Abstract
Introduction
Heart failure (HF) is a heterogeneous clinical syndrome and affects millions of people all over the world. HF occurs when the cardiac overload and injury, which is a worldwide complaint. The aim of this study was to screen and verify hub genes involved in developmental HF as well as to explore active drug molecules.
Methods
The expression profiling by high throughput sequencing of GSE141910 dataset was downloaded from the Gene Expression Omnibus (GEO) database, which contained 366 samples, including 200 heart failure samples and 166 non heart failure samples. The raw data was integrated to find differentially expressed genes (DEGs) and were further analyzed with bioinformatics analysis. Gene ontology (GO) and REACTOME enrichment analyses were performed via ToppGene; protein–protein interaction (PPI) networks of the DEGs was constructed based on data from the HiPPIE interactome database; modules analysis was performed; target gene—miRNA regulatory network and target gene—TF regulatory network were constructed and analyzed; hub genes were validated; molecular docking studies was performed.
Results
A total of 881 DEGs, including 442 up regulated genes and 439 down regulated genes were observed. Most of the DEGs were significantly enriched in biological adhesion, extracellular matrix, signaling receptor binding, secretion, intrinsic component of plasma membrane, signaling receptor activity, extracellular matrix organization and neutrophil degranulation. The top hub genes ESR1, PYHIN1, PPP2R2B, LCK, TP63, PCLAF, CFTR, TK1, ECT2 and FKBP5 were identified from the PPI network. Module analysis revealed that HF was associated with adaptive immune system and neutrophil degranulation. The target genes, miRNAs and TFs were identified from the target gene—miRNA regulatory network and target gene—TF regulatory network. Furthermore, receiver operating characteristic (ROC) curve analysis and RT-PCR analysis revealed that ESR1, PYHIN1, PPP2R2B, LCK, TP63, PCLAF, CFTR, TK1, ECT2 and FKBP5 might serve as prognostic, diagnostic biomarkers and therapeutic target for HF. The predicted targets of these active molecules were then confirmed.
Conclusion
The current investigation identified a series of key genes and pathways that might be involved in the progression of HF, providing a new understanding of the underlying molecular mechanisms of HF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-021-02146-8.
Keywords: Heart failure, Differentially expressed genes, Molecular docking, Enrichment analysis, Prognosis
Introduction
Heart failure (HF) is a cardiovascular disease characterized by tachycardia, tachypnoea, pulmonary rales, pleural effusion, raised jugular venous pressure, peripheral oedema and hepatomegaly [1]. Morbidity and mortality linked with HF is a prevalent worldwide health problem holding a universal position as the leading cause of death [2]. The numbers of cases of HF are rising globally and it has become a key health issue. According to a survey, the prevalence HF is expected to exceed 50% of the global population [3]. Research suggests that modification in multiple genes and signaling pathways are associated in controlling the advancement of HF. However, a lack of investigation on the precise molecular mechanisms of HF development limits the treatment efficacy of the disease at present.
Previous study showed that HF was related to the expression of MECP2 [4] RBM20 [5], CaMKII [6], troponin I [7] and SERCA2a [8]. Toll-Like receptor signaling pathway [9], activin type II receptor signaling pathway [10], CaMKII signaling pathways [11], Drp1 signaling pathways [12] and JAK-STAT signaling pathway [13] were liable for progression of HF. More investigations are required to focus on treatments that enhance the outcome of patients with HF, to strictly make the diagnosis of the disease based on screening of biomarkers. These investigations can upgrade prognosis of patients by lowering the risk of advancement of HF and related complications. So it is essential to recognize the mechanism and find biomarkers with a good specificity and sensitivity.
The recent high-throughput RNA sequencing data has been widely employed to screen the differentially expressed genes (DEGs) between normal samples and HF samples in human beings, which makes it accessible for us to further explore the entire molecular alterations in HF at multiple levels involving DNA, RNA, proteins, epigenetic alterations, and metabolism [14]. However, there still exist obstacles to put these RNA seq data in application in clinic for the reason that the number of DEGs found by expression profiling by high throughput sequencing were massive and the statistical analyses were also too sophisticated [15–19]
In this study, first, we had chosen dataset GSE141910 from Gene Expression Omnibus (GEO) (http:// www.ncbi.nlm.nih.gov/geo/) [20]. Second, we applied for limma tool in R software to obtain the differentially expressed genes (DEGs) in this dataset. Third, the ToppGene was used to analyze these DEGs including biological process (BP), cellular component (CC) and molecular function (MF) REACTOME pathways. Fourth, we established protein–protein interaction (PPI) network and then applied Cytotype PEWCC1 for module analysis of the DEGs which would identify some hub genes. Fifth, we established target gene—miRNA regulatory network and target gene—TF regulatory network. In addition, we further validated the hub genes by receiver operating characteristic (ROC) curve analysis and RT-PCR analysis. Finally, we performed molecular docking studies for over expressed hub genes. Results from the present investigation might provide new vision into potential prognostic and therapeutic targets for HF.
Materials and methods
Data resource
Expression profiling by high throughput sequencing with series number GSE141910 based on platform GPL16791 was downloaded from the GEO database. The dataset of GSE141910 contained 200 heart failure samples and 166 non heart failure samples. It was downloaded from the GEO database in NCBI based on the platform of GPL16791 Illumina HiSeq 2500 (Homo sapiens).
Identification of DEGs in HF
DEGs of dataset GSE141910 between HF groups and non heart failure groups were respectively analyzed using the limma package in R [21]. Fold changes (FCs) in the expression of individual genes were calculated and DEGs with P < 0.05, |log FC|> 1.158 for up regulated genes and |log FC|< − 0.83 for down regulated genes were considered to be significant. Hierarchical clustering and visualization were used by Heat-map package of R.
Functional enrichment analysis
Gene Ontology (GO) analysis and REACTOME pathway analysis were performed to determine the functions of DEGs using the ToppGene (ToppFun) (https://toppgene.cchmc.org/enrichment.jsp) [22] GO terms (http://geneontology.org/) [23] included biological processes (BP), cellular components (CC) and molecular functions (MF) of genomic products. REACTOME (https://reactome.org/) [24] analyzes pathways of important gene products. ToppGene is a bioinformatics database for analyzing the functional interpretation of lists of proteins and genes. The cutoff value was set to P < 0.05.
Protein–protein interaction network construction and module screening
PPI networks are used to establish all protein coding genes into a massive biological network that serves an advance compassionate of the functional system of the proteome [25]. The HiPPIE interactome (https://cbdm.uni-mainz.de/hippie/) [26] database furnish information regarding predicted and experimental interactions of proteins. In the current investigation, the DEGs were mapped into the HiPPIE interactome database to find significant protein pairs with a combined score of > 0.4. The PPI network was subsequently constructed using Cytoscape software, version 3.8.2 (www.cytoscape.org) [27]. The nodes with a higher node degree [28], higher betweenness centrality [29], higher stress centrality [30] and higher closeness centrality [31] were considered as hub genes. Additionally, cluster analysis for identifying significant function modules with a degree cutoff > 2 in the PPI network was performed using the PEWCC1 (http://apps.cytoscape.org/apps/PEWCC1) [32] in Cytoscape.
Target gene—miRNA regulatory network construction
The miRNet database (https://www.mirnet.ca/) [33] contains information on miRNA and the regulated genes. Using information collected from the miRNet database, hub genes were matched with their associated miRNA. The target gene—miRNA regulatory network then was constructed using Cytoscape software. MiRNAs and target are selected based on highest node degree.
Target gene—TF regulatory network construction
The NetworkAnalyst database (https://www.networkanalyst.ca/) [34] contains information on TF and the regulated genes. Using information collected from the NetworkAnalyst database, hub genes were matched with their associated TF. The target gene—TF regulatory network then was constructed using Cytoscape software. TFs and target genes are selected based on highest node degree.
Receiver operating characteristic (ROC) curve analysis
Then ROC curve analysis was implementing to classify the sensitivity and specificity of the hub genes for HF diagnosis and we investigated how large the area under the curve (AUC) was by using the statistical package pROC in R software [35].
RT-PCR analysis
H9C2 cells (ATCC) were cultured in Dulbecco’s minimal essential medium (DMEM) (Sigma-Aldrich) supplemented with 10% fetal calf serum (Sigma-Aldrich) and 1% streptomycin (Sigma-Aldrich) at 37 °C in 5% CO2. HL-1 cells (ATCC) was culture in Claycomb medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Sigma-Aldrich), 1% streptomycin (Sigma-Aldrich), 1% glutamax (Sigma-Aldrich) and 0.1 mM norepinephrine (Sigma-Aldrich) at 37 °C in 5% CO2. Total RNA was isolated from cell culture of H9C2 for HF and HL-1 for normal control using the TRI Reagent (Sigma, USA). cDNA was synthesized using 2.0 μg of total RNA with the Reverse transcription cDNA kit (Thermo Fisher Scientific, Waltham, MA, USA). The 7 Flex real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA) was employed to detect the relative mRNA expression. The relative expression levels were determined by the 2-ΔΔCt method and normalized to internal control beta-actin [36]. All RT-PCR reactions were performed in triplicate. The primers used to explore mRNA expression of ten hub genes were listed in Table 1.
Table 1.
The sequences of primers for quantitative RT-PCR
| Genes | Forward primers | Reverse primers |
|---|---|---|
| ESR1 | CCTCTGGCTACCATTATGGG | AGTCATTGTGTCCTTGAATGC |
| PYHIN1 | GCAAGATCAGTACGACAGAG | AGATAACTGAGCAACCTGTG |
| PPP2R2B | ACCAGAGACTATCTGACCG | GTAGTCATGAACCTGGTATGTC |
| LCK | CTAGTCCGGCTTTATGCAG | AAATCTACTAGGCTCCCGT |
| TP63 | ATTCAATGAGGGACAGATTGC | GGGTCTTCTACATACTGGGC |
| PCLAF | GACCAATATAAACTGTGGCGGG | CCAGGGTAAACAAGGAGACGTT |
| CFTR | CTGTGGCCTTGGTTTACTG | CTCTGATCTCTGTACTTCACCA |
| TK1 | AGATTCAGGTGATTCTCGGG | ACTTGTACTGGGCGATCTG |
| ECT2 | GCTGTATTGTACGAGTATGCT | GTCACCAATTTGACAAGCTC |
| FKBP5 | CCTAAGTTTGGCATTGACCC | CCAAGATTCTTTGGCCTTCTC |
Identification of candidate small molecules
SYBYL-X 2.0 perpetual drug design software has been used for surflex-docking studies of the designed novel molecules and the standard on over expressed genes of PDB protein. Using ChemDraw Software, all designed molecules and standards were sketched, imported and saved using open babel free software in sdf. template. The protein of over expressed genes of ESR1, LCK, PPP2R2B, TP63 and their co-crystallised protein of PDB code 4PXM, 1KSW, 2HV7, 3VD8 and 6RU6 were extracted from Protein Data Bank [37–40]. Optimizations of the designed molecules were performed by standard process by applying Gasteiger Huckel (GH) charges together with the TRIPOS force field. In addition, energy minimization was achieved using MMFF94s and MMFF94 algorithm methods. The preparation of the protein was done after protein incorporation. The co-crystallized ligand and all water molecules have been eliminated from the crystal structure; more hydrogen’s were added and the side chain was set, TRIPOS force field was used for the minimization of structure. The interaction efficiency of the compounds with the receptor was expressed in kcal/mol units by the Surflex-Dock score. The best location was integrated into the molecular region by the interaction between the protein and the ligand. Using Discovery Studio Visualizer, the visualisation of ligand interaction with receptor is performed.
Results
Identification of DEGs in HF
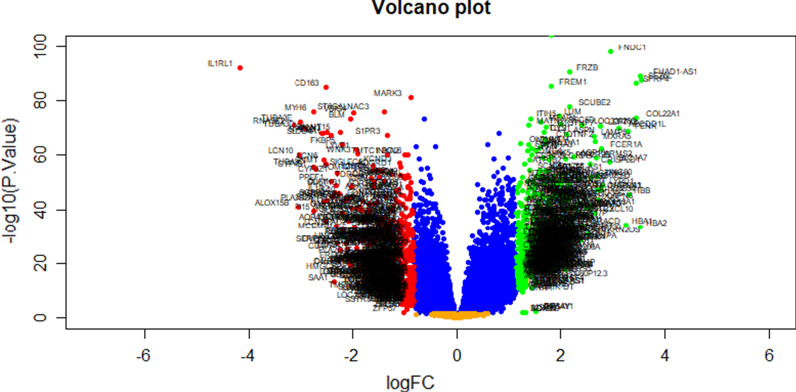
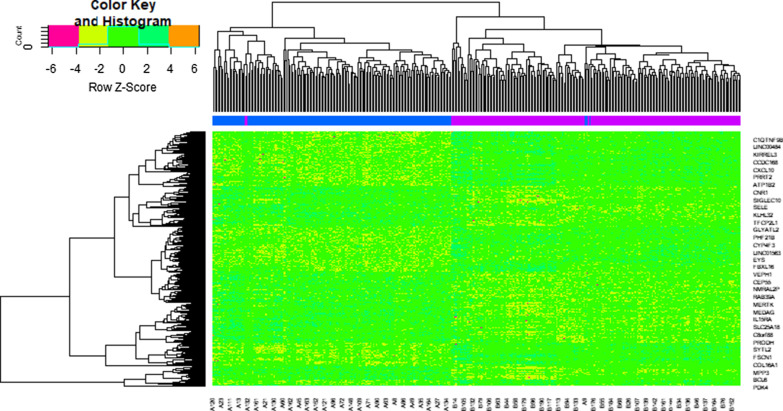
We identified 881 DEGs in the GSE141910 dataset using the limma package in R. Based on the limma analysis, using the adj P val < 0.05, |log FC|> 1.158 for up regulated genes and |log FC|< − 0.83 for down regulated genes, a total of 881 DEGs were identified, consisting of 442 genes were up regulated and 439 genes were down regulated. The DEGs are listed in Additional file 1: Table S1. The volcano plot for DEGs is illustrated in Fig. 1. Figure 2 is the hierarchical clustering heat-map.
Fig. 1.
Volcano plot of differentially expressed genes. Genes with a significant change of more than two-fold were selected. Green dot represented up regulated significant genes and red dot represented down regulated significant genes
Fig. 2.
Heat map of differentially expressed genes. Legend on the top left indicate log fold change of genes. (A1 – A200 = heart failure samples; B1 – B166 = non heart failure samples)
Functional enrichment analysis
Results of GO analysis showed that the up regulated genes were significantly enriched in BP, CC, and MF, including biological adhesion, regulation of immune system process, extracellular matrix, cell surface, signaling receptor binding and molecular function regulator (Table 2); the down regulated genes were significantly enriched in BP, CC, and MF, including secretion, defense response, intrinsic component of plasma membrane, whole membrane, signaling receptor activity and molecular transducer activity (Table 2). Pathway analysis showed that the up regulated genes were significantly enriched in extracellular matrix organization and immunoregulatory interactions between a lymphoid and a non-lymphoid cell (Table 3); the down regulated genes were significantly enriched in neutrophil degranulation and SLC-mediated transmembrane transport (Table 3).
Table 2.
The enriched GO terms of the up and down regulated differentially expressed genes
| GO ID | CATEGORY | GO Name | P Value | FDR B&H | FDR B&Y | Bonferroni | Gene Count | Gene |
|---|---|---|---|---|---|---|---|---|
| Up regulated genes | ||||||||
| GO:0022610 | BP | biological adhesion | 1.32E−13 | 3.37E−10 | 3.08E−09 | 6.75E−10 | 72 | HLA-DQA1, DACT2, CD83, MDK, UBASH3A, ITGBL1, FAP, MFAP4, SERPINE2, NRXN2, COL14A1, CCR7, ALOX15, COL1A1, LAMB4, COL8A2, STAB2, COL16A1, COMP, TBX21, FERMT1, XG, CCDC80, APOA1, PODXL2, ZAP70, HAPLN1, TENM4, SKAP1, CNTNAP2, PDE5A, CARD11, CTNNA2, SLAMF7, ATP1B2, CX3CR1, LRRC15, IDO1, MYOC, SIGLEC8, ISLR, SMOC2, ITGAL, ITGB7, FREM1, PTN, KIRREL3, NTM, GLI2, FBLN7, DPT, NT5E, ECM2, LCK, OMG, OPCML, TGFB2, RASGRP1, CD2, CD3E, THBS4, CD5, CD6, THY1, TIGIT, CD27, CD40LG, ROBO2, GREM1, LY9, HBB, LEF1 |
| GO:0002682 | BP | regulation of immune system process | 2.20E−10 | 2.25E−07 | 2.05E−06 | 1.12E−06 | 72 | IL34, HLA-DQA1, ESR1, TLR7, CD83, IL17D, MDK, UBASH3A, TNFRSF4, PYHIN1, ZBP1, FCER1A, MS4A2, FCER2, FCN1, CCR7, SMPD3, CCL24, SCARA3, ALOX15, COL1A1, IL31RA, TBX21, XG, CXCL14, APOA1, ZAP70, SH2D1B, SKAP1, PDE5A, CARD11, SLAMF7, CTSG, CX3CR1, IDO1, CXCL10, ITGAL, ACE, SIT1, ITGB7, PTN, TBC1D10C, FCRL3, BPI, GLI2, KLRB1, NPPA, CAMK4, LCK, TGFB2, RASGRP1, CD1C, CD1E, CD2, CD3D, CD3E, THBS4, CD3G, CD247, CD5, CD6, THY1, TIGIT, MS4A1, CD27, GPR68, CD40LG, CD48, GREM1, SH2D1A, LEF1, LRRC17 |
| GO:0031012 | CC | extracellular matrix | 1.09E−20 | 2.77E−18 | 1.89E−17 | 5.54E−18 | 52 | MATN2, COL22A1, MDK, COLQ, MFAP4, SERPINE2, HMCN2, AEBP1, FCN1, CMA1, CTHRC1, COL14A1, SCARA3, COL1A1, LAMB4, COL8A2, COL9A1, COL9A2, COL10A1, MXRA5, FMOD, COL16A1, COMP, CCDC80, APOA1, HAPLN1, CTSG, ADAMTSL2, LRRC15, ASPN, MYOC, NDP, SMOC2, FREM1, PTN, SSC5D, SULF1, DPT, NPPA, ADAMTSL1, ECM2, OGN, ITIH5, TGFB2, LEFTY2, EYS, THBS4, P3H2, LTBP2, GREM1, LUM, LRRC17 |
| GO:0009986 | CC | cell surface | 5.07E−17 | 8.61E−15 | 5.86E−14 | 2.58E−14 | 63 | HYAL4, NRG1, HLA-DQA1, CD83, TNFRSF4, ITGBL1, FAP, SERPINE2, FCER1A, MS4A2, FCER2, FCN1, CXCL9, CCR7, IL31RA, SFRP4, STAB2, DUOX2, APOA1, ACKR4, FCRL6, SCUBE2, CNTNAP2, SLAMF7, CTSG, IL2RB, CX3CR1, LRRC15, CXCL10, NDP, ITGAL, ACE, ITGB7, GFRA3, PTN, PROM1, SSC5D, FCRL3, SULF1, MRC2, NTM, CLEC9A, NT5E, TGFB2, LHCGR, CD1C, HHIP, CD1E, CD2, CD3D, CD3E, CD3G, CD5, CD6, THY1, TIGIT, MS4A1, CD27, CD40LG, CD48, ROBO2, GREM1, LY9 |
| GO:0005102 | MF | signaling receptor binding | 1.36E−09 | 5.99E−07 | 4.41E−06 | 1.20E−06 | 73 | IL34, NRG1, HLA-DQA1, ESR1, GDF6, PENK, TAC4, KDM5D, IL17D, MDK, ITGBL1, FAP, SERPINE2, FCER2, NRXN2, FCN1, CLEC11A, UCHL1, AGTR2, CXCL9, NGEF, CTHRC1, C1QTNF2, CCL22, CCL24, CXCL11, COL16A1, COMP, WNT10B, WNT9A, CXCL14, APOA1, FCRL6, GNA14, OASL, RASL11B, LRRC15, CXCL10, ADAM18, MYOC, SYTL2, NDP, ACE, GDNF, ITGB7, GFRA3, PTN, LYPD1, SCG2, NPPA, NPPB, MCHR1, ECM2, CMTM2, ESM1, LCK, OGN, TGFB2, LEFTY2, CD2, CD3E, THBS4, CD3G, THY1, TIGIT, MS4A1, C1QTNF9, CD40LG, LTB, GREM1, SYTL1, LEF1, LGI1 |
| GO:0098772 | MF | molecular function regulator | 3.79E−04 | 1.59E−02 | 1.17E−01 | 3.34E−01 | 58 | IL34, NRG1, ESR1, GDF6, PENK, TAC4, IL17D, MDK, KCNIP1, SERPINE2, MYOZ1, NRXN2, CLEC11A, SCN2B, AGTR2, CXCL9, NGEF, CCL22, CCL24, CXCL11, HTR2B, PI16, SCG5, WNT10B, WNT9A, CXCL14, APOA1, LRRC55, PPP2R2B, ATP1B2, CXCL10, NDP, BIRC7, GDNF, PTN, TBC1D10C, LYPD1, SCG2, NPPA, NPPB, AZIN2, CMTM2, OGN, RGS4, ITIH5, TGFB2, LEFTY2, RASGRP1, THBS4, THY1, CD27, C1QTNF9, CD40LG, RGS17, LTB, GREM1, LEF1, INKA1 |
| Down regulated genes | ||||||||
| GO:0046903 | BP | secretion | 1.07E−11 | 5.64E−08 | 5.16E−07 | 5.64E−08 | 78 | SERPINA3, HK3, SYN3, ACP3, TRPC4, CFTR, CD109, HMOX2, CHI3L1, F5, F8, F13A1, S100A8, S100A9, SAA1, FCER1G, MGST1, PIK3C2A, HP, AGTR1, PLA2G2A, CCR1, FGF10, C1QTNF1, PLA2G4F, FGR, MERTK, SERPINF2, ALOX5, SYT13, IL17RB, CNR1, ALOX15B, FLT3, ANPEP, P2RY12, ANXA3, FPR1, CR1, SLC1A1, SLC2A1, ARG1, ARNTL, SLC11A1, SLC22A16, LGI3, NSG1, ATP2A2, IL10, SIGLEC9, GPR84, NHLRC2, SSTR5, HPSE, KCNB1, IL1R2, PTX3, GLUL, SYN2, BANK1, WNK3, KNG1, CRISPLD2, CACNA1E, CD177, SIGLEC14, EDN1, EDN2, EDNRB, THBS1, RNASE2, CD38, TLR2, SERPINE1, ELANE, STEAP3, IL1RL1, MCEMP1 |
| GO:0006952 | BP | defense response | 1.04E−06 | 1.63E−04 | 1.49E−03 | 5.50E−03 | 65 | SERPINA3, EREG, VSIG4, TMIGD3, CLEC7A, RAET1E, CHI3L1, F8, CD163, S100A8, S100A9, SAA1, FCER1G, HP, HPR, AGTR1, PLA2G2A, CCR1, FGR, SERPINF2, ALOX5, ALOX5AP, IL17RB, CNR1, SELE, ADAMTS4, ANXA3, FPR1, APOB, SAMHD1, CR1, FCN3, AQP4, ARG1, SLC11A1, MARCO, IL10, BCL6, IL18R1, GGT5, IL1R2, PTX3, SIGLEC10, KNG1, CACNA1E, CD177, SOCS3, SIGLEC14, ADAMTS5, LBP, S1PR3, EDN1, EDNRB, FOSL1, THBS1, RNASE2, NAMPT, TLR2, SERPINE1, ELANE, IRAK3, ELF3, IL1RL1, CALCRL, OSMR |
| GO:0031226 | CC | intrinsic component of plasma membrane | 1.74E−10 | 4.55E−08 | 3.11E−07 | 9.10E−08 | 74 | TPO, EREG, OPN4, TRPC4, CFTR, TMIGD3, KCNIP2, CD163, FCER1G, SCN3A, AGTR1, CCR1, C1QTNF1, MERTK, SYT13, IL17RB, CNR1, TRHDE, SELE, LRRC8E, FLT3, SLC4A7, P2RY12, SLC31A2, CR1, LGR5, AQP3, AQP4, SLC1A1, SLC2A1, SLC5A1, MSR1, SLC11A1, SIGLEC7, ART3, SLCO2A1, ATP2A2, MARCO, GABRR2, SIGLEC9, SLCO4A1, GPR84, SSTR2, SSTR5, IL18R1, LAPTM5, GGT5, SLC52A3, LYVE1, KCNA7, KCNB1, KCND3, NECTIN1, KCNK1, KCNK3, KCNS2, ADGRD1, CACNA1E, GPR4, GPR12, SLC38A4, GPR183, GPRC5A, RGR, S1PR3, RHAG, EDNRB, TGFBR3, TLR2, LGR6, CALCRL, OSMR, HAS2, CDH16 |
| GO:0098805 | CC | whole membrane | 1.91E−03 | 4.99E−02 | 3.41E−01 | 9.97E−01 | 51 | EREG, SYN3, ACP3, TRPC4, CFTR, CD109, HMOX2, CD163, FCER1G, MGST1, PLA2G4F, GPAT2, MOG, CNR1, SELE, ANPEP, P2RY12, ANXA3, FPR1, APOB, SCGN, CR1, AQP4, SLC1A1, SLC2A1, ARG1, MSR1, SLC11A1, NSG1, RAB39A, MARCO, SIGLEC9, GPR84, HPSE, LAPTM5, KCND3, SYN2, SLC9A7, WASF1, CD177, SIGLEC14, GRB14, STEAP4, EDNRB, GRIP1, CD38, TLR2, STEAP3, HAS2, SERPINA5, MCEMP1 |
| GO:0038023 | MF | signaling receptor activity | 2.36E−04 | 1.97E−02 | 1.49E−01 | 2.53E−01 | 55 | EREG, OPN4, CLEC7A, FCER1G, FCGR3A, AGTR1, CCR1, ADGRF5, ADGRF4, MERTK, IL17RB, CNR1, SELE, FLT3, MYOT, ANPEP, P2RY12, ANXA3, FPR1, CR1, LGR5, SIGLEC7, MARCO, PALLD, IL10, GABRR2, IL15RA, GPR82, DNER, PAQR5, GPR84, IL20RA, SSTR2, SSTR5, IL18R1, LYVE1, IL1R2, NECTIN1, ADGRD1, GPR4, GPR12, NPTX2, GPR183, GPRC5A, PKHD1L1, RGR, S1PR3, EDNRB, TGFBR3, TLR2, SERPINE1, IL1RL1, LGR6, CALCRL, OSMR |
| GO:0060089 | MF | molecular transducer activity | 4.71E−04 | 2.10E−02 | 1.59E−01 | 5.04E−01 | 58 | EREG, OPN4, CLEC7A, FCER1G, FCGR3A, AGTR1, CCR1, ADGRF5, ADGRF4, MERTK, IL17RB, CNR1, SELE, FLT3, MYOT, ANPEP, P2RY12, ANXA3, FPR1, CR1, LGR5, SIGLEC7, MARCO, PALLD, IL10, GABRR2, IL15RA, GPR82, DNER, PAQR5, GPR84, IL20RA, STOX1, SSTR2, SSTR5, IL18R1, BLM, CDKL5, LYVE1, IL1R2, NECTIN1, ADGRD1, GPR4, GPR12, NPTX2, GPR183, GPRC5A, PKHD1L1, RGR, S1PR3, EDNRB, TGFBR3, TLR2, SERPINE1, IL1RL1, LGR6, CALCRL, OSMR |
Biological Process(BP), Cellular Component(CC) and Molecular Functions (MF)
Table 3.
The enriched pathway terms of the up and down regulated differentially expressed genes
| Pathway ID | Pathway name | P-value | FDR B&H | FDR B&Y | Bonferroni | Gene count | Gene |
|---|---|---|---|---|---|---|---|
| Up regulated genes | |||||||
| 1270244 | Extracellular matrix organization | 3.33E−08 | 1.80E−05 | 1.23E−04 | 1.80E−05 | 24 | COL22A1, MFAP4, CMA1, COL14A1, COL1A1, COL8A2, COL9A1, COL9A2, COL10A1, FMOD, COL16A1, COMP, HAPLN1, ADAMTS14, CTSG, ASPN, ITGAL, ITGB7, CAPN6, TGFB2, P3H2, TLL2, LTBP2, LUM |
| 1269201 | Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell | 8.13E−06 | 7.31E−04 | 5.03E−03 | 4.39E−03 | 13 | SH2D1B, SLAMF7, SIGLEC8, ITGAL, ITGB7, KLRB1, CD1C, CD3D, CD3E, CD3G, CD247, CD40LG, SH2D1A |
| 1269544 | GPCR ligand binding | 3.92E−04 | 1.51E−02 | 1.04E−01 | 2.12E−01 | 22 | GNG8, PENK, F2RL2, AGTR2, APLNR, CXCL9, CCR7, CXCL11, OXER1, HTR2A, HTR2B, WNT10B, WNT9A, ACKR4, CRHBP, S1PR5, FZD2, CX3CR1, CXCL10, MCHR1, LHCGR, GPR68 |
| 1268749 | Metabolism of Angiotensinogen to Angiotensins | 5.26E−04 | 1.85E−02 | 1.27E−01 | 2.84E−01 | 4 | CMA1, CTSG, ACE, GZMH |
| 1269868 | Muscle contraction | 3.44E−02 | 4.03E−01 | 1.00E+00 | 1.00E+00 | 9 | KCNIP1, RYR3, SCN2B, ATP1A4, ATP1B2, MYL1, KCNK17, NPPA, TNNI1 |
| 1269340 | Hemostasis | 6.57E−02 | 5.21E−01 | 1.00E+00 | 1.00E+00 | 20 | GNG8, CEACAM3, F2RL2, SERPINE2, APOA1, GNA14, PDE5A, ATP1B2, IL2RB, CTSW, ISLR, ITGAL, LCK, TGFB2, LEFTY2, RASGRP1, CD2, P2RX6, CD48, HBB |
| 1269171 | Adaptive Immune System | 1.32E−01 | 6.87E−01 | 1.00E+00 | 1.00E+00 | 23 | NRG1, HLA-DQA1, ZAP70, SH2D1B, CARD11, SLAMF7, SIGLEC8, ITGAL, ITGB7, ASB18, IER3, MRC2, KLRB1, LCK, RASGRP1, CD1C, CD3D, CD3E, CD3G, CD247, FBXL16, CD40LG, SH2D1A |
| Down regulated genes | |||||||
| 1457780 | Neutrophil degranulation | 4.82E−06 | 3.14E−03 | 2.22E−02 | 3.14E−03 | 28 | SERPINA3, HK3, ACP3, HMOX2, CHI3L1, S100A8, S100A9, FCER1G, MGST1, HP, FGR, ALOX5, ANPEP, FPR1, CR1, ARG1, SLC11A1, SIGLEC9, GPR84, HPSE, PTX3, CRISPLD2, CD177, SIGLEC14, RNASE2, TLR2, ELANE, MCEMP1 |
| 1269907 | SLC-mediated transmembrane transport | 6.91E−04 | 4.74E−02 | 3.35E−01 | 4.51E−01 | 16 | HK3, SLC7A11, SLC4A7, SLC1A1, SLC2A1, SLC5A1, SLC11A1, SLC22A16, SLCO2A1, SLCO4A1, GCKR, LCN15, SLC9A7, SLC25A18, SLC38A4, RHAG |
| 1269545 | Class A/1 (Rhodopsin-like receptors) | 8.72E−04 | 4.74E−02 | 3.35E−01 | 5.69E−01 | 17 | OPN4, SAA1, AGTR1, CCR1, CNR1, P2RY12, FPR1, SSTR2, SSTR5, KNG1, GPR4, GPR183, RGR, S1PR3, EDN1, EDN2, EDNRB |
| 1269340 | Hemostasis | 2.18E−03 | 7.11E−02 | 5.02E−01 | 1.00E+00 | 26 | SERPINA3, CD109, SLC7A11, F5, F8, F13A1, SERPINB8, FCER1G, FGR, MERTK, SERPINF2, SELE, P2RY12, APOB, KIF18B, ATP2A2, NHLRC2, KNG1, PDE11A, CD177, DOCK9, GRB7, GRB14, THBS1, SERPINE1, SERPINA5 |
| 1269903 | Transmembranetransport of small molecules | 4.89E−03 | 1.28E−01 | 9.01E−01 | 1.00E+00 | 26 | HK3, TRPC4, CFTR, HMOX2, SLC7A11, ABCB1, SLC4A7, AQP3, AQP4, SLC1A1, SLC2A1, SLC5A1, SLC11A1, SLC22A16, SLCO2A1, ATP2A2, GABRR2, SLCO4A1, GCKR, LCN15, SLC9A7, WNK3, SLC25A18, SLC38A4, RHAG, STEAP3 |
| 1269203 | Innate Immune System | 9.62E−03 | 1.96E−01 | 1.00E+00 | 1.00E+00 | 42 | SERPINA3, EREG, HK3, MARK3, ACP3, CLEC7A, HMOX2, CHI3L1, S100A8, S100A9, SAA1, FCER1G, MGST1, FCGR3A, HP, GRAP2, PLA2G2A, FGF5, FGF10, FGR, ALOX5, ANPEP, FPR1, APOB, CR1, FCN3, ARG1, SLC11A1, SIGLEC9, GPR84, HPSE, PTX3, WASF1, CRISPLD2, CD177, SIGLEC14, LBP, RNASE2, TLR2, ELANE, IRAK3, MCEMP1 |
| 1269310 | Cytokine Signaling in Immune system | 8.42E−02 | 4.76E−01 | 1.00E+00 | 1.00E+00 | 23 | EREG, MARK3, F13A1, SAA1, FGF5, CCR1, FGF10, ALOX5, IL17RB, FLT3, FPR1, SAMHD1, IL10, IL15RA, IL20RA, BCL6, IL18R1, IL1R2, SOCS3, LBP, IRAK3, IL1RL1, OSMR |
Protein–protein interaction (PPI) network and module analysis
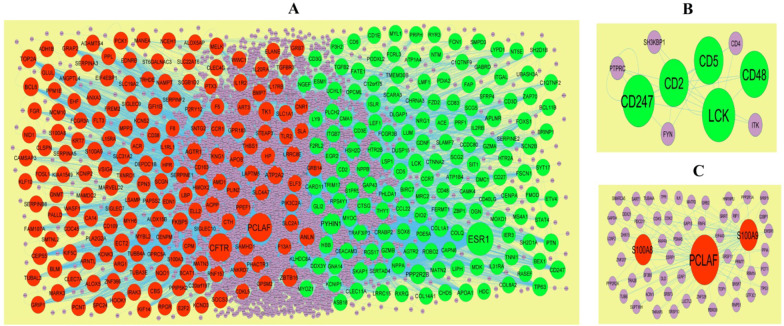
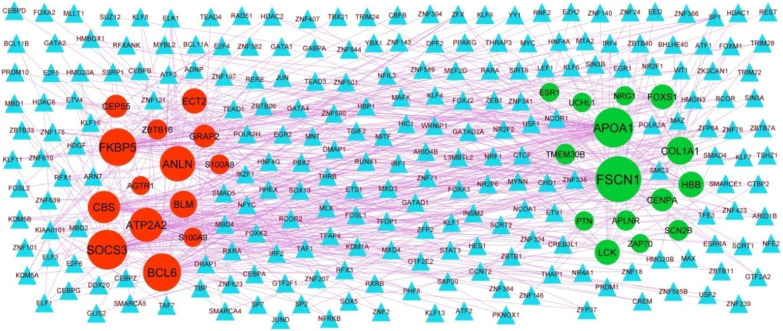
Based on the HiPPIE interactome database, the PPI network for the DEGs (including 6541 nodes and 13,909 edges) was constructed (Fig. 3A). Up regulated gene with higher node degree, higher betweenness centrality, higher stress centrality and higher closeness centrality were as follows: ESR1, PYHIN1, PPP2R2B, LCK, TP63 and so on. Down regulated genes had higher node degree, higher betweenness centrality, higher stress centrality and higher closeness centrality were as follows PCLAF, CFTR, TK1, ECT2, FKBP5 and so on. The node degree, betweenness centrality, stress centrality and closeness centrality are listed in Table 4.
Fig. 3.
PPI network and the most significant modules of DEGs. A The PPI network of DEGs was constructed using Cytoscape. B The most significant module was obtained from PPI network with 4 nodes and 6 edges for up regulated genes. C The most significant module was obtained from PPI network with 6 nodes and 10 edges for down regulated genes. Up regulated genes are marked in green; down regulated genes are marked in red
Table 4.
Topology table for up and down regulated genes
| Regulation | Node | Degree | Betweenness | Stress | Closeness |
|---|---|---|---|---|---|
| Up | ESR1 | 1094 | 0.250896 | 7.4E+08 | 0.392769 |
| Up | PYHIN1 | 342 | 0.054258 | 1.1E+08 | 0.339882 |
| Up | PPP2R2B | 199 | 0.023115 | 35,268,762 | 0.346839 |
| Up | LCK | 162 | 0.033618 | 18,589,354 | 0.353915 |
| Up | TP63 | 142 | 0.018577 | 39,426,608 | 0.319664 |
| Up | CD247 | 129 | 0.013832 | 18,625,274 | 0.317784 |
| Up | PTN | 105 | 0.016638 | 36,892,166 | 0.300662 |
| Up | APLNR | 103 | 0.016611 | 40,715,208 | 0.288093 |
| Up | APOA1 | 100 | 0.018834 | 13,935,332 | 0.315866 |
| Up | CENPA | 98 | 0.009846 | 31,131,318 | 0.301786 |
| Up | SKAP1 | 97 | 0.01599 | 10,109,482 | 0.313338 |
| Up | FSCN1 | 88 | 0.010226 | 9,387,016 | 0.335367 |
| Up | SCN2B | 86 | 0.01089 | 24,233,242 | 0.284756 |
| Up | TMEM30B | 79 | 0.016411 | 10,490,230 | 0.270807 |
| Up | FOXS1 | 79 | 0.009408 | 26,398,078 | 0.293247 |
| Up | COL1A1 | 76 | 0.010472 | 9,315,670 | 0.308098 |
| Up | ZAP70 | 75 | 0.007096 | 5,315,196 | 0.317614 |
| Up | UCHL1 | 74 | 0.009753 | 9,381,024 | 0.331525 |
| Up | HBB | 72 | 0.009984 | 14,209,592 | 0.304767 |
| Up | NRG1 | 70 | 0.01151 | 13,449,066 | 0.29526 |
| Up | LEF1 | 61 | 0.007998 | 18,260,094 | 0.290744 |
| Up | NT5E | 60 | 0.009599 | 11,470,634 | 0.301563 |
| Up | MDK | 59 | 0.006889 | 7,533,352 | 0.305822 |
| Up | ISLR | 58 | 0.010139 | 9,992,806 | 0.294012 |
| Up | FATE1 | 57 | 0.011609 | 9,248,788 | 0.281581 |
| Up | LRRC15 | 56 | 0.010069 | 4,772,792 | 0.299094 |
| Up | MATN2 | 54 | 0.004491 | 9,580,600 | 0.286792 |
| Up | LIPH | 54 | 0.008197 | 6,873,658 | 0.282347 |
| Up | MYOC | 49 | 0.005138 | 12,313,276 | 0.291029 |
| Up | SCARA3 | 49 | 0.006492 | 11,494,220 | 0.290151 |
| Up | NPPA | 46 | 0.009502 | 4,868,574 | 0.307143 |
| Up | CD83 | 43 | 0.005149 | 5,484,906 | 0.272841 |
| Up | COL14A1 | 41 | 0.006129 | 5,699,128 | 0.292893 |
| Up | CTSG | 40 | 0.003821 | 1,913,996 | 0.296155 |
| Up | SFRP4 | 40 | 0.004006 | 7,876,966 | 0.282518 |
| Up | TRAF3IP3 | 38 | 0.006447 | 4,263,002 | 0.290602 |
| Up | CLEC11A | 38 | 0.005085 | 3,520,012 | 0.283596 |
| Up | ATP1B4 | 38 | 0.005722 | 2,476,482 | 0.245939 |
| Up | CD3E | 37 | 0.003892 | 1,537,326 | 0.297624 |
| Up | SH2D1A | 37 | 0.003969 | 2,117,220 | 0.308113 |
| Up | DDX3Y | 37 | 0.003754 | 1,632,916 | 0.32095 |
| Up | PRPH | 37 | 0.001871 | 1,994,266 | 0.301494 |
| Up | BIRC7 | 35 | 0.004835 | 2,567,254 | 0.28549 |
| Up | CARD11 | 35 | 0.002249 | 2,510,852 | 0.292173 |
| Up | RXRG | 35 | 0.002394 | 7,134,478 | 0.26225 |
| Up | CCL22 | 34 | 0.005706 | 3,916,024 | 0.281423 |
| Up | CD27 | 33 | 0.003915 | 2,076,446 | 0.294263 |
| Up | GZMB | 32 | 0.003277 | 6,141,522 | 0.285602 |
| Up | THY1 | 32 | 0.003561 | 1,793,684 | 0.291509 |
| Up | CHRNA3 | 32 | 0.004247 | 3,415,720 | 0.236631 |
| Up | LSP1 | 32 | 0.003371 | 5,734,010 | 0.281836 |
| Up | IL2RB | 31 | 0.002351 | 1,938,172 | 0.305808 |
| Up | HTR2B | 30 | 0.004018 | 2,165,998 | 0.27434 |
| Up | DLGAP1 | 29 | 0.003816 | 6,072,882 | 0.277754 |
| Up | TRIM17 | 29 | 0.002943 | 5,543,810 | 0.275137 |
| Up | CTNNA2 | 29 | 0.003554 | 3,511,510 | 0.30254 |
| Up | SERPINE2 | 28 | 0.002577 | 3,031,536 | 0.27426 |
| Up | CD1E | 28 | 0.003282 | 3,419,392 | 0.255229 |
| Up | MRC2 | 28 | 0.003395 | 2,950,548 | 0.296276 |
| Up | C1QTNF2 | 28 | 0.003203 | 2,505,388 | 0.270147 |
| Up | SH2D1B | 27 | 0.001864 | 1,043,570 | 0.29028 |
| Up | BRINP1 | 27 | 0.001516 | 4,402,948 | 0.27726 |
| Up | PDIA2 | 27 | 0.001967 | 2,895,182 | 0.286453 |
| Up | CHD5 | 27 | 0.001918 | 5,223,636 | 0.286503 |
| Up | FAP | 27 | 0.003531 | 5,820,086 | 0.268583 |
| Up | IL31RA | 26 | 0.002073 | 1,606,954 | 0.263603 |
| Up | GAP43 | 25 | 0.002745 | 2,793,268 | 0.279858 |
| Up | CD5 | 25 | 0.001695 | 655,904 | 0.295861 |
| Up | UBASH3A | 25 | 0.001652 | 1,347,264 | 0.290951 |
| Up | ROBO2 | 25 | 0.002959 | 3,726,616 | 0.267048 |
| Up | ITGB7 | 24 | 0.002686 | 3,351,660 | 0.276124 |
| Up | HTR2A | 24 | 0.002571 | 2,472,010 | 0.275833 |
| Up | MOXD1 | 24 | 0.002391 | 2,492,756 | 0.259926 |
| Up | ASB18 | 24 | 9.77E−04 | 3,202,162 | 0.273001 |
| Up | CD2 | 23 | 0.002221 | 926,408 | 0.287954 |
| Up | BCL11B | 23 | 8.18E−04 | 1,888,298 | 0.28836 |
| Up | STAT4 | 23 | 0.001786 | 2,435,506 | 0.277166 |
| Up | NGEF | 23 | 0.001809 | 1,548,206 | 0.277636 |
| Up | SMPD3 | 23 | 0.002518 | 2,175,774 | 0.281 |
| Up | FZD2 | 22 | 0.003673 | 3,239,390 | 0.250335 |
| Up | DUSP15 | 22 | 0.001253 | 2,474,532 | 0.284472 |
| Up | CD3D | 21 | 0.001725 | 1,111,132 | 0.288589 |
| Up | SYT17 | 21 | 0.002549 | 2,482,574 | 0.285802 |
| Up | FCGR3B | 21 | 0.002748 | 1,492,022 | 0.282286 |
| Up | EGR2 | 21 | 0.002934 | 3,438,856 | 0.266406 |
| Up | ZBP1 | 21 | 0.001876 | 2,664,938 | 0.26006 |
| Up | CAMK4 | 21 | 0.001773 | 3,472,134 | 0.272716 |
| Up | DMC1 | 20 | 0.002511 | 4,659,098 | 0.254277 |
| Up | GDNF | 20 | 0.002515 | 3,274,958 | 0.244751 |
| Up | FCN1 | 20 | 0.002571 | 1,243,380 | 0.236742 |
| Up | LUM | 20 | 0.002276 | 1,515,870 | 0.283903 |
| Up | GZMA | 20 | 0.001051 | 3,258,230 | 0.276498 |
| Up | TGFB2 | 20 | 0.002259 | 1,632,566 | 0.277119 |
| Up | SLAMF7 | 20 | 0.00211 | 1,035,482 | 0.271708 |
| Up | MS4A1 | 20 | 0.002857 | 1,169,078 | 0.288398 |
| Up | ETV4 | 20 | 0.001747 | 1,674,080 | 0.301883 |
| Up | GLI2 | 20 | 0.001398 | 2,977,194 | 0.285902 |
| Up | PHLDA1 | 19 | 4.37E−04 | 818,256 | 0.298194 |
| Up | COL8A2 | 19 | 0.00147 | 1,089,762 | 0.273835 |
| Up | GABRD | 19 | 0.002826 | 2,629,544 | 0.25748 |
| Up | LMF1 | 19 | 0.004342 | 2,024,228 | 0.265132 |
| Up | F2RL2 | 19 | 0.001554 | 790,338 | 0.282933 |
| Up | LYPD1 | 19 | 0.003123 | 3,995,458 | 0.266276 |
| Up | CAPN6 | 19 | 0.001415 | 3,046,736 | 0.267802 |
| Up | SOX8 | 19 | 0.003361 | 2,763,024 | 0.251306 |
| Up | IER3 | 18 | 0.001921 | 3,613,164 | 0.282982 |
| Up | BEX1 | 18 | 0.001034 | 1,286,206 | 0.273606 |
| Up | COLQ | 18 | 0.001173 | 1,414,826 | 0.261234 |
| Up | NTM | 18 | 0.00284 | 2,486,684 | 0.275102 |
| Up | RPS4Y1 | 18 | 0.001013 | 1,200,768 | 0.287713 |
| Up | FERMT1 | 18 | 0.001713 | 4,279,868 | 0.270315 |
| Up | RGS17 | 18 | 0.002928 | 3,868,106 | 0.249895 |
| Up | TNNI1 | 17 | 0.001349 | 1,550,004 | 0.266765 |
| Up | MYOZ1 | 17 | 0.00128 | 2,111,156 | 0.283203 |
| Up | KLHDC8A | 17 | 0.001147 | 7,007,508 | 0.251036 |
| Up | MYL1 | 17 | 7.90E−04 | 1,213,666 | 0.289945 |
| Up | DIO2 | 16 | 0.001161 | 1,959,228 | 0.279416 |
| Up | ITGAL | 16 | 0.001182 | 1,521,116 | 0.271527 |
| Up | CRABP2 | 16 | 4.13E−04 | 675,182 | 0.272171 |
| Up | HSH2D | 16 | 0.001425 | 889,856 | 0.26034 |
| Up | CD48 | 3 | 0 | 0 | 0.265422 |
| Up | CD3G | 2 | 0 | 0 | 0.23833 |
| Up | LY9 | 2 | 0 | 0 | 0.240141 |
| Up | SIT1 | 2 | 0 | 0 | 0.264221 |
| Up | ATP1A4 | 2 | 1.16E−04 | 79,140 | 0.235049 |
| Up | FMOD | 2 | 3.96E−05 | 20,526 | 0.240707 |
| Up | CCDC80 | 2 | 3.58E−05 | 499,704 | 0.288908 |
| Up | CCR7 | 2 | 0 | 0 | 0.244312 |
| Up | KCNIP1 | 1 | 0 | 0 | 0.219995 |
| Up | CD6 | 1 | 0 | 0 | 0.22832 |
| Up | FCRL3 | 1 | 0 | 0 | 0.241062 |
| Up | SERTAD4 | 1 | 0 | 0 | 0.257531 |
| Up | PRF1 | 1 | 0 | 0 | 0.222162 |
| Up | C1QTNF9 | 1 | 0 | 0 | 0.226548 |
| Up | OPCML | 1 | 0 | 0 | 0.215756 |
| Up | ESM1 | 1 | 0 | 0 | 0.213551 |
| Up | CD40LG | 1 | 0 | 0 | 0.240053 |
| Up | S1PR5 | 1 | 0 | 0 | 0.24224 |
| Up | AGTR2 | 1 | 0 | 0 | 0.259256 |
| Up | NPPB | 1 | 0 | 0 | 0.211726 |
| Up | SCG5 | 1 | 0 | 0 | 0.238721 |
| Up | PDE5A | 1 | 0 | 0 | 0.243548 |
| Up | RYR3 | 1 | 0 | 0 | 0.274755 |
| Up | RASEF | 1 | 0 | 0 | 0.274755 |
| Up | PODXL2 | 1 | 0 | 0 | 0.213106 |
| Up | OGN | 1 | 0 | 0 | 0.226548 |
| Up | PLCH2 | 1 | 0 | 0 | 0.238721 |
| Up | SCG2 | 1 | 0 | 0 | 0.267704 |
| Up | P3H2 | 1 | 0 | 0 | 0.207132 |
| Up | C12orf75 | 1 | 0 | 0 | 0.217608 |
| Up | ACE | 1 | 0 | 0 | 0.241159 |
| Up | GNA14 | 1 | 0 | 0 | 0.217608 |
| Up | HDC | 1 | 0 | 0 | 0.216614 |
| Up | CMA1 | 1 | 0 | 0 | 0.226713 |
| Up | CEACAM3 | 1 | 0 | 0 | 0.265519 |
| Down | PCLAF | 817 | 0.135529 | 4.95E+08 | 0.365547 |
| Down | CFTR | 800 | 0.168404 | 4.5E+08 | 0.378823 |
| Down | TK1 | 188 | 0.034997 | 43,663,230 | 0.331089 |
| Down | ECT2 | 164 | 0.020509 | 39,431,940 | 0.325989 |
| Down | FKBP5 | 157 | 0.028064 | 15,963,868 | 0.346288 |
| Down | ANLN | 153 | 0.021564 | 38,168,832 | 0.325066 |
| Down | ATP2A2 | 148 | 0.027131 | 19,656,040 | 0.363859 |
| Down | BCL6 | 142 | 0.022279 | 29,419,916 | 0.314181 |
| Down | TOP2A | 132 | 0.018571 | 16,838,266 | 0.361426 |
| Down | ZBTB16 | 132 | 0.025165 | 14,500,206 | 0.349976 |
| Down | S100A9 | 124 | 0.01355 | 11,186,464 | 0.352219 |
| Down | CEP55 | 123 | 0.019583 | 21,505,878 | 0.316891 |
| Down | BLM | 108 | 0.014259 | 18,458,556 | 0.321945 |
| Down | AGTR1 | 100 | 0.019518 | 14,083,216 | 0.313564 |
| Down | SAMHD1 | 94 | 0.011463 | 12,340,270 | 0.337357 |
| Down | S100A8 | 88 | 0.011637 | 8,662,548 | 0.361486 |
| Down | GRAP2 | 86 | 0.011721 | 16,819,438 | 0.305936 |
| Down | CBS | 83 | 0.011248 | 20,466,334 | 0.301591 |
| Down | SOCS3 | 83 | 0.011071 | 9,067,820 | 0.324888 |
| Down | GFI1B | 80 | 0.011791 | 21,469,034 | 0.299012 |
| Down | APOB | 78 | 0.014102 | 9,290,092 | 0.319133 |
| Down | PCK1 | 77 | 0.004102 | 12,732,476 | 0.305408 |
| Down | MARK3 | 76 | 0.008497 | 19,265,788 | 0.304512 |
| Down | HMOX2 | 75 | 0.011098 | 15,258,770 | 0.312053 |
| Down | PCNT | 74 | 0.011297 | 9,190,430 | 0.312261 |
| Down | PIK3C2A | 69 | 0.005568 | 8,768,122 | 0.313053 |
| Down | KIF14 | 69 | 0.01035 | 12,506,564 | 0.304668 |
| Down | WASF1 | 67 | 0.009478 | 18,219,554 | 0.29633 |
| Down | ARNTL | 65 | 0.00974 | 19,494,854 | 0.295526 |
| Down | ALOX5 | 65 | 0.010921 | 7,343,824 | 0.306424 |
| Down | MCM10 | 64 | 0.006773 | 8,807,396 | 0.306438 |
| Down | THBS1 | 64 | 0.008915 | 6,203,038 | 0.312694 |
| Down | VSIG4 | 64 | 0.010353 | 10,534,518 | 0.302037 |
| Down | WWC1 | 64 | 0.007241 | 14,604,594 | 0.301647 |
| Down | MELK | 63 | 0.008554 | 18,629,232 | 0.283953 |
| Down | P2RY12 | 63 | 0.008962 | 9,063,088 | 0.286641 |
| Down | PPL | 62 | 0.007851 | 16,137,388 | 0.297313 |
| Down | MYBL2 | 59 | 0.006189 | 16,766,208 | 0.291964 |
| Down | FAM107A | 59 | 0.006172 | 12,510,302 | 0.289688 |
| Down | GRIP1 | 58 | 0.008069 | 3,384,760 | 0.320055 |
| Down | ELF3 | 56 | 0.004945 | 7,205,142 | 0.309118 |
| Down | PALLD | 55 | 0.00509 | 15,467,012 | 0.293274 |
| Down | CTH | 54 | 0.007754 | 5,179,060 | 0.296908 |
| Down | EIF4EBP1 | 53 | 0.005367 | 9,748,852 | 0.303946 |
| Down | KNG1 | 53 | 0.007017 | 4,204,766 | 0.304243 |
| Down | GLUL | 51 | 0.00728 | 10,616,752 | 0.30116 |
| Down | SLC2A1 | 51 | 0.004557 | 8,307,646 | 0.303974 |
| Down | HP | 51 | 0.006741 | 3,398,298 | 0.314741 |
| Down | RPGR | 50 | 0.004441 | 10,097,488 | 0.29384 |
| Down | TLR2 | 50 | 0.00754 | 8,322,330 | 0.294595 |
| Down | GRB7 | 49 | 0.004147 | 4,832,846 | 0.308113 |
| Down | PPEF1 | 49 | 0.001983 | 3,327,232 | 0.298276 |
| Down | TXNRD1 | 49 | 0.00627 | 2,860,122 | 0.328561 |
| Down | NAMPT | 48 | 0.005035 | 10,420,710 | 0.290538 |
| Down | BMP7 | 47 | 0.007622 | 4,442,306 | 0.286981 |
| Down | CA14 | 47 | 0.005218 | 4,884,858 | 0.279189 |
| Down | CCR1 | 46 | 0.008305 | 11,217,842 | 0.27812 |
| Down | CDC45 | 45 | 0.004479 | 3,231,162 | 0.30618 |
| Down | ARG1 | 45 | 0.004931 | 2,347,856 | 0.32381 |
| Down | SPC24 | 43 | 0.005356 | 6,589,710 | 0.294834 |
| Down | FGR | 43 | 0.003434 | 3,020,950 | 0.303452 |
| Down | KIF5C | 42 | 0.004876 | 2,365,086 | 0.319586 |
| Down | IL1R2 | 42 | 0.006825 | 9,489,620 | 0.289265 |
| Down | SERPINA3 | 42 | 0.005518 | 4,047,110 | 0.293168 |
| Down | DEPDC1B | 42 | 0.002978 | 9,632,346 | 0.260838 |
| Down | SLC4A7 | 41 | 0.006314 | 2,752,106 | 0.316232 |
| Down | SERPINA5 | 41 | 0.003604 | 13,750,404 | 0.273206 |
| Down | MPP3 | 40 | 0.008262 | 9,328,402 | 0.297003 |
| Down | NCEH1 | 40 | 0.009405 | 3,554,728 | 0.304214 |
| Down | SLC1A1 | 38 | 0.008678 | 2,278,154 | 0.320573 |
| Down | CLSPN | 38 | 0.003668 | 3,801,300 | 0.294343 |
| Down | BCAT1 | 38 | 0.0052 | 9,066,238 | 0.269657 |
| Down | MYH6 | 38 | 0.005049 | 1,731,786 | 0.308578 |
| Down | IL20RA | 37 | 0.005252 | 8,769,348 | 0.267901 |
| Down | HOOK1 | 37 | 0.005558 | 7,195,380 | 0.279177 |
| Down | FLT3 | 37 | 0.002948 | 1,938,440 | 0.292408 |
| Down | ADAMTS4 | 37 | 0.005524 | 2,338,476 | 0.307519 |
| Down | CAMSAP3 | 36 | 0.003339 | 4,795,892 | 0.29578 |
| Down | PLA2G2A | 35 | 0.003637 | 1,686,594 | 0.300565 |
| Down | FOSL1 | 34 | 0.004151 | 10,955,318 | 0.269402 |
| Down | NQO1 | 34 | 0.001945 | 5,351,260 | 0.289201 |
| Down | ELANE | 34 | 0.005024 | 2,289,646 | 0.302834 |
| Down | KCND3 | 34 | 0.002555 | 9,153,178 | 0.28203 |
| Down | EPN3 | 34 | 0.005073 | 7,423,282 | 0.280302 |
| Down | GPR183 | 34 | 0.003642 | 4,243,792 | 0.256783 |
| Down | CD109 | 34 | 0.006381 | 3,655,940 | 0.303565 |
| Down | TUBA3E | 34 | 0.003459 | 6,711,886 | 0.289048 |
| Down | TGFBR3 | 33 | 0.005143 | 1,983,082 | 0.267386 |
| Down | NID1 | 33 | 0.004536 | 1,702,236 | 0.311503 |
| Down | STEAP3 | 33 | 0.004665 | 2,788,716 | 0.285365 |
| Down | AMD1 | 32 | 0.005714 | 3,154,782 | 0.29099 |
| Down | EDNRB | 31 | 0.003092 | 7,273,156 | 0.265368 |
| Down | IL17RB | 31 | 0.004227 | 6,381,040 | 0.261527 |
| Down | SLC19A2 | 30 | 0.004653 | 2,505,974 | 0.281302 |
| Down | SLC22A16 | 30 | 0.004545 | 3,831,872 | 0.240618 |
| Down | PHACTR3 | 29 | 0.002193 | 6,417,862 | 0.280976 |
| Down | LAPTM5 | 29 | 0.003298 | 2,735,158 | 0.274317 |
| Down | ANGPTL4 | 29 | 0.003467 | 1,447,446 | 0.325163 |
| Down | PPM1E | 29 | 0.002894 | 5,733,032 | 0.270427 |
| Down | E2F2 | 28 | 0.002816 | 5,508,320 | 0.28041 |
| Down | SERPINE1 | 28 | 0.001474 | 2,497,574 | 0.271302 |
| Down | ACPP | 28 | 0.003084 | 2,749,550 | 0.291223 |
| Down | KRT7 | 28 | 0.002861 | 1,288,592 | 0.315774 |
| Down | SERPINB8 | 28 | 0.002944 | 3,167,812 | 0.28186 |
| Down | FREM2 | 28 | 0.003954 | 3,395,758 | 0.276661 |
| Down | RNF157 | 28 | 0.002172 | 6,196,626 | 0.265551 |
| Down | PPIP5K2 | 28 | 0.003886 | 8,572,108 | 0.270014 |
| Down | F8 | 27 | 0.002839 | 4,879,016 | 0.274836 |
| Down | TUBAL3 | 27 | 0.002055 | 1,052,840 | 0.318915 |
| Down | ELL2 | 26 | 0.003971 | 6,281,508 | 0.255859 |
| Down | GRB14 | 25 | 0.002326 | 3,092,024 | 0.28378 |
| Down | IRAK3 | 25 | 0.00257 | 6,900,170 | 0.265897 |
| Down | MANEA | 25 | 0.004508 | 5,075,608 | 0.263869 |
| Down | CLEC7A | 25 | 0.004246 | 4,293,212 | 0.277095 |
| Down | KLF10 | 24 | 0.001607 | 3,013,994 | 0.281339 |
| Down | GNMT | 24 | 0.00165 | 3,015,768 | 0.269136 |
| Down | ART3 | 24 | 0.002904 | 2,401,360 | 0.255748 |
| Down | LRRC8E | 24 | 0.003739 | 4,188,308 | 0.288665 |
| Down | SLA | 23 | 0.001714 | 1,003,510 | 0.289329 |
| Down | CLEC4G | 23 | 0.002667 | 2,376,260 | 0.277495 |
| Down | TUBB4A | 5 | 1.28E−04 | 181,440 | 0.250652 |
| Down | CD38 | 4 | 0 | 0 | 0.268176 |
| Down | FCGR3A | 4 | 1.01E−04 | 73,756 | 0.268385 |
| Down | F5 | 3 | 8.10E−07 | 708 | 0.248641 |
| Down | EHF | 2 | 7.11E−06 | 4180 | 0.254842 |
| Down | KIAA1549 | 2 | 4.16E−04 | 193,604 | 0.261391 |
| Down | S100A3 | 2 | 1.84E−05 | 45,822 | 0.254376 |
| Down | ADH1B | 2 | 3.40E−05 | 28,184 | 0.233546 |
| Down | PAPSS2 | 2 | 1.05E−05 | 8726 | 0.251868 |
| Down | PTX3 | 1 | 0 | 0 | 0.19143 |
| Down | IL15RA | 1 | 0 | 0 | 0.234199 |
| Down | EDN1 | 1 | 0 | 0 | 0.209723 |
| Down | SERPINF2 | 1 | 0 | 0 | 0.232451 |
| Down | ZNF366 | 1 | 0 | 0 | 0.282018 |
| Down | ACR | 1 | 0 | 0 | 0.214588 |
| Down | MATN3 | 1 | 0 | 0 | 0.222881 |
| Down | CNR1 | 1 | 0 | 0 | 0.216205 |
| Down | LBP | 1 | 0 | 0 | 0.240053 |
| Down | ALOX5AP | 1 | 0 | 0 | 0.23456 |
| Down | SCGN | 1 | 0 | 0 | 0.23353 |
| Down | MAMDC2 | 1 | 0 | 0 | 0.248745 |
| Down | CDKL5 | 1 | 0 | 0 | 0.219891 |
| Down | CENPM | 1 | 0 | 0 | 0.231833 |
| Down | KCNIP2 | 1 | 0 | 0 | 0.219995 |
| Down | CPM | 1 | 0 | 0 | 0.24533 |
| Down | GPSM2 | 1 | 0 | 0 | 0.245855 |
| Down | LSAMP | 1 | 0 | 0 | 0.215756 |
| Down | KCNK3 | 1 | 0 | 0 | 0.219353 |
| Down | ALOX15B | 1 | 0 | 0 | 0.234981 |
| Down | ST6GALNAC3 | 1 | 0 | 0 | 0.233263 |
| Down | GPRC5A | 1 | 0 | 0 | 0.274755 |
| Down | SLC31A2 | 1 | 0 | 0 | 0.215287 |
| Down | MARVELD2 | 1 | 0 | 0 | 0.218671 |
| Down | SNTG2 | 1 | 0 | 0 | 0.229 |
| Down | TRHDE | 1 | 0 | 0 | 0.208786 |
| Down | SIGLEC7 | 1 | 0 | 0 | 0.245229 |
| Down | SMTNL2 | 1 | 0 | 0 | 0.265519 |
| Down | ANXA3 | 1 | 0 | 0 | 0.274755 |
| Down | F13A1 | 1 | 0 | 0 | 0.248745 |
| Down | ANKRD7 | 1 | 0 | 0 | 0.233438 |
| Down | KCNS2 | 1 | 0 | 0 | 0.219721 |
| Down | SIGLEC9 | 1 | 0 | 0 | 0.227565 |
| Down | SIGLEC10 | 1 | 0 | 0 | 0.282018 |
| Down | C20orf197 | 1 | 0 | 0 | 0.282018 |
| Down | SCGB1D2 | 1 | 0 | 0 | 0.226548 |
| Down | IL1RL1 | 1 | 0 | 0 | 0.21698 |
| Down | PLIN2 | 1 | 0 | 0 | 0.241935 |
| Down | CD163 | 1 | 0 | 0 | 0.239403 |
| Down | HPR | 1 | 0 | 0 | 0.240053 |
Additionally, two significant modules, including module 1 (10 nodes and 24 edges) and module 2 (5 nodes and 10 edges) (Fig. 3B) and module 3 (55 nodes and 115 edges), were acquired by PEWCC1 plug-in (Fig. 3C). Furthermore, GO terms and REACTOME pathways were significantly enriched by module 1, including adaptive immune system, immunoregulatory interactions between a lymphoid and a non-lymphoid cell, hemostasis, biological adhesion and regulation of immune system process. Meanwhile, the nodes in module 2 were significantly enriched in GO terms and REACTOME pathways, including neutrophil degranulation and secretion.
Target gene—miRNA regulatory network construction
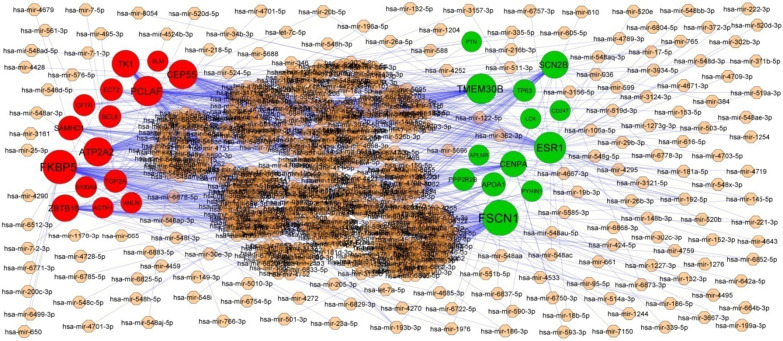
Associations between 2063 miRNAs and their 319 target genes were collected from the target gene—miRNA regulatory network (Fig. 4). MiRNAs of hsa-mir-4533, hsa-mir-548ac, hsa-mir-548i, hsa-mir-5585-3p, hsa-mir-6750-3p, hsa-mir-200c-3p, hsa-mir-1273 g-3p, hsa-mir-1244, hsa-mir-4789-3p and hsa-mir-766-3p, which exhibited a high degree of interaction, were selected from this network. Furthermore, the results also showed that FSCN1 was the target of hsa-mir-4533, ESR1 was the target of hsa-mir-548ac, TMEM30B was the target of hsa-mir-548i, SCN2B was the target of hsa-mir-5585-3p, CENPA was the target of hsa-mir-6750-3p, FKBP5 was the target of hsa-mir-200c-3p, PCLAF was the target of hsa-mir-1273g-3p, CEP55 was the target of hsa-mir-1244, ATP2A2 was the target of hsa-mir-4789-3p and TK1 was the target of hsa-mir-766-3p, and are listed in Table 5.
Fig. 4.
Target gene—miRNA regulatory network between target genes. The light orange color diamond nodes represent the key miRNAs; up regulated genes are marked in green; down regulated genes are marked in red
Table 5.
miRNA—target gene and TF—target gene interaction
| Regulation | Target Genes | Degree | MicroRNA | Regulation | Target Genes | Degree | TF |
|---|---|---|---|---|---|---|---|
| Up | FSCN1 | 99 | hsa-mir-4533 | Up | FSCN1 | 62 | ESRRA |
| Up | ESR1 | 72 | hsa-mir-548ac | Up | APOA1 | 48 | RERE |
| Up | TMEM30B | 64 | hsa-mir-548i | Up | COL1A1 | 21 | HMG20B |
| Up | SCN2B | 46 | hsa-mir-5585-3p | Up | HBB | 16 | THRAP3 |
| Up | CENPA | 35 | hsa-mir-6750-3p | Up | LCK | 15 | ATF1 |
| Up | APOA1 | 22 | hsa-mir-6722-5p | Up | FOXS1 | 14 | YBX1 |
| Up | PPP2R2B | 14 | hsa-mir-149-3p | Up | CENPA | 10 | SAP30 |
| Up | TP63 | 12 | hsa-mir-1178-3p | Up | SCN2B | 5 | RCOR2 |
| Up | PYHIN1 | 5 | hsa-mir-205-3p | Up | TMEM30B | 5 | ZNF24 |
| Up | APLNR | 2 | hsa-mir-10b-5p | Up | APLNR | 4 | FOXJ2 |
| Up | PTN | 1 | hsa-mir-155-5p | Up | NRG1 | 2 | SUZ12 |
| Up | LCK | 1 | hsa-mir-335-5p | Up | PTN | 2 | L3MBTL2 |
| Up | CD247 | 1 | hsa-mir-346 | Up | UCHL1 | 2 | MAZ |
| Down | FKBP5 | 88 | hsa-mir-200c-3p | Up | ESR1 | 1 | EZH2 |
| Down | PCLAF | 62 | hsa-mir-1273g-3p | Up | ZAP70 | 1 | ZFX |
| Down | CEP55 | 57 | hsa-mir-1244 | Down | SOCS3 | 48 | MXD3 |
| Down | ATP2A2 | 55 | hsa-mir-4789-3p | Down | BCL6 | 44 | ARID4B |
| Down | TK1 | 45 | hsa-mir-766-3p | Down | FKBP5 | 43 | CBFB |
| Down | ZBTB16 | 43 | hsa-mir-1976 | Down | ANLN | 38 | TAF7 |
| Down | SAMHD1 | 26 | hsa-mir-3124-3p | Down | ATP2A2 | 35 | CREM |
| Down | TOP2A | 17 | hsa-mir-186-5p | Down | CBS | 31 | IKZF1 |
| Down | BCL6 | 13 | hsa-mir-339-5p | Down | BLM | 19 | ZNF501 |
| Down | ECT2 | 13 | hsa-mir-132-3p | Down | ECT2 | 15 | KLF16 |
| Down | CFTR | 9 | hsa-mir-145-5p | Down | CEP55 | 10 | FOSL2 |
| Down | S100A9 | 7 | hsa-mir-4679 | Down | GRAP2 | 10 | CEBPD |
| Down | AGTR1 | 5 | hsa-mir-410-3p | Down | ZBTB16 | 4 | TRIM28 |
| Down | ANLN | 5 | hsa-mir-503-5p | Down | S100A8 | 3 | STAT3 |
| Down | BLM | 3 | hsa-mir-193b-3p | Down | S100A9 | 2 | CEBPG |
| Down | AGTR1 | 1 | EZH2 |
Target gene—TF regulatory network construction
Associations between 330 TFs and their 247 target genes were collected from the target gene—TF regulatory network (Fig. 5). TFs of ESRRA, RERE, HMG20B, THRAP3, ATF1, MXD3, ARID4B, CBFB, TAF7 and CREM, which exhibited a high degree of interaction, were selected from this network. Furthermore, the results also showed that FSCN1 was the target of ESRRA, APOA1 was the target of RERE, COL1A1 was the target of HMG20B, HBB was the target of THRAP3, LCK was the target of ATF1, SOCS3 was the target of MXD3, BCL6 was the target of ARID4B, FKBP5 was the target of CBFB, ANLN was the target of TAF7 and ATP2A2 was the target of CREM, and are listed in Table 5.
Fig. 5.
Target gene—TF regulatory network between target genes. The sky blue color triangle nodes represent the key TFs; up regulated genes are marked in green; down regulated genes are marked in red
Receiver operating characteristic (ROC) curve analysis
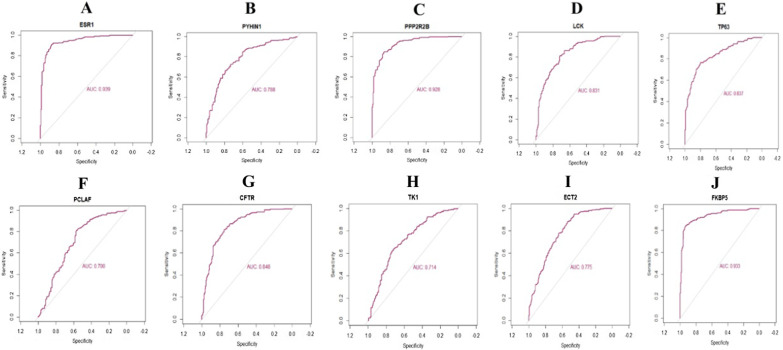
First of all, we performed the ROC curve analysis among 10 hub genes based on the GSE141910. The results showed that ESR1, PYHIN1, PPP2R2B, LCK, TP63, PCLAF, CFTR, TK1, ECT2 and FKBP5 achieved an AUC value of > 0.7, demonstrating that these ten genes have high sensitivity and specificity for HF, suggesting they can be served as biomarkers for the diagnosis of HF (Fig. 6).
Fig. 6.
ROC curve analyses of hub genes. A ESR1, B PYHIN1, C PPP2R2B, D LCK, E TP63, F PCLAF, G CFTR, H TK1, I ECT2, J FKBP5
RT-PCR analysis
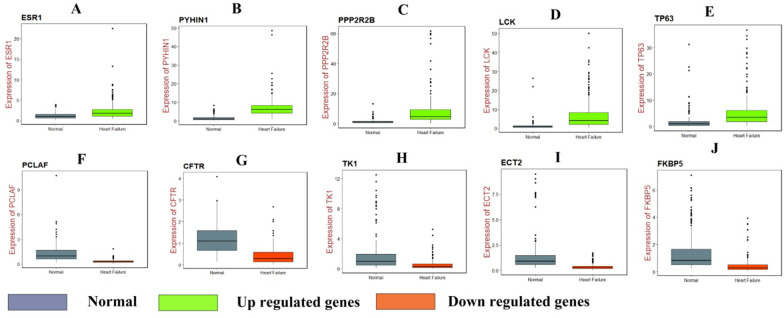
RT-PCR was used to validate the hub genes between normal and HF cell lines. The results suggested that the mRNA expression level of ESR1, PYHIN1, PPP2R2B, LCK and TP63 were significantly increased in HF compared with that in normal, while PCLAF, CFTR, TK1, ECT2 and FKBP5 were significantly decreased in HF compared with that in normal and are shown in Fig. 7.
Fig. 7.
RT-PCR analyses of hub genes. A ESR1, B PYHIN1, C PPP2R2B, D LCK, E TP63, F PCLAF, G) CFTR H TK1, I ECT2, J FKBP5
Identification of candidate small molecules
In the present study docking simulations are performed to spot the active site and foremost interactions accountable for complex stability with the receptor binding sites. In heart failure recognized over expressed genes and their proteins of x-ray crystallographic structure are chosen from PDB for docking studies. Most generally, medications containing benzothiadiazine ring hydrochlorothiazide are used in heart failure either alone or in conjunction with other drugs, based on this the molecules containing heterocyclic ring of benzothiadiazine are designed and hydrochlorothiazide is uses as a reference standard. Docking experiments using Sybyl-X 2.1.1. drug design perpetual software were used on the designed molecules. Docking studies were performed in order to understand the biding interaction of standard hydrochlorothiazide and designed molecules on over expressed protein. The X- RAY crystallographic structure of one proteins from each over expressed genes of ESR1, LCK, PPP2R2B, PYHIN1, TP63 and their co-crystallised protein of PDB code 4PXM, 1KSW, 2HV7, 3VD8 and 6RU6 respectively were selected for the docking studies to identify and predict the potential molecule based on the binding score with the protein and successful in heart failure. For the docking tests, a total of 34 molecules were built and the molecule with binding score greater than 5 is believed to be good. The designed molecules obtained docking score of 5 to 7 were HIM10, HTZ5, HIM6, HTZ31, HIM3, HIM14, HIM1, HIM7 and HIM11, HIM16, HTZ9, HIM17, HIM12, HTZ12, HIM6, HTZ7, HIM10, HTZ3 and HIM8, HTZ9, HIM6, HIM4, HIM13, HTZ16, HIM9, HIM7, HTZ5, HIM16, HTZ7, HIM10, HIM5, HIM12, HIM15, HTZ12, HIM3, HIM14 and HIM14, HIM6, HIM17, HTZ7, HIM10, HIM1, HTZ9, HIM3, HIM16, HIM15, HIM8, HIM9, HIM7, HTZ10, HTZ3, HTZ5, HTZ1, HIM13, HTZ4, HIM11, HTZ12, HTZ14, HIM2 and HIM7, HTZ13, HTZ5, HIM15, HIM12, HIM6, HTZ11, HIM14, HTZ9, HIM11, HIM13, HIM9, HIM8, HIM10, HIM1, HIM5, HIM4, HTZ12, HIM2, HIM17, HIM3, HTZ1, HTZ8, HIM3, HTZ14, HTZ3 with proteins 4PXM and, 1KSW and 2HV7 and 3VD8 and 6RUR respectively (Fig. 8). The molecules obtained binding score of less than 5 were HTZ13, HTZ12, HTZ10, HIM3, HIM15, HIM16, HIM13, HIM8, HTZ16, HIM2, HIM4, HIM17, HTZ17, HIM11, HTZ5, HTZ3, HIM9, HTZ15, HTZ5, HTZ9, HTZ11, HIM5, HTZ8 and HTZ14, HIM14, HTZ13, HIM13, HTZ16, HIM2, HIM3, HTZ10, HIM7, HIM1, HTZ1, HTZ4, HIM8, HIM5, HTZ2, HIM9, HTZ5, HTZ15, HTZ3, HIM4, HIM15, HTZ17, HTZ8, HTZ11 and HTZ14, HIM2, HIM1, HTZ11, HIM17, HTZ13, HTZ4, HTZ2, HIM3, HTZ15, HTZ8, HTZ17, HTZ1, HTZ3 and HTZ8, HIM4, HTZ16, HTZ15, HIM5, HTZ11, HTZ13, HIM3, HTZ17, HTZ2 and HTZ7, HTZ4, HTZ2, HTZ17, HTZ15 with proteins 4PXM and, 1KSW and 2HV7 and 3VD8 and 6RUR respectively. The molecules obtained very less binding score are HTZ1, HIM12, HTZ2, HTZ4 with protein 4PXM and the standard hydrochlorothiazide (HTZ) obtained less binding score with all proteins, the values are depicted in Table 6.
Fig. 8.
Structures of designed molecules
Table 6.
Docking results of Designed Molecules on Over Expressed Proteins
| Sl. No/ Code |
Over expressed gene: ESR1 | Over expressed gene: LCK | Over expressed gene: PPP2R2B | Over expressed gene: PYHIN 1 | Over expressed gene: TP63 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDB: 4PXM | PDB:1KSW | PDB: 2HV7 | PDB: 3VD8 | PDB: 6RU6 | |||||||||||
| Total Score | Crash (-Ve) |
Polar | Total Score | Crash (-Ve) |
Polar | Total Score | Crash (−Ve) |
Polar | Total Score | Crash (−Ve) |
Polar | Total Score | Crash (−Ve) |
Polar | |
| HIM1 | 5.097 | − 4.375 | 0.114 | 4.258 | − 1.555 | 1.784 | 4.904 | − 0.870 | 0.004 | 5.794 | − 0.514 | 3.828 | 5.770 | − 1.440 | 2.231 |
| HIM2 | 4.057 | − 6.172 | 0.039 | 4.624 | − 0.997 | 1.959 | 4.906 | − 0.468 | 1.517 | 5.052 | − 0.780 | 3.415 | 5.414 | − 1.529 | 2.444 |
| HIM3 | 5.353 | − 5.309 | 0.161 | 4.578 | − 1.492 | 1.790 | 5.042 | − 1.845 | 1.073 | 5.680 | − 0.804 | 3.956 | 5.350 | − 1.170 | 2.316 |
| HIM4 | 3.976 | − 5.132 | 0.167 | 3.839 | − 1.635 | 1.196 | 6.328 | − 1.172 | 1.128 | 4.966 | − 0.666 | 1.818 | 5.627 | − 1.416 | 2.320 |
| HIM5 | 2.707 | − 7.759 | 0.179 | 4.067 | − 0.997 | 1.987 | 5.254 | − 0.674 | 1.618 | 4.563 | − 1.068 | 2.935 | 5.698 | − 1.240 | 2.485 |
| HIM6 | 5.948 | − 3.902 | 1.796 | 5.229 | − 0.707 | 3.656 | 6.766 | − 1.424 | 1.858 | 6.670 | − 0.941 | 5.519 | 6.218 | − 1.468 | 2.578 |
| HIM7 | 5.019 | − 7.055 | 0.203 | 4.382 | − 2.443 | 3.329 | 6.028 | − 0.629 | 1.660 | 5.374 | − 1.876 | 0.670 | 6.627 | − 1.484 | 2.579 |
| HIM8 | 4.429 | − 3.983 | 0.344 | 4.150 | − 4.382 | 4.210 | 6.794 | − 1.279 | 1.129 | 5.468 | − 0.769 | 3.444 | 5.842 | − 2.088 | 2.272 |
| HIM9 | 3.722 | − 5.956 | 0.197 | 4.051 | − 2.006 | 0.002 | 6.116 | − 0.597 | 1.717 | 5.407 | − 0.565 | 1.370 | 5.877 | − 2.054 | 0.792 |
| HIM10 | 6.771 | − 3.977 | 1.836 | 5.176 | − 3.512 | 4.023 | 5.332 | − 1.378 | 3.349 | 6.071 | − 0.923 | 3.854 | 5.825 | − 0.966 | 3.672 |
| HIM11 | 3.775 | − 6.079 | 0.898 | 6.998 | − 2.086 | 3.842 | 8.678 | − 1.065 | 2.876 | 5.087 | − 0.881 | 1.854 | 5.948 | − 1.015 | 1.202 |
| HIM12 | 0.190 | − 8.149 | 0.022 | 5.302 | − 2.305 | 3.475 | 5.227 | − 1.636 | 0.710 | 7.322 | − 1.128 | 4.099 | 6.237 | − 2.562 | 2.171 |
| HIM13 | 4.523 | − 4.537 | 0.014 | 4.840 | − 0.664 | 3.305 | 6.181 | − 2.966 | 3.523 | 5.281 | − 0.503 | 3.981 | 5.905 | − 1.136 | 2.218 |
| HIM14 | 5.247 | − 3.183 | 0.000 | 4.888 | − 1.296 | 2.563 | 5.037 | − 0.377 | 1.647 | 7.057 | − 0.799 | 4.256 | 6.116 | − 1.366 | 2.438 |
| HIM15 | 4.633 | − 4.173 | 0.180 | 3.756 | − 0.710 | 2.072 | 5.188 | − 1.559 | 1.143 | 5.570 | − 1.125 | 3.718 | 6.238 | − 1.708 | 2.443 |
| HIM16 | 4.588 | − 2.883 | 0.000 | 6.027 | − 1.099 | 3.903 | 5.606 | − 0.987 | 4.197 | 5.661 | − 0.926 | 2.751 | 7.263 | − 1.533 | 4.212 |
| HIM17 | 3.944 | − 4.806 | 0.236 | 5.329 | − 0.590 | 2.798 | 4.830 | − 1.682 | 1.617 | 6.234 | − 0.830 | 3.912 | 5.366 | − 1.257 | 3.022 |
| HTZ1 | 0.593 | − 7.518 | 0 | 4.221 | − 0.692 | 1.975 | 3.993 | − 0.539 | 0.003 | 5.284 | − 0.566 | 1.432 | 5.227 | − 1.045 | 0.903 |
| HTZ2 | − 1.770 | − 8.477 | 0.000 | 4.055 | − 1.438 | 2.877 | 4.388 | − 1.665 | 1.170 | 4.100 | − 0.546 | 3.017 | 4.563 | − 0.976 | 1.266 |
| HTZ3 | 4.649 | − 5.870 | 0.148 | 5.104 | − 0.861 | 3.922 | 4.243 | − 1.539 | 1.933 | 5.304 | − 1.370 | 1.398 | 5.138 | − 1.217 | 1.930 |
| HTZ4 | − 3.169 | − 12.002 | 0.482 | 4.173 | − 1.898 | 1.864 | 4.654 | − 1.627 | 1.128 | 5.163 | − 0.745 | 1.304 | 5.084 | − 1.143 | 1.018 |
| HTZ5 | 4.021 | − 12.325 | 0.246 | 3.215 | − 1.481 | 4.232 | 3.256 | − 6.374 | 2.317 | 2.382 | − 5.263 | 1.238 | 4.623 | − 0.951 | 1.280 |
| HTZ6 | 6.605 | − 3.866 | 1.6487 | 4.004 | − 1.104 | 2.851 | 5.834 | − 1.310 | 3.111 | 5.286 | − 1.530 | 1.436 | 6.336 | − 2.326 | 3.781 |
| HTZ7 | 4.977 | − 5.434 | 0.655 | 5.197 | − 2.040 | 3.250 | 5.352 | − 1.371 | 1.172 | 6.138 | − 1.734 | 1.627 | 4.908 | − 1.057 | 1.335 |
| HTZ8 | 1.025 | − 8.223 | 0.000 | 3.549 | − 1.310 | 2.403 | 4.024 | − 3.825 | 2.440 | 4.980 | − 0.593 | 1.474 | 5.164 | − 1.291 | 0.999 |
| HTZ9 | 3.386 | − 7.041 | 0.194 | 5.567 | − 1.622 | 3.057 | 6.792 | − 2.581 | 1.088 | 5.794 | − 0.683 | 1.774 | 6.053 | − 1.408 | 1.037 |
| HTZ10 | 4.744 | − 5.463 | 0.837 | 4.520 | − 2.469 | 3 | 7.758 | − 1.518 | 3.765 | 5.345 | − 1.133 | 3.661 | 7.507 | − 2.080 | 4.086 |
| HTZ11 | 2.991 | − 6.177 | 0 | 3.453 | − 0.721 | 1.266 | 4.841 | − 1.738 | 0.045 | 4.368 | − 0.805 | 1.074 | 6.176 | − 1.380 | 1.511 |
| HTZ12 | 4.810 | − 6.157 | 0.275 | 5.296 | − 2.814 | 3.605 | 5.138 | − 1.840 | 2.189 | 5.083 | − 0.870 | 1.536 | 5.592 | − 1.321 | 1.525 |
| HTZ13 | 4.868 | − 3.837 | 0 | 4.863 | − 0.535 | 2.405 | 4.656 | − 0.681 | 3.152 | 4.246 | − 2.335 | 0.529 | 6.404 | − 0.975 | 2.954 |
| HTZ14 | 5.646 | − 3.473 | 0 | 4.948 | − 0.801 | 2.324 | 4.953 | − 1.672 | 1.066 | 5.058 | − 1.174 | 1.121 | 5.114 | − 1.299 | 1.296 |
| HTZ15 | 3.428 | − 4.957 | 0.348 | 3.949 | − 0.614 | 1.873 | 4.049 | − 0.787 | 1.224 | 4.796 | − 1.066 | 1.489 | 3.510 | − 0.607 | 0.461 |
| HTZ16 | 4.227 | − 4.787 | 0.298 | 4.654 | − 1.534 | 2.096 | 6.143 | − 1.204 | 2.879 | 4.854 | − 0.994 | 1.564 | 7.102 | − 0.917 | 3.001 |
| HTZ17 | 3.784 | − 5.018 | 0.380 | 3.661 | − 0.897 | 1.676 | 4.016 | − 1.179 | 1.384 | 4.039 | − 0.569 | 2.706 | 4.256 | − 1.040 | 1.236 |
|
HTZ STD |
4.722 | − 1.084 | 1.063 | 3.319 | − 0.890 | 3.033 | 3.564 | − 0.272 | 2.367 | 3.394 | − 0.882 | 1.169 | 4.237 | − 0.801 | 1.855 |
Discussion
HF is the most prevalent form of cardiovascular disease among the elderly. A complete studies of HF, comprising pathogenic factors, pathological processes, clinical manifestations, early clinical diagnosis, clinical prevention, and drug therapy targets urgency to be consistently analyzed. In the present investigation, bioinformatics analysis was engaged to explore HF biomarkers and the pathological processes in myocardial tissues, acquired from HF groups and non heart failure groups. We analyzed GSE141910 expression profiling by high throughput sequencing obtained 881 different genes between HF groups and non heart failure groups, 442 up regulated and 439 down regulated genes. HBA2 and HBA1 have a key role in hypertension [41], but these genes might be linked with development HF. SFRP4 was linked with progression of myocardial ischemia [42]. Emmens et al. [43] and Broch et al. [44] found that PENK (proenkephalin) and IL1RL1 were up regulated in HF. ALOX15B has lipid accumulation and inflammation activity and is highly expressed in atherosclerosis [45]. Studies have shown that expression of MYH6 was associated with hypertrophic cardiomyopathy [46].
In functional enrichment analysis, some genes involved with regulation of cardiovascular system processes were enriched in HF. Liu et al. [47], Kosugi et al. [48], McMacken et al. [49], Pan and Zhang [50], Li et al. [51] and Jiang et al. [52] presented that expression of HLA-DQA1, KDM5D, UCHL1, SAA1, ARG1 and LYVE1 were associated with progression of cardiomyopathy. Hou et al. [53] and Olesen et al. [54] demonstrated that DACT2 and KCND3 were found to be substantially related to atrial fibrillation. Ge and Concannon [55], Ferjeni et al. [56], Anquetil et al. [57], Glawe et al. [58], Kawabata et al. [59], Li et al. [60], Buraczynska et al. [61], Amini et al. [62], Yang et al. [63], Du Toit et al. [64], Hirose et al. [65], Zhang et al. [66], Griffin et al. [67], Zouidi et al. [68], Trombetta et al. [69], Alharbi et al. [70], Ikarashi et al. [71], Dharmadhikari et al. [72], Sutton et al. [73] and Deng et al. [74] reported that UBASH3A, ZAP70, IDO1, ITGAL (integrin subunit alpha L). ITGB7, RASGRP1, CNR1, SLC2A1, SLC11A1, GPR84, SSTR5, KCNB1, GLUL (glutamate-ammonia ligase), BANK1, CACNA1E, LGR5, AQP3, SIGLEC7, SSTR2 and DNER (delta/notch like EGF repeat containing) could be an index for diabetes, but these genes might be responsible for progression of HF. Experiments show that expression of FAP (fibroblast activation protein alpha) [75], THBS4 [76], CD27 [77], LEF1 [78], CTHRC1 [79], ESR1 [80], CXCL9 [81], SERPINA3 [82], TRPC4 [83], F13A1 [84], PIK3C2A [85], KCNIP2 [86] and GPR4 [87] contributed to myocardial infarction. MFAP4 [88], ALOX15 [89], COL1A1 [90], APOA1 [91], PDE5A [92], CX3CR1 [93], THY1 [94], GREM1 [95], FMOD (fibromodulin) [96], NPPA (natriuretic peptide A) [97], LTBP2 [98], LUM (lumican) [99], IL34 [100], NRG1 [101], CXCL14 [102], CXCL10 [103], ACE (angiotensin I converting enzyme) [104], CFTR (ystic fibrosis transmembrane conductance regulator) [105], S100A8 [106], S100A9 [106], HP (haptoglobin) [107], AGTR1 [108], ATP2A2 [109], IL10 [110], EDN1 [111], TLR2 [112], MCEMP1 [113], TPO (thyroid peroxidase) [114], CD163 [115], IL18R1 [116], KCNA7 [117] and CALCRL (calcitonin receptor like receptor) [118] have an important role in HF. Li et al. [119], Deckx et al. [120], Ichihara et al. [121] and Paik et al. [122] showed that the SERPINE2, OGN (osteoglycin), AGTR2 and WNT10B promoted cardiac interstitial fibrosis. Cai et al. [123], Mo et al. [124], Sun et al. [125], Martinelli et al. [126], Zhao et al. [127], Assimes et al. [128] and Piechota et al. [129] showed that CCR7, FCN1, ESM1, F8 (coagulation factor VIII), C1QTNF1, ALOX5 and MSR1 were an important target gene for coronary artery disease. STAB2 have been suggested to be associated with venous thromboembolic disease [130]. Genes such as COMP (cartilage oligomeric matrix protein) [131], CHI3L1 [132], PLA2G2A [133], P2RY12 [134], CR1 [135], HPSE (heparanase) [136], PTX3 [137] and SERPINE1 [138] were related to atherosclerosis. CCDC80 [139], CMA1 [140], MDK (midkine) [141], GNA14 [142], SCG2 [143], NPPB (natriuretic peptide B) [144], FGF10 [145], ARNTL (aryl hydrocarbon receptor nuclear translocator like) [146], WNK3 [147], EDNRB (endothelin receptor type B) [148], THBS1 [149], SELE (selectin E) [150], SLC4A7 [151], AQP4 [152] and KCNK3 [153] are thought to be responsible for progression of hypertension, but these genes might to be associated with progression of HF. CNTNAP2 [154], GLI2 [155], DPT (dermatopontin) [156], AEBP1 [157], ITIH5 [158], CXCL11 [159], GDNF (glial cell derived neurotrophic factor) [160], MCHR1 [161], FLT3 [162], ELANE (elastase, neutrophil expressed) [163], OSMR (oncostatin M receptor) [164] and IL15RA [165] are involved in development of obesity, but these genes might be key for progression of HF. CTSG (cathepsin G) is a protein coding gene plays important roles in aortic aneurysms [166]. Evidence from Safa et al. [167], Chen et al. [168], Zhou et al. [169], Hu et al. [170], Lou et al. [171], Zhang et al. [172] and Chen et al. [173] study indicated that the expression of CCL22, CCR1, FPR1, KNG1, CRISPLD2, CD38 and GPRC5A were linked with progression of ischemic heart disease. Li et al. [174] showed that STEAP3 expression can be associated with cardiac hypertrophy progression.
The HiPPIE interactome database was used to construct the PPI network, and modules analysis was performed. We finally screened out up regulated hub genes and down regulated hub genes, including ESR1, PYHIN1, PPP2R2B, LCK, TP63, PCLAF, CD247, CD2, CD5, CD48, CFTR, TK1, ECT2, FKBP5, S100A9 and S100A8 from the PPI network and its modules. TP63 might serve as a potential prognostic factor in cardiomyopathy [175]. The expression of FKBP5 is related to the progression of coronary artery disease [176]. CD247 plays a central role in hypertension [177], but this gene might be involved in the HF. PYHIN1, PPP2R2B, LCK (LCK proto-oncogene, Src family tyrosine kinase), PCLAF (PCNA clamp associated factor), TK1, ECT2, CD2, CD5 and CD48 might be the novel biomarker for HF.
The miRNet database and NetworkAnalyst database were used to construct the target gene—miRNA regulatory network and target gene—TF regulatory network. We finally screened out target genes, miRNA, TFs, including FSCN1, ESR1, TMEM30B, SCN2B, CENPA, FKBP5, PCLAF, CEP55, ATP2A2, TK1, APOA1, COL1A1, HBB, LCK, SOCS3, BCL6, ANLN, hsa-mir-4533, hsa-mir-548ac, hsa-mir-548i, hsa-mir-5585-3p, hsa-mir-6750-3p, hsa-mir-200c-3p, hsa-mir-1273 g-3p, hsa-mir-1244, hsa-mir-4789-3p, hsa-mir-766-3p, ESRRA, RERE, HMG20B, THRAP3, ATF1, MXD3, ARID4B, CBFB, TAF7 and CREM from the target gene—miRNA regulatory network and target gene—TF regulatory network. SCN2B [178] and SOCS3 [179] are considered as a markers for HF and might be a new therapeutic target. BCL6 levels are correlated with disease severity in patients with atherosclerosis [180]. A previous study showed that hsa-mir-1273 g-3p [181], hsa-mir-4789-3p [182] and ATF1 [183] could involved in hypertension, but these markers might be responsible for progression of HF. hsa-miR-518f, was demonstrated to be associated with cardiomyopathy [184]. An evidence demonstrating a role for ESRRA (estrogen related receptor alpha) [185] and THRAP3 [186] in diabetes, but these genes might be liable for development of HF. FSCN1, TMEM30B, CENPA (centromere protein A), CEP55, HBB (hemoglobin subunit beta), ANLN (anillin actin binding protein), hsa-mir-4533, hsa-mir-548ac, hsa-mir-548i, hsa-mir-5585-3p, hsa-mir-6750-3p, hsa-mir-200c-3p, hsa-mir-1244, RERE(arginine-glutamic acid dipeptide repeats), HMG20B, MXD3, ARID4B, CBFB (core-binding factor subunit beta), TAF7 and CREM (cAMP response element modulator) might be the novel biomarker for HF.
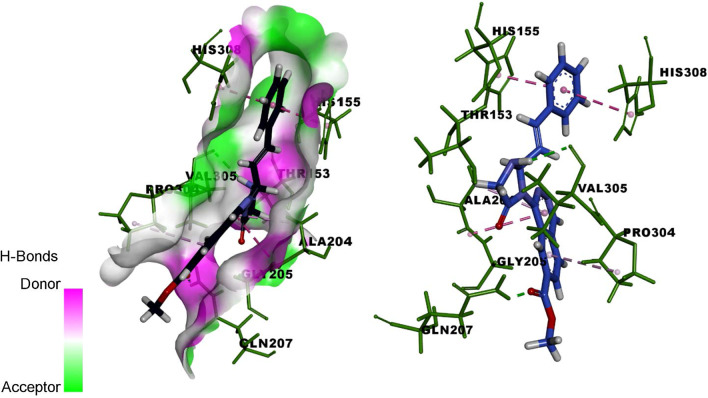
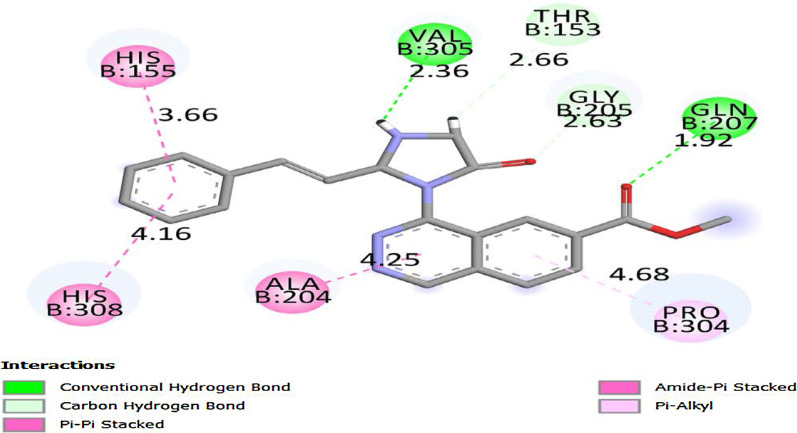
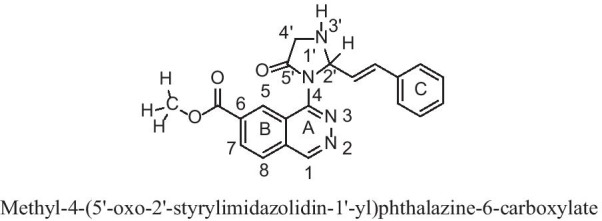
The molecules HIM6, HIM10 obtained good binding score of more 5 to 6.999 with all proteins and the molecules HIM11, HIM12, HIM14, HTZ9, HTZ10 and HTZ12 obtained binding score above 5 and less than 9 with PDB protein code of 2HV7, 3VD8 and 6RUR respectively. The molecule HIM11 obtained highest binding score of 8.678 with 2HV7 and its interaction with amino acids are molecule HIM11 (Fig. 9) has obtained with a high binding score with PDB protein 2HV7, the interactions of molecule is the C6 side chin acyl carbonyl C=O formed hydrogen bond interaction with amino acid GLN-207 with bond length 1.92 A° and 3’ N–H group of imidazole ring formed hydrogen bond interaction with VAL-305 with bond length 2.36 A° respectively. It also formed other interactions of carbon hydrogen bond of –CH3 group of carboxylate at C6 with PRO-304 and amide-pi stacked and pi–pi stacked interaction of electrons of aromatic ring A with ALA-204 and ring C with HIS-155 and HIS-308. Molecule formed pi-alkyl interaction of ring B with PRO-304 and all interactions with amino acids and bond length are depicted by 3D and 2D figures (Fig. 10 and Fig. 11).
Fig. 9.
Structure of active designed molecule of HIM11
Fig.10.
3D binding of molecule HIM11 with 2HV7
Fig.11.
2D binding of molecule HIM11 with 2HV7
Conclusions
The present investigation aimed at characterizing the expression profiling by high throughput sequencing of the HF patients. Our bioinformatics analyses revealed key gene signatures as candidate biomarkers in HF. Hub genes (ESR1, PYHIN1, PPP2R2B, LCK, TP63, PCLAF, CFTR, TK1, ECT2 and FKBP5) were diagnosed as an essential genetic factors in HF. In general, DEGs linked with HF genes, including already known markers of HF and other HF related diseases, and novel biomarkers, were diagnosed. Potential implicated miRNAs and TFs were also diagnosed. The diagnosed hub genes might represent candidate diagnostic and prognostic biomarkers, and therapeutic targets. The current investigation reported novel genes and signaling pathways in HF, and further investigation is required.
Supplementary Information
The statistical metrics for key differentially expressed genes (DEGs).
Acknowledgements
I thank Michael Patrick Morley, Perelman School of Medicine at the University of Pennsylvania, Penn Cardiovascular Institute, Philadelphia, USA, very much, the author who deposited their profiling by high throughput sequencing dataset GSE141910, into the public GEO database.
Authors' contributions
VK—Methodology and validation. BV—Writing original draft, and review and editing. CV—Software and investigation. SK—Supervision and resources. AT—Formal analysis and validation. All authors read and approved the final manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are available in the GEO (Gene Expression Omnibus) (https://www.ncbi.nlm.nih.gov/geo/) repository. [(GSE141910) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE141910].
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent because this study does not contain human or animals participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xanthopoulos A, Triposkiadis F, Starling RC. Heart failure with preserved ejection fraction: classification based upon phenotype is essential for diagnosis and treatment. Trends Cardiovasc Med. 2018;28(6):392–400. doi: 10.1016/j.tcm.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Wang F, Cao Q, Li Z, Huang L, Chen S. The effect of Mecp2 on heart failure. Cell Physiol Biochem. 2018;47(6):2380–2387. doi: 10.1159/000491610. [DOI] [PubMed] [Google Scholar]
- 5.Ma J, Lu L, Guo W, Ren J, Yang J. Emerging role for RBM20 and its splicing substrates in cardiac function and heart failure. Curr Pharm Des. 2016;22(31):4744–4751. doi: 10.2174/1381612822666160701145322. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63(3):476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure. Circulation. 1997;96(9):2953–2958. doi: 10.1161/01.cir.96.9.2953. [DOI] [PubMed] [Google Scholar]
- 8.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477(7366):601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, Xiao C, Zlotoff D, Bezzerides V, Houstis N, et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11(482):eaau8680. doi: 10.1126/scitranslmed.aau8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Feng Z. The role of toll-like receptor signaling in the progression of heart failure. Mediators Inflamm. 2018;2018:9874109. doi: 10.1155/2018/9874109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhang L, Zhang Y, Fan X, Yang W, Yu B, Kou J, Li F. YiQiFuMai powder injection attenuates coronary artery ligation-induced heart failure through improving mitochondrial function via regulating ROS generation and CaMKII signaling pathways. Front Pharmacol. 2019;10:381. doi: 10.3389/fphar.2019.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Tian Y, Hu S, Bi S, Li S, Hu Y, Kou J, Qi J, Yu B. Extract of Sheng–Mai–San ameliorates myocardial ischemia-induced heart failure by modulating Ca2+-calcineurin-mediated Drp1 signaling pathways. Int J Mol Sci. 2017;18(9):1825. doi: 10.3390/ijms18091825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booz GW, Day JN, Baker KM. Interplay between the cardiac renin angiotensin system and JAK-STAT signaling: role in cardiac hypertrophy, ischemia/reperfusion dysfunction, and heart failure. J Mol Cell Cardiol. 2002;34(11):1443–1453. doi: 10.1006/jmcc.2002.2076. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Li B, Jiang H. Identification of time-series differentially expressed genes and pathways associated with heart failure post-myocardial infarction using integrated bioinformatics analysis. Mol Med Rep. 2019;19(6):5281–5290. doi: 10.3892/mmr.2019.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Morley M, Brandimarto J, Hannenhalli S, Hu Y, Ashley EA, Tang WH, Moravec CS, Margulies KB, Cappola TP, et al. RNA-Seq identifies novel myocardial gene expression signatures of heart failure. Genomics. 2015;105(2):83–89. doi: 10.1016/j.ygeno.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiano C, Costa V, Aprile M, Grimaldi V, Maiello C, Esposito R, Soricelli A, Colantuoni V, Donatelli F, Ciccodicola A, et al. Heart failure: Pilot transcriptomic analysis of cardiac tissue by RNA-sequencing. Cardiol J. 2017;24(5):539–553. doi: 10.5603/CJ.a2017.0052. [DOI] [PubMed] [Google Scholar]
- 17.Rahman MR, Islam T, Zaman T, Shahjaman M, Karim MR, Huq F, Quinn JMW, Holsinger RMD, Gov E, Moni MA. Identification of molecular signatures and pathways to identify novel therapeutic targets in Alzheimer's disease: Insights from a systems biomedicine perspective. Genomics. 2020;112(2):1290–1299. doi: 10.1016/j.ygeno.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Rahman MR, Islam T, Turanli B, Zaman T, Faruquee HM, Rahman MM, Mollah MNH, Nanda RK, Arga KY, Gov E, et al. Network-based approach to identify molecular signatures and therapeutic agents in Alzheimer's disease. Comput Biol Chem. 2019;78:431–439. doi: 10.1016/j.compbiolchem.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Rahman MR, Petralia MC, Ciurleo R, Bramanti A, Fagone P, Shahjaman M, Wu L, Sun Y, Turanli B, Arga KY, et al. Comprehensive analysis of RNA-Seq gene expression profiling of brain transcriptomes reveals novel genes, regulators, and pathways in autism spectrum disorder. Brain Sci. 2020;10(10):747. doi: 10.3390/brainsci10100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clough E, Barrett T. The gene expression omnibus database. Methods Mol Biol. 2016;1418:93–110. doi: 10.1007/978-1-4939-3578-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas PD. The gene ontology and the meaning of biological function. Methods Mol Biol. 2017;1446:15–24. doi: 10.1007/978-1-4939-3743-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37(Web Server issue):W305–W311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, Haw R, Jassal B, Korninger F, May B, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2018;46(D1):D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong J, Li L, Zhimin L, Yan J, Ji D, Chen Y, Yuanyuan W, Chen X, Shao H, Wang J, et al. Potential protein biomarkers for systemic lupus erythematosus determined by bioinformatics analysis. Comput Biol Chem. 2019;83:107135. doi: 10.1016/j.compbiolchem.2019.107135. [DOI] [PubMed] [Google Scholar]
- 26.Alanis-Lobato G, Andrade-Navarro MA, Schaefer MH. HIPPIE v2.0: enhancing meaningfulness and reliability of protein-protein interaction networks. Nucleic Acids Res. 2017;45(D1):D408–D414. doi: 10.1093/nar/gkw985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Przulj N, Wigle DA, Jurisica I. Functional topology in a network of protein interactions. Bioinformatics. 2004;20(3):340–348. doi: 10.1093/bioinformatics/btg415. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen TP, Liu WC, Jordán F. Inferring pleiotropy by network analysis: linked diseases in the human PPI network. BMC Syst Biol. 2011;5:179. doi: 10.1186/1752-0509-5-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z, Zhang B. Fast network centrality analysis using GPUs. BMC Bioinformatics. 2011;12:149. doi: 10.1186/1471-2105-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fadhal E, Gamieldien J, Mwambene EC. Protein interaction networks as metric spaces: a novel perspective on distribution of hubs. BMC Syst Biol. 2014;8:6. doi: 10.1186/1752-0509-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaki N, Efimov D, Berengueres J. Protein complex detection using interaction reliability assessment and weighted clustering coefficient. BMC Bioinformatics. 2013;14:163. doi: 10.1186/1471-2105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan Y, Xia J. miRNet-functional analysis and visual exploration of miRNA-target interactions in a network context. Methods Mol Biol. 2018;1819:215–233. doi: 10.1007/978-1-4939-8618-7_10. [DOI] [PubMed] [Google Scholar]
- 34.Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47:W234–W241. doi: 10.1093/nar/gkz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Liao C, Sitzmann M, Pugliese A, Nicklaus MC. Software and resources for computational medicinal chemistry. Future Med Chem. 2011;3(8):1057–1085. doi: 10.4155/fmc.11.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okawa T, Aramaki Y, Yamamoto M, Kobayashi T, Fukumoto S, Toyoda Y, Henta T, Hata A, Ikeda S, Kaneko M, et al. Design, synthesis, and evaluation of the highly selective and potent g-protein-coupled receptor Kinase 2 (GRK2) inhibitor for the potential treatment of heart failure. J Med Chem. 2017;60(16):6942–6990. doi: 10.1021/acs.jmedchem.7b00443. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Liu Y, Yu H, Zhang Y, Hsu AC, Zhang M, Gou Y, Sun W, Wang F, Li P, et al. Design, synthesis and biological evaluation of novel pyxinol derivatives with anti-heart failure activity. Biomed Pharmacother. 2021;133:111050. doi: 10.1016/j.biopha.2020.111050. [DOI] [PubMed] [Google Scholar]
- 41.Hill QA, Farrar L, Lordan J, Gallienne A, Henderson S. A combination of two novel alpha globin variants Hb Bridlington (HBA1) and Hb Taybe (HBA2) resulting in severe hemolysis, pulmonary hypertension, and death. Hematology. 2015;20(1):50–52. doi: 10.1179/1607845414Y.0000000164. [DOI] [PubMed] [Google Scholar]
- 42.Zeng W, Cao Y, Jiang W, Kang G, Huang J, Xie S. Knockdown of Sfrp4 attenuates apoptosis to protect against myocardial ischemia/reperfusion injury. J Pharmacol Sci. 2019;140(1):14–19. doi: 10.1016/j.jphs.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Emmens JE, Ter Maaten JM, Damman K, van Veldhuisen DJ, de Boer RA, Struck J, Bergmann A, Sama IE, Streng KW, Anker SD, et al. Proenkephalin, an opioid system surrogate, as a novel comprehensive renal marker in heart failure. Circ Heart Fail. 2019;12(5):e005544. doi: 10.1161/CIRCHEARTFAILURE.118.005544. [DOI] [PubMed] [Google Scholar]
- 44.Broch K, Ueland T, Yndestad A, Aukrust P, Gullestad L. Heart failure biomarkers: focus on interleukin-1 receptor-like 1-based blood tests. Drugs Today (Barc) 2012;48(7):479–491. doi: 10.1358/dot.2012.48.7.1811719. [DOI] [PubMed] [Google Scholar]
- 45.Magnusson LU, Lundqvist A, Karlsson MN, Skålén K, Levin M, Wiklund O, Borén J, Hultén LM. Arachidonate 15-lipoxygenase type B knockdown leads to reduced lipid accumulation and inflammation in atherosclerosis. PLoS ONE. 2012;7(8):e43142. doi: 10.1371/journal.pone.0043142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellana S, Mastroianno S, Palumbo P, Palumbo O, Biagini T, Leone MP, De Luca G, Potenza DR, Amico CM, Mazza T, et al. Sudden death in mild hypertrophic cardiomyopathy with compound DSG2/DSC2/MYH6 mutations: Revisiting phenotype after genetic assessment in a master runner athlete. J Electrocardiol. 2019;53:95–99. doi: 10.1016/j.jelectrocard.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Liu W, Li WM, Sun NL. Relationship between HLA-DQA1 polymorphism and genetic susceptibility to idiopathic dilated cardiomyopathy. Chin Med J (Engl) 2004;117(10):1449–1452. [PubMed] [Google Scholar]
- 48.Kosugi M, Otani M, Kikkawa Y, Itakura Y, Sakai K, Ito T, Toyoda M, Sekita Y, Kimura T. Mutations of histone demethylase genes encoded by X and Y chromosomes, Kdm5c and Kdm5d, lead to noncompaction cardiomyopathy in mice. Biochem Biophys Res Commun. 2020 doi: 10.1016/j.bbrc.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 49.McMacken G, Lochmüller H, Bansagi B, Pyle A, Lochmüller A, Chinnery PF, et al. Behr syndrome and hypertrophic cardiomyopathy in a family with a novel UCHL1 deletion. J Neurol. 2020;267(12):3643–3649. doi: 10.1007/s00415-020-10059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan XY, Zhang ZW. MFGE8, ALB, APOB, APOE, SAA1, A2M, and C3 as novel biomarkers for stress cardiomyopathy. Cardiovasc Ther. 2020;2020:1615826. doi: 10.1155/2020/1615826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C, Wang L, Li Y, Feng Z, Wang Q, Luo W. Common variants in the ARG1 gene contribute to the risk of dilated cardiomyopathy in the Han Chinese population. Genet Test Mol Biomarkers. 2020;24(9):584–591. doi: 10.1089/gtmb.2020.0080. [DOI] [PubMed] [Google Scholar]
- 52.Jiang X, Cui J, Yang C, Song Y, Yuan J, Liu S, Hu F, Yang W, Qiao S. Elevated lymphatic vessel density measured by Lyve-1 expression in areas of replacement fibrosis in the ventricular septum of patients with hypertrophic obstructive cardiomyopathy (HOCM) Heart Vessels. 2020;35(1):78–85. doi: 10.1007/s00380-019-01463-5. [DOI] [PubMed] [Google Scholar]
- 53.Hou J, Huang S, Long Y, Huang J, Yang S, Yao J, Chen G, Yue Y, Liang M, Mei B, et al. DACT2 regulates structural and electrical atrial remodeling in atrial fibrillation. J Thorac Dis. 2020;12(5):2039–2048. doi: 10.21037/jtd-19-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olesen MS, Refsgaard L, Holst AG, Larsen AP, Grubb S, Haunsø S, Svendsen JH, Olesen SP, Schmitt N, Calloe K. A novel KCND3 gain-of-function mutation associated with early-onset of persistent lone atrial fibrillation. Cardiovasc Res. 2013;98(3):488–495. doi: 10.1093/cvr/cvt028. [DOI] [PubMed] [Google Scholar]
- 55.Ge Y, Concannon P. Molecular-genetic characterization of common, noncoding UBASH3A variants associated with type 1 diabetes. Eur J Hum Genet. 2018;26(7):1060–1064. doi: 10.1038/s41431-018-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferjeni Z, Bouzid D, Fourati H, Stayoussef M, Abida O, Kammoun T, Hachicha M, Penha-Gonçalves C, Masmoudi H. Association of TCR/CD3, PTPN22, CD28 and ZAP70 gene polymorphisms with type 1 diabetes risk in Tunisian population: family based association study. Immunol Lett. 2015;163(1):1–7. doi: 10.1016/j.imlet.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Anquetil F, Mondanelli G, Gonzalez N, Rodriguez Calvo T, Zapardiel Gonzalo J, Krogvold L, Dahl-Jørgensen K, Van den Eynde B, Orabona C, Grohmann U, et al. Loss of IDO1 expression from human pancreatic β-cells precedes their destruction during the development of type 1 diabetes. Diabetes. 2018;67(9):1858–1866. doi: 10.2337/db17-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glawe JD, Patrick DR, Huang M, Sharp CD, Barlow SC, Kevil CG. Genetic deficiency of Itgb2 or ItgaL prevents autoimmune diabetes through distinctly different mechanisms in NOD/LtJ mice. Diabetes. 2009;58(6):1292–1301. doi: 10.2337/db08-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawabata Y, Nishida N, Awata T, Kawasaki E, Imagawa A, Shimada A, Osawa H, Tanaka S, Takahashi K, Nagata M, et al. Genome-wide association study confirming a strong effect of HLA and identifying variants in CSAD/lnc-ITGB7-1 on chromosome 12q13.13 associated with susceptibility to fulminant type 1 diabetes. Diabetes. 2019;68(3):665–675. doi: 10.2337/db18-0314. [DOI] [PubMed] [Google Scholar]
- 60.Li JY, Tao F, Wu XX, Tan YZ, He L, Lu H. Common RASGRP1 gene variants that confer risk of type 2 diabetes. Genet Test Mol Biomarkers. 2015;19(8):439–443. doi: 10.1089/gtmb.2015.0005. [DOI] [PubMed] [Google Scholar]
- 61.Buraczynska M, Wacinski P, Zukowski P, Dragan M, Ksiazek A. Common polymorphism in the cannabinoid type 1 receptor gene (CNR1) is associated with microvascular complications in type 2 diabetes. J Diabetes Complications. 2014;28(1):35–39. doi: 10.1016/j.jdiacomp.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Amini S, Javanmardi M, Mokarizadeh A, Maroofi F, Jalali C, Azadi NA, Mohammadi H, Abdi M. Association of HaeIII single nucleotide polymorphisms in the SLC2A1 gene with risk of diabetic nephropathy; evidence from Kurdish patients with type 2 diabetes mellitus. QJM. 2016;109(6):399–404. doi: 10.1093/qjmed/hcv149. [DOI] [PubMed] [Google Scholar]
- 63.Yang JH, Downes K, Howson JM, Nutland S, Stevens HE, Walker NM, Todd JA. Evidence of association with type 1 diabetes in the SLC11A1 gene region. BMC Med Genet. 2011;12:59. doi: 10.1186/1471-2350-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du Toit E, Browne L, Irving-Rodgers H, Massa HM, Fozzard N, Jennings MP, Peak IR. Effect of GPR84 deletion on obesity and diabetes development in mice fed long chain or medium chain fatty acid rich diets. Eur J Nutr. 2018;57(5):1737–1746. doi: 10.1007/s00394-017-1456-5. [DOI] [PubMed] [Google Scholar]
- 65.Hirose H, Yamasaki T, Ogino M, Mizojiri R, Tamura-Okano Y, Yashiro H, Muraki Y, Nakano Y, Sugama J, Hata A, et al. Discovery of novel 5-oxa-2,6-diazaspiro[3.4]oct-6-ene derivatives as potent, selective, and orally available somatostatin receptor subtype 5 (SSTR5) antagonists for treatment of type 2 diabetes mellitus. Bioorg Med Chem. 2017;25(15):4175–4193. doi: 10.1016/j.bmc.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Zhang YX, Liu Y, Dong J, Wang YX, Wang J, Zhuang GQ, Han SJ, Guo QQ, Luo YX, Zhang J, et al. An exploratory study of the association between KCNB1 rs1051295 and type 2 diabetes and its related traits in Chinese Han population. PLoS ONE. 2013;8(2):e56365. doi: 10.1371/journal.pone.0056365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Griffin JWD, Liu Y, Bradshaw PC, Wang K. In silico preliminary association of ammonia metabolism genes GLS, CPS1, and GLUL with risk of Alzheimer's Disease, Major Depressive Disorder, and Type 2 Diabetes. J Mol Neurosci. 2018;64(3):385–396. doi: 10.1007/s12031-018-1035-0. [DOI] [PubMed] [Google Scholar]
- 68.Zouidi F, Stayoussef M, Bouzid D, Fourati H, Abida O, João C, Ayed MB, Fakhfakh R, Thouraya K, Monjia H, et al. Association of BANK1 and cytokine gene polymorphisms with type 1 diabetes in Tunisia. Gene. 2014;536(2):296–301. doi: 10.1016/j.gene.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Trombetta M, Bonetti S, Boselli M, Turrini F, Malerba G, Trabetti E, Pignatti P, Bonora E, Bonadonna RC. CACNA1E variants affect beta cell function in patients with newly diagnosed type 2 diabetes the Verona newly diagnosed type 2 diabetes study (VNDS) 3. PLoS ONE. 2012;7(3):e32755. doi: 10.1371/journal.pone.0032755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alharbi KK, Ali Khan I, Syed R, Alharbi FK, Mohammed AK, Vinodson B, Al-Daghri NM. Association of JAZF1 and TSPAN8/LGR5 variants in relation to type 2 diabetes mellitus in a Saudi population. Diabetol Metab Syndr. 2015;7:92. doi: 10.1186/s13098-015-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikarashi N, Mizukami N, Kon R, Kaneko M, Uchino R, Fujisawa I, Fukuda N, Sakai H, Kamei J. Study of the mechanism underlying the onset of diabetic xeroderma focusing on an aquaporin-3 in a streptozotocin-induced diabetic mouse model. Int J Mol Sci. 2019;20(15):3782. doi: 10.3390/ijms20153782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dharmadhikari G, Stolz K, Hauke M, Morgan NG, Varki A, de Koning E, Kelm S, Maedler K. Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes. Sci Rep. 2017;7:45319. doi: 10.1038/srep45319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sutton BS, Palmer ND, Langefeld CD, Xue B, Proctor A, Ziegler JT, Haffner SM, Norris JM, Bowden DW. Association of SSTR2 polymorphisms and glucose homeostasis phenotypes: the Insulin Resistance Atherosclerosis Family Study. Diabetes. 2009;58(6):1457–1462. doi: 10.2337/db08-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng Z, Shen J, Ye J, Shu Q, Zhao J, Fang M, Zhang T. Association between single nucleotide polymorphisms of delta/notch-like epidermal growth factor (EGF)-related receptor (DNER) and Delta-like 1 Ligand (DLL 1) with the risk of type 2 diabetes mellitus in a Chinese Han population. Cell Biochem Biophys. 2015;71(1):331–335. doi: 10.1007/s12013-014-0202-3. [DOI] [PubMed] [Google Scholar]
- 75.Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, Galuppo P, Bauersachs J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 76.Cui J, Randell E, Renouf J, Sun G, Green R, Han FY, Xie YG. Thrombospondin-4 1186G>C (A387P) is a sex-dependent risk factor for myocardial infarction: a large replication study with increased sample size from the same population. Am Heart J. 2006;152(3):543.e1–543.e5435. doi: 10.1016/j.ahj.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Li W, Zhang F, Ju C, Lv S, Huang K. The role of CD27-CD70 signaling in myocardial infarction and cardiac remodeling. Int J Cardiol. 2019;278:210–216. doi: 10.1016/j.ijcard.2018.11.132. [DOI] [PubMed] [Google Scholar]
- 78.Cho HM, Lee KH, Shen YM, Shin TJ, Ryu PD, Choi MC, Kang KS, Cho JY. Transplantation of hMSCs genome edited with LEF1 improves cardio-protective effects in myocardial infarction. Mol Ther Nucleic Acids. 2020;19:1186–1197. doi: 10.1016/j.omtn.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz-Villalba A, Romero JP, Hernández SC, Vilas-Zornoza A, Fortelny N, Castro-Labrador L, San Martin-Uriz P, Lorenzo-Vivas E, García-Olloqui P, Palacio M, et al. Single-Cell RNA sequencing analysis reveals a crucial role for CTHRC1 (collagen triple helix repeat containing 1) cardiac fibroblasts after myocardial infarction. Circulation. 2020;142(19):1831–1847. doi: 10.1161/CIRCULATIONAHA.119.044557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puzianowska-Kuźnicka M. ESR1 in myocardial infarction. Clin Chim Acta. 2012;413(1–2):81–87. doi: 10.1016/j.cca.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 81.Lin CF, Su CJ, Liu JH, Chen ST, Huang HL, Pan SL. Potential effects of CXCL9 and CCL20 on cardiac fibrosis in patients with myocardial infarction and isoproterenol-treated rats. J Clin Med. 2019;8(5):659. doi: 10.3390/jcm8050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao L, Chen M, Yang X. Serpina3 in myocardial infarction. Int J Cardiol. 2020;306:8. doi: 10.1016/j.ijcard.2020.02.045. [DOI] [PubMed] [Google Scholar]
- 83.Jung C, Gené GG, Tomás M, Plata C, Selent J, Pastor M, Fandos C, Senti M, Lucas G, Elosua R, et al. A gain-of-function SNP in TRPC4 cation channel protects against myocardial infarction. Cardiovasc Res. 2011;91(3):465–471. doi: 10.1093/cvr/cvr083. [DOI] [PubMed] [Google Scholar]
- 84.Ansani L, Marchesini J, Pestelli G, Luisi GA, Scillitani G, Longo G, Milani D, Serino ML, Tisato V, Gemmati D. F13A1 gene variant (V34L) and residual circulating FXIIIA levels predict short- and long-term mortality in acute myocardial infarction after coronary angioplasty. Int J Mol Sci. 2018;19(9):2766. doi: 10.3390/ijms19092766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan B, Liu M, Yang Y, Liu L, Meng F. Low expression of PIK3C2A gene: a potential biomarker to predict the risk of acute myocardial infarction. Medicine (Baltimore) 2019;98(14):e15061. doi: 10.1097/MD.0000000000015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X, Liao C, Sun K, Liu L, Xu D. A soluble epoxide hydrolase inhibitor upregulated KCNJ12 and KCNIP2 by downregulating MicroRNA-29 in a mouse model of myocardial infarction. Heart Surg Forum. 2020;23(5):E579–E585. doi: 10.1532/hsf.2999. [DOI] [PubMed] [Google Scholar]
- 87.Fukuda H, Ito S, Watari K, Mogi C, Arisawa M, Okajima F, Kurose H, Shuto S. Identification of a potent and selective GPR4 antagonist as a drug lead for the treatment of myocardial infarction. ACS Med Chem Lett. 2016;7(5):493–497. doi: 10.1021/acsmedchemlett.6b00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang HB, Yang J, Shuai W, Yang J, Liu LB, Xu M, Tang QZ. Deletion of microfibrillar-associated protein 4 attenuates left ventricular remodeling and dysfunction in heart failure. J Am Heart Assoc. 2020;9(17):e015307. doi: 10.1161/JAHA.119.015307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H, Okada S, Tateno K, Moriya J, Yokoyama M, et al. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J Exp Med. 2009;206(7):1565–1574. doi: 10.1084/jem.20082596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hua X, Wang YY, Jia P, Xiong Q, Hu Y, Chang Y, Lai S, Xu Y, Zhao Z, Song J. Multi-level transcriptome sequencing identifies COL1A1 as a candidate marker in human heart failure progression. BMC Med. 2020;18(1):2. doi: 10.1186/s12916-019-1469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gombos T, Förhécz Z, Pozsonyi Z, Jánoskuti L, Prohászka Z, Karádi I. Long-term survival and apolipoprotein A1 level in chronic heart failure: interaction with tumor necrosis factor α -308 G/A polymorphism. J Card Fail. 2017;23(2):113–120. doi: 10.1016/j.cardfail.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 92.Westermann D, Becher PM, Lindner D, Savvatis K, Xia Y, Fröhlich M, Hoffmann S, Schultheiss HP, Tschöpe C. Selective PDE5A inhibition with sildenafil rescues left ventricular dysfunction, inflammatory immune response and cardiac remodeling in angiotensin II-induced heart failure in vivo. Basic Res Cardiol. 2012;107(6):308. doi: 10.1007/s00395-012-0308-y. [DOI] [PubMed] [Google Scholar]
- 93.Koller L, Blum S, Korpak M, Richter B, Goliasch G, Zorn G, Brekalo M, Maurer G, Wojta J, Pacher R, et al. Predictive power of the fractalkine receptor CX3CR1 on CD4 T cells in patients with chronic heart failure. Int J Cardiol. 2014;171(1):96–97. doi: 10.1016/j.ijcard.2013.11.082. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Song D, Mao L, Abraham DM, Bursac N. Lack of Thy1 defines a pathogenic fraction of cardiac fibroblasts in heart failure. Biomaterials. 2020;236:119824. doi: 10.1016/j.biomaterials.2020.119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mueller KA, Tavlaki E, Schneider M, Jorbenadze R, Geisler T, Kandolf R, Gawaz M, Mueller II, Zuern CS. Gremlin-1 identifies fibrosis and predicts adverse outcome in patients with heart failure undergoing endomyocardial biopsy. J Card Fail. 2013;19(10):678–684. doi: 10.1016/j.cardfail.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Andenæs K, Lunde IG, Mohammadzadeh N, Dahl CP, Aronsen JM, Strand ME, Palmero S, Sjaastad I, Christensen G, Engebretsen KVT, et al. The extracellular matrix proteoglycan fibromodulin is upregulated in clinical and experimental heart failure and affects cardiac remodeling. PLoS ONE. 2018;13(7):e0201422. doi: 10.1371/journal.pone.0201422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abuzaanona A, Lanfear D. Pharmacogenomics of the natriuretic peptide system in heart failure. Curr Heart Fail Rep. 2017;14(6):536–542. doi: 10.1007/s11897-017-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bai Y, Zhang P, Zhang X, Huang J, Hu S, Wei Y. LTBP-2 acts as a novel marker in human heart failure—a preliminary study. Biomarkers. 2012;17(5):407–415. doi: 10.3109/1354750X.2012.677860. [DOI] [PubMed] [Google Scholar]
- 99.Engebretsen KV, Lunde IG, Strand ME, Waehre A, Sjaastad I, Marstein HS, Skrbic B, Dahl CP, Askevold ET, Christensen G, et al. Lumican is increased in experimental and clinical heart failure, and its production by cardiac fibroblasts is induced by mechanical and proinflammatory stimuli. FEBS J. 2013;280(10):2382–2398. doi: 10.1111/febs.12235. [DOI] [PubMed] [Google Scholar]
- 100.Tao R, Fan Q, Zhang H, Xie H, Lu L, Gu G, Wang F, Xi R, Hu J, Chen Q, et al. Prognostic significance of interleukin-34 (IL-34) in patients with chronic heart failure with or without renal insufficiency. J Am Heart Assoc. 2017;6(4):e004911. doi: 10.1161/JAHA.116.004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Keulenaer GW, Feyen E, Dugaucquier L, Shakeri H, Shchendrygina A, Belenkov YN, Brink M, Vermeulen Z, Segers VFM. Mechanisms of the multitasking endothelial protein NRG-1 as a compensatory factor during chronic heart failure. Circ Heart Fail. 2019;12(10):e006288. doi: 10.1161/CIRCHEARTFAILURE.119.006288. [DOI] [PubMed] [Google Scholar]
- 102.Zeng L, Gu N, Chen J, Jin G, Zheng Y. IRX1 hypermethylation promotes heart failure by inhibiting CXCL14 expression. Cell Cycle. 2019;18(23):3251–3262. doi: 10.1080/15384101.2019.1673099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Altara R, Manca M, Hessel MH, Gu Y, van Vark LC, Akkerhuis KM, Staessen JA, Struijker-Boudier HA, Booz GW, Blankesteijn WM. CXCL10 is a circulating inflammatory marker in patients with advanced heart failure: a pilot study. J Cardiovasc Transl Res. 2016;9(4):302–314. doi: 10.1007/s12265-016-9703-3. [DOI] [PubMed] [Google Scholar]
- 104.Petrie MC, Padmanabhan N, McDonald JE, Hillier C, Connell JM, McMurray JJ. Angiotensin converting enzyme (ACE) and non-ACE dependent angiotensin II generation in resistance arteries from patients with heart failure and coronary heart disease. J Am Coll Cardiol. 2001;37(4):1056–1061. doi: 10.1016/s0735-1097(01)01111-1. [DOI] [PubMed] [Google Scholar]
- 105.Meissner A, Yang J, Kroetsch JT, Sauvé M, Dax H, Momen A, Noyan-Ashraf MH, Heximer S, Husain M, et al. Tumor necrosis factor-α-mediated downregulation of the cystic fibrosis transmembrane conductance regulator drives pathological sphingosine-1-phosphate signaling in a mouse model of heart failure. Circulation. 2012;125(22):2739–2750. doi: 10.1161/CIRCULATIONAHA.111.047316. [DOI] [PubMed] [Google Scholar]
- 106.Wei X, Wu B, Zhao J, Zeng Z, Xuan W, Cao S, Huang X, Asakura M, Xu D, Bin J, et al. Myocardial hypertrophic preconditioning attenuates cardiomyocyte hypertrophy and slows progression to heart failure through upregulation of S100A8/A9. Circulation. 2015;131(17):1506–1517. doi: 10.1161/CIRCULATIONAHA.114.013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS) Ann Med. 2009;41(7):522–532. doi: 10.1080/07853890903089453. [DOI] [PubMed] [Google Scholar]
- 108.de Denus S, Dubé MP, Fouodjio R, Huynh T, LeBlanc MH, Lepage S, Sheppard R, Giannetti N, Lavoie J, Mansour A, et al. A prospective study of the impact of AGTR1 A1166C on the effects of candesartan in patients with heart failure. Pharmacogenomics. 2018;19(7):599–612. doi: 10.2217/pgs-2018-0004. [DOI] [PubMed] [Google Scholar]
- 109.Angrisano T, Schiattarella GG, Keller S, Pironti G, Florio E, Magliulo F, Bottino R, Pero R, Lembo F, Avvedimento EV, et al. Epigenetic switch at atp2a2 and myh7 gene promoters in pressure overload-induced heart failure. PLoS ONE. 2014;9(9):e106024. doi: 10.1371/journal.pone.0106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amir O, Rogowski O, David M, Lahat N, Wolff R, Lewis BS. Circulating interleukin-10: association with higher mortality in systolic heart failure patients with elevated tumor necrosis factor-alpha. Isr Med Assoc J. 2010;12(3):158–162. [PubMed] [Google Scholar]
- 111.Taylor MR, Slavov D, Humphrey K, Zhao L, Cockroft J, Zhu X, Lavori P, Bristow MR, Mestroni L, Lazzeroni LC. Pharmacogenetic effect of an endothelin-1 haplotype on response to bucindolol therapy in chronic heart failure. Pharmacogenet Genomics. 2009;19(1):35–43. doi: 10.1097/FPC.0b013e328317cc57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liang S, Xinyong C, Hongmin Z, Jing W, Lang H, Ping Z. TLR2 and TLR3 expression as a biomarker for the risk of doxorubicin-induced heart failure. Toxicol Lett. 2018;295:205–211. doi: 10.1016/j.toxlet.2018.06.1219. [DOI] [PubMed] [Google Scholar]
- 113.Raman K, O'Donnell MJ, Czlonkowska A, Duarte YC, Lopez-Jaramillo P, Peñaherrera E, Sharma M, Shoamanesh A, Skowronska M, Yusuf S, et al. Peripheral blood MCEMP1 gene expression as a biomarker for stroke prognosis. Stroke. 2016;47(3):652–658. doi: 10.1161/STROKEAHA.115.011854. [DOI] [PubMed] [Google Scholar]
- 114.Wells BJ, Hueston WJ. Are thyroid peroxidase antibodies associated with cardiovascular disease risk in patients with subclinical hypothyroidism? Clin Endocrinol (Oxf) 2005;62(5):580–584. doi: 10.1111/j.1365-2265.2005.02262.x. [DOI] [PubMed] [Google Scholar]
- 115.Ptaszynska-Kopczynska K, Marcinkiewicz-Siemion M, Lisowska A, Waszkiewicz E, Witkowski M, Jasiewicz M, Miklasz P, Jakim P, Galar B, Musial WJ, et al. Alterations of soluble TWEAK and CD163 concentrations in patients with chronic heart failure. Cytokine. 2016;80:7–12. doi: 10.1016/j.cyto.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 116.Grisoni ML, Proust C, Alanne M, Desuremain M, Salomaa V, Kuulasmaa K, Cambien F, Nicaud V, Wiklund PG, Virtamo J, et al. Lack of association between polymorphisms of the IL18R1 and IL18RAP genes and cardiovascular risk: the MORGAM Project. BMC Med Genet. 2009;10:44. doi: 10.1186/1471-2350-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bardien-Kruger S, Wulff H, Arieff Z, Brink P, Chandy KG, Corfield V. Characterisation of the human voltage-gated potassium channel gene, KCNA7, a candidate gene for inherited cardiac disorders, and its exclusion as cause of progressive familial heart block I (PFHBI) Eur J Hum Genet. 2002;10(1):36–43. doi: 10.1038/sj.ejhg.5200739. [DOI] [PubMed] [Google Scholar]
- 118.Totsune K, Takahashi K, Mackenzie HS, Murakami O, Arihara Z, Sone M, Mouri T, Brenner BM, Ito S. Increased gene expression of adrenomedullin and adrenomedullin-receptor complexes, receptor-activity modifying protein (RAMP)2 and calcitonin-receptor-like receptor (CRLR) in the hearts of rats with congestive heart failure. Clin Sci (Lond) 2000;99(6):541–546. doi: 10.1042/CS20000108. [DOI] [PubMed] [Google Scholar]
- 119.Li X, Wang G, QiLi M, Liang H, Li T, E X, Feng Y, Zhang Y, Liu X, Qian M, et al. Aspirin Reduces Cardiac Interstitial Fibrosis by Inhibiting Erk1/2-Serpine2 and P-Akt Signalling Pathways. Cell Physiol Biochem. 2018;45(5):1955–1965. 10.1159/000487972 [DOI] [PubMed]
- 120.Deckx S, Heggermont W, Carai P, Rienks M, Dresselaers T, Himmelreich U, van Leeuwen R, Lommen W, van der Velden J, Gonzalez A, et al. Osteoglycin prevents the development of age-related diastolic dysfunction during pressure overload by reducing cardiac fibrosis and inflammation. Matrix Biol. 2018;66:110–124. doi: 10.1016/j.matbio.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Ichihara S, Senbonmatsu T, Price E, Jr, Ichiki T, Gaffney FA, Inagami T. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation. 2001;104(3):346–351. doi: 10.1161/01.cir.104.3.346. [DOI] [PubMed] [Google Scholar]
- 122.Paik DT, Rai M, Ryzhov S, Sanders LN, Aisagbonhi O, Funke MJ, Feoktistov I, Hatzopoulos AK. Wnt10b gain-of-function improves cardiac repair by arteriole formation and attenuation of fibrosis. Circ Res. 2015;117(9):804–816. doi: 10.1161/CIRCRESAHA.115.306886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cai W, Tao J, Zhang X, Tian X, Liu T, Feng X, Bai J, Yan C, Han Y. Contribution of homeostatic chemokines CCL19 and CCL21 and their receptor CCR7 to coronary artery disease. Arterioscler Thromb Vasc Biol. 2014;34(9):1933–1941. doi: 10.1161/ATVBAHA.113.303081. [DOI] [PubMed] [Google Scholar]
- 124.Mo XG, Liu W, Yang Y, Imani S, Lu S, Dan G, Nie X, Yan J, Zhan R, Li X, et al. NCF2, MYO1F, S1PR4, and FCN1 as potential noninvasive diagnostic biomarkers in patients with obstructive coronary artery: a weighted gene co-expression network analysis. J Cell Biochem. 2019;120(10):18219–18235. doi: 10.1002/jcb.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun H, Fang F, Li K, Zhang H, Zhang M, Zhang L, Li J, Qin Y, Wei Y. Circulating ESM-1 levels are correlated with the presence of coronary artery disease in patients with obstructive sleep apnea. Respir Res. 2019;20(1):188. doi: 10.1186/s12931-019-1143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martinelli N, Girelli D, Lunghi B, Pinotti M, Marchetti G, Malerba G, Pignatti PF, Corrocher R, Olivieri O, Bernardi F. Polymorphisms at LDLR locus may be associated with coronary artery disease through modulation of coagulation factor VIII activity and independently from lipid profile. Blood. 2010;116(25):5688–5697. doi: 10.1182/blood-2010-03-277079. [DOI] [PubMed] [Google Scholar]
- 127.Zhao X, Li Y, Yan Y, Ma X, Guo C. Methylation of CpG sites in C1QTNF1 (C1q and tumor necrosis factor related protein 1) differs by gender in acute coronary syndrome in Han population: a case-control study. Genes Genomics. 2020;42(6):681–689. doi: 10.1007/s13258-020-00936-6. [DOI] [PubMed] [Google Scholar]
- 128.Assimes TL, Knowles JW, Priest JR, Basu A, Volcik KA, Southwick A, Tabor HK, Hartiala J, Allayee H, Grove ML, et al. Common polymorphisms of ALOX5 and ALOX5AP and risk of coronary artery disease. Hum Genet. 2008;123(4):399–408. doi: 10.1007/s00439-008-0489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Piechota M, Banaszewska A, Dudziak J, Slomczynski M, Plewa R. Highly upregulated expression of CD36 and MSR1 in circulating monocytes of patients with acute coronary syndromes. Protein J. 2012;31(6):511–518. doi: 10.1007/s10930-012-9431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Desch KC, Ozel AB, Halvorsen M, Jacobi PM, Golden K, Underwood M, Germain M, Tregouet DA, Reitsma PH, Kearon C, et al. Whole-exome sequencing identifies rare variants in STAB2 associated with venous thromboembolic disease. Blood. 2020;136(5):533–541. doi: 10.1182/blood.2019004161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fu Y, Gao C, Liang Y, Wang M, Huang Y, Ma W, Li T, Jia Y, Yu F, Zhu W, et al. Shift of macrophage phenotype due to cartilage oligomeric matrix protein deficiency drives atherosclerotic calcification. Circ Res. 2016;119(2):261–276. doi: 10.1161/CIRCRESAHA.115.308021. [DOI] [PubMed] [Google Scholar]
- 132.Wu S, Hsu LA, Cheng ST, Teng MS, Yeh CH, Sun YC, Huang HL, Ko YL. Circulating YKL-40 level, but not CHI3L1 gene variants, is associated with atherosclerosis-related quantitative traits and the risk of peripheral artery disease. Int J Mol Sci. 2014;15(12):22421–22437. doi: 10.3390/ijms151222421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Monroy-Muñoz IE, Angeles-Martinez J, Posadas-Sánchez R, Villarreal-Molina T, Alvarez-León E, Flores-Dominguez C, Cardoso-Saldaña G, Medina-Urrutia A, Juárez-Rojas JG, Posadas-Romero C, et al. PLA2G2A polymorphisms are associated with metabolic syndrome and type 2 diabetes mellitus. Results from the genetics of atherosclerotic disease Mexican study. Immunobiology. 2017;222(10):967–972. doi: 10.1016/j.imbio.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 134.Pi S, Mao L, Chen J, Shi H, Liu Y, Guo X, Li Y, Zhou L, He H, Yu C, et al. The P2RY12 receptor promotes VSMC-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy. 2020 doi: 10.1080/15548627.2020.1741202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Vries MA, Trompet S, Mooijaart SP, Smit RA, Böhringer S, Castro Cabezas M, Jukema JW. Complement receptor 1 gene polymorphisms are associated with cardiovascular risk. Atherosclerosis. 2017;257:16–21. doi: 10.1016/j.atherosclerosis.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Osterholm C, Folkersen L, Lengquist M, Pontén F, Renné T, Li J, Hedin U. Increased expression of heparanase in symptomatic carotid atherosclerosis. Atherosclerosis. 2013;226(1):67–73. doi: 10.1016/j.atherosclerosis.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 137.Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20(2):35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 138.Koch W, Schrempf M, Erl A, Mueller JC, Hoppmann P, Schömig A, Kastrati A. 4G/5G polymorphism and haplotypes of SERPINE1 in atherosclerotic diseases of coronary arteries. Thromb Haemost. 2010;103(6):1170–1180. doi: 10.1160/TH09-10-0702. [DOI] [PubMed] [Google Scholar]
- 139.Sasagawa S, Nishimura Y, Sawada H, Zhang E, Okabe S, Murakami S, Ashikawa Y, Yuge M, Kawaguchi K, Kawase R, et al. Comparative transcriptome analysis identifies CCDC80 as a novel gene associated with pulmonary arterial hypertension. Front Pharmacol. 2016;7:142. doi: 10.3389/fphar.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu Y, Li Q, Yang K, Xiao C. Association of CMA1 gene tag single nucleotide polymorphisms with essential hypertension in Yi population from Yunnan. Chinese. 2014;31(4):449–454. doi: 10.3760/cma.j.issn.1003-9406.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 141.Sato W, Sato Y. Midkine in nephrogenesis, hypertension and kidney diseases. Br J Pharmacol. 2014;171(4):879–887. doi: 10.1111/bph.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kohara K, Tabara Y, Nakura J, Imai Y, Ohkubo T, Hata A, Soma M, Nakayama T, Umemura S, Hirawa N, et al. Identification of hypertension-susceptibility genes and pathways by a systemic multiple candidate gene approach: the millennium genome project for hypertension. Hypertens Res. 2008;31(2):203–212. doi: 10.1291/hypres.31.203. [DOI] [PubMed] [Google Scholar]
- 143.Wen G, Wessel J, Zhou W, Ehret GB, Rao F, Stridsberg M, Mahata SK, Gent PM, Das M, Cooper RS, et al. An ancestral variant of Secretogranin II confers regulation by PHOX2 transcription factors and association with hypertension. Hum Mol Genet. 2007;16(14):1752–1764. doi: 10.1093/hmg/ddm123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seidelmann SB, Vardeny O, Claggett B, Yu B, Shah AM, Ballantyne CM, Selvin E, MacRae CA, Boerwinkle E, Solomon SD. An NPPB promoter polymorphism associated with elevated N-terminal pro-B-type natriuretic peptide and lower blood pressure, hypertension, and mortality. J Am Heart Assoc. 2017;6(4):e005257. doi: 10.1161/JAHA.116.005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shi L, Tian C, Sun L, Cao F, Meng Z. The lncRNA TUG1/miR-145-5p/FGF10 regulates proliferation and migration in VSMCs of hypertension. Biochem Biophys Res Commun. 2018;501(3):688–695. doi: 10.1016/j.bbrc.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 146.Woon PY, Kaisaki PJ, Bragança J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104(36):14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leng Q, Kahle KT, Rinehart J, MacGregor GG, Wilson FH, Canessa CM, Lifton RP, Hebert SC. WNK3, a kinase related to genes mutated in hereditary hypertension with hyperkalaemia, regulates the K+ channel ROMK1 (Kir1.1) J Physiol. 2006;571(Pt 2):275–286. doi: 10.1113/jphysiol.2005.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zicha J, Dobešová Z, Zídek V, Silhavý J, Simáková M, Mlejnek P, Vaněčková I, Kuneš J, Pravenec M. Pharmacogenetic analysis of captopril effects on blood pressure: possible role of the Ednrb (endothelin receptor type B) candidate gene. Physiol Res. 2014;63(2):263–265. doi: 10.33549/physiolres.932732. [DOI] [PubMed] [Google Scholar]
- 149.Maloney JP, Stearman RS, Bull TM, Calabrese DW, Tripp-Addison ML, Wick MJ, Broeckel U, Robbins IM, Wheeler LA, Cogan JD, et al. Loss-of-function thrombospondin-1 mutations in familial pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L541–L554. doi: 10.1152/ajplung.00282.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ouyang Y, Wu H, Tan A, Yang H, Gao Y, Li H, Lu S, Hu Y, Tang X, Zhang H. E-selectin gene polymorphism (A561C) and essential hypertension. Meta-analysis in the Chinese population. Herz. 2015;40(Suppl 2):197–202. doi: 10.1007/s00059-014-4122-1. [DOI] [PubMed] [Google Scholar]
- 151.Wang L, Li H, Yang B, Guo L, Han X, Li L, Li M, Huang J, Gu D. The hypertension risk variant Rs820430 functions as an enhancer of SLC4A7. Am J Hypertens. 2017;30(2):202–208. doi: 10.1093/ajh/hpw127. [DOI] [PubMed] [Google Scholar]
- 152.Kerty E, Heuser K, Indahl UG, Berg PR, Nakken S, Lien S, Omholt SW, Ottersen OP, Nagelhus EA. Is the brain water channel aquaporin-4 a pathogenetic factor in idiopathic intracranial hypertension? Results from a combined clinical and genetic study in a Norwegian cohort. Acta Ophthalmol. 2013;91(1):88–91. doi: 10.1111/j.1755-3768.2011.02231.x. [DOI] [PubMed] [Google Scholar]
- 153.Lambert M, Capuano V, Boet A, Tesson L, Bertero T, Nakhleh MK, Remy S, Anegon I, Pechoux C, Hautefort A, et al. Characterization of Kcnk3-mutated rat, a novel model of pulmonary hypertension. Circ Res. 2019;125(7):678–695. doi: 10.1161/CIRCRESAHA.119.314793. [DOI] [PubMed] [Google Scholar]
- 154.Buchner DA, Geisinger JM, Glazebrook PA, Morgan MG, Spiezio SH, Kaiyala KJ, Schwartz MW, Sakurai T, Furley AJ, Kunze DL, et al. The juxtaparanodal proteins CNTNAP2 and TAG1 regulate diet-induced obesity. Mamm Genome. 2012;23(7–8):431–442. doi: 10.1007/s00335-012-9400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shi Y, Long F. Hedgehog signaling via Gli2 prevents obesity induced by high-fat diet in adult mice. Elife. 2017;6:e31649. doi: 10.7554/eLife.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Unamuno X, Gómez-Ambrosi J, Ramírez B, Rodríguez A, Becerril S, Valentí V, Moncada R, Silva C, Salvador J, et al. Dermatopontin, a novel adipokine promoting adipose tissue extracellular matrix remodelling and inflammation in obesity. J Clin Med. 2020;9(4):1069. doi: 10.3390/jcm9041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang L, Reidy SP, Nicholson TE, Lee HJ, Majdalawieh A, Webber C, Stewart BR, Dolphin P, Ro HS. The role of AEBP1 in sex-specific diet-induced obesity. Mol Med. 2005;11(1–12):39–47. doi: 10.2119/2005-00021.Ro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Anveden Å, Sjöholm K, Jacobson P, Palsdottir V, Walley AJ, Froguel P, Al-Daghri N, McTernan PG, Mejhert N, Arner P, et al. ITIH-5 expression in human adipose tissue is increased in obesity. Obesity (Silver Spring) 2012;20(4):708–714. doi: 10.1038/oby.2011.268. [DOI] [PubMed] [Google Scholar]
- 159.Kochumon S, Madhoun AA, Al-Rashed F, Azim R, Al-Ozairi E, Al-Mulla F, Ahmad R. Adipose tissue gene expression of CXCL10 and CXCL11 modulates inflammatory markers in obesity: implications for metabolic inflammation and insulin resistance. Ther Adv Endocrinol Metab. 2020 doi: 10.1177/2042018820930902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mwangi SM, Nezami BG, Obukwelu B, Anitha M, Marri S, Fu P, Epperson MF, Le NA, Shanmugam M, Sitaraman SV, et al. Glial cell line-derived neurotrophic factor protects against high-fat diet-induced obesity. Am J Physiol Gastrointest Liver Physiol. 2014;306(6):G515–G525. doi: 10.1152/ajpgi.00364.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Högberg T, Frimurer TM, Sasmal PK. Melanin concentrating hormone receptor 1 (MCHR1) antagonists-Still a viable approach for obesity treatment? Bioorg Med Chem Lett. 2012;22(19):6039–6047. doi: 10.1016/j.bmcl.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 162.Mazzarella L, Botteri E, Matthews A, Gatti E, Di Salvatore D, Bagnardi V, Breccia M, Montesinos P, Bernal T, Gil C, et al. Obesity is a risk factor for acute promyelocytic leukemia: evidence from population and cross-sectional studies and correlation with FLT3 mutations and polyunsaturated fatty acid metabolism. Haematologica. 2020;105(6):1559–1566. doi: 10.3324/haematol.2019.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mirea AM, Stienstra R, Kanneganti TD, Tack CJ, Chavakis T, Toonen EJM, Joosten LAB. Mice deficient in the IL-1β activation genes Prtn3, Elane, and Casp1 are protected against the development of obesity-induced NAFLD. Inflammation. 2020;43(3):1054–1064. doi: 10.1007/s10753-020-01190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Piquer-Garcia I, Campderros L, Taxerås SD, Gavaldà-Navarro A, Pardo R, Vila M, Pellitero S, Martínez E, Tarascó J, Moreno P, et al. A role for oncostatin M in the impairment of glucose homeostasis in obesity. J Clin Endocrinol Metab. 2020;105(3):e337–e348. doi: 10.1210/clinem/dgz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Quinn LS, Anderson BG. Interleukin-15, IL-15 receptor-alpha, and obesity: concordance of laboratory animal and human genetic studies. J Obes. 2011;2011:456347. doi: 10.1155/2011/456347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang J, Sukhova GK, Liu J, Ozaki K, Lesner A, Libby P, Kovanen PT, Shi GP. Cathepsin G deficiency reduces periaortic calcium chloride injury-induced abdominal aortic aneurysms in mice. J Vasc Surg. 2015;62(6):1615–1624. doi: 10.1016/j.jvs.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Safa A, Rashidinejad HR, Khalili M, Dabiri S, Nemati M, Mohammadi MM, Jafarzadeh A. Higher circulating levels of chemokines CXCL10, CCL20 and CCL22 in patients with ischemic heart disease. Cytokine. 2016;83:147–157. doi: 10.1016/j.cyto.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 168.Chen C, Peng H, Zeng Y, Dong G. CD14, CD163, and CCR1 are involved in heart and blood communication in ischemic cardiac diseases. J Int Med Res. 2020;48(9):300060520951649. doi: 10.1177/0300060520951649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou QL, Teng F, Zhang YS, Sun Q, Cao YX, Meng GW. FPR1 gene silencing suppresses cardiomyocyte apoptosis and ventricular remodeling in rats with ischemia/reperfusion injury through the inhibition of MAPK signaling pathway. Exp Cell Res. 2018;370(2):506–518. doi: 10.1016/j.yexcr.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 170.Hu Z, Liu J, Song Z, Hou Q, Fan X, Hou D. Variants in the Atherogenic ALOX5AP, THBD, and KNG1 genes potentiate the risk of ischemic stroke via a genetic main effect and epistatic interactions in a Chinese Population. J Stroke Cerebrovasc Dis. 2015;24(9):2060–2068. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 171.Lou Y, Wang S, Qu J, Zheng J, Jiang W, Lin Z, Zhang S. miR-424 promotes cardiac ischemia/reperfusion injury by direct targeting of CRISPLD2 and regulating cardiomyocyte pyroptosis. Int J Clin Exp Pathol. 2018;11(7):3222–3235. [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang X, Li L, Zhang Q, Wei Q, Lin J, Jia J, Zhang J, Yan T, Lv Y, Jiang X, et al. CD38 causes autophagic flux inhibition and cardiac dysfunction through a transcriptional inhibition pathway under hypoxia/ischemia conditions. Front Cell Dev Biol. 2020;8:191. doi: 10.3389/fcell.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen W, Li S, Liu Q, Yang Y, Wei L, Lu Y. MicroRNA-342-5p protects against myocardial ischemia-reperfusion injury by targeting the GPRC5A pathway. Pharmazie. 2020;75(6):271–274. doi: 10.1691/ph.2020.0383. [DOI] [PubMed] [Google Scholar]
- 174.Li PL, Liu H, Chen GP, Li L, Shi HJ, Nie HY, Liu Z, Hu YF, Yang J, Zhang P, et al. STEAP3 (six-transmembrane epithelial antigen of prostate 3) inhibits pathological cardiac hypertrophy. Hypertension. 2020;76(4):1219–1230. doi: 10.1161/HYPERTENSIONAHA.120.14752. [DOI] [PubMed] [Google Scholar]
- 175.Poloni G, Calore M, Rigato I, Marras E, Minervini G, Mazzotti E, Lorenzon A, Li Mura IEA, Telatin A, Zara I, et al. A targeted next-generation gene panel reveals a novel heterozygous nonsense variant in the TP63 gene in patients with arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16(5):773–780. doi: 10.1016/j.hrthm.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 176.Brandt J, Warnke K, Jörgens S, Arolt V, Beer K, Domschke K, Haverkamp W, Kuhlmann SL, Müller-Nordhorn J, Rieckmann N, et al. Association of FKBP5 genotype with depressive symptoms in patients with coronary heart disease: a prospective study. J Neural Transm (Vienna) 2020;127(12):1651–1662. doi: 10.1007/s00702-020-02243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL; PhysGen Knockout Program. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63(3):559–564. 10.1161/HYPERTENSIONAHA.113.02191 [DOI] [PMC free article] [PubMed]
- 178.Mishra S, Undrovinas NA, Maltsev VA, Reznikov V, Sabbah HN, Undrovinas A. Post-transcriptional silencing of SCN1B and SCN2B genes modulates late sodium current in cardiac myocytes from normal dogs and dogs with chronic heart failure. Am J Physiol Heart Circ Physiol. 2011;301(4):H1596–H1605. doi: 10.1152/ajpheart.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gao J, Guo Y, Chen Y, Zhou J, Liu Y, Su P. Adeno-associated virus 9-mediated RNA interference targeting SOCS3 alleviates diastolic heart failure in rats. Gene. 2019;697:11–18. doi: 10.1016/j.gene.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 180.Wei Y, Zhu M, Corbalán-Campos J, Heyll K, Weber C, Schober A. Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of microRNA-155 on atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):796–803. doi: 10.1161/ATVBAHA.114.304723. [DOI] [PubMed] [Google Scholar]
- 181.Wu N, Jin L, Cai J. Profiling and bioinformatics analyses reveal differential circular RNA expression in hypertensive patients. Clin Exp Hypertens. 2017;39(5):454–459. doi: 10.1080/10641963.2016.1273944. [DOI] [PubMed] [Google Scholar]
- 182.Li Z, Chyr J, Jia Z, Wang L, Hu X, Wu X, Song C. Identification of hub genes associated with hypertension and their interaction with miRNA based on weighted gene coexpression network analysis (WGCNA) analysis. Med Sci Monit. 2020;26:e923514. doi: 10.12659/MSM.923514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang S, Gao Y, Liu G, Li J, Shi K, Du B, Si D, Yang P. The human ATF1 rs11169571 polymorphism increases essential hypertension risk through modifying miRNA binding. FEBS Lett. 2015;589(16):2087–2093. doi: 10.1016/j.febslet.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 184.Sun D, Li C, Liu J, Wang Z, Liu Y, Luo C, Chen Y, Wen S. Expression profile of microRNAs in hypertrophic cardiomyopathy and effects of microRNA-20 in inducing cardiomyocyte hypertrophy through regulating gene MFN2. DNA Cell Biol. 2019;38(8):796–807. doi: 10.1089/dna.2019.4731. [DOI] [PubMed] [Google Scholar]
- 185.Larsen LH, Rose CS, Sparsø T, Overgaard J, Torekov SS, Grarup N, Jensen DP, Albrechtsen A, Andersen G, Ek J, et al. Genetic analysis of the estrogen-related receptor alpha and studies of association with obesity and type 2 diabetes. Int J Obes (Lond) 2007;31(2):365–370. doi: 10.1038/sj.ijo.0803408. [DOI] [PubMed] [Google Scholar]
- 186.Choi JH, Choi SS, Kim ES, Jedrychowski MP, Yang YR, Jang HJ, Suh PG, Banks AS, Gygi SP, Spiegelman BM. Thrap3 docks on phosphoserine 273 of PPARγ and controls diabetic gene programming. Genes Dev. 2014;28(21):2361–2369. doi: 10.1101/gad.249367.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The statistical metrics for key differentially expressed genes (DEGs).
Data Availability Statement
The datasets supporting the conclusions of this article are available in the GEO (Gene Expression Omnibus) (https://www.ncbi.nlm.nih.gov/geo/) repository. [(GSE141910) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE141910].