, 32, 47–83. 10.1111/j.1745-9125.1994.tb01146.x [DOI] [Google Scholar]
- Tucker-Drob EM, & Harden KP (2012). Intellectual interest mediates Gene × Socioeconomic status interaction on adolescent academic achievement. Child Development, 83, 743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Jones AP, Paul JF, Moffitt TE, & Plomin R (2008). Heritability of antisocial behaviour at 9: Do callous-unemotional traits matter? Developmental Science, 11, 17–22. 10.1111/j.1467-7687.2007.00648.x [DOI] [PubMed] [Google Scholar]
- Yanovitzky I (2005). Sensation seeking and adolescent drug use: The mediating role of association with deviant peers and pro-drug discussions. Health Communication, 17, 67–89. 10.1207/s15327027hc1701_5 [DOI] [PubMed] [Google Scholar]
- Zuckerman M (1971). Dimensions of sensation seeking. Journal of Consulting and Clinical Psychology, 36, 45–52. 10.1037/h0030478 [DOI] [PubMed] [Google Scholar]


