Abstract
Introduction
Urban environmental exposures might contribute to the incidence of Alzheimer's disease (AD). Our aim was to identify structural brain imaging correlates of urban environmental exposures in cognitively unimpaired individuals at increased risk of AD.
Methods
Two hundred twelve participants with brain scans and residing in Barcelona, Spain, were included. Land use regression models were used to estimate residential exposure to air pollutants. The daily average noise level was obtained from noise maps. Residential green exposure indicators were also generated. A cerebral 3D‐T1 was acquired to obtain information on brain morphology. Voxel‐based morphometry statistical analyses were conducted to determine the areas of the brain in which regional gray matter (GM) and white matter (WM) volumes were associated with environmental exposures.
Results
Exposure to nitrogen dioxide was associated with lower GM volume in the precuneus and greater WM volume in the splenium of the corpus callosum and inferior longitudinal fasciculus. In contrast, exposure to fine particulate matter was associated with greater GM in cerebellum and WM in the splenium of corpus callosum, the superior longitudinal fasciculus, and cingulum cingulate gyrus. Noise was positively associated with WM volume in the body of the corpus callosum. Exposure to greenness was associated with greater GM volume in the middle frontal, precentral, and the temporal pole.
Discussion
In cognitively unimpaired adults with increased risk of AD, exposure to air pollution, noise, and green areas are associated with GM and WM volumes of specific brain areas known to be affected in AD, thus potentially conferring a higher vulnerability to the disease.
Keywords: air pollution, Alzheimer's disease, brain imaging, greenness, noise, prevention, risk factors
1. INTRODUCTION
Individuals living in urban areas are often exposed to high levels of air pollutants and noise, and experience a lack of exposure to natural outdoor environments. 1 Long‐term exposure to air pollution and noise, and the lack of green space, have been associated with premature all‐cause mortality and other detrimental health effects. 2 , 3 , 4 , 5 , 6 , 7 , 8 Additionally, a growing body of evidence suggests that such exposures are environmental risk factors for brain diseases. 9
Air pollution is a well‐known environmental hazard and a major environmental health problem. It has been shown to have adverse health effects through mechanisms such as inflammation and oxidative stress. 10 , 11 Epidemiological studies suggest that exposure to ambient air pollution has neurological effects, and might have a causal influence via harmful effects on the brain, accelerating cognitive aging. 12 , 13 , 14 Likewise, exposure to high noise levels has been associated with adverse impacts on quality of life and health, but only very few epidemiological studies have reported a link between high levels of traffic‐related noise and neurodegenerative disorders. 15 , 16 , 17 , 18 , 19 Contrary to air pollution exposure, an accumulating body of evidence suggests that being exposed to urban green spaces might have health‐promoting effects, including improved mental health and reduced morbidity and mortality. 5 , 7 , 20 , 21 , 22 Exposure to green spaces has been linked to cognitive development in early stages of life, which can have long‐term effects in cognitive function in adults and cognitive decline in the elderly, 23 but the number of studies examining such associations in the adult population is still very limited. 24
Some studies have gone a step beyond the evaluation of the impact of air pollution, noise, and green spaces on neurodevelopment or neurodegenerative disorders, and have evaluated the impact on the brain structure to further understand the potential mechanisms by which urban environmental exposures influence dementia risk, which is especially relevant in individuals at increased risk of such diseases. For instance, exposure to fine particles has been related to brain structural and functional alterations in children, 25 , 26 and most recently, a few studies have shown that exposure to ambient air particles might have a negative impact on the brain of older adults. 27 , 28 , 29 On the contrary, literature on the potential impact of greenspace exposure on the brain is scarce, but suggestive of a potential beneficial effect on brain structure development, especially in children. 22 Notably, there is no research on the potential impact of exposure to noise on brain structure.
In a previous study, 30 we looked at the association between air pollutants with cognitive performance and brain structure in healthy individuals at risk for Alzheimer's disease (AD) dementia. We found that higher exposure to air pollutants was associated with lower cortical thickness in brain regions known to be affected by AD and that increasing greenness indicators was associated with greater thickness in these same areas. In this hypothesis‐driven study, we focused our attention on a single brain region of interest: the so‐called “AD signature,” which is composed of cortical regions known to suffer atrophy in AD dementia. However, these results prompted us to question whether urban exposures might be associated to volumetric changes in other brain regions, including white matter bundles, in a more exploratory and regionally unbiased manner.
The aim of our study was to identify structural brain imaging correlates of urban environmental exposures (including air pollution, noise, and green space) in cognitively unimpaired individuals at increased risk of AD. To this end, we performed a whole brain analysis to detect areas where gray matter (GM) or white matter (WM) volumes were associated with exposure levels.
2. METHODS
2.1. Study participants
We conducted a cross‐sectional analysis in the context of the ALFA (Alzheimer and Families) cohort. The details of the recruitment and participants’ characteristics have been extensively described elsewhere. 31 Briefly, the ALFA study includes a total of 2743 cognitively unimpaired men and women, aged 45 to 74, many of them kindred of AD patients, which have been broadly characterized at multiple levels (sociodemographic, clinic, epidemiologic, genetic, among others). Compared to the general population, a higher frequency of the apolipoprotein E (APOE) ε4 allele has been observed among the ALFA participants (14% vs. 19%, respectively; P < .001), confirming enrichment for AD risk. Exclusion criteria of participants included cognitive performance falling outside the established cutoffs: Mini‐Mental State Examination 32 , 33 (MMSE) < 26; Memory Impairment Screen 34 , 35 (MIS) < 6; Time‐Orientation subtest of the Barcelona Test II 36 (TO‐BTII) < 68; semantic fluency 37 , 38 (animals; SF) < 12; Clinical Dementia Rating scale 39 (CDR) > 0.
RESEARCH IN CONTEXT
Systematic Review: The authors used PubMed to identify previous studies examining urban environmental exposures in association with brain volumes and Alzheimer's disease (AD) risk.
Interpretation: We observe that in cognitively unimpaired adults with increased risk of AD, exposure to air pollution (NO2) is associated with decreased gray matter volumes of specific brain areas known to be affected in AD, like the precuneus, thus potentially conferring a higher vulnerability to the disease. Additional specific cerebral correlates of PM2.5, noise, and green spaces were found.
Future Directions: Evaluating the association between exposure to air pollutants and core biomarkers (in blood or cerebrospinal fluid) of AD to better understand the mechanisms underlying the observed associations.
From the 2743 ALFA participants, there were 658 cognitively unimpaired subjects that had submitted to a magnetic resonance imaging (MRI) scan at the time point of the study. Of those, we had complete information on pollutant exposures of 228 participants, those living in the city of Barcelona at recruitment, having not changed their residence for at least the previous 3 years, and having reliable geocoded data to allow allocating an exposure to them based on the address of residence. Finally, 14 subjects had to be discarded for other reasons: incidental findings, poor image quality, and outlier values of pollutant exposures, which could have biased the linear regressions performed in voxel‐based morphometry (VBM) analyses, defined as values over 3.5 standard deviations from mean value. Therefore, a total of 212 subjects fulfilled all the inclusion criteria and were included in the present analysis. The local Ethics Committee approved the ALFA study, and all subjects and their accompanying close relative signed an informed consent form.
2.2. Exposure assessment
2.2.1. Residential exposure to air pollutants
Air pollution measurements were collected as a part of the air pollution monitoring campaign of the European Study of Cohorts for Air Pollution Effects (ESCAPE; http://www.escapeproject.eu/). 40 , 41 In the city of Barcelona, nitrogen dioxide (NO2) and fine particulate matter (PM2.5 : particulate matter with aerodynamic diameter less than 2.5 μm) were measured in three different seasons (warm, cold, and one intermediate temperature season) to model an annual average for 2009. Following well‐established protocols, 40 , 41 we used a land use regression (LUR) model, based on geographical information system (GIS) and statistical methods, to estimate individual levels of air pollution at the participants’ residential addresses reported in 2013 through 2014 (baseline).
2.2.2. Residential exposure to environmental noise levels
Noise exposure was estimated using Barcelona's municipal strategic noise map in 2012 (Strategic Noise Map of Barcelona 2012–2017) as provided by the city council (one assessment every 5 years under the European Noise Directive). The map was developed with a comprehensive set of standardized noise measurements, according to the Environmental Noise Directive 2002/49/EC (European Commission). The daily average noise level at the street nearest to each participant's residential address was registered. Total noise levels based on Lden index (day‐evening‐night sound average) were estimated.
2.2.3. Residential exposure to green spaces
We assessed exposure to residential green space within a buffer of 300 meters around a participant's residence using as indicator the surrounding greenness. To assess surrounding greenness, we used the normalized difference vegetation index (NDVI). The NDVI is an indicator of greenness and is based on land surface reflectance of visible (red) and near‐infrared parts of the spectrum. 42 The NDVI came from one imagery in the greenest season of the year (April–July), and provides information on the actual greenness captured by satellite, including small shrubss, grass, and so on, independently of the type of land use. Its values range from −1 to 1, with higher positive numbers indicating more greenness (i.e., photosynthetically active vegetation). The index was derived from the Landsat 4‐5 TM data at 30 m × 30 m resolution. The Landsat 5 imagery data atmospherically corrected was acquired for 26/07/2009 covering the Barcelona city area. Exposure to greenness indicator within a 300‐meter buffer was the main variable regarding exposure to green spaces.
Because indices related to noise and green spaces exposure showed skewed distributions, variables were transformed to force their symmetry using Box‐Cox 43 or Wand‐Marron‐Rupert 44 transformations. Additionally, subjects with outlier values (as defined as more than 3.5 standard deviations [SD] from sample's mean value) in any of the indices, were excluded from the corresponding analyses by means of a nulling regressor. 45 In total, one individual was excluded from the analysis regarding exposure to PM2.5, and two subjects were excluded from the analysis of noise and surrounding greenness indicators.
2.3. Brain image data acquisition
MRI was conducted in a 3.0‐T scanner (GE Discovery MR750 W) at baseline. Structural 3D high‐resolution T1‐weighted images were collected using a fast spoiled gradient‐echo sequence implementing the following parameters: voxel size = 1 mm3 isotropic, repetition time = 6.16 ms, echo time = 2.38 ms, inversion time = 450 ms, matrix size = 256 × 256 × 174, and flip angle = 12°.
2.4. Structural image analysis
Structural images were visually inspected to discard movement and other artifacts. We used the standard preprocessing pipeline for VBM as implemented in SPM12. 46 Briefly, T1 images were segmented into tissues; GM and WM maps were normalized to Montreal Neurological Institute by means of the DARTEL algorithm, multiplied by Jacobians determinants to encode volume changes as intensity variations voxel by voxel and smoothed with an 8 mm full width half maximum Gaussian kernel. We used general linear models (GLM) to assess, in a voxel basis, to what extent and where in the brain GM or WM volumes were associated with each particular exposure, correcting for age, sex, years of education, and total intracranial volume (TIV). The threshold for statistical significance was set to P < .001, uncorrected for multiple comparison considering only clusters whose size was above 300 voxels (1 cm3). Sensitivity analyses including the number of APOE ε4 alleles, smoker status (two: ever and current smoker), body mass index (BMI; kg/m2), and physical activity (metabolic equivalents/day) as covariates (separately) were also performed.
3. RESULTS
3.1. Descriptive analysis
Main characteristics of ALFA study participants and of the subset of participants from Barcelona included in the present analysis have been previously presented in detail 30 , 47 , 48 (more details in Molinuevo et al. 31 ). There were no statistically significant differences regarding age, sex, and years of education between the ALFA study cohort and the participants residing in Barcelona included in the present analysis (for further details see Table 1 in Crous‐Bou et al. 30 ). Briefly, mean age of the 212 participants included in the present analysis was 58.6 years (SD = 7.5), 59% were female, and participants had a mean of 14 years of education (SD = 3.3). More than 80% of the participants included in the present study had a family history of AD; additionally, 49.6% of the participants were carriers of the APOE ε4 allele. There were no statistically significant differences regarding age, sex, and years of education between the ALFA study cohort and the participants included in the present analysis (data not shown; presented in Crous‐Bou et al. 30 and Molinuevo et al. 31 ).
TABLE 1.
Correlation coefficients (r) between urban environmental exposures. All correlations were significant at P < .05 and survived Bonferroni's correction (nominal P < .0083 after correcting for multiple comparisons)
| Correlation coefficients (r) between pollutant exposures | ||||
|---|---|---|---|---|
| NO2 | 1 | |||
| PM2.5 | 0.680 | 1 | ||
| NDVI 300 m | –0.532 | –0.257 | 1 | |
| Lden | 0.618 | 0.501 | –0.151 | 1 |
| NO2 | PM2.5 | NDVI 300 m | Lden | |
Abbreviations: Lden index, day‐evening‐night sound average; NDVI, normalized difference vegetation index; NO2, nitrogen dioxide; PM2.5, fine particulate matter.
Figure 1 displays the distribution of urban environmental exposures along the sample. Table 1 shows the correlation coefficient between exposures. We observed a moderate‐to‐high correlation among exposures, indicating they probably share common sources, mainly road traffic. As expected, all correlations were positive except for green space exposure indicators, which were negatively correlated with the rest of the exposures. Table 2 includes the distribution of confounder analyses by pollutant tertiles and the corresponding P value for the one‐way analysis of variance. Covariables used in the VBM analyses, as well as other potential confounders (smoking status, BMI, or physical activity), across pollutant tertiles were included to check for potential unbalanced distributions. Neither age, education, nor physical activity showed significant differences across pollutant tertiles.
FIGURE 1.
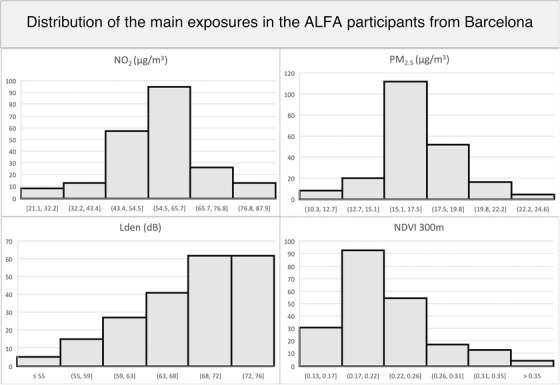
Distribution of pollutants and noise exposures in the sample. ALFA, Alzheimer and Families study; Lden index, day‐evening‐night sound average; NDVI, normalized difference vegetation index; NO2, nitrogen dioxide; PM2.5, fine particulate matter.
TABLE 2.
Distribution of potential confounders by pollutant terciles
| Distribution of potential confounders by pollutant terciles and ANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AGE | TIV | Education | Physical activity | Sex | APOE | Smoker | ||||
| (years) | (cc) | (years) | (mets/day) | BMI | male/total | ε4 carrier | Ever | Current | ||
| NO2 | Tercile 1: | 58.2 (8.0) | 1454.2 (167.7) | 14.0 (3.6) | 2512 (1889) | 26.0 (3.7) | 26 M / 71 | 32 / 71 | 58 / 71 | 21 / 71 |
| Tercile 2: | 59.0 (7.4) | 1493.3 (153.2) | 13.8 (3.2) | 2720 (2351) | 27.1 (3.5) | 30 M / 70 | 40 / 70 | 56 / 70 | 15 / 70 | |
| Tercile 3: | 58.7 (7.2) | 1523.7 (164.2) | 14.5 (3.1) | 2483 (2250) | 27.0 (4.3) | 30 M / 71 | 35 / 71 | 63 / 71 | 23 / 71 | |
| ANOVA | 0.808 | 0.039 * | 0.517 | 0.779 | 0.16 | |||||
| PM2.5 | Tercile 1: | 58.4 (8.1) | 1453.4 (155.7) | 13.9 (3.4) | 2442 (1991) | 26.5 (3.7) | 27 M / 71 | 34 / 71 | 55 / 71 | 18 / 71 |
| Tercile 2: | 59.4 (7.5) | 1505.1 (162.0) | 14.1 (3.4) | 2875 (2184) | 26.4 (3.9) | 32 M / 70 | 38 / 71 | 60 / 70 | 15 / 70 | |
| Tercile 3: | 58.1 (6.9) | 1513.0 (168.7) | 14.3 (3.2) | 2401 (2304) | 27.3 (3.9) | 27 M / 71 | 35 / 70 | 62 / 71 | 26 / 71 | |
| ANOVA | 0.554 | 0.062 | 0.723 | 0.357 | 0.322 | |||||
| Lden | Tercile 1: | 57.9 (7.3) | 1497.0 (164.1) | 14.1 (3.3) | 2735 (2389) | 27.0 (3.8) | 34 M / 71 | 39 / 71 | 57 / 71 | 17 / 71 |
| Tercile 2: | 58.1 (8.0) | 1448.8 (144.1) | 14.2 (3.5) | 2417 (1843) | 25.9 (3.5) | 21 M / 70 | 33 / 70 | 59 / 70 | 21 / 70 | |
| Tercile 3: | 59.9 (7.3) | 1526.0 (174.3) | 14.0 (3.3) | 2561 (2242) | 27.4 (4.1) | 31 M / 71 | 35 / 71 | 61 / 71 | 21 / 71 | |
| ANOVA | 0.238 | 0.017 * | 0.981 | 0.682 | 0.045 * | |||||
| NDVI 300 m | Tercile 1: | 58.9 (7.5) | 1498.2 (168.6) | 14.1 (3.1) | 2504 (2237) | 27.2 (4.2) | 30 M / 71 | 34 / 71 | 60 / 71 | 23 / 71 |
| Tercile 2: | 59.2 (7.2) | 1495.0 (159.1) | 14.1 (3.3) | 2650 (2057) | 26.5 (3.6) | 26 M / 71 | 38 / 71 | 58 / 71 | 16 / 71 | |
| Tercile 3: | 57.7 (7.9) | 1478.2 (164.6) | 14.2 (3.7) | 2561 (2219) | 26.6 (3.8) | 30 M / 70 | 35 / 70 | 59 / 70 | 20 / 70 | |
| ANOVA | 0.48 | 0.739 | 0.977 | 0.923 | 0.511 | |||||
Abbreviations: ANOVA, analysis of variance; APOE, apolipoprotein E; BMI, body mass index; Lden index, day‐evening‐night sound average; NDVI, normalized difference vegetation index; NO2, nitrogen dioxide; PM2.5, fine particulate matter; TIV, total intracranial volume.
Notes: Continuous values displayed as mean (standard deviation). Discrete values in number of cases/total. TIV in cubic centimeters. Physical activity in mets (metabolic equivalents). ANOVA rows: P‐value of one‐way ANOVA of variables across pollutant terciles. (*) for significant ANOVAs at P < 0.05 uncorrected by multiple comparisons. Only TIV for NO2 and Lden and BMI for Lden showed significant differences across pollutant terciles. Those differences were disregarded (refer to main text for the rationale). Limit values of central tercile: NO2 = [52.8,60.6]; PM2.5 = [16.2,17.5]; Lden = [66,70] and NDVI = [0.19,0.22].
3.2. Brain structural changes related to urban environmental exposures
The results of the association between exposure to air pollutants, noise, and surrounding greenness with GM and WM volumes are presented in Figure 2 and Table 3.
FIGURE 2.
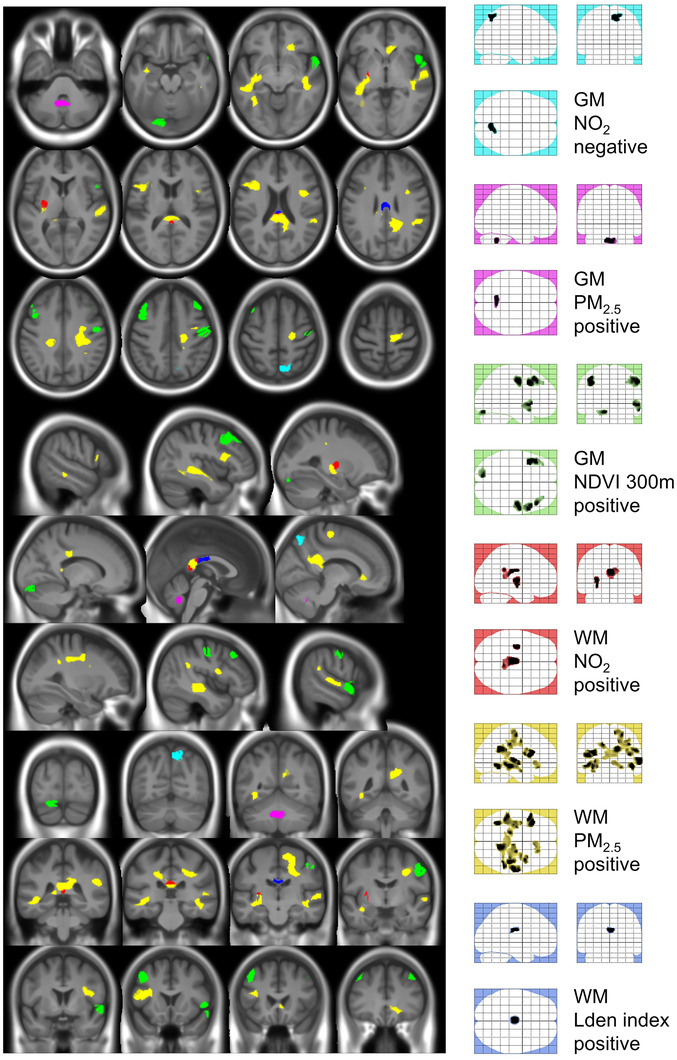
Impact of exposure to air pollutants, noise, and proximity to green areas on gray matter and white matter volume. Colored glass brains in the right column offer a global view of each contribution and act as a legend for color codes: cyan for negative association between NO2 and GM, magenta for positive association between PM2.5 and GM, green for positive association between NDVI 300 m and GM, red for positive association between NO2 and WM, yellow for positive association between PM2.5 and WM, and blue for positive association between Lden index and WM. Refer to Table 3 for detailed description of the clusters. Cluster colors correspond to glass‐brain legend colors and color code in Table 2 for an easier identification. Positive and negative indicate the sign of the association. GM, gray matter; Lden index, day‐evening‐night sound average; NDVI, normalized difference vegetation index; NO2, nitrogen dioxide; PM2.5, fine particulate matter; WM, white matter.
TABLE 3.
Results of the VBM analysis (cluster information and statistical significance) on the correlation between exposure to nitrogen oxides and GM volume (A), and WM volume (B)
| A) GRAY MATTER | |||||||
|---|---|---|---|---|---|---|---|
| cluster size | % cluster | aal label | x | y | x | T max | |
| NO2 | |||||||
| (negative | 484 | 61,6 | Precuneus R | 12 | ‐66 | 54 | 3,80 |
| association) | 38,4 | Parietal Sup R | |||||
| cluster size | % cluster | aal label | x | y | x | T max | |
| PM2.5 | |||||||
| (positive | 417 | 47,0 | Vermis 9 | ‐5 | ‐56 | ‐36 | 3,44 |
| association) | 31,7 | Cerebelum 9 L | |||||
| 10,1 | Cerebelum 9 R | ||||||
| cluster size | % cluster | AAL label | x | y | x | T max | |
| NDVI 300 m | |||||||
| (positive | 441 | 81,0 | Frontal Mid L | ‐39 | 11 | 41 | 4,55 |
| association) | 19,0 | Precentral L | |||||
| 730 | 52,3 | Temporal Pole Sup R | 57 | 11 | ‐5 | 4,13 | |
| 14,4 | Frontal Inf Oper R | ||||||
| 10,4 | Temporal Sup R | ||||||
| 10,3 | Insula R | ||||||
| 528 | 87,3 | Precentral R | 56 | ‐8 | 45 | 3,95 | |
| B) WHITE MATTER | |||||||
|---|---|---|---|---|---|---|---|
| cluster size | % cluster | JHU & AAL label | x | y | x | T max | |
| NO2 | |||||||
| (positive | 892 | 91,7 | Splenium CC | 0 | ‐21 | 23 | 3,82 |
| association) | 306 | 62,4 | Putamen L | ‐29 | ‐14 | 0 | 3,79 |
| 26,8 | Inf Long Fasc L | ||||||
| cluster size | % cluster | JHU & AAL label | x | y | x | T max | |
| PM2.5 | |||||||
| (positive | 881 | 53,0 | Frontal Inf Oper L | ‐38 | 18 | 21 | 4,29 |
| association) | 27,7 | Sup Long Fasc L | |||||
| 14,5 | Frontal Inf Tri L | ||||||
| 1187 | 52,6 | Temporal Sup R | 62 | ‐18 | 6 | 4,24 | |
| 42,9 | Inf Long Fasc R | ||||||
| 2131 | 69 | Cing Cingulate Gyrus R | 14 | ‐48 | 27 | 4,10 | |
| 11,0 | Cingulum Post R | ||||||
| 1652 | 82,7 | Inf Long Fasc L | ‐38 | ‐57 | ‐6 | 3,78 | |
| 1954 | 77,3 | Sup Long Fasc R | 38 | ‐27 | 38 | 3,67 | |
| 350 | 76,3 | Sup Long Fasc R | 39 | 3 | 17 | 3,64 | |
| 11,7 | Frontal Inf Oper R | ||||||
| 347 | 99,4 | Cing Cingulate Gyrus L | ‐17 | ‐29 | 36 | 3,61 | |
| 412 | 77,2 | Forceps Minor | 23 | 30 | ‐11 | 3,45 | |
| 10,2 | Olfactory R | ||||||
| cluster size | % cluster | JHU & AAL label | x | y | x | T max | |
| Lden index | |||||||
| (positive association) | 324 | 100 | Body CC | 0 | ‐18 | 23 | 4,44 |
Notes: Statistical threshold was set to P < .001 (uncorrected) with a minimum extension cluster of 300 voxels (1 cm3). “%cluster” refers to the percentage of the cluster extension that is into the anatomical region determined by the atlas label. Residual contributions (below 10%) are not listed, because they were assumed to be spreading effects due to the smoothing procedure. WM labels in italics correspond to juxta‐cortical voxels of WM, which have been labelled according to the closest GM AAL area. Colors of labels correspond to cluster color in Figure 2 for an easier identification.
Abbreviations: AAL: Anatomical Automatic Labelling (GM); CC: Corpus callosum; GM, gray matter; JHU: Johns Hopkins University WM atlas; Lden index, day‐evening‐night sound average; NDVI, normalized difference vegetation index; WM, white matter.
3.2.1. Air pollutants
Exposure to increasing concentrations of NO2 was associated with lower GM volume in the precuneus but with higher WM volume in the splenium of the corpus callosum, left inferior longitudinal fasciculi, and putamen (Table 3; Figure 2). Increasing exposure to PM2.5 was associated with greater GM volume in cerebellum. Additionally, increasing levels of PM2.5 was associated with greater WM volume in extensive areas of the ventral segment of the cingulum bundle. Moreover, a positive association between levels of PM2.5 and WM volume was found in the anterior segment of the corpus callosum, the superior longitudinal fasciculus, and the WM adjacent to the anterior bilateral insula and the olfactory cortex (Table 3; Figure 2).
3.2.2. Noise levels
We observed that exposure to increasing noise levels was associated with greater WM volume in the body of the corpus callosum at the level of auditory cortex (Table 3; Figure 2).
3.2.3. Exposure to green spaces
Increasing greenness index was associated with greater GM volume in the left middle frontal, precentral cortices, as well as the temporal pole (Table 3; Figure 2).
All analyses were repeated including separately the number of APOE ε4 alleles, the smoking status, BMI, and physical activity as covariates, with similar results (results not shown).
4. DISCUSSION
In a population of cognitively unimpaired adults at increased risk of AD, exposure to air pollution was associated with reductions in GM volume and increments in WM volume of specific brain areas known to be affected in AD. 49 , 50 This result suggests that increasing air pollution levels might confer a higher vulnerability to the neurodegenerative effects of AD pathology. Specifically, exposure to increasing levels of NO2 were associated with reduced GM volume in the precuneus, while exposures to increasing levels of PM2.5 were associated with greater WM in a great part of the splenium of corpus callosum, cingulum, and the superior longitudinal fasciculi. Exposure to increasing noise levels was associated with greater volume of the corpus callosum. Finally, being exposed to increasing surrounding greenness was associated with increased GM volume in the left middle frontal, precentral cortex, as well as in the right temporal pole and surrounding areas.
4.1. Brain correlates of air pollution
Epidemiological studies suggest that exposure to ambient air pollution might have harmful effects on the brain and its structure. 12 , 13 , 14 Increased exposure to fine particles has been associated with brain structural and functional alterations in children, specifically associated with reduced cerebral cortex thickness in the precuneus and rostral middle frontal regions. 25 , 26 Additionally, few studies have shown that exposure to ambient air particles might have a direct impact on the brain of older adults and consequently, are potentially harmful for the brain. 27 , 28 , 29 In the recent review study by de Prado Bert et al., 27 the authors summarized the main findings from environmental epidemiology studies and neuroimaging. The selected studies revealed that both GM and WM might be targets of exposure to air pollutants. Specifically, VBM analysis in elderly populations showed a link between exposure to PM2.5 and decreased GM volumes, primarily in the dorsolateral and medial prefrontal cortex, which also show reduction in volumes with aging. Additionally, higher exposures to fine particles was associated with reduction of WM volumes in frontal, parietal, and temporal areas. However, such associations were observed in older populations (mean age over 70 years). 27 , 28 The association between greater exposure to particulate matter and lower GM volume was also presented in the recent pooled analysis by Power et al. 29 However, no associations with other brain volume measures or markers cerebrovascular and small vessel disease were observed. 29
We found, in cognitively unimpaired individuals at higher risk of developing AD, an association between increasing levels of air pollutants and lower GM volume, primarily in the precuneus, and higher WM volume, mainly in the corpus callosum. These associations were observed for all air pollutants, with particulate matter showing the most significant effects. The precuneus, along with the posterior cingulate cortex, is a major hub of the brain, which is strongly interconnected with the hippocampal formation. It is involved in a variety of complex functions including reflective, self‐related processing, awareness and conscious information processing, episodic memory, and visuospatial processing. 51 It also shows greater activity during resting compared to responding to an external task and constitutes a central node of the so called “resting state network.” Relevant to AD, the precuneus and cortical regions of the default mode network are particularly vulnerable to early amyloid deposition, 52 which is associated with aberrant brain functioning even in cognitively unimpaired individuals. 53 , 54 At early stages of AD progression, prominent atrophy and metabolic abnormalities emerge in the precuneus and associated posterior cortical and medial temporal regions related to symptom severity. 55 We also observed a positive association between NDVI and GM volume in areas related to the default mode network, such as the temporal pole and the temporal superior gyrus. Interestingly, increased GM volume with higher levels of PM2.5 was also in the bilateral lobule IX of the cerebellum. While the cerebellum has been classically thought to be devoted exclusively to motor control, recent evidence has shown that it appears an integral part of brain networks subserving cognition and emotion. 56 In particular, the cerebellar lobule IX also participates in the default mode network. 57 Taken together, this pattern suggests an impact of air pollutants in areas of the default mode network. Rather than neuronal loss, lower GM volume in cognitively unimpaired subjects may reflect compensatory mechanisms associated to shrinkage of neurons, reductions of synaptic spines, and dendritic arborization, and lower numbers of synapses. 58 Even in the absence of neuronal death, lower GM volume is considered to be a marker of lower resilience for the brain to cope with the deleterious effects of aging or disease. 59 Such a finding in a group of cognitively unimpaired individuals suggests that subjects exposed to greater levels of pollutants might present lower brain resilience to the effects of AD pathology in areas showing early alterations in AD.
On the other hand, the prominent positive association between pollutant levels and WM volume has a less straightforward interpretation. Both increments and decrements in WM have been observed in association with air pollutants. 27 Particulate matter has been reported to enter the central nervous system through olfactory nerves or across the blood‐brain barrier (BBB) and trigger neuroinflammatory processes. 60 , 61 , 62 , 63 , 64 Potentially associated with the olfactory system, we observed a positive association between levels of PM2.5 and WM volume in the olfactory cortex. With respect to the BBB, it has been shown that, even when intact, blood‐borne substances can be transferred to some brain areas, including the hippocampus, the insula, and the corpus callosum, presumably through leaky vessels in the subfornical organs or the choroid plexus. 65 The corpus callosum is the largest fiber tract in the brain, connecting the two cerebral hemispheres, and thereby facilitating the integration of motor and sensory information from the two sides of the body as well as influencing higher cognition associated with executive function, social interaction, and language. 66 Although structural alteration in AD mainly involves the cerebral GM, changes in the corpus callosum have been reported already in the early stages of AD, which likely contribute to the clinical presentation in AD. 67 Relevant to this, with higher levels of air pollutants we also observed increased WM volume in the splenium of the corpus callosum, the ventral segment of the cingulum bundle that connects the hippocampal formation with the posterior cingulate cortex and the WM adjacent to the anterior bilateral insula. Once into the central nervous system, air pollutants might trigger inflammatory mechanisms, exacerbate the innate immune response to AD pathology, 68 or even accelerate the buildup of amyloid beta (Aβ) deposits in the brain. 13 , 69 , 70 , 71 , 72
Therefore, it could be hypothesized that a neuroinflammatory response may underlie the observed greater WM volume with increasing pollutant levels. The presence of foreign particles in these regions would activate the brain innate immune system and trigger a transient inflammatory response including subclinical brain swelling and/or edema, resulting in a net increase in volume of the affected WM tracts that interconnect cortical and cerebellar regions of the default mode network. Such a chronic neuroinflammatory response in WM might impair the connectivity of distal cerebral regions and eventually lead to a compensatory mechanism involving changes in GM volume. Given the relatively high correlation among some of the exposures (particularly PM2.5, NO2 and, negatively, NDVI), it would be challenging to attribute effects to individual pollutants. The fact that associations were found to be more significant with some pollutants might be due to significance threshold effects. Taken together, our hypothesis is that the presence of pollutants in the brain triggers neuroinflammatory and compensatory mechanisms in the brain that could explain the observed effects, in both directions. These effects may be particularly prominent in a sample of middle‐ to late‐age individuals enriched for risk factors of AD as in this work. Even though not directly measured here, this sample is expected to harbor a significantly higher prevalence of AD pathology compared to the general population of the same age. However, other mechanisms linked to air pollutants cannot be discarded, such as oxidative stress, which can lead to the production of aberrant proteins, oxidative byproducts, and DNA oxidation that can alter epigenetic and genetic regulation. 72 Further studies are needed to assess whether increased levels of air pollutants are directly associated to higher levels of AD biomarkers.
4.2. Brain correlates of noise levels
Even though few epidemiological studies have reported a link between increasing levels of traffic‐related noise and neurodegenerative disorders, 15 , 16 , 17 , 18 , 19 to the best of our knowledge, our study is the first to evaluate the potential impact of exposure to noise on brain structure. Due to the Lden index determined at participants’ residential address, it could be hypothesized that the incidence of noise in brain structure may be related to a poorer quality night's rest. In a recently published review, Huang et al. 73 highlight that the underlying mechanisms in the association between exposure to noise and dementia‐related outcomes is still unclear; however, they point toward several related pathways such as neuroinflammation, excitotoxicity, psychological stress, and oxidative stress injury in the auditory cortex and hippocampus. They suggest that these changes could further lead to a reduction in neurogenesis, necrosis or apoptosis, Aβ deposition, and tau hyperphosphorylation, and eventually may result in degenerative dementia. 73
Similar to our results on air pollution, we observed that increasing exposure to noise levels was associated with increased WM volume in the body of the corpus callosum. Moreover, these areas overlap with some of the results shown in relation to exposure to air pollutants. Noise levels in urban areas mainly come from traffic; consequently, it is difficult to disentangle if this is a specific effect of noise or if this is due to the effect of air pollutants.
4.3. Brain correlates of exposure to green space
Contrary to air pollution exposure, an accumulating body of evidence suggests that being exposed to natural environments, including urban green spaces, might have health‐promoting effects. 5 , 20 , 21 , 22 Literature on the potential impact of greenspace exposure on the brain is scarce, but suggestive of a potential beneficial effect on brain development, particularly in children. 22
Our results show that increasing greenness is associated with greater GM volume in the dorsolateral frontal cortex as well as in the temporal pole, two brain regions with a prominent involvement in memory also known to be affected by AD pathophysiology. 49 Additionally, lower greenness being associated with increased WM volume could be interpreted in the context of the inverse correlation between greenness indicators and air pollutants.
4.4. Limitations of the present study
Our study is not free of limitations, being the first one in its cross‐sectional design. The inclusion criteria of the study led to a relatively small number of participants, which might limit the statistical power to detect potential effects of urban environmental exposures on the brain. However, this is a very thoroughly characterized population, which provides detailed information on the evaluated outcomes, the exposures, and also clinical and epidemiological co‐variables. The study sample originated in a relatively restricted geographical area; however, exposure levels showed a wide range of values, and the distribution of exposures was not uniform, making the statistical weight of extreme values in correlation analyses higher.
Exposure to air pollution was estimated based on participants’ residential address. Unfortunately, we did not have access to participants’ daily mobility and workplace exposure, which may lead to exposure misclassification, and thereby, we might be underestimating the association between environmental exposures and the outcomes assessed. 74 , 75 It is worth mentioning that several studies have shown that adding daily mobility does not improve the risk estimates, and that the spatial distribution of air pollution in Barcelona has been reasonably consistent over the past 20 years, with the exception of some temporal variation with pollutant levels that was seen before and after the global economic crisis in 2009. Because the temporal variation affected every area/subject equally, we use the spatial distribution to differentiate exposure to air pollution among participants. Therefore, the timing of the assessment is not so critical. Total noise levels in our population essentially came from traffic, so we could not distinguish the effects of traffic noise from the effect of other sources of noise. Additionally, because we measured traffic‐related noise, areas with high levels of noise also may have high levels of air pollutants. Furthermore, in the present study we could not distinguish the effects of different green space types because urban green space had a significant weight in the total amount of green space exposure. Besides, surrounding greenness, based on the NDVI, does not distinguish among types of green space. Finally, the sample here studied is highly enriched for AD risk factors such as family history of AD and APOE ε4 carriership. This, in combination with their age range, strongly suggests that a significant percentage will harbor AD pathology, which can precede clinical symptoms by decades. Thus, our results are not representative of the general population but, on the other hand, it may also have improved our capacity to detect the cerebral structural effects of urban ambient exposures with underlying AD pathology. However, the lack of AD biomarkers in this sample prevents us from being able to draw conclusions on the effects of pollutants in association with AD pathology.
Even though we adjusted the analysis by potential confounders, other variables of interest could not be included due to unavailability. This could be of relevance for socioeconomic status, for example. Unfortunately, this information was not collected for participants at recruitment. However, we are including level of education in all our analyses, which highly correlates with socioeconomic status, and consequently might account for some of the confounding related to socioeconomic status. Additionally, it has been previously reported that neighborhood socioeconomic position is not necessarily associated with higher exposure to pollutants in the city of Barcelona. 76 Even though we do have available information on other variables related to sociodemographic and lifestyle characteristics of our participants (detailed in Table 1 of Crous‐Bou et al. 30 ), we did not consider including them in the present analysis because those are not usual confounders in neuroimaging studies. However, we have performed additional analyses including covariables such as physical activity and smoking, and our main results remained essentially the same.
It is worth mentioning that the exposures are correlated. Thus, the results we present here might be an overlap among different exposures and might limit our ability to distinguish which is the exposure driving the main effects.
Despite these limitations, for the first time we were able to evaluate different urban‐related exposures within the same study population in relation to brain structure. The choice of a voxel‐based statistical approach implied to perform prospective analyses on topographic effects in the brain due to the assessment of different pollutants. That allowed us to find relevant associations in previously unreported brain regions, which would have been unseen if a predefined region of interest approach had been used instead. The counterpart of this choice was the need to use a slightly more lenient statistical threshold that increased the risk of false positives due to multiple comparisons. Otherwise, a highly restrictive statistical threshold to prevent false positives performing as much analyses as independent measures in the brain, would have masked most of the positive associations. Still, we have used standard thresholds for statistical significance for this kind of analysis.
Finally, it is crucial to perform studies on the impact of long‐term exposure to environmental elements, as well as longitudinal studies, to properly evaluate brain correlates of urban environmental exposures, especially when including middle‐age, cognitively unimpaired individuals (i.e., cognitive decline has not occurred yet).
Our results show that urban environmental exposures might have a direct impact on the volume of specific brain areas potentially related to AD. 49 However, no differences in cognition were observed in our population, 30 suggesting that the increased risk of cognitive decline has not happened yet. In this study, we sought effects of urban environmental exposures across the whole brain in a more exploratory manner that complements our previous hypothesis‐driven results. The fact that participants of our study are cognitively unimpaired suggests that environmental exposures might have an impact very early in the disease continuum by increasing vulnerability in cognitively unimpaired subjects. The alterations observed in brain structure and diffusivity in relation to urban environmental exposures might contribute to cognitive decline in the future, which makes it necessary to follow‐up these individuals to confirm such a hypothesis.
In conclusion, in a population of cognitively unimpaired adults with increased risk of AD, greater exposure to air pollution and noise is associated with lower GM volumes of specific brain areas vulnerable in aging and AD. These results suggest that exposure to such pollutants might lead to a lower brain resilience to the effects of AD pathology. On the other hand, higher exposure to these pollutants was associated with greater WM volume, which is compatible with the presence of neuroinflammatory effects. Further research is needed to confirm the mechanisms involved in such associations.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was conducted in accordance with the directives of the Spanish Law 14/2007, of 3rd of July, on Biomedical Research (Ley 14/2007 de Investigación Biomédica). It was approved by the IRB Parc de Salut Mar, Barcelona. All participants accepted the study procedures by signing an informed consent form. Clinicaltrials.gov Identifier: NCT01835717
AVAILABILITY OF DATA AND MATERIAL
The data that support the findings of this study are available on upon reasonable request from the corresponding author, JDG.
CONFLICTS OF INTEREST
JLM is currently a full‐time employee of Lundbeck and prior to that served as a consultant or on advisory boards for the following for‐profit companies, or has given lectures in symposia sponsored by the following for‐profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, ProMIS Neurosciences.
ACKNOWLEDGMENTS
This publication is part of the ALFA study (ALzheimer and FAmilies). The authors would like to express their most sincere gratitude to the ALFA project participants, without whom this research would have not been possible.
Collaborators of the ALFA study are: Annabella Beteta, Anna Brugulat‐Serrat, Raffaele Cacciaglia, Noemí Carranza, Carme Deulofeu, Ruth Dominguez, Chema González de Echávarri, Oriol Grau‐Rivera, Laura Hernandez, Gema Huesa, Jordi Huguet, Iva Knezevic, María León, Paula Marne, Tania Menchón, Marta Milà‐Alomà, Carolina Minguillon, Maria Pascual, Albina Polo, Sandra Pradas, Aleix Sala‐Vila, Gemma Salvadó, Gonzalo Sánchez‐Benavides, Mahnaz Shekari, Anna Soteras, Marc Suárez‐Calvet, Laia Tenas, Natàlia Vilor‐Tejedor, Marc Vilanova. The research leading to these results has received funding from “la Caixa” Foundation (ID 100010434) under agreement LCF/PR/GN17/10300004. Additional funding is obtained from the Universities and Research Secretariat, Ministry of Business and Knowledge of the Catalan Government under the grant no. 2017‐SGR‐892. MG holds a Miguel Servet fellowship (Grants CP19/00183) funded by Acción Estratégica de Salud–Instituto de Salud Carlos III, Spain. Juan D. Gispert and Eider M. Arenaza‐Urquijo hold a ‘Ramón y Cajal’ fellowship from the Spanish Ministry of Science, Innovation and Universities (RYC‐2013‐ 13054 and RYC2018‐026053‐I, respectively).
Falcón C, Gascon M, Molinuevo JL, et al. Brain correlates of urban environmental exposures in cognitively unimpaired individuals at increased risk for Alzheimer's disease: A study on Barcelona's population. Alzheimer's Dement. 2021;13:e12205. 10.1002/dad2.12205
Contributor Information
Juan Domingo Gispert, Email: jdgispert@barcelonabeta.org.
Marta Crous‐Bou, Email: mcrous@barcelonabeta.org.
REFERENCES
- 1. Nieuwenhuijsen MJ. Urban and transport planning, environmental exposures and health‐new concepts, methods and tools to improve health in cities. Environ Health. 2016;15(Suppl 1):38. 10.1186/s12940-016-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang M, Beelen R, Stafoggia M, et al. Long‐term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: results from the ESCAPE and TRANSPHORM projects. Environ Int. 2014;66:97‐106. 10.1016/j.envint.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 3. Hoek G, Krishnan RM, Beelen R, et al. Long‐term air pollution exposure and cardio‐ respiratory mortality: a review. Environ Heal. 2013;12:43. 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halonen JI, Hansell AL, Gulliver J, et al. Road traffic noise is associated with increased cardiovascular morbidity and mortality and all‐cause mortality in London. Eur Heart J. 2015;36:2653‐2661. 10.1093/eurheartj/ehv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gascon M, Triguero‐Mas M, Martínez D, et al. Residential green spaces and mortality: a systematic review. Environ Int. 2016;86:60‐67. 10.1016/j.envint.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 6. Nieuwenhuijsen M, Gascon M, Martinez D, et al. Air Pollution, Noise, Blue Space, and Green Space and Premature Mortality in Barcelona: a Mega Cohort. Int J Environ Res Public Health. 2018;15:2405. 10.3390/ijerph15112405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rojas‐Rueda D, Nieuwenhuijsen MJ, Gascon M, et al. Green spaces and mortality: a systematic review and meta‐analysis of cohort studies. Lancet Planet Health. 2019;3(11):e469‐e477. 10.1016/S2542-5196(19)30215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fong KC, Hart JE, James P. A review of epidemiologic studies on greenness and health: updated literature through. Curr Environ Heal Reports. 2017;5:77‐87. 10.1007/s40572-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972‐984. 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holgate ST. “Every breath we take: the lifelong impact of air pollution” ‐ a call for action. Clin Med. 2017;17:8‐12. 10.7861/clinmedicine.17-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Møller P, Jacobsen NR, Folkmann JK, et al. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010;44:1‐46. 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- 12. Maher BA, Ahmed IAM, Karloukovski V, et al. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci. 2016;113:10797‐10801. 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calderón‐Garcidueñas L, Solt AC, Henríquez‐Roldán C, et al. Long‐term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood‐Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid β‐42 and α‐Synuclein in Children and Young Adult. Toxicol Pathol. 2008;36:289‐310. 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 14. Campbell A, Oldham M, Becaria A, et al. Particulate Matter in Polluted Air May Increase Biomarkers of Inflammation in Mouse Brain. Neurotoxicology. 2005;26:133‐140. 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 15. Oudin A, Forsberg B, Adolfsson AN, et al. Traffic‐Related Air Pollution and Dementia Incidence in Northern Sweden: a Longitudinal Study. Environ Health Perspect. 2016;124:306‐312. 10.1289/ehp.1408322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tzivian L, Dlugaj M, Winkler A, et al. Long‐Term Air Pollution and Traffic Noise Exposures and Mild Cognitive Impairment in Older Adults: a Cross‐Sectional Analysis of the Heinz Nixdorf Recall Study. Environ Health Perspect. 2016;124:1361‐1368. 10.1289/ehp.1509824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A, Schwartz J. Traffic‐Related Air Pollution and Cognitive Function in a Cohort of Older Men. Environ Health Perspect. 2011;119:682‐687. 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilker EH, Preis SR, Beiser AS, et al. Long‐term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015;46:1161‐1166. 10.1161/STROKEAHA.114.008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wellenius GA, Boyle LD, Coull BA, et al. Residential Proximity to Nearest Major Roadway and Cognitive Function in Community‐Dwelling Seniors: results from the MOBILIZE Boston Study. J Am Geriatr Soc. 2012;60:2075‐2080. 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee ACK, Maheswaran R. The health benefits of urban green spaces: a review of the evidence. J Public Health (Bangkok). 2011;33:212‐222. 10.1093/pubmed/fdq068. [DOI] [PubMed] [Google Scholar]
- 21. Gascon M, Triguero‐Mas M, Martínez D, et al. Mental Health Benefits of Long‐Term Exposure to Residential Green and Blue Spaces: a Systematic Review. Int J Environ Res Public Health. 2015;12:4354‐4379. 10.3390/ijerph120404354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dadvand P, Pujol J, Macià D, et al. The Association between Lifelong Greenspace Exposure and 3‐Dimensional Brain Magnetic Resonance Imaging in Barcelona Schoolchildren. Environ Health Perspect. 2018;126:027012. 10.1289/EHP1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Preuß M, Nieuwenhuijsen M, Marquez S, et al. Low Childhood Nature Exposure is Associated with Worse Mental Health in Adulthood. Int J Environ Res Public Health. 2019;16:1809. 10.3390/ijerph16101809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keijzer CDe, Gascon M, Nieuwenhuijsen MJ. Long‐Term Green Space Exposure and Cognition Across the Life Course : a Systematic Review. Curr Environ Health Rep. 2016;3(4):468‐477. 10.1007/s40572-016-0116-x. [DOI] [PubMed] [Google Scholar]
- 25. Guxens M, Lubczyńska MJ, Muetzel RL, et al. Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School‐Age Children. Biol Psychiatry. 2018;84:295‐303. 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 26. Pujol J, Martínez‐Vilavella G, Macià D, et al. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage. 2016;129:175‐184. 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 27.de Prado Bert P, Mercader EMH, Pujol J, Sunyer J, Mortamais M. The Effects of Air Pollution on the Brain: a Review of Studies Interfacing Environmental Epidemiology and Neuroimaging. Curr Environ Heal Reports. 2018;5:351. 10.1007/S40572-018-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casanova R, Wang X, Reyes J, et al. A Voxel‐Based Morphometry Study Reveals Local Brain Structural Alterations Associated with Ambient Fine Particles in Older Women. Front Hum Neurosci. 2016;10:495. 10.3389/fnhum.2016.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Power MC, Lamichhane AP, Liao D, et al. The Association of Long‐Term Exposure to Particulate Matter Air Pollution with Brain MRI Findings: the ARIC Study. Environ Health Perspect. 2018;126:027009. 10.1289/EHP2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crous‐Bou M, Gascon M, Gispert JD, et al. Impact of urban environmental exposures on cognitive performance and brain structure of healthy individuals at risk for Alzheimer's dementia. Environ Int. 2020;138:105546. 10.1016/j.envint.2020.105546. [DOI] [PubMed] [Google Scholar]
- 31. Molinuevo JL, Gramunt N, Gispert JD, et al. The ALFA project: a research platform to identify early pathophysiological features of Alzheimer's disease. Alzheimer's. Dement Transl Res Clin Interv. 2016;2:82‐92. 10.1016/j.trci.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33. Blesa R, Pujol M, Aguilar M, et al. Clinical validity of the “mini‐mental state” for Spanish speaking communities. Neuropsychologia. 2001;39:1150‐1157. 10.1016/s0028-3932(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 34. Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the Memory Impairment Screen. Neurology. 1999;52:231 LP ‐ 231. 10.1212/WNL.52.2.231. [DOI] [PubMed] [Google Scholar]
- 35. Böhm P, Peña‐Casanova J, Gramunt N, Manero RM, Terrón C, Quiñones‐Ubeda S. Spanish version of the Memory Impairment Screen (MIS): normative data and discriminant validity. Neurologia. 2005;20:402‐411. [PubMed] [Google Scholar]
- 36. Quiñones Ubeda S, Desenvolupament, normalització i validació de la versió estàndard de la segona versió del Test Barcelona. 2009.
- 37. Ramier A, Hecaen H. Rôle respectif des atteintes frontales et de la latéralisation lésionnelle dans les déficits de la fluence verbale. Rev Neurol (Paris). 1970;123:17‐22. [PubMed] [Google Scholar]
- 38. Peña‐Casanova J, Quiñones‐Ubeda S, Gramunt‐Fombuena N, et al. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for verbal fluency tests. Arch Clin Neuropsychol. 2009;24:395‐411. 10.1093/arclin/acp042. [DOI] [PubMed] [Google Scholar]
- 39. Morris J. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43. 10.1212/WNL.43.11.2412-A. [DOI] [PubMed] [Google Scholar]
- 40. Beelen R, Hoek G, Vienneau D, et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe – The ESCAPE project. Atmos Environ. 2013;72:10‐23. 10.1016/J.ATMOSENV.2013.02.037. [DOI] [Google Scholar]
- 41. Eeftens M, Beelen R, de Hoogh K, et al. Development of Land Use Regression Models for PM 2.5, PM 2.5 Absorbance, PM 10 and PM coarse in 20 European Study Areas; Results of the ESCAPE Project. Environ Sci Technol. 2012;46:11195‐11205. 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- 42. Weier J, Herring D, NASA Earth Observatory: Measuring vegetation (NDVI & EVI). 2000.
- 43. Box GEP, Cox DR, An Analysis of Transformations. vol. 26. 1964. [Google Scholar]
- 44. Wand MP, Marron JS, Ruppert D. Transformations in density estimation. J Am Stat Assoc. 1991;86:343‐353. 10.1080/01621459.1991.10475041. [DOI] [Google Scholar]
- 45. Lemieux L, Salek‐Haddadi A, Lund TE, Laufs H, Carmichael D. Modelling large motion events in fMRI studies of patients with epilepsy. Magn Reson Imaging. 2007;25:894‐901. 10.1016/j.mri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 46. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95‐113. 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 47. Vert C, Sánchez‐Benavides G, Martínez D, et al. Effect of long‐term exposure to air pollution on anxiety and depression in adults: a cross‐sectional study. Int J Hyg Environ Health. 2017;220:1074‐1080. 10.1016/j.ijheh.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 48. Gascon M, Sánchez‐Benavides G, Dadvand P, et al. Long‐term exposure to residential green and blue spaces and anxiety and depression in adults: a cross‐sectional study. Environ Res. 2018;162:231‐239. 10.1016/j.envres.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 49. Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid‐positive individuals. Cereb Cortex. 2009;19:497‐510. 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jack CR, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138:3747‐3759. 10.1093/brain/awv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang S, Li C‐SR. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59:3548‐3562. 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Collij LE, Heeman F, Salvadó G, et al. Multitracer model for staging cortical amyloid deposition using PET imaging. Neurology. 2020;95:e1538‐53. 10.1212/WNL.0000000000010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sperling RA, Laviolette PS, O'Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178‐188. 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Skouras S, Torner J, Andersson P, et al. Earliest amyloid and tau deposition modulate the influence of limbic networks during closed‐loop hippocampal downregulation. Brain. 2020;143:976‐992. 10.1093/brain/awaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709‐7717. 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The Theory and Neuroscience of Cerebellar Cognition. Annu Rev Neurosci. 2019;42:337‐364. 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]
- 57. Habas C, Kamdar N, Nguyen D, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586‐8594. 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187‐221. [DOI] [PubMed] [Google Scholar]
- 59. Bartrés‐Faz D, Arenaza‐Urquijo EM. Structural and Functional Imaging Correlates of Cognitive and Brain Reserve Hypotheses in Healthy and Pathological Aging. Brain Topogr. 2011;24:340‐357. 10.1007/s10548-011-0195-9. [DOI] [PubMed] [Google Scholar]
- 60. Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. 2012;2012:782462. 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allen JL, Oberdorster G, Morris‐Schaffer K, et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2017;59:140‐154. 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheng H, Saffari A, Sioutas C, Forman HJ, Morgan TE, Finch CE. Nanoscale Particulate Matter from Urban Traffic Rapidly Induces Oxidative Stress and Inflammation in Olfactory Epithelium with Concomitant Effects on Brain. Environ Health Perspect. 2016;124:1537‐1546. 10.1289/EHP134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jayaraj RL, Rodriguez EA, Wang Y, Block ML. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: the Neuroinflammation Hypothesis. Curr Environ Heal Reports. 2017;4:166‐179. 10.1007/s40572-017-0142-3. [DOI] [PubMed] [Google Scholar]
- 64. Kristiansson M, Sörman K, Tekwe C, Calderón‐Garcidueñas L. Urban air pollution, poverty, violence and health – Neurological and immunological aspects as mediating factors. Environ Res. 2015;140:511‐513. 10.1016/j.envres.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 65. Ueno M, Chiba Y, Matsumoto K, et al. Blood‐brain barrier damage in vascular dementia. Neuropathology. 2016;36:115‐124. 10.1111/neup.12262. [DOI] [PubMed] [Google Scholar]
- 66. Edwards TJ, Sherr EH, Barkovich AJ, Richards LJ. Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain. 2014;137:1579‐1613. 10.1093/brain/awt358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Di Paola M, Spalletta G, Caltagirone C. In vivo structural neuroanatomy of corpus callosum in Alzheimer's disease and mild cognitive impairment using different MRI techniques: a review. J Alzheimers Dis. 2010;20:67‐95. 10.3233/JAD-2010-1370. [DOI] [PubMed] [Google Scholar]
- 68. Jew K, Herr D, Wong C, et al. Selective memory and behavioral alterations after ambient ultrafine particulate matter exposure in aged 3xTgAD Alzheimer's disease mice. Part Fibre Toxicol. 2019;16:45. 10.1186/s12989-019-0323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim SH, Knight EM, Saunders EL, et al. Rapid doubling of Alzheimer's amyloid‐β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000Research. 2012;1:70. 10.12688/f1000research.1‐70.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Calderón‐Garcidueñas L, González‐Maciel A, Reynoso‐Robles R, et al. Alzheimer's disease and alpha‐synuclein pathology in the olfactory bulbs of infants, children, teens and adults ≤ 40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ Res. 2018;166:348‐362. 10.1016/j.envres.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 71. Calderón‐Garcidueñas L, Franco‐Lira M, Mora‐Tiscareño A, Medina‐Cortina H, Torres‐Jardón R, Kavanaugh M. Early Alzheimer's and Parkinson's disease pathology in urban children: friend versus Foe responses–it is time to face the evidence. Biomed Res Int. 2013:161687. 10.1155/2013/161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mir RH, Sawhney G, Pottoo FH, et al. Role of environmental pollutants in Alzheimer's disease: a review. Environ Sci Pollut Res. 2020;27:44724‐44742. 10.1007/s11356-020-09964-x. [DOI] [PubMed] [Google Scholar]
- 73. Huang L, Zhang Y, Wang Y, Lan Y. Relationship Between Chronic Noise Exposure, Cognitive Impairment, and Degenerative Dementia: update on the Experimental and Epidemiological Evidence and Prospects for Further Research. J Alzheimer's Dis. 2021;79:1409‐1427. 10.3233/JAD-201037. [DOI] [PubMed] [Google Scholar]
- 74. Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med. 1998;55:651‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time‐series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419‐426. 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Temam S, Burte E, Adam M, et al. Socioeconomic position and outdoor nitrogen dioxide (NO2) exposure in Western Europe: a multi‐city analysis. Environ Int. 2017;101:117‐124. 10.1016/j.envint.2016.12.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on upon reasonable request from the corresponding author, JDG.


