Abstract
Background and Aims
This analysis examined the long-term safety and efficacy of ozanimod in patients with moderately to severely active ulcerative colitis [UC] with ≥ 4 years of follow-up in the phase 2 TOUCHSTONE open-label extension [OLE].
Methods
Patients receiving placebo or ozanimod HCl 0.5 mg or 1 mg during the double-blind period could enter the OLE [ozanimod HCl 1 mg daily]. Partial Mayo score [pMS] clinical response and remission were assessed through OLE week 200 and summarized descriptively using observed cases [OC] and non-responder imputation [NRI]. Endoscopy was required at OLE week 56 and the end of treatment. Parameters associated with endoscopy were summarized at weeks 56 and 104 [OC], and week 56 [NRI]. C-reactive protein and faecal calprotectin were assessed. Adverse events were monitored throughout the study.
Results
Of 197 patients receiving double-blind treatment, 170 entered the OLE. Discontinuation rates were 28% at year 1 and 15–18% annually through year 4. Partial Mayo measures indicated clinical response and remission rates at OLE week 200 of 93.3% and 82.7%, respectively, using OC and 41% and 37% with the more conservative NRI analysis. At weeks 56 and 104, respectively, histological remission rates were 46.3% and 38.5%, and endoscopic improvement rates were 46.4% and 46.5% [OC]. No new safety signals were identified during ≥ 4 years of follow-up.
Conclusions
There was a high rate of continued study participation and long-term benefit with ozanimod HCl 1 mg daily based on clinical, histological and biomarker measures in patients with moderately to severely active UC in the TOUCHSTONE OLE. [NCT02531126]
Keywords: Ozanimod, ulcerative colitis, clinical trial
1. Introduction
Ulcerative colitis [UC] is a chronic inflammatory bowel disease [IBD] associated with significant morbidity and reduced quality of life.1,2 UC is characterized by a chronic course of remissions and exacerbations, and patients with active disease are at an increased risk for dysplasia as a result of uncontrolled inflammation, as well as comorbid psychological conditions such as anxiety and depression.1 An important therapeutic goal in UC is achievement and long-term maintenance of steroid-free remission, defined based on symptoms and endoscopic findings.1,3 Mucosal healing, an important therapeutic objective, has traditionally been evaluated with endpoints based on endoscopic scores only. Recent studies, however, suggest that histological remission, defined as Geboes index score of < 2.0, rather than endoscopic remission may be more predictive of disease outcomes in UC.4
Current treatments for UC include non-specific anti-inflammatory agents, immunosuppressants, and targeted therapy with biologics and small molecules.1,5,6 Biological agents require parenteral administration, and are associated with lack or loss of response.3,7–9 The oral small-molecule Janus kinase inhibitor tofacitinib has been linked to safety concerns, including infections and thromboembolic events.10,11 Thus, there is a high unmet need for a safe and effective oral therapy for UC that provides a durable treatment response.
The sphingosine-1-phosphate [S1P] receptor subtype 1 [S1P1] plays an important role in the inflammatory response.12–16 By preventing trafficking of disease-exacerbating lymphocytes to the gut, ozanimod may provide immunomodulatory effects and moderate disease processes. S1P-receptor modulation has been shown to be safe and efficacious in other chronic immune-mediated inflammatory conditions, including relapsing forms of multiple sclerosis [MS] and Crohn’s disease.17–21
Ozanimod [RPC1063] is a potent S1P1 receptor modulator that binds selectively with high affinity to the S1P receptor subtypes S1P1 and S1P5,22 both of which are involved in immune regulation.14 Ozanimod was approved in 2020 in the USA23 and EU24 for the treatment of relapsing forms of MS based on data from two phase 3 clinical trials.20,25 Ozanimod is also being developed for patients with moderately to severely active IBD, including UC and Crohn’s disease, based on favourable activity and safety data from preclinical and phase 1 studies.22,26,27 The randomized, placebo-controlled phase 2 TOUCHSTONE study evaluated the efficacy and safety of ozanimod HCl doses of 0.5 mg [equivalent to ozanimod 0.46 mg] and 1 mg [equivalent to ozanimod 0.92 mg] orally once daily compared with placebo during induction and maintenance periods in patients with moderately to severely active UC. Treatment with ozanimod HCl 1 mg daily resulted in a significantly higher rate of UC clinical remission compared with placebo at 8 weeks during the induction period [primary endpoint].28 The higher rates of clinical remission, clinical response, endoscopic improvement and histological remission and the lower Mayo Clinic scores observed at week 8 were maintained through week 32 of the maintenance period. Indeed, the proportion of patients in clinical remission was higher at week 32 than at week 8, suggesting that extended treatment may incrementally improve efficacy.28,29
Herein, we report the long-term efficacy and safety of ozanimod HCl 1 mg orally once daily with 4 years [200 weeks] of follow-up during the open-label extension [OLE] period of the TOUCHSTONE study.
2. Materials and Methods
2.1. Study design
TOUCHSTONE [NCT01647516] was a randomized, double-blind, placebo-controlled phase 2 trial that included a 5-week screening, 9-week induction [including an initial 7-day dose-escalation period], 24-week maintenance and optional OLE period in patients with moderately to severely active UC [NCT02531126]. The TOUCHSTONE study design and the results from the induction and maintenance periods have been published previously.28 Eligible patients were 18–75 years of age with a diagnosis of UC confirmed by endoscopic and histological evidence. Additional eligibility criteria and the exclusion criteria have been published.28
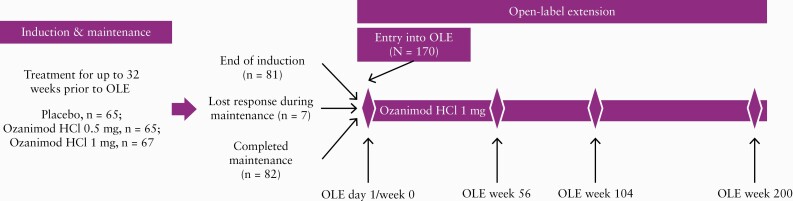
Patients with moderately to severely active UC [Mayo score 6–12 with endoscopic subscore of ≥ 2] who enrolled into the main study could enter the optional OLE and receive treatment with ozanimod HCl 1 mg orally once daily if they were non-responders at the end of the 9-week induction period, lost their response during the subsequent 24-week maintenance period, or completed maintenance treatment. After entering the OLE period, patients who had not shown clinical improvement, based on investigator assessment, within 8 weeks were discontinued from the study. In 2019, the sponsor made the decision to close the phase 2 TOUCHSTONE study after all active patients had completed at least 4 years [200 weeks] of follow-up. Eligible patients who remained in the study at the time of study closure also were provided the opportunity to roll over into the phase 3 OLE study [Figure 1].
Figure 1.
TOUCHSTONE study design.
After entering the OLE period, patients received a 7-day dose-escalating ozanimod regimen, consisting of the 0.25 mg dose for 4 days, followed by the 0.5 mg dose for 3 days, and then 1 mg daily over the duration of the OLE period. The objectives of the study during the OLE period were to assess the long-term safety and efficacy of ozanimod HCl 1 mg daily.
The study was designed by the sponsor and authors and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines as described in International Conference on Harmonization Guideline E6. In addition, the study complied with all local regulatory guidelines and requirements for data protection. All patients provided written informed consent.
2.2. Assessments and measurements
2.2.1. Efficacy measures
Patients recorded their stool frequency and rectal bleeding in daily diaries through the end of the OLE. This information was added to severity scores from physician global assessment [PGA] of disease to comprise a partial Mayo score [pMS], and to the PGA and findings on endoscopy to comprise the total Mayo score. Parameters comprising the various endpoints assessed are summarized in Supplementary Table 1.
2.2.2. Partial Mayo scores
Partial Mayo scores ranged from 0 to 9 and were assessed at OLE weeks 4 and 8 and at 12-week intervals thereafter. Clinical remission was defined as a pMS of ≤ 2 points with no individual subscore of > 1 point, and clinical response was defined as a reduction from baseline in pMS of ≥ 2 points and ≥ 30% and either a reduction in rectal bleeding score [RBS] of ≥ 1 point or an absolute RBS of ≤ 1 point.
2.2.3. Total Mayo scores
A three-component Mayo score included the subcomponents of stool frequency score [SFS], RBS and endoscopy, the four-component Mayo score included these as well as the PGA. Scores on the four subcomponents ranged from 0 to 3, with the total scores ranging up to 9 for the three-component and up to 12 for the four-component Mayo assessments, respectively. Higher scores indicate greater disease severity. Clinical remission and clinical response were defined using both the three-component and the four-component Mayo score as described in Supplementary Table 1. Clinical remission was defined as a total Mayo score of ≤ 2 points with no individual subscore of > 1 point [four-component] and as an RBS of 0, SFS of ≤ 1 with a decrease in SFS of ≥ 1 point from baseline, and an endoscopy score of ≤ 1 [three-component]. Clinical response was defined as a reduction in total Mayo score of ≥ 3 points and ≥ 30% from baseline and a reduction in RBS of ≥ 1 point or absolute RBS of ≤ 1 point [four-component] and as a reduction from baseline in the nine-point Mayo score of ≥ 2 points and ≥ 35% and either a reduction from baseline in RBS of ≥ 1 point or absolute RBS of ≤ 1 point [three-component].
2.2.4. Endoscopy and timing considerations
Endoscopy assessment timing was required in the final protocol at OLE week 56 and at the end of treatment or study for all enrolled patients. Because endoscopy assessments beyond week 56 were limited based on protocol requirements, the parameters that are associated with endoscopy (i.e. four- or three-component total Mayo score, endoscopy improvement per independent central evaluation at Robarts Clinical Trials [London, Ontario, Canada] and histology) were reported based on assessments at OLE weeks 56 and 104. Histological remission was defined as a Geboes index score of < 2.0; endoscopic improvement was defined as an endoscopic subscore of ≤ 1 point; endoscopic remission was defined as an endoscopy subscore of 0; and mucosal healing was defined as an endoscopy subscore of 0 and a Geboes index score < 2.0. Efficacy measurements with assessments up to OLE week 200 included frequency of clinical remission or clinical response based on the pMS, frequency of patients with SFS and RBS of 0 or 0/1, and changes from OLE baseline in pMS. Efficacy measures with limited data after OLE week 104 included clinical remission or response based on four- or three-component Mayo scores, histological remission, endoscopic improvement, mucosal healing and change from OLE baseline in total Mayo score.
2.2.5. Biomarkers
The biomarkers C-reactive protein [CRP] and faecal calprotectin [FCP] were assessed prior to ozanimod initiation, and at OLE week 8 and the end of treatment/study, and at safety follow-up day 30. CRP was also assessed at OLE week 4 and at 12-week intervals after OLE week 8.
2.2.6. Safety
Adverse events were monitored throughout the study and at the 30- and 90-day safety follow-up visits; blood chemistry and haematological assessments were conducted at OLE weeks 4 and 8, at 12-week intervals thereafter, at the end of study and at the 30-day safety follow-up visit.
Safety measures included incidence and type of treatment-emergent adverse events [TEAEs], discontinuations due to TEAEs and serious TEAEs. The incidence of TEAEs of special interest also was assessed, including events potentially associated with modulation of S1P receptors [bradycardia, heart conduction abnormalities, pulmonary toxicity, increases in hepatic aminotransferases, macular oedema] and events potentially related to immune modulation [infections, malignancies]. Vital signs, Holter monitoring, electrocardiograms [ECGs], pulmonary function tests, optical coherence tomography [OCT], blood chemistry and haematology panels, coagulation panels and urinalysis were assessed at baseline and various time points during the study. In addition, to monitor for macular oedema, patients were questioned about visual signs or symptoms at each study visit and instructed to inform the investigator if symptoms developed between visits. For symptomatic patients, OCT and ophthalmological examination including dilated ophthalmoscopy were performed, and patients whose ophthalmic evaluations revealed abnormalities were followed until values returned to baseline. Changes in absolute lymphocyte count also were assessed.
2.3. Statistical analyses
Analyses were performed on all randomized patients who received at least one dose of study drug in the OLE. OLE baseline was defined as the last assessment prior to the first dose of ozanimod regardless of study phase. No formal hypothesis testing was performed. The proportions of patients in partial Mayo clinical remission or with partial Mayo clinical response were summarized descriptively using observed cases and non-responder imputation [NRI] analyses. Because amendments to the study protocol modified the requirement for endoscopy during the OLE, the proportions of patients in clinical remission [based on the four- or three-component Mayo score], clinical response [four- or three-component Mayo] and endoscopic endpoints [histological remission, endoscopic improvement, endoscopic remission and mucosal healing] were descriptively summarized using observed cases and NRI at OLE weeks 56 and 104. Change from baseline in Mayo score, SFS and RBS scores over time, and CRP biomarker levels over time were calculated using observed cases. Safety endpoints are presented descriptively.
3. Results
3.1. Patient disposition and baseline characteristics
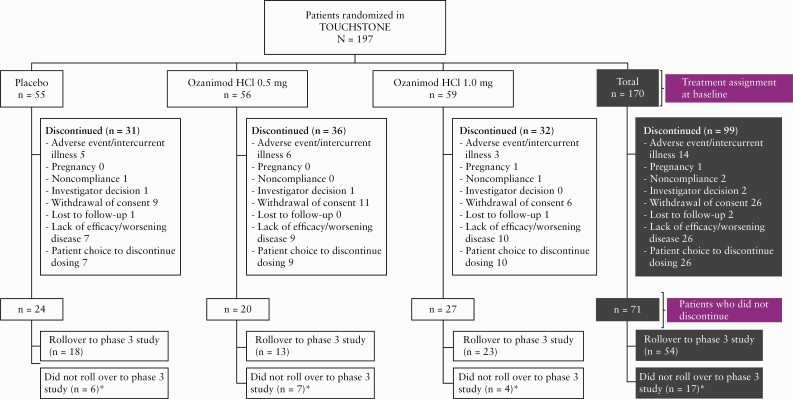
Of 197 patients in TOUCHSTONE, 170 entered the OLE period and received daily ozanimod HCl 1 mg [104 were non-responders and 66 were responders; 55, 56, and 59 were originally randomized to placebo, 0.5 mg ozanimod HCl and 1 mg ozanimod HCl, respectively]. Of these, 81 patients [78 non-responders, three responders, no remitters] entered the OLE period at the end of the induction period, seven [all non-responders] entered during the maintenance period, and 82 (22 non-responders and 60 responders [of whom 29 were remitters]) entered at the end of the maintenance period [three of the 22 non-responders were responders in the induction phase, entered the OLE at the end of maintenance with missing data and were included as responders; Figure 1]. At the time of this analysis [data cutoff, November 15, 2019], 99 patients [58%] had discontinued the OLE study, with 28% of the patients discontinuing in the first year, and an annual discontinuation rate of 15– 18% for existing patients in years 2–4 [Table 1]. Reasons for discontinuation are shown in Figure 2. At study closure, of the 71 UC patients eligible to roll over into the phase 3 OLE study, 54 did so as a joint decision of patients and the treating investigators [Figure 2].
Table 1.
Annual patient discontinuations over the 4-year OLE
| Completed, n | Discontinued/entered, n/N | Cumulative discontinuations, n | Discontinuation rate, % | |
|---|---|---|---|---|
| OLE day 1/week 0 | 170 | — | — | |
| OLE week 56 | 123 | 47/170 | 47 | 27.6 |
| OLE week 104 | 102 | 21/123 | 68 | 17.1 |
| OLE week 152 | 84 | 18/102 | 86 | 17.6 |
| OLE week 200 | 71 | 13/84 | 99 | 15.5 |
OLE, open-label extension.
Figure 2.
Patient disposition.
*In 2019, the sponsor made the decision to close the phase 2 TOUCHSTONE study after all active patients had completed at least 4 years [200 weeks] of follow-up.
Of the 170 patients in the OLE, the mean [range] age of patients at baseline for the double-blind study [induction period] was 40.4 [18–73] years, 57.6% of patients were male and 92.4% of patients were white [Table 2]. The mean [SD] duration of disease from diagnosis to the baseline of the double-blind study was 5.9 [5.29] years, and most patients [81.8%] had not received prior anti-tumour necrosis factor [anti-TNF] therapy.
Table 2.
Patient demographics and baseline characteristics [ITT population]
| Characteristic | Total, N = 170 |
|---|---|
| Sex, n [%] | |
| Female | 72 [42.4] |
| Male | 98 [57.6] |
| Age [years], mean [SD] | 40.4 [11.76] |
| Race, n [%] | |
| White | 157 [92.4] |
| Black | 3 [1.8] |
| Other | 10 [5.9] |
| BMI [kg/m2], mean [SD] | 25.0 [4.96] |
| Years since UC diagnosis, mean [SD] | 5.9 [5.29] |
| Prior anti-TNF treatment, n [%] | |
| Yes | 31 [18.2] |
| No | 139 [81.8] |
| Partial Mayo score at OLE baseline, median [range] | 6.0 [3–9] |
| Total Mayo score at OLE baseline, median [range] | 8.0 [5–12] |
BMI, body mass index; ITT, intent to treat; OLE, open-label extension; SD, standard deviation; SEM, standard error of the mean; TNF, tumour necrosis factor; UC, ulcerative colitis.
The mean exposure to ozanimod over the course of the study was 2.8 person-years [478.7 total person-years].
3.2. Efficacy
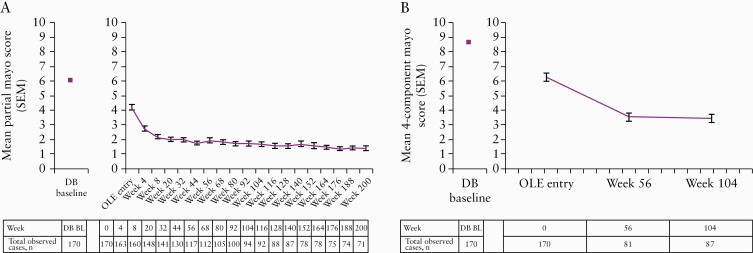
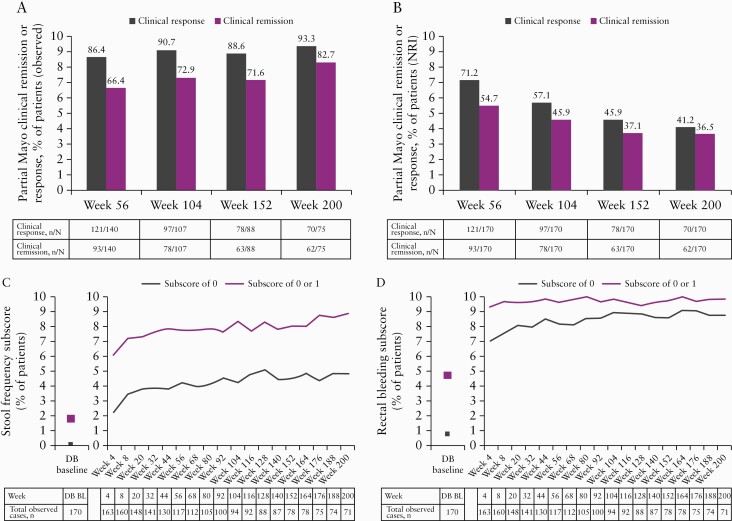
Observed scores for the partial and total Mayo scores decreased substantially over time, with pMS plateauing at < 2 by OLE week 8 and remaining stable over time [Figure 3]. The percentages of patients in partial Mayo clinical response and remission were 86.4% and 66.4%, respectively, at OLE week 56 and 93.3% and 82.7%, respectively, at OLE week 200 [Figure 4A]. When using NRI, partial Mayo clinical response and remission were 71.2% and 54.7%, respectively, at OLE week 56 and 41.2% and 36.5%, respectively, at week 200 [Figure 4B].
Figure 3.
Absolute mean [SEM] partial Mayo [A] and four-component Mayo [B] scores over time during OLE [observed cases analysis].
BL, baseline; DB, double-blind; OLE, open-label extension; SEM, standard error of the mean.
Figure 4.
Percentages of patients with partial Mayo clinical response or remission based on observed cases [A] or based on NRI [B], patients with a Mayo stool frequency subscore of 0 or a subscore of 0 or 1 [observed cases] [C], and patients with a Mayo rectal bleeding subscore of 0 or a subscore of 0 or 1 [observed cases] [D] in the OLE phase.
BL, baseline; DB, double-blind; NRI, non-responder imputation; OLE, open-label extension.
The percentages of patients with SFS of 0 or 0/1 were higher at OLE week 4 [23.3% and 62.0%, respectively] compared with double-blind baseline [0.6% and 16.5%, respectively] and continued to increase during the OLE period until week 200 [47.9% and 88.7%, respectively; Figure 4C]. Similarly, the percentages of patients with RBS of 0 or 0/1 were higher at OLE week 4 [71.2% and 93.9%, respectively] compared with double-blind baseline [7.6% and 46.5%] and continued to increase slightly over time until week 200 [87.3% and 98.6%, respectively; Figure 4D].
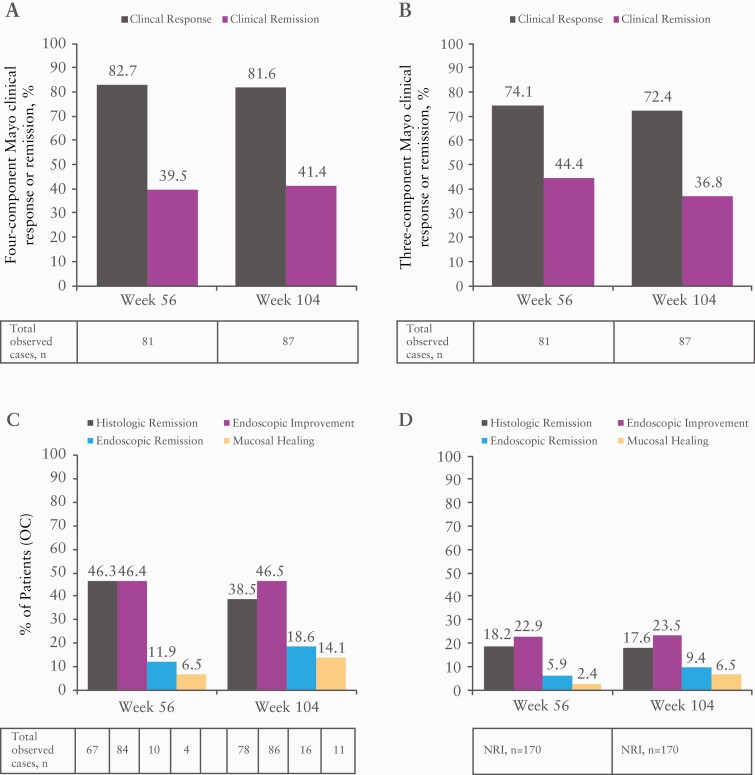
Clinical response and remission using a four- and three-component Mayo score, and endoscopic endpoints in observed cases at OLE weeks 56 and 104 are shown in Figure 5. Results obtained by OLE week 56 were generally maintained to week 104 for each endpoint. Using NRI, the clinical response and remission at OLE week 56 were 39.4% and 18.8%, respectively on the four-component Mayo measure, and 35.3% and 21.2%, respectively on the three-component Mayo measure [data not shown]. The percentage of patients achieving histological remission, endoscopic improvement, endoscopic remission and mucosal healing at week 56 [using NRI] was 18.2%, 22.9%, 5.9% and 2.4%, respectively.
Figure 5.
Percentages of patients achieving four-component Mayo clinical response or remission [A], three-component Mayo clinical response or remission [B], and histological remission, endoscopic improvement, endoscopic remission and mucosal healing based on OC [C], and based on NRI [D] at weeks 56 and 104 in the OLE phase.
NRI, non-responder imputation OC, observed cases; OLE, open-label extension.
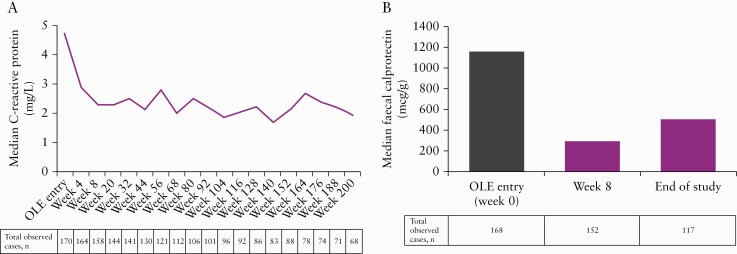
The median CRP concentration was reduced by 25% from pretreatment [OLE baseline] to OLE week 4 (median [min, max] CRP at OLE baseline: 4.7 mg/L [0.1, 131]; at OLE week 4: 2.9 mg/L [0.1, 152]), and by 35% from pretreatment to week 8 (median OLE week 8: 2.3 mg/L [0.1, 84]), and this reduction was maintained over time through week 200 [Figure 6A]. Of the 158 patients with available data, 47 [29.7%] achieved CRP < 10 at OLE week 8. FCP was measured at week 8 and at the end of the study. The median FCP concentration was reduced by 68% from pretreatment to OLE week 8 (OLE baseline median [min, max]: 1159.0 µg/g [10, 12 364]; OLE week 8: 288.5 µg/g [10, 6464]) and by 63% at the end of the study (end of study median [min, max]: 502.0 µg/g [10, 109 336]; Figure 6B). Of 160 patients with available data, 69 [43.1%] achieved FCP < 250 at OLE week 8. FCP concentrations were highly variable, which may account for the change seen between the week 8 and end of study visits.
Figure 6.
Median C-reactive protein concentrations over time [A] and faecal calprotectin levels at baseline and week 8 and end of the study [B] based on observed cases in the OLE phase.
OLE, open-label extension.
3.3. Safety
The most common TEAEs were UC [6.5%], hypertension [5.9%], upper respiratory infection [5.9%] and increased gamma glutamyltransferase [5.3%] [Table 3]. Two patients experienced macular oedema or thickening due to retinal vein thrombosis that did not require study discontinuation. One patient experienced two episodes of mild or moderate localized herpes zoster infection [no prior history of herpes zoster infection] [n = 1; ozanimod HCl 0.5 mg], increased hepatic enzymes [n = 1; ozanimod HCl 0.5 mg] and cholestasis [n = 1; placebo] during the OLE.
Table 3.
Overview of adverse events during the OLE period
| Total [N = 170] | |
|---|---|
| Mean person-years of exposure [SD] | 2.8 [1.85] |
| Total person-years of exposure | 478.7 |
| TEAEs in ≥ 5% of patients in any group, n [%] | |
| Ulcerative colitis | 11 [6.5] |
| Hypertension | 10 [5.9] |
| Upper respiratory tract infection | 10 [5.9] |
| Gamma-glutamyltransferase increased | 9 [5.3] |
| Anaemia | 8 [4.7] |
| Back pain | 7 [4.1] |
| Nasopharyngitis | 7 [4.1] |
| Headache | 7 [4.1] |
| Alanine aminotransferase increased | 6 [3.5] |
| Lymphocyte count decreased | 6 [3.5] |
| Bronchitis | 4 [2.4] |
| Viral respiratory tract infection | 4 [2.4] |
| SAEs in > 1 patient, n [%]* | |
| Ulcerative colitis | 6 [3.5] |
| Anaemia | 2 [1.2] |
| Ischaemic stroke | 2 [1.2] |
OLE, open-label extension; SAE, serious adverse event; SD, standard deviation; TEAE, treatment-emergent adverse event.
*The following SAEs occurred in one patient each: acute coronary syndrome, adenocarcinoma, ascites, autoimmune haemolytic anaemia, basal cell carcinoma, colitis, colon adenoma, dehydration, erysipelas, haemolytic anaemia, hypochromic anaemia, hyperbilirubinaemia, idiopathic pulmonary fibrosis, inguinal hernia, interstitial lung disease, intestinal obstruction, jaundice, joint dislocation, nephrolithiasis, pleurisy, pneumonia, pneumococcal pneumonia, prostate cancer, pulmonary bulla, pulmonary microemboli, rheumatoid arthritis, schizophrenia, spinal column stenosis, spontaneous abortion, umbilical hernia, viral gastroenteritis, wrist fracture.
Nine patients [5.3%] had a TEAE of lymphopaenia or reduced lymphocyte counts; these events were considered to be mild in two patients, moderate in six patients and severe in one patient. One patient with decreased lymphocyte counts had an adverse event of gastrointestinal infection during the same time period that was of moderate severity and considered unrelated to treatment. Overall, the mean absolute blood lymphocyte count decreased from OLE baseline [1.910 × 109 cells/L, SD = 0.855] to OLE week 4 [0.990 × 109 cells/L; SD = 0.552] and remained steady thereafter.
TEAEs leading to study drug discontinuation during the OLE were reported for 17 patients [10%; eight originally in the ozanimod HCl 0.5 mg group, four in the ozanimod HCl 1 mg group and five in the placebo group during the double-blind treatment period]. These events included UC [n = 4; discontinuation due to pleurisy also reported in one of the four patients], adenocarcinoma of unknown origin [n = 1], cholestasis [n = 1], colitis [n = 1], dysplasia [n = 1], erysipelas [n = 1], haemolytic anaemia and jaundice [n = 1], hypochromic anaemia [n = 1], hyperbilirubinaemia and autoimmune haemolytic anaemia [n = 1], interstitial lung disease [n = 1], ischaemic stroke [n = 2], spontaneous abortion [n = 1] and thrombocytopenia [n = 1].
The most commonly reported serious adverse events [SAEs] were UC [n = 6], anaemia [n = 2] and ischaemic stroke [n = 2] [Table 3]; none of these were considered related to study treatment as assessed by the study investigator. SAEs considered potentially related to study treatment by the investigator that were reported in patients originally randomized to ozanimod HCl 0.5 mg were adenocarcinoma [of unknown origin] and ascites [both occurring in one patient who discontinued treatment], pneumococcal pneumonia, pneumonia, and hyperbilirubinaemia [n = 1 each]; those occurring in patients originally randomized to ozanimod HCl 1 mg included haemolytic anaemia and jaundice [both occurring in the same patient] and spontaneous abortion in a 29-year-old woman. Study medication was discontinued immediately upon a positive pregnancy test, 43 days after her last menstrual period. She experienced a miscarriage 18 days later, which was 61 days after her last menstrual period. This event was considered by the investigator to be ‘possibly’ related to study medication. No SAEs considered potentially related to study treatment by the investigator were reported in patients originally randomized to placebo. No serious abnormalities in cardiac chronotropy or adverse effects on cardiac conduction were observed. No clinically significant elevations in hepatic transaminases and no evidence of serious hepatocellular injury were observed. No safety concerns related to the study drug were noted for any of the haematological assessments; however, one case of lymphopaenia was classified as severe, but was not associated with infection and did not result in study discontinuation. Three patients had a serious infection during the OLE, but none was associated with grade 4 lymphopenia [absolute lymphocyte count < 200]. One death was reported due to the above-mentioned mucinous adenocarcinoma of unknown origin with metastasis to the liver in a patient who had been in the study for more than 2 years.
4. Discussion
In the randomized, phase 2 TOUCHSTONE OLE, long-term treatment with oral ozanimod HCl 1 mg daily resulted in durable efficacy based on clinical, endoscopic, histological and biomarker measures with up to 4 years of OLE treatment in patients with moderate-to-severe UC. Patient retention rates over this long-term study were high. Remission and response rates based on endoscopic and histological measures using observed-case and NRI analyses, including clinical remission [four- and three-component Mayo], clinical response [four- and three-component Mayo], histological remission and endoscopic improvement, were achieved by a considerable proportion of patients by OLE week 56 and the observed case analysis shows that improvements were generally maintained to week 104. Using the more conservative NRI analysis, 71.2% and 54.7% of patients achieved partial Mayo clinical response and remission, respectively, by OLE week 56, and 41.2% and 36.5% of patients maintained these benefits through OLE week 200. In addition, biomarker data support the clinical findings, as decreases in CRP and FCP were noted from baseline to week 8, similar to the outcomes observed for SFS or RBS; decreases in CRP values were also maintained through week 200.
In the induction and maintenance phases of the TOUCHSTONE study, rates of clinical and histological remission with ozanimod HCl 1 mg daily were higher at week 32 than at week 8.28 The current results expand on this with remission and response rates that were high throughout the OLE portion of the study, suggesting that the long-term treatment of 1 mg daily ozanimod HCl provides continued clinical and histological benefit in UC. Moreover, the percentages of patients reporting normal [SFS of 0] or near-normal stool frequency [SFS of 0 or 1] and no or limited rectal bleeding [RBS of 0, or ‘0 or 1’] increased from double-blind study baseline to OLE week 4, with a high proportion of patients having improved symptoms continuing through week 200. Similarly, mean partial and total Mayo scores decreased from study baseline at OLE entry and continued to decrease through the observed OLE time points [OLE week 200 for pMS and OLE week 104 for total Mayo score].
Endoscopic improvement may be an important treatment target for UC, and endoscopically determined improvement in mucosal appearance has been associated with more favourable long-term outcomes in patients with UC.30 Accumulating evidence also indicates that histological healing is an important indicator of mucosal recovery and is associated with improved disease outcomes.31,32 In this study, rates of endoscopic improvement and histological remission at OLE week 56 [46.4% and 46.3%, respectively] represented a substantial increase over those reported at week 32 in the induction period of the trial [33% and 31%, respectively].28 These improvements were maintained to OLE week 104 [the last assessment time point with sufficient patient numbers for analysis], and mirror the reported sustained improvements in clinical symptoms of disease as measured by pMS. In a preliminary analysis of data from the 170 patients who entered the OLE, patients in histological remission upon entry into the OLE [n = 46] were four times more likely to be in clinical remission at OLE weeks 44 and 80, underlining the potential importance of histopathology as a treatment target in UC.29
The biomarkers CRP and FCP may be useful measures in the follow-up of patients with UC, and levels are related to disease outcomes.33 In the present study, both CRP and FCP levels reflected clinical and histological findings. CRP levels decreased quickly after ozanimod initiation and remained low throughout the remainder of the study. FCP levels also decreased from pretreatment to week 8 and remained low at the end of study assessment. These data provide an additional confirmatory measure of long-term improvements with continued ozanimod treatment and suggest the effects of ozanimod are mediated through reducing the pro-inflammatory response.
Long-term treatment with ozanimod was well tolerated, and the results build on the efficacy findings in the induction and maintenance phases. Consistent with the core study, the dose escalation during the initiation of ozanimod therapy successfully mitigated the risk of bradycardia, which also reflects the recent approval of ozanimod for MS in both the USA and the EU, where first-dose monitoring is not required for all patients initiating ozanimod.23,24 There was no signal that long-term use of ozanimod increased the risks of clinically significant infections [including opportunistic infections], bradyarrhythmia, hepatic or pulmonary dysfunction, macular oedema, or malignancy. Because S1P1 modulation reduces lymphocyte migration from lymphoid organs, a decrease in peripheral lymphocyte count is an expected pharmacodynamic effect of ozanimod treatment. In this study, a decreased lymphocyte count was reported as a treatment-related adverse event in nine patients, but there were no instances of serious or opportunistic infections associated with these events. During the OLE period, only 10% of patients discontinued because of a TEAE.
Of 170 patients enrolled in the OLE, 71 [42%] remained in the study and received ozanimod for at least 3.8 years. The annual discontinuation rate of 15–28% during the OLE period of TOUCHSTONE compares favourably with real-world discontinuation rates with approved therapies for UC. One retrospective database study of TNF inhibitor use reported 12-month discontinuation rates with adalimumab and infliximab of 52% and 45%, respectively.34
Limitations of the current study should be considered. The OLE population included patients who had initially received either placebo or two different doses of ozanimod. Data for endoscopy-based outcomes such as remission or response based on the three- or four-component Mayo score, endoscopic improvement and histological remission were limited after week 104 of OLE treatment. Moreover, endoscopic assessments were only able to be analysed in observed cases, and the results demonstrated that response and remission rates by four- or three-component Mayo scores were lower than those by pMS, suggesting a potential selection bias for more symptomatic patients who agreed to an endoscopy. As this was a long-term clinical study, premature discontinuations resulted in incomplete patient data; in this analysis, patients with missing data were imputed as non-responders. Data were not collected to allow assessment of steroid-free remission or steroid use/tapering over time during the OLE. In addition, FCP assessments were limited beyond OLE week 8, precluding assessment of the kinetics of FCP over the 200-week period. Finally, there was no placebo group with which to compare safety data.
5. Conclusion
Data from the OLE period of the randomized, placebo-controlled, phase 2 TOUCHSTONE study support a favourable benefit–risk profile of ozanimod HCl 1 mg daily in patients with moderately to severely active UC, and resulted in a high rate of continued study participation. Consistent efficacy outcomes across clinical, endoscopic, histological and biomarker measures suggest that long-term use of ozanimod may reduce inflammation and lead to histological and endoscopic disease remission, manifested as sustained improvement in clinical symptoms of disease. No new safety signals were identified with ≥ 4 years of follow-up. The efficacy and safety of oral ozanimod HCl 1 mg daily are being further characterized in a randomized, placebo-controlled phase 3 study [True North, clinicaltrials.gov identifier: NCT02435992].
Supplementary Material
Acknowledgments
The authors would like to thank Yin Yang, of Bristol Myers Squibb, for her contributions to statistical analyses. Support for third-party writing assistance for this manuscript was provided by Cindy Gobbel, PhD, and Traci Stuve, MA, of Peloton Advantage, LLC, an OPEN Health company, and was funded by Bristol Myers Squibb. William Sandborn is funded in part by the NIDDK-funded San Diego Digestive Diseases Research Center [P30 DK120515].
Conference: These data were presented in part at the following conferences: ECCO, Copenhagen, Denmark, 2019; DDW, San Diego, CA, USA, 2019; UEGW, Barcelona, Spain, 2019; UEGW, virtual, 2020.
Funding
This study was sponsored by Bristol Myers Squibb.
Conflict of Interest
William Sandborn reports: research grants from AbbVie, Amgen, Atlantic Healthcare Limited, Celgene/Receptos, Genentech, Gilead Sciences, Janssen, Lilly, Pfizer, Prometheus Laboratories [now Prometheus Biosciences], Takeda; consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories], Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials [owned by Health Academic Research Trust, HART], Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, TiGenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Progenity, Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories], Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Escalier Biosciences—prior employee, stock options; Iveric Bio—consultant, stock options; Oppilan Pharma—consultant, stock options; Progenity—consultant, stock; Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories]—employee, stock options; Ventyx Biosciences—stock options; Vimalan Biosciences—stock options. Brian Feagan has consulted for AbbVie, ActoGeniX, Albireo, Amgen, AstraZeneca, Avaxia Biologics, Baxter, Biogen Idec, Boehringer Ingelheim, BMS, Calypso, Celgene, Elan, EnGene, Ferring Pharma, Roche/Genentech, GiCare, Gilead, Given Imaging, GSK, Ironwood, Janssen, Johnson & Johnson, Lexicon, Lilly, Merck, Millennium, Nektar, Novo Nordisk, Pfizer, Prometheus, Protagonist, Sanofi, and UCB and is a director at Robarts Clinical Trials. Stephen Hanauer has consulted for Celgene, AbbVie, Janssen, UCB, Shire, Actavis, Salix, BMS, Merck, Pfizer, Boehringer Ingelheim, Sanofi-Aventis, Ferring, and Caremark. Severine Vermeire reports grant support from AbbVie, Pfizer, J&J, and Takeda; consultancy fees from AbbVie, MSD, Takeda, Ferring, Genentech/Roche, Shire, Pfizer Inc, Galapagos, Mundipharma, Hospira, Celgene, Second Genome, Progenity, Lilly, Arena, Gilead, and Janssen. Subrata Ghosh reports consulting fees from Janssen, AbbVie, Takeda, Pfizer, Galapagos, Gilead, BMS, Boehringer-Ingelheim, and Celgene, speaker fees from Abbvie, Janssen, Takeda, and Pfizer, and research grants from AbbVie. Wenzhong Liu, AnnKatrin Petersen, Lorna Charles, Vivian Huang and Keith Usiskin: employees of Bristol Myers Squibb. Doug Wolf has received research funding from AbbVie, Amgen, Elan, Given Imaging, Genentech, Janssen, Millennium, Pfizer, Prometheus, Celgene, and UCB, has lectured for AbbVie, Janssen, Prometheus, Santarus, Salix, Takeda, and UCB, and consulted for AbbVie, Genentech, Given Imaging, Janssen Prometheus, Salix, Takeda, and UCB. Geert D’Haens has consulted for AbbVie, Ablynx, Amakem, AM Pharma, Avaxia, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Cosmo, Covidien/Medtronic, Ferring, Dr. Falk Pharma, EnGene, Galapagos, Genentech/Roche, Gilead, GlaxoSmithKline, Hospira, Immunic, Johnson & Johnson, Lycera, Medimetriks, Millennium/Takeda, Mitsubishi Pharma, Merck Sharp & Dohme, Mundipharma, Novo Nordisk, Otsuka, Pfizer, Prometheus Laboratories/Nestle, Protagonist, Receptos, Robarts Clinical Trials, Salix, Sandoz, SetPoint, Shire, Teva, Tigenix, Tillotts, TopiVert, Versant, and Vifor; is a speaker for AbbVie, Biogen, Ferring, Johnson & Johnson, Merck Sharp & Dohme, Mundipharma, Norgine, Pfizer, Millennium/Takeda, Tillotts, and Vifor; is a director at Robarts Clinical Trials; and is a shareholder at EnGene.
Data Sharing Statement
Data requests may be submitted to Celgene, a Bristol Myers Squibb Company, at https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.
Author Contributions
Conception or design of the work: WS, BF, SH, AP. Data collection: WS, BF, SV, WJL, AP, KU, DW, GDH. Data analysis and interpretation: WS, BF, SH, SV, SG, WJL, AP, LC, VH, KU, GDH. Drafting the article: WS, BF, SG, WJL, AP, VH, KU, DW. Critical revision of the article: WS, BF, SH, SV, SG, WJL, AP, LC, VH, KU, DW, GDH. All authors had full access to all the data and had final responsibility for the decision to submit for publication.
References
- 1. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 2. Calvet X, Argüelles-Arias F, López-Sanromán A, et al. . Patients’ perceptions of the impact of ulcerative colitis on social and professional life: results from the UC-LIFE survey of outpatient clinics in Spain. Patient Prefer Adherence 2018;12:1815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danese S, Allez M, van Bodegraven AA, et al. . Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis 2019;37:266–83. [DOI] [PubMed] [Google Scholar]
- 4. Narang V, Kaur R, Garg B, et al. . Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intest Res 2018;16:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Currò D, Pugliese D, Armuzzi A. Frontiers in drug research and development for inflammatory bowel disease. Front Pharmacol 2017;8:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 7. Moss AC, Brinks V, Carpenter JF. Review article: Immunogenicity of anti-TNF biologics in IBD - the role of patient, product and prescriber factors. Aliment Pharmacol Ther 2013;38:1188–97. [DOI] [PubMed] [Google Scholar]
- 8. Thomas SS, Borazan N, Barroso N, et al. . Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. a systematic review and meta-analysis. BioDrugs 2015;29:241–58. [DOI] [PubMed] [Google Scholar]
- 9. Fine S, Papamichael K, Cheifetz AS. Etiology and management of lack or loss of response to anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2019;15:656–65. [PMC free article] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Panés J, Sands BE, et al. . Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol 2019;12:1756284819848631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brinkmann V, Baumruker T. Pulmonary and vascular pharmacology of sphingosine 1-phosphate. Curr Opin Pharmacol 2006;6:244–50. [DOI] [PubMed] [Google Scholar]
- 13. Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol 2007;8:1295–301. [DOI] [PubMed] [Google Scholar]
- 14. Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev 2017;16:495–503. [DOI] [PubMed] [Google Scholar]
- 15. Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu Rev Biochem 2013;82:637–62. [DOI] [PubMed] [Google Scholar]
- 16. Suh JH, Degagné É, Gleghorn EE, et al. . Sphingosine-1-phosphate signaling and metabolism gene signature in pediatric inflammatory bowel disease: a matched-case control pilot study. Inflamm Bowel Dis 2018;24:1321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kappos L, Radue EW, O’Connor P, et al. . A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- 18. Kappos L, Bar-Or A, Cree BAC, et al. . Siponimod versus placebo in secondary progressive multiple sclerosis (expand): a double-blind, randomised, phase 3 study. Lancet 2018;391:1263–73. [DOI] [PubMed] [Google Scholar]
- 19. Cohen JA, Arnold DL, Comi G, et al. . Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (radiance): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:373–81. [DOI] [PubMed] [Google Scholar]
- 20. Cohen JA, Comi G, Selmaj KW, et al. . Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (radiance): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol 2019;18:1021–33. [DOI] [PubMed] [Google Scholar]
- 21. Feagan BG, Sandborn WJ, Danese S, et al. . Ozanimod induction therapy for patients with moderate to severe crohn’s disease: a single-arm, phase 2, prospective observer-blinded endpoint study. The Lancet Gastroenterology & Hepatology 2020;5:819–28. [DOI] [PubMed] [Google Scholar]
- 22. Scott FL, Clemons B, Brooks J, et al. . Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol 2016;173:1778–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeposia [package insert]. Princeton, NJ: Bristol Myers Squibb; 2020. [Google Scholar]
- 24. Zeposia [summary of product characteristics]. Utrecht: Celgene Distribution B.V.; 2020. [Google Scholar]
- 25. Comi G, Kappos L, Selmaj KW, et al. . Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (sunbeam): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol 2019;18:1009–20. [DOI] [PubMed] [Google Scholar]
- 26. Tran JQ, Hartung JP, Peach RJ, et al. . Results from the first-in-human study with ozanimod, a novel, selective sphingosine-1-phosphate receptor modulator. J Clin Pharmacol 2017;57:988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tran JQ, Hartung JP, Olson AD, et al. . Cardiac safety of ozanimod, a novel sphingosine-1-phosphate receptor modulator: results of a thorough QT/QTc study. Clin Pharmacol Drug Dev 2018;7:263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sandborn WJ, Feagan BG, Wolf DC, et al. . Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med 2016;374:1754–62. [DOI] [PubMed] [Google Scholar]
- 29. Sandborn W, Feagan B, D’Haens G, et al. . Histological remission is predictive of improved clinical outcomes in patients with ulcerative colitis: results from the touchstone open-label extension [abstract]. J Crohns Colitis 2017;11:S9. [Google Scholar]
- 30. Colombel JF, Rutgeerts P, Reinisch W, et al. . Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- 31. Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol 2014;12:929–34.e2. [DOI] [PubMed] [Google Scholar]
- 32. Bryant RV, Burger DC, Delo J, et al. . Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016;65:408–14. [DOI] [PubMed] [Google Scholar]
- 33. Sandborn WJ, Panés J, Zhang H, et al. . Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology 2016;150:96–102. [DOI] [PubMed] [Google Scholar]
- 34. Null KD, Xu Y, Pasquale MK, et al. . Ulcerative colitis treatment patterns and cost of care. Value Health 2017;20:752–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.