Abstract
With solid tumor cancer survivorship increasing, the number of patients requiring post-treatment surveillance also continues to increase. This highlights the need for evidence-based cancer surveillance guidelines. Ideally, these guidelines would be based on combined high-quality data from randomized controlled trials (RCTs). We present a systematic review of published cancer surveillance RCTs in which we sought to determine the feasibility of data pooling for guideline development.
We carried out a systematic search of medical databases for RCTs in which adult patients with solid tumors that had undergone surgical resection with curative intent and had no metastatic disease at presentation, were randomized to different surveillance regimens that assessed effectiveness on overall survival (OS). We extracted study characteristics and primary and secondary outcomes, and assessed risk of bias and validity of evidence with standardized checklist tools.
Our search yielded 32,216 articles for review and 18 distinct RCTs were included in the systematic review. The 18 trials resulted in 23 comparisons of surveillance regimens. There was a highlevel of variation between RCTs, including the study populations evaluated, interventions assessed and follow-up periods for the primary outcome. Most studies evaluated colorectal cancer patients (11/18, [61%]). The risk of bias and validity of evidence were variable and inconsistent across studies.
This review demonstrated that there is tremendous heterogeneity among RCTs that evaluate effectiveness of different postoperative surveillance regimens in cancer patients, rendering the consolidation of data to inform high-quality cancer surveillance guidelines unfeasible. Future RCTs in the field should focus on consistent methodology and primary outcome definition.
Key words: Tumor, survival, surveillance, randomized controlled trials, systematic review
Introduction
Surgical intervention is the primary mode of cure for many types of solid tumors including breast, colorectal, testicular and non-small cell lung cancers.1 Following surgery, the risk of local recurrence or metastases remains a concern. Therefore, the use of post-operative surveillance protocols, which include follow-up appointments, biochemical tests and surveillance imaging in likely areas of recurrence or metastases, have become the standard of care in the management of solid cancers.2 However, the development of guidelines for post-operative surveillance protocols that effectively balance survival benefit with cost effectiveness have been challenged by the lack of evidence on which to base them.2,3
Due to advancements in imaging modalities and molecular diagnostic tests to detect relapsed disease, clinicians have an expanding repertoire of surveillance options. However, adverse effects of surveillance programs that make use of more intensive imaging modalities and/or more frequent follow-up visits are noteworthy. Patients have expressed concern over harmful levels of radiation exposure used in advanced imaging techniques, as well as the direct and indirect costs associated with more frequent follow-up visits.2,4-7 Moreover, as direct costs to the healthcare system for more intensive surveillance programs are also notable, The American Society of Clinical Oncology (ASCO) recommends not to perform additional surveillance testing or imaging in asymptomatic patients if evidence suggests these tests do not improve outcomes.7-10
The National Comprehensive Cancer Network (NCCN) develops and updates guidelines on best-practice surveillance for various cancers based on the best available evidence.11,12 In recent years, organizations and guideline developers such as the NCCN and the World Health Organization (WHO) are integrating randomized controlled trial (RCT) results via systematic reviews and meta-analyses, to inform high-quality evidence-based recommendations. 13 However, a recent systematic review of North American and European cancer surveillance guidelines found that only 35% of guidelines were informed by systematic reviews and no guidelines referenced a meta-analysis in their development.3 In order to conduct a meaningful meta-analysis that may inform guideline development, certain conditions must be met, including homogeneity with respect to study design, populations, and periods of follow-up.13-15 If studies are highly heterogenous it may not be appropriate to conduct a meta-analysis, thereby preventing organizations from developing high-quality evidence-based guidelines. 16 In order to inform future cancer surveillance guidelines, we carried out a systematic review of studies in which cancer patients were randomized to different surveillance regimens with a primary or secondary outcome of overall survival (OS), and assessed between-study heterogeneity, study risk of bias and validity.
Methods of research
This systematic review adheres to and is reported according to the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review protocol was submitted for registration on the International Prospective Register of Systematic Reviews (PROSPERO) (ID:150689).
Eligibility criteria
The study population included solid cancer patients, 18 years of age or older that had previously undergone surgical resection of their tumors and no metastatic disease at presentation. All oncology subspecialties were eligible. All RCTs, including cluster RCTs, that evaluated the efficacy of a post-operative surveillance regimen were eligible for inclusion. Eligible studies assessed OS as one of their primary or secondary outcomes, as the ASCO previously determined this outcome to be the most important in cancer treatment. 17 Only published journal articles in the English language were considered, with no lower limit on the study publication year.
Information sources
A comprehensive and systematic literature search of MEDLINE (OVID Interface, 1946 to present), EMBASE (OVID interface 1974 to present), and CENTRAL databases was conducted on 21 August 2019. Additionally, reference lists of included articles were scanned for potentially eligible titles.
Search
The search strategy was developed uniquely for each medical database by combining exploded medical indexing terms (MeSH® terms for MEDLINE and CENTRAL, EMTree® terms for EMBASE) and keywords using Boolean operators ‘OR’ and ‘AND’. The search included terms to filter for RCTs as developed by the Scottish Intercollegiate Guidelines Network.18 The full search strategy can be found in Appendix 1.
Study selection
Duplicate removal was performed in two rounds. The first round of duplicate removal was conducted manually on RefWorks by one author (BL). Following this, remaining articles were uploaded onto the DistillerSR online software (www.evidencepartners.com) to create a study database. The second round of duplicate removal utilized DistillerSR’s duplicate identification feature, and two authors (BL and VG) independently audited the integrity of this feature prior to deletion. Author pairs (BL and PS/BL and HB/BL and PT/LN and AB/ML and PT/VG and PS/VG and IM/PS and PW) independently screened titles and abstracts to determine if the articles should be considered for inclusion. The following inclusion questions were used: i) Does this article describe a RCT?; ii) Do the participants of this trial have a solid tumor?; iii) Was the cancer treated with surgery with curative intent prior to inclusion in the RCT?; iv) Does the RCT assess different postoperative follow-up regimens?; v) Is there any other reason for exclusion of the study?. The full texts of included abstracts were then reviewed in duplicate (VG and IM/PS and PW) for inclusion using the aforementioned inclusion questions, in addition to the following questions: Were the participants of this trial nonmetastatic at the time of inclusion in the RCT; and vii) Is overall survival evaluated as a primary or secondary outcome?. These two inclusion questions were added at the full-text screening stage as this information was found to only be available in the full text. All discrepancies were resolved by consensus with the senior author (MG).
Data collection process and data items
Data was extracted in duplicate (VG and IM) on DistillerSR using uniquely developed Study Characteristics, Risk of Bias and Validity of Evidence, and Statistical Reporting data collection forms (Appendix 2). Additional information on RCTs was obtained from protocols or dissertations as required. Trial authors were contacted on multiple occasions for any missing data. Data extraction forms were pilot tested on two randomly selected full texts to ensure that reviewers extracted data consistently and to ensure the forms were unambiguous and free from errors.
Study characteristics
Study characteristics including year of publication, country of origin, type of cancer, RCT design, characteristics of the interventions, primary and secondary outcomes, sample size, follow-up time period, and if the study was single- or multi-center were extracted from each article.
Studies were also classified into categories in duplicate (VG and IM) based on the type of interventions assessed. If a trial was factorial in design, each comparison within the trial was categorized independently. Due to the large variation in surveillance programs, categories were predefined as per below: i) Biological tests - any study that evaluates more intensive versus less intensive or sensitive (including a reduced number or none at all) laboratory tests; ii) Frequency - any study that evaluates more frequent versus less frequent (including a reduced number or none at all) clinic visits; iii) Imaging - any study that evaluates more intensive versus less intensive (including a reduced number or none at all) imaging modalities; iv) Practitioner type - any study that evaluates specialist (medical oncology or surgeon)-led versus primary care physician or nurse practitioner-led.
Interventions within each RCT were then categorized as either the more intensive- or less intensive-group (control group). We defined more intensive surveillance as the most comprehensive treatment of the treatment groups in any given trial (i.e., the treatment that is: more frequent, contains more invasive surveillance component(s) or is specialist-led). Any disagreements were reconciled with the senior author (MG).
Individual study outcomes
Statistical information including event rates and related effect measures for OS were extracted from each article. Event rates were calculated from OS percentages when raw results were not reported in the RCT article. When trials presented more than one hypothesis test, we reported the most adjusted result. Each comparison in a factorial trial was evaluated independently. The most recently published article was used for OS results when updated results were published for the same trial. Results were considered statistically significant if they were indicated as such by the RCT authors.
Risk of bias and validity of data in individual studies and across studies
Author pairs (VG and IM) individually assessed the risk of bias of the included studies using the Risk of Bias and Validity of Evidence form. Part A of this form follows the Cochrane Collaboration’s Risk of Bias tool.19 Any disagreements were reconciled with the senior author (MG).
Author pairs (VG and IM) individually assessed included studies on the internal and external validity of evidence using the Risk of Bias and Validity of Evidence form. Part B of this form follows the 2010 CONSORT checklist for the reporting of randomized trials. 20 Studies were marked in each element as sufficiently and appropriately reporting details of the checklist item (Yes), not reporting the checklist item (No), insufficiently reporting details of the checklist item (Insufficient details), or as not applicable to the study (NA). Any disagreements were reconciled with the senior author (MG).
Results
Study selection
Our search of databases yielded 32,197 potentially eligible studies titles, with an additional 19 records identified through reference list screening. Following duplicate removal, 25,825 titles remained for screening. 25,772 studies were excluded following title and abstract screening due to not being RCTs, patients not having sarcoma or carcinoma, patients not treated with surgery, or the RCT not assessing different post-operative follow-up regimens. Fifty-three articles remained for full text screening. Thirtytwo articles were excluded at this final stage, resulting in 21 articles reporting on 18 distinct trials included in the review.21-41 Two RCTs published extended follow-up time frame results,27,33 and one RCT published an additional cost-analysis of results.24 Other reasons for exclusion included the article being a conference abstract or preliminary results from a trial that did not report OS. Study selection is presented in Figure 1.
Figure 1.
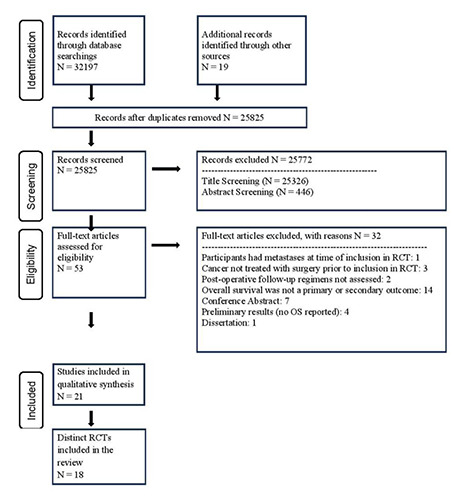
PRISMA flow diagram.
Study characteristics
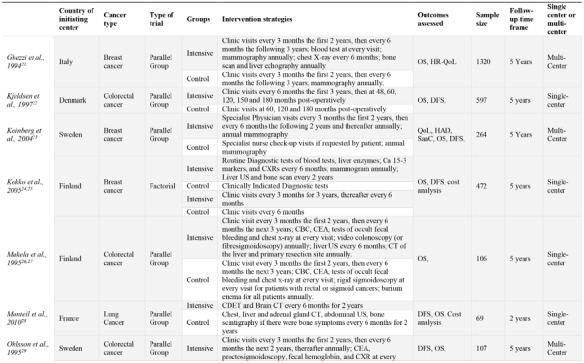
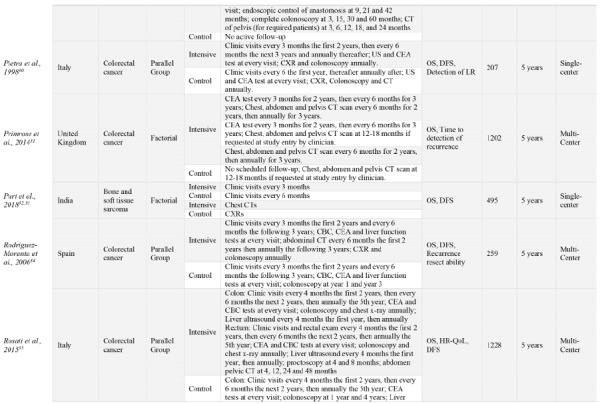
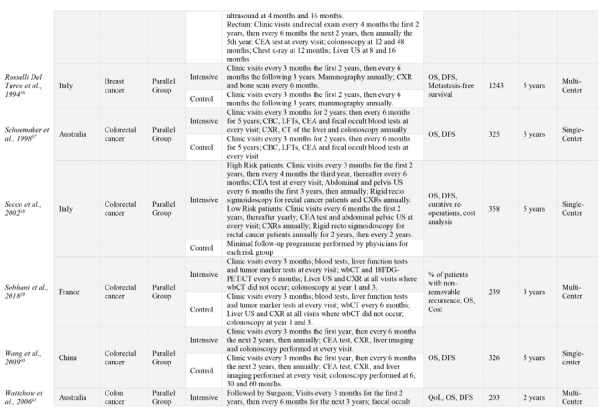
The 18 RCTs comprised a total of 9020 participants from 11 different countries. Most trials’ coordinating centers or initiating sites were based in Europe, 15/18 (83%)21-23,25,27-31,34-39 with the remaining sites in China (1/18, [6%]),40 India (1/18, [6%]),32 and Australia (2/18, [11%]).37,41 Half of the trials (50%) were singlecenter studies. Fourteen RCTs (78%) were published prior to 2010.21,22,24,25,27,29,30,34,36-38,40,41 The most common type of cancer studied was colorectal cancer, (11/18 [61%]),22,27,29-31,34,35,37-40 and one RCT included patients with only colon cancer and not rectal cancer due to slightly different follow-up programs between these cancers.41 The remaining types of cancers studied included breast (4/17 [22%]),21,23,25,36 lung (1/18 [6%]),28 and bone and soft-tissue sarcomas (1/18 [6%]).32 Fifteen RCTs were parallel group trials, (83%), with the other three of factorial design.25,30,32 OS follow-up periods ranged from two- to five-years of follow-up. In addition to OS, common outcomes assessed included disease free survival (DFS), cost analysis and quality of life (QoL), among others. Included studies characteristics are show in Table 1.
Study outcomes
Due to the multiple comparisons made in factorial trials and one trial using different follow-up protocols for high-risk and lowrisk patients,37 a total of 23 surveillance intensity comparisons were assessed in the 18 included trials. One comparison (4%) was classified as a biological test intervention, eight (35%) were classified as frequency interventions, 12 (52%) were classified as imaging interventions, and two (9%) were classified as practitioner type interventions. Even when grouping surveillance protocols by the type of intervention, there was a high-level of variation in the intervention strategies assessed. Often, comparisons in the imaging category evaluated the presence of an imaging test versus not using that imaging modality at all. OS results for each comparison are presented in Table 2.
OS event rates could be extracted from all but one RCT (two comparisons).28 Only 8/23 (35%) comparisons reported effect measures. All RCTs described the significance of intensive intervention strategies by evaluating p-values, however, four comparisons simply indicated results were not significant but did not provide point estimates.25,28,36 Furthermore, the methods to test significance varied between RCTs.
Three comparisons (13%) reported that a more intensive surveillance program significantly improved OS outcomes. All three comparisons were classified as frequency interventions, evaluated 5-year OS and involved colorectal cancer patients.30,38 No significant differences in more intensive versus less intensive protocols were reported in any other intervention category.
Risk of bias in included studies
Blinding of patients and research personnel was not possible due to the nature of the interventions; therefore, all studies had a high risk of performance bias. Many trials did not clearly report if the assessment of outcomes was blinded and if results were selectively reported. The risk of biases of included trials are presented in Figure 2.
Figure 2.
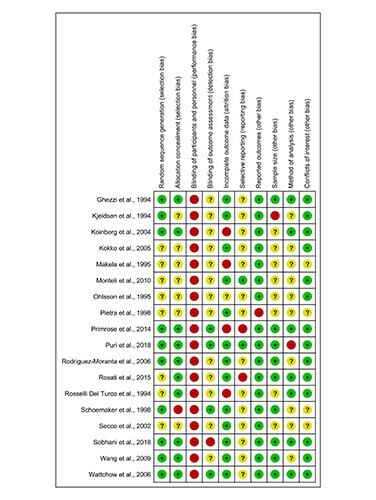
Risk of bias assessment of included studies. Listed in alphabetical order and assessed following the Cochrane Collaboration’s Risk of Bias tool17. Unclear = ?, Low risk of bias = +, High risk of bias = -
Table 1.
Study characteristics of all included studies. Ordered alphabetically. Intensive group is defined as the most comprehensive treatment of the treatment groups in any given trial (i.e., the treatment that is: more frequent, contains more invasive surveillance component(s) or is specialist-led).

Validity of evidence in included studies
Most studies detailed interventions and eligibility criteria to ensure trials were replicable. However, sufficient details regarding randomization procedures were often not reported. Furthermore, although primary and secondary outcomes were often defined, details of analysis plans and presentation of results did not meet the 2010 CONSORT guidelines in many trials. Trials included in this review often failed to report absolute and relative effect sizes. Validity of evidence for included trials is presented in Table 3.
Discussion
Summary of results
The purpose of this study was to evaluate the available RCTs that assessed the effectiveness of post-operative surveillance protocols on OS in cancer patients. Following a systematic search of the literature, 18 distinct RCTs evaluated this question, contributing a total of 23 comparisons of less intensive versus more intensive surveillance interventions. There was a high-level of heterogeneity among included studies, with variations in populations assessed, interventions used, and follow-up time periods. Only 8/23 (35%) comparisons reported effect measures on the outcome of OS, and only three comparisons indicated more intensive surveillance programs benefited OS outcomes.
Implications
Routine follow-up after tumor resection surgery varies significantly, even within cancer types. Guidelines have been found to be inconsistent due to the high variability in research and minimal high-quality data.3 Systematic differences between RCTs prevents meta-analyses from being performed in order to inform the development of high-quality evidence based guidelines.14-16 No two trials evaluated identical interventions in post-operative surveillance, emphasizing that clinical equipoise exists with respect to how patients are followed after definitive treatment in each cancer subtype. Although we attempted to organize the studies using the categories of biological test, frequency, imaging and practitioner type interventions, variability persisted between interventions even within each category. Frequency interventions included RCTs that conducted clinic visits every three to every six months in intensive groups, compared to clinic visits every six months to no clinic visits at all in control groups. Evidently, an intensive intervention in one RCT could be considered a control in another RCT. Similarly, imaging modalities were prioritized differently between RCTs, with some RCTs using CXRs, CT scans and colonoscopies as the ‘intensive’ imaging interventions and other RCTs using the aforementioned imaging modalities as the ‘control’.
Investigating surveillance strategies by cancer type did not appreciably reduce heterogeneity in surveillance interventions. Systematic differences in RCTs included the primary outcome, whereby some RCTs evaluated OS after two years and others after five years. This variation could lead to innate differences in survival outcomes.14,16,42 Ultimately these fundamental differences in RCT design highlights the need for surveillance research evaluating OS to follow standard methods of assessment and standard definitions of a primary outcome.
Relation to previous literature
Previous systematic reviews and meta-analyses of colorectal cancer demonstrated that intensive follow-up after curative resection for colorectal cancer improved five-year OS.42-46 Limitations of the aforementioned studies include limited quantities of eligible studies and the high risk of bias in the included trials. Furthermore, many of the systematic differences observed in the compilation of our review, including variations in the primary outcome and intervention types, are prevalent within these meta-analyses thereby raising concerns about the validity of the results.42,43,46 Although three comparisons in our review indicated that five-year OS could be improved with more intensive surveillance protocols in colorectal cancer patients, the remaining ten comparisons of colorectal cancer patients indicated that a more intensive surveillance protocol did not improve OS.
Table 2.
Reported statistical results for all include comparisons. Ordered by intervention categorization. Intensive group is defined as the most comprehensive treatment of the treatment groups in any given trial (i.e., the treatment that is: more frequent, contains more invasive surveillance component(s) or is specialist-led).
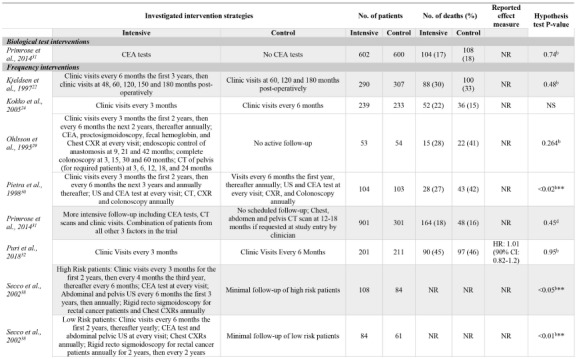
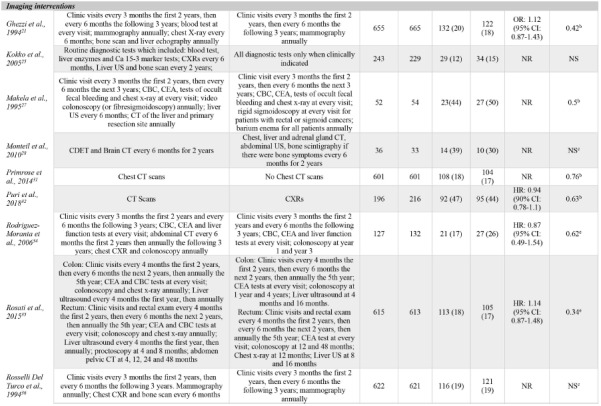
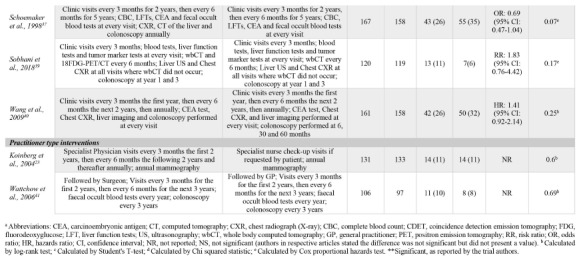
Table 3.
Validity of evidence of included studies. Listed in alphabetical order and assessed following the 2010 Consolidated Standards of Reporting Trials (CONSORT) tool.20
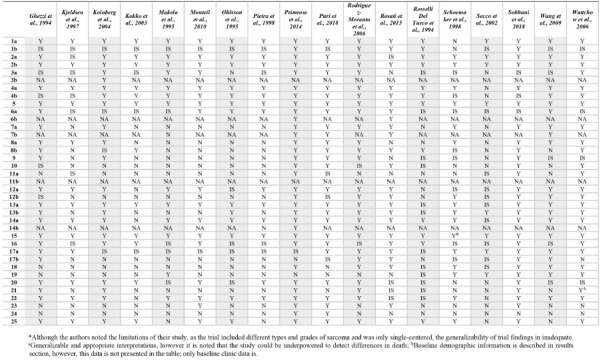
Although the risk of bias was generally low across all included studies, the validity of evidence is questionable. In the evaluation of binary outcomes such as OS, the 2010 CONSORT guidelines recommends reporting both the absolute and relative effect sizes.20 Only eight of 23 comparisons (seven trials) reported a relative effect size and no trial reported the absolute effect size. Although our review was interested in varying surveillance protocol’s effect on OS, many trials identified OS as a secondary outcome and indicated they were underpowered to detect differences in death between intervention groups. The external validity is further questioned in many trials due to the lack of generalizability of results. Half of the included trials were conducted at a single-center alone, thereby preventing generalizability to various populations. A recent systematic review reported that single-center trials estimated significantly larger treatment effects compared to multi-center trials, cautioning against forming recommendations based on single- center trials alone.47 In the assessment of validity of included trials, it is evident that large RCTs powered to evaluate discrete intervention types for each cancer subtype should be conducted.
The publication date of many of the included trials predates advancements made in both RCT reporting and cancer management. Previous CONSORT checklists were not as comprehensive as the 2010 update.20 Fourteen of 18 RCTs included in our review were published prior to 2010, possibly attributing to the lack of detailed information on checklist items such as randomization procedures, effect measures, and discussions of limitations in many of the articles. In fact, four trials were published prior to 1996, preceding any development of CONSORT guidelines. The wide range in publication dates may also prevent comparisons between RCTs due to changes in methods of trial design and interventions available for use. Use of certain imaging modalities may not have been a feasible intervention in older trials.48 Furthermore, many of the included trials predate advancements in the management of cancer, including wider tumor resection and the use of combined therapies, which have been documented to improve survival.49
While this review focused on OS as the most important outcome in cancer treatment, additional outcomes including costs to the healthcare system and patient’s quality of life should also be considered when conducting and evaluating RCTs and developing guidelines for post-operative surveillance in cancer patients. A systematic review of breast cancer surveillance programs reported that the adoption of less intensive surveillance programs that do not compromise OS can result in savings of $8,000 USD per patient per quality adjusted life year.50 Furthermore, patient anxiety and burdens due to more frequent follow-up visits could be reduced by a less intensive surveillance program.
Limitations
Despite the large number of RCTs included in this review, this study has some limitations. All patients in the RCTs were postoperative patients who were treated for a solid tumor, excluding many cancer types that are primarily treated by chemotherapy and radiation. The variability between RCTs in respect to intervention design, populations and tumor characteristics, and follow-up periods prevents the integration of evidence and thereby the development of high-quality, evidence-based recommendations. Furthermore, this review did not have any publication date restrictions. Considering advancements in cancer management and diagnostic tests to detect malignant disease, guideline developers may only be interested in the most up-to-date RCT evidence when refining post-operative surveillance guidelines. Moreover, there is great variation in respect to how metastatic disease is treated in different cancer subtypes, possibly affecting proposed follow-up protocols. Due to the lack of detailed reporting on many 2010 CONSORT checklist items, both the internal and external validity should be questioned for all included trials. We recommend that the critical appraisal of RCTs within this review is taken into consideration when developing cancer type specific, large multi-center RCTs.
Conclusions
In this systematic review, we found tremendous heterogeneity among published cancer surveillance RCTs with respect to surveillance protocols, including definitions of ‘intensive’ surveillance, and RCT design. Research on patient-important outcomes should be standardized to allow for further high-quality studies and metaanalyses and ultimately the development of evidence-based clinical guidelines. Optimal surveillance protocols for each cancer type should continue to be investigated in large RCTs powered to evaluate standardized definitions of OS, while also considering other patient-important outcomes and health system costs.
Funding Statement
Funding: McMaster is the sponsor of this review. This study did not receive any funding.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-9. [DOI] [PubMed] [Google Scholar]
- 2.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol 2008;26:4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med 2017;177:701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokolenko AP, Imyanitov EN. Molecular diagnostics in clinical oncology. Front Mol Biosci 2018;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner DJ, Hall EJ. Computed tomography - an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [DOI] [PubMed] [Google Scholar]
- 6.Longo C, Deber R, Fitch M, et al. An examination of cancer patients’ monthly ‘out-of-pocket’costs in Ontario, Canada. Eur J Cancer Care (Engl) 2007;16:500-7. [DOI] [PubMed] [Google Scholar]
- 7.Goel A, Christy ME, Virgo KS, et al. Costs of follow-up after potentially curative treatment for extremity soft-tissue sarcoma. Int J Oncol 2004;25:429-35. [PubMed] [Google Scholar]
- 8.Tzeng C-WD, Abbott DE, Cantor SB, et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: a cost-effectiveness analysis. Ann Surg Oncol 2013;20:2197-203. [DOI] [PubMed] [Google Scholar]
- 9.Wu JX, Beni CE, Zanocco KA, et al. Cost-effectiveness of longterm every three-year versus annual postoperative surveillance for low-risk papillary thyroid cancer. Thyroid 2015;25:797-803. [DOI] [PubMed] [Google Scholar]
- 10.American Society of Clinical Oncology. Five things physicians and patients should question; Published online 2013. Available from: https://www.choosingwisely.org/wp-content/uploads/2015/02/ASCO-Choosing-Wisely-List.pdf [Google Scholar]
- 11.National Comprehensive Cancer Network. Development and Update of the NCCN Guidelines.; Published online 2020. Available from: https://www.nccn.org/professionals/development.aspx [Google Scholar]
- 12.Wright JG. A practical guide to assigning levels of evidence. JBJS. 2007;89:1128-30. [DOI] [PubMed] [Google Scholar]
- 13.Kanters S, Ford N, Druyts E, et al. Use of network meta-analysis in clinical guidelines. Bull World Health Organ 2016;94:782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari M, Devereaux P, Montori V, et al. Users’ guide to the surgical literature: how to use a systematic literature review and metaanalysis. Can J Surg. 2004;47:60. [PMC free article] [PubMed] [Google Scholar]
- 15.Greenland S. A critical look at some popular meta-analytic methods [invited commentary]. Am J Epidemiol 1994;140:290-6. [DOI] [PubMed] [Google Scholar]
- 16.Haidich AB. Meta-analysis in medical research. Hippokratia 2010;14:29-37. [PMC free article] [PubMed] [Google Scholar]
- 17.American Society of Clinical Oncology. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J Clin Oncol 1996;14:671-9. [DOI] [PubMed] [Google Scholar]
- 18.Scottish Intercollegiate Guidelines. Search Filters; Published online 2019. Available from: https://www.sign.ac.uk/search-filters.html [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28-55. [DOI] [PubMed] [Google Scholar]
- 21.Ghezzi P, Magnanini S, Rinaldini M, et al. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients: a multicenter randomized controlled trial. JAMA 1994;271:1587-92. [DOI] [PubMed] [Google Scholar]
- 22.Kjeldsen B, Kronborg O, Fenger C, Jørgensen O. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666-9. [PubMed] [Google Scholar]
- 23.Koinberg I-L, Fridlund B, Engholm G-B, Holmberg L. Nurse-led follow-up on demand or by a physician after breast cancer surgery: a randomised study. Eur J Oncol Nurs 2004;8:109-17. [DOI] [PubMed] [Google Scholar]
- 24.Kokko R, Hakama M, Holli K. Role of chest X-ray in diagnosis of the first breast cancer relapse: a randomized trial. Breast Cancer Res Treat 2003;81:33-9. [DOI] [PubMed] [Google Scholar]
- 25.Kokko R, Hakama M, Holli K. Follow-up cost of breast cancer patients with localized disease after primary treatment: a randomized trial. Breast Cancer Res Treat 2005;93:255-60. [DOI] [PubMed] [Google Scholar]
- 26.Mäkelä J, Laitinen S, Kairaluoma M. Early results of follow-up after radical resection for colorectal cancer. Preliminary results of a prospective randomized trial. Surg Oncol 1992;1:157-61. [DOI] [PubMed] [Google Scholar]
- 27.Mäkelä JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer: results of a prospective randomized trial. Arch Surg 1995;130:1062-7. [DOI] [PubMed] [Google Scholar]
- 28.Monteil J, Vergnenègre A, Bertin F, et al. Randomized follow-up study of resected NSCLC patients: conventional versus 18F-DG coincidence imaging. Anticancer Res 2010;30:3811-6. [PubMed] [Google Scholar]
- 29.Ohlsson B, Breland U, Ekberg H, et al. Follow-up after curative surgery for colorectal carcinoma. Dis Colon Rectum 1995;38:619-26. [DOI] [PubMed] [Google Scholar]
- 30.Pietra N, Sarli L, Costi R, et al. Role of follow-up in management of local recurrences of colorectal cancer. Dis Colon Rectum 1998;41:1127-33. [DOI] [PubMed] [Google Scholar]
- 31.Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311:263-70. [DOI] [PubMed] [Google Scholar]
- 32.Puri A, Ranganathan P, Gulia A, et al. Does a less intensive surveillance protocol affect the survival of patients after treatment of a sarcoma of the limb? updated results of the randomized toss study. Bone Jt J 2018;100:262-8. [DOI] [PubMed] [Google Scholar]
- 33.Puri A, Gulia A, Hawaldar R, et al. Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. Clin Orthop Relat Res 2014;472:1568-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Moranta F, Saló J, Arcusa À, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol 2006;24:386-93. [DOI] [PubMed] [Google Scholar]
- 35.Rosati G, Ambrosini G, Barni S, et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol 2016;27:274-80. [DOI] [PubMed] [Google Scholar]
- 36.Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer: a randomized trial. JAMA-J Am Med Assoc-US Ed. 1994;271:1593-7. [DOI] [PubMed] [Google Scholar]
- 37.Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology 1998;114:7-14. [DOI] [PubMed] [Google Scholar]
- 38.Secco GB, Fardelli R, Gianquinto D, et al. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol 2002;28:418-23. [DOI] [PubMed] [Google Scholar]
- 39.Sobhani I, Itti E, Luciani A, et al. Colorectal cancer (CRC) monitoring by 6-monthly 18FDG-PET/CT: an open-label multicenter randomised trial. Ann Oncol 2018;29:931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Cui Y, Huang W-S, et al. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointest Endosc 2009;69:609-15. [DOI] [PubMed] [Google Scholar]
- 41.Wattchow DA, Weller DP, Esterman A, et al. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer 2006;94:1116-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pita-Fernández S, Alhayek-Ai M, Gonzalez-Martin C, et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol 2015;26:644-56. [DOI] [PubMed] [Google Scholar]
- 43.Renehan AG, Egger M, Saunders MP, O’Dwyer S. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 2002;324:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 2003;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeffery M Hickey BE Hider PN.. Follow??up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 2007;50:1783-99. [DOI] [PubMed] [Google Scholar]
- 47.Unverzagt S, Prondzinsky R, Peinemann F. Single-center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol 2013;66:1271-80. [DOI] [PubMed] [Google Scholar]
- 48.Pelc NJ. Recent and future directions in CT imaging. Ann Biomed Eng 2014;42:260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palumbo MO, Kavan P, Miller W, et al. Systemic cancer therapy: achievements and challenges that lie ahead. Front Pharmacol 2013;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Augestad KM, Norum J, Dehof S, et al. Cost-effectiveness and quality of life in surgeon versus general practitioner-organised colon cancer surveillance: a randomised controlled trial. BMJ Open 2013;3:e002391. [DOI] [PMC free article] [PubMed] [Google Scholar]


