Abstract
Declining female fertility has become a global health concern. It results partially from an abnormal circadian clock caused by unhealthy diet and sleep habits in modern life. The circadian clock system is a hierarchical network consisting of central and peripheral clocks. It not only controls the sleep–wake and feeding–fasting cycles but also coordinates and maintains the required reproductive activities in the body. Physiologically, the reproductive processes are governed by the hypothalamic–pituitary–gonadal (HPG) axis in a time-dependent manner. The HPG axis releases hormones, generates female characteristics, and achieves fertility. Conversely, an abnormal daily rhythm caused by aberrant clock genes or abnormal environmental stimuli contributes to disorders of the female reproductive system, such as polycystic ovarian syndrome and premature ovarian insufficiency. Therefore, breaking the “time code” of the female reproductive system is crucial. In this paper, we review the interplay between circadian clocks and the female reproductive system and present its regulatory principles, moving from normal physiology regulation to disease etiology.
Keywords: Circadian clock, hypothalamic–pituitary–gonadal axis, female reproductive disorders, clock genes
The female reproductive system consists of various secretory organs that release hormones and generate female characteristics as well as achieve fertility. Physiologically, the whole system is governed by the hypothalamic–pituitary–gonadal (HPG) axis in a time-dependent manner. In the early stage of the menstrual cycle, a rise in follicle-stimulating hormone (FSH) prompts estrogen, which later stimulates luteinizing hormone (LH) secretion. About 36 to 40 hours after LH surges at midcycle, the dominant follicle releases an oocyte (1, 2), and all hormones return to base levels. However, this periodic regulation is vulnerable to disruption from pathological circumstances, such as eating and sleeping disorders (3). With long-term exposure, these could lead to an irregular menstrual cycle, which is also a signal of multiple reproductive diseases.
The intrinsic clock oscillator in all organisms works to adapt to these disruptive challenges (4). Its mechanism is indeed the circadian clock, which is entrained by external cues and rhythmically regulates the vast majority of physiological and behavioral processes of the human body. During the life of a female, circadian control is exerted at all levels, from follicle development (5, 6) to coordination of hormone homeostasis (7), and from embryo implantation (8, 9) to the final delivery (10). In humans, optimal fitness requires synchronized oscillation between the internal circadian rhythm and external environmental cycles. Once it is misaligned, the rhythm is lost and eventually diseases show up. Thereby, a novel therapeutic option, termed “chronotherapy,” is put forward to use the concept of time in disease treatment, further emphasizing the status of circadian rhythms (11).
Regarding biological rhythms in humans, apart from sleep and endocrine activities, reproductive rhythms are the best understood. Recent work has implicated the circadian clock genes in regulating processes in the hypothalamus, pituitary, ovary, and some endocrine organs, all of which are critical in hormone secretion (12-17). Some evidence indicates that disruption to circadian rhythms can increase the risk of irregular estrous cyclicity and polycystic ovarian syndrome (PCOS) (10, 18). In this review, we focus on the relationship between the HPG axis and the circadian clock with elusive interplay and discuss the underlying mechanisms. Furthermore, we present the regulatory principles of circadian rhythmicity, moving from normal physiology regulation to disease etiology of the female reproductive system.
The Circadian Clock System
Most organisms experience daily changes in their environment, such as light, temperature, and food. To better adapt to these precise and regular alternations, organisms, ranging from fungi and bacteria to plants and mammals, present with daily biological processes and physiological rhythms (19-22). The external cues, termed zeitgebers (a German word, literally “time-givers”), entrain the circadian rhythm and further activate the regulation of the endogenous timing system. The normal function of the system is based on a hierarchical network of central and peripheral clocks. Generally, the central clocks are located in the suprachiasmatic nucleus (SCN) in the hypothalamus and regulate and convey rhythmic information to “downstream” clocks in peripheral tissues and organs, resulting in specific biochemistry, physiology, and behavior (23).
In essence, the circadian clock is a rhythmic transcriptional and translational feedback loop (TTFL). In the main loop in mammals, the transcriptional activators, CLOCK and BMAL1, accumulate in the nucleus and dimerize to bind to the E/E′-box containing enhancers or promoters of the transcriptional suppressor genes PER and CRY. Then, PER and CRY proteins physically interact and translocate to the nucleus to suppress their genes and the activities of BMAL1 and CLOCK. Once PER and CRY levels sufficiently degrade, a new cycle starts. In addition, a second feedback loop is formed by the nuclear receptors REVERB and ROR (24, 25). They compete to bind with the promoter and enhancer regions of the target gene (eg, BMAL1) and play an inhibitory and activation role in transcription, respectively (26). The CLOCK-BMAL1 target gene DBP forms another loop, and it competes with the REV-ERB and ROR target gene NFIL3 to regulate the expression of clock genes. Ultimately, all loops mentioned above can control the expression of clock-controlled genes (CCGs), which mediate circadian output. Accordingly, TTFL forms the foundation of the physiological activity of different tissues and organs, thus leading to cyclic variations in gene expression and tissue function. Abnormal clock genes or environmental stimuli interfere with the feedback loop and are associated with diseases of multiple systems, most prominently metabolic, cardiovascular, and mental disorders and infertility (3, 27).
Chronobiology in the HPG Axis
A wide variety of processes in the HPG axis and its accessory organs display biological rhythm, which is typically reflected by periodic hormones during menstruation. One is the estrogen production initiated by FSH, and the other is the postovulatory progesterone secretion from the corpus luteum. Both of the steroid hormones are regulated by the circadian clock system, the impairment of which can lead to ovarian dysfunction and infertility (8, 28). In the following sections, we summarize the current chronobiological knowledge of the HPG axis, which shows diurnal rhythms in humans, and demonstrate the role of the circadian clock system in these processes.
The Circadian Clock System From Hypothalamus to Pituitary
In the hypothalamus, the SCN generates timed signals to activate gonadotropin-releasing hormone (GnRH) neurons and stimulate LH release from pituitary gonadotrope cells. There are 2 peptidergic pathways that the SCN regulates in GnRH neurons: vasoactive intestinal peptide (VIP)–containing neurons project directly to GnRH neurons, and arginine vasopressin (AVP)–containing neurons project to anteroventral periventricular nucleus (AVPV) Kisspeptin neurons to evoke the preovulatory GnRH/LH stimulation (29-35). Regardless of receiving afferent signals from the SCN, AVPV Kisspeptin neurons were reported to host an estradiol (E2)-sensitive circadian oscillator (36, 37). In murine AVPV explants, sustained autonomous oscillations were observed for up to 4 days (36). In vivo, AVPV Kisspeptin neurons presented daily rhythm related to the estrous cycle—a phase delayed by 2.8 hours at diestrus when circulating E2 is low, compared with proestrus when circulating E2 is high (36). Similarly, apart from the SCN regulation, GnRH neurons express oscillations of endogenous clocks, both in vivo and in the immortalized GnRH-secreting GT1-7 cell line (35, 38, 39). Hence, an intrinsic circadian time-keeping apparatus exists in hypothalamic extra-SCN cells. Normal circadian clock expression is required for the response activity to SCN. One study hypothesized that BMAL1 within GnRH or Kisspeptin neurons provides temporal gating of the LH surge (40). However, unexpectedly, its conditional deletion in Kisspeptin or GnRH neurons did not affect the occurrence of the LH surge (41). In contrast, deleting BMAL1 in global somatic cells presented undetectable LH, along with variable effects on the estrous cycle (42). This suggested that the extra-SCN circadian oscillators in the hypothalamus represent a potential time-keeping system that phases the daily changes in the upstream signals and maintains a steady state.
In the pituitary, gonadotropins are stimulated by the pulsatile release of GnRH. Logically, it is not surprising that the response sensitivity rhythm of gonadotrope cells is modulated by the circadian clock system (43, 44). Previous data suggested that BMAL1–/– females lack both LH and FSH surges. A further study focusing on BMAL1 in gonadotrope cells found only a slight elevation in LH but no noticeable effect on reproductive deficits (45), suggesting that BMAL1 is not necessary in pituitary gonadotrope cells. Currently, there are few studies on the chronobiology of the pituitary, and future studies should pay more attention to its role in the female reproductive system (Fig. 1).
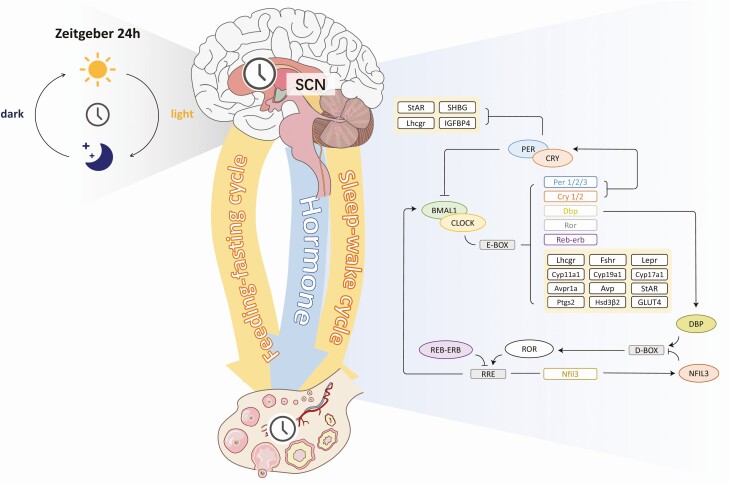
Figure 1.
Organization of the circadian clock system within the female hypothalamic–pituitary–gonadal (HPG) axis. The light entrains the central clock in the suprachiasmatic nucleus (SCN), which drives the daily rhythms of the sleep-wake and feeding-fasting cycles, as well as hormone release within the HPG axis. The circadian clock works at the HPG axis by means of transcription and translation feedback loops. In the main loop, the transcriptional activators CLOCK-BMAL1 activate the transcription of PER, CRY, DBP, ROR, and REV-ERB genes. PER and CRY proteins physically interact and suppress the activities of CLOCK-BMAL1. REVERB and ROR compete to bind with the promoter and enhancer regions of the BMAL1 and play an inhibitory and activation role in transcription, respectively. DBP forms another loop, and it competes with the REV-ERB and ROR target gene NFIL3 to regulate the expression of the clock genes. All these loops control the expression of clock-controlled genes (CCGs), which mediate various reproductive processes. (The yellow box shows the CCGs mentioned in this article.)
The Circadian Clock System in the Ovary
Ovaries have 2 interrelated functions: the generation of mature oocytes and the production of hormones that create an internal environment where fertilization and subsequent implantation can occur. Daily oscillations of clock genes were recently demonstrated in the ovaries, especially in cells within the follicles, including granulosa cells, theca cells, and oocytes (46-49).
In granulosa cells, periodic fluctuations in ovarian steroids, including E2 and small amounts of progesterone, have been observed. The circadian clock regulates this rhythmic hormone fluctuation pattern via aromatase. The latest study using a BMAL1 deletion model showed that Cyp19a1 is involved in the E2 circadian process (50). A previous study confirmed this notion and showed that its promoter region possesses an E-box site that is recognized and bound by clock genes to initiate later transcription (51). Cyp19a1 itself also demonstrates a circadian rhythm. It was identified to peak at ZT16 during the dark period in a 12-hour light/12-hour dark cycle (52). Besides that, the circadian clock also participates in progesterone production, and steroid hormone–related enzyme (StAR) is a key enzyme in this process (8). The clock proteins PER and CRY have an inhibitory effect on StAR (53, 54), whereas the CLOCK-BMAL1 heterodimer activates its transcription via the E-box elements and thus achieves progesterone secretion and implantation (8, 9, 55). Apart from that, during hormone synthesis in granulosa cells, some other genes are also affected by the clock, including the leptin receptor (Lepr), FSH receptor (Fshr), Hsd3β2, Cyp11a1, and Ptgs2 (50).
Rhythmic characteristics also exist in ovarian theca cells, which produce androstenedione with LH stimulation. Physiologically, ovarian sensitivity to LH is determined by the LH receptor (Lhcgr), which presents a daily rhythm under the direct control of the molecular clock (8, 56). This fact is supported by decreased Lhcgr expression levels in response to PER2 or CLOCK siRNA treatment and the altered mRNA abundance patterns in theca cell BMAL1 deletion mice (53, 57). Moreover, the circadian clock affects theca cell steroidogenesis. With the loss of BMAL1 function in theca cells, the synthesis of androstenedione and testosterone is notably impaired, along with decreased mRNA levels of Cyp17a1 (58), which converts progesterone into androgen (59). This implies that clock genes and CCGs within theca cells play a crucial part in maintaining androgen homeostasis and probably participate in the etiology of some reproductive diseases.
In oocytes, classical clock genes are rhythmically transcribed (60), and it has been previously documented that transcript levels vary with the ovarian development stage: the amount of CLOCK, BMAL1, CRY1, and PER1 proteins are significantly higher in rodent oocytes at the germinal vesicle (GV) stage than at the metaphase II (MII) stage (61, 62). Furthermore, the meiotic process is well regulated by the clock gene CRY1, whose knockdown in GV oocytes slows the process. However, its function does not depend on circadian clock regulation (62) (Fig. 1).
Chronodisruption in the HPG Axis
Circadian clock disorders exacerbated by ambient light, time of eating, and nightshift work are increasingly common in modern society. Desynchrony of biological rhythms, especially the disrupted homeostatic oscillations in the HPG axis, can cause dramatic consequences.
Polycystic Ovarian Syndrome
PCOS is a common disorder detected in 10% of women of reproductive age and is characterized by hyperandrogenism, insulin resistance, and oligo-anovulation (63). These 3 characteristics interrelate in PCOS pathogenesis. Evidence suggests that excessive insulin usually accompanies hyperandrogenism (63), and it is widely accepted that insulin resistance inhibits liver sex hormone–binding globulin (SHBG) production and enhances androgen production, which suppresses follicular maturity and diminishes the likelihood of ovulation (64). Even so, the initial cause of this disease remains unclear. Recent studies have confirmed that biorhythm disorders give rise to metabolic and reproductive characteristics of PCOS (65).
Accumulating epidemiological evidence supports that misalignment of sleep–wake behavior is an important contributor to insulin resistance in PCOS. Early studies suggest that sleep disturbances, especially the difficulty of achieving and maintaining sleep, are twice as common in PCOS (66). In healthy adults, 5 days of insufficient sleep was proven to lead to a 20% decrease in insulin sensitivity (67), and in girls with PCOS, a disrupted morning rhythm is associated with worse insulin sensitivity (68). These phenomena have also been found in rodents. Continuous light exposure could induce PCOS-like ovarian changes and glucose metabolism disorders with decreased islet beta-cell function (69). Likewise, a constant darkness treatment led to consistent results, along with arrhythmic BMAL1 expression that promoted insulin resistance via GLUT4 (65). More importantly, such PCOS-like alternations are reversible. Treating with melatonin or restoring normal light/dark exposure can alleviate these hallmarks, including hyperinsulinemia and hyperandrogenism (65). Obstructive sleep apnea (OSA), a condition characterized by recurrent upper airway instability during sleep, has been a recent focus of research, as it affects almost one-third of PCOS patients (70). Although OSA is prevalent in sleep and metabolic disorders, the causality between PCOS and OSA is controversial. A previous study argued that sleep fragmentation induced by OSA in PCOS exacerbates insulin resistance and contributes to the development of type 2 diabetes (71). In contrast, insulin resistance and postprandial hyperglycemia can be relieved after OSA treatment with continuous positive airway pressure (72). Even so, its molecular mechanism is still inconclusive.
A large amount of evidence strongly links the relationship between the circadian clock and androgen production. The clock protein PER2 displays a morning peak (73) similar to the fluctuation pattern of dehydroepiandrosterone, dehydroepiandrosterone sulfate, androstenedione, and dihydrotestosterone (DHT) (74). Accordingly, PER2 is probably involved in androgen production. This hypothesis has been supported by the fact that decreased PER2 levels promote androgen production via insulin-like growth factor-binding protein 4 (IGFBP4) and SHBG in the liver in irregular daylight (65). In turn, the hyperandrogenic status owns the capacity to reset the circadian clock system programming. In rodents exposed to DHT at weaning, the internal circadian organization is weakened and characterized by disrupted PER1 phase distribution and discordant peripheral synchrony (75). In human granulosa cells, the testosterone stimulation can alter the oscillating pattern of PER2 and StAR (76). Thereby, the interplay between the circadian clock and steroidogenesis may participate in the pathophysiological regulation of PCOS.
Oligo-anovulation is a typical feature of PCOS, owing to impaired oocyte development induced partially by circadian rhythm disruption (65). Its classical ovarian morphology is the increased number of atretic antral follicles (77, 78), triggered by the apoptosis of granulosa cells (79, 80). A recent study confirmed that hyperinsulinemia and hyperandrogenism share a bidirectional link, and both of them promote abnormal clock gene expression and induce granulosa cell apoptosis (65). Despite the relationship between circadian rhythm and apoptosis having been revealed in multiple cell types (81, 82), few studies have focused on ovarian granulosa cells (Fig. 2; Table 1).
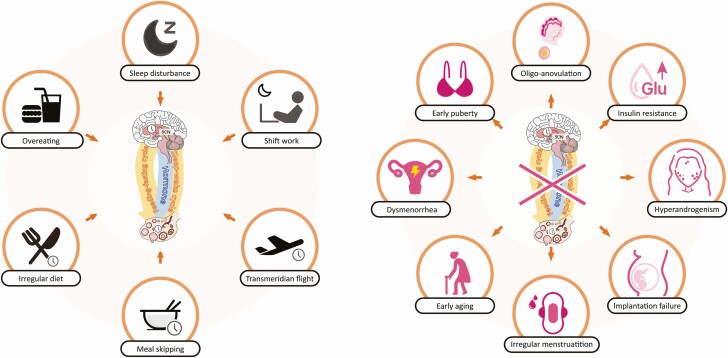
Figure 2.
Chronodisruption caused by environmental cues within the female HPG axis. (A) Environmental cues that lead to circadian rhythm disruption within the HPG axis. (B) Circadian clock misalignment induces multiple pathophysiological alternations in the female reproductive system.
Table 1.
Effect of mutated clock genes and the potential diseases in the female reproductive system
| Mutated genes | Effect | Potential disease | References |
|---|---|---|---|
| BMAL1 | Irregular estrous cycles | POI; PCOS | (55) |
| Abnormal StAR expression | POI | (8) | |
| Insulin resistance | PCOS | (65) | |
| CLOCK | Irregular estrous cycle | POI; PCOS | (28) |
| Undetectable LH surges | POI; PCOS | (28) | |
| PER | Excessive androgen | PCOS | (65) |
| Irregular estrous cycle | POI; PCOS | (54) | |
| Lower reproductive rate with fewer litters | POI | (54) | |
| CRY | Reduced oocyte maturation ability | POI; PCOS | (62) |
| Irregular estrous cycle | POI; PCOS | (84) | |
| Infertility | POI; PCOS | (84) |
Premature Ovarian Insufficiency
Premature ovarian insufficiency (POI) is a heterogeneous disease termed as insufficient ovarian activity before 40 years of age with high gonadotropins and low estrogen (83). Few studies have proposed a correlation with clock genes. However, the declining ovarian reserve has been observed in multiple clock gene–deficient models and shiftwork women and results from endogenous or environmentally imposed circadian misalignment (6, 49).
PER and CRY are probably involved in the occurrence of POI, owing to their association with senescence (54, 84). PER or CRY rhythm disruption accelerates aging in the reproductive capacity, and the level of PER1 has also been noted to decline during the physiological aging process (85). In rodents, middle-aged (9- to 12-month-old) PER mutant females display a significantly lower reproductive rate with fewer litters. Their estrous cycles, characterized by prolongation and acyclicity, are similar to that of 13- to 16-month-old females (54). Moreover, a recent study generated mice with PER1 and PER2 double knockout that display decreased fertility since approximately 20 weeks old, with notably fewer litters born from 32 weeks old and onwards (6). It seems that there exists a synergistic effect between PER1 and PER2 in the early onset of fertility decline. Likewise, CRY presents similar functions in early aging phenotypes (84), and, interestingly, such conditions could be restored. When tuning the light–dark cycles closely to the endogenous one inherent in CRY-deficient females, the estrous cycles recovered and fertility improved (84). Hence, reproductive aging could benefit from the optimal timing of environmental signals. Furthermore, as evidenced by significantly reduced follicle number in PER1 and PER2 double mutated mice (6), clock-related advanced aging may be due to impaired follicular development. This implies that altered PER and CRY circadian rhythms and circadian regulatory circuits could be an important factor contributing to POI.
CLOCK and BMAL1 participate in reproductive processes in mammals, and their deficiency exhibits ovulation dysfunction and decreased fertility (55, 86), suggesting that they may induce POI. CLOCK affects the possibility of ovulation and conception, mediated by the LH surge (28). It has been reported that CLOCK mutant mice display an extended, irregular estrous cycle without an LH surge on the afternoon of proestrus (28). Moreover, within their hypothalamus, the expression of both AVP in the SCN and AVP 1a receptor (Avpr1a) is decreased (87). After supplementing AVP, the LH surge could be reversed to some extent (87). Thereby, CLOCK is essential for the LH surge through the regulation of hypothalamic AVP signaling and may be involved in POI. In addition, BMAL1 maintains proper fertility capacity via downstream hormone signaling molecules. Cyp17a1 and Cyp19a1 are essential downstream factors of BMAL1, presenting a circadian rhythm and producing androgens and estrogens, respectively (51, 58). StAR is the other essential enzyme downstream of BMAL1 (8, 9, 55, 88). It was reported that the ovaries from StAR knockout mice retain only a few scattered follicles with abundant stromal cells, coinciding with the features of POI (89). This phenotype can, in part, explain the fertility decline disease in humans—congenital lipoid adrenal hyperplasia. This disease induced by a StAR mutation and failure to move cholesterol into the mitochondria leads to accumulated cholesterol esters that damage steroidogenic capacity and result in POI (90, 91) (Fig. 2 and Table 1).
Premenstrual Syndrome
Premenstrual syndrome (PMS) is a mood disorder with a high prevalence of sleep disturbances (92). Although the current study was at its initial stage, it documented that sleep disturbance and the subsequent suppression in nocturnal melatonin underlie the circadian rhythm disruption in PMS (93, 94). Additionally, evening light therapy that phase shifts the melatonin rhythm could effectively improve the anxiety and mood of these patients (95). It indicated that melatonin-specific treatment might hold new promise for patients with PMS in the future (Fig. 2).
Dysmenorrhea
Dysmenorrhea, also known as menstrual cramps, is a rhythmic pain just before or during menstruation. A meta-analysis from 1990 to 2018 revealed a negative relationship between meal skipping and the severity of primary dysmenorrheal (96). Likewise, data from Palestine and China both drew similar conclusions and showed that skipping breakfast was the strongest predictor of this disorder (97, 98). Recently, a hypothesis was put forward that a disrupted feeding rhythm in adolescence could trigger endometriosis, which is the most common cause of secondary dysmenorrheal (99). This novel conception implies that the immature reproductive system in young ages may be susceptible to interference by external cues, but how exactly they impinge on later disorders encourages more work (Fig. 2).
Central Precocious Puberty
Resulting from the premature activation of the HPG axis, central precocious puberty is commonly defined as puberty that starts at an unusually early age (before 8 years in girls) (100). During puberty, testis development in male golden hamsters has been proven to be photoperiodically controlled (101). However, little evidence proves the role of biorhythm in female puberty. Melatonin functions to restrain pubertal onset (102), which is consistent with a recent study in which melatonin was low and periods of sleep were short in precocious puberty patients (103). In contrast, another study held the opposite notion in its 24-hour profile where there was no difference between patients with precocious puberty and those with normal puberty (104, 105). Hence, larger sample size population studies in the future need to clarify the relevance, and female animal models are needed to reveal the underlying mechanism.
Current Progress in Circadian Medicine for the Female Reproductive System
Circadian medicine is a strategy to treat diseases by adapting to the body’s original circadian rhythms. It includes light exposure modulation, behavioral modulation, pharmacological interventions, and chronotherapy.
As mentioned above, light therapy relieves anxiety in PMS (95). Similarly, for PCOS, several pieces of evidence support the efficacy of light exposure modulation. In rodents, the PCOS model has been successfully built by continuous light exposure (69, 106). The increased prevalence of PCOS in night-shift workers has been proven in a clinic survey, indicating the risk of artificial lighting (18). In line with the finding, artificial light exposure at night while sleeping increased the risk of being overweight or obese (107), which is also characteristic of PCOS (63). Hence, as the main input for the SCN, light modulating can reset circadian synchrony (108) and is consequently a potential treatment strategy for PCOS.
Behavioral modulation is another treatment. Owing to the strong relevance between disturbed sleep and PCOS (66, 109), the guideline has suggested screening PCOS patients for symptoms suggestive of OSA and, when identified, providing appropriate treatment (110). Individualized nutrition therapy is another core intervention. In addition to various dietary strategies of meal composition (107, 111-113), 8-hour time-restricted feeding has been proposed as a novel intervention that effectively reduces body fat and improves menstruation, hyperandrogenemia, and insulin resistance (114).
When lifestyle modification alone is insufficient, pharmacological interventions for better managing the diseases are unavoidable. As a chronobiotic agent, melatonin has a special place in PCOS. Early studies have identified its potential as a therapeutic agent to improve in vitro fertilization outcomes in PCOS patients (115, 116). Currently, its favorable effect on PCOS has also been identified in a randomized controlled trial to improve hirsutism and serum tumor necrosis factor-α and total antioxidant capacity levels (117). Furthermore, in terms of metabolism, melatonin can improve lipid metabolism in PCOS patients with endometrial cancer (118). For other diseases such as PMS and precocious puberty, where melatonin levels are lower than in the normal population, melatonin supplementation or receptor agonists may be helpful (93, 94, 103). Besides, some circadian molecule drugs that enhance the activity of transcription factors such as PER2, CRY, REV-ERBα, and RORα (119) are currently under development for various diseases. Among them, CRY has been identified to alleviate PCOS-induced damage to ovarian tissue (120), insulin resistance (121), and reproductive disturbances (122) in rats. Therefore, it is possible to enhance CRY activity by small molecule agonists, affecting its circadian rhythm within the HPG axis to treat and control PCOS. Last but not least, the concept of chronotherapy was put forward in the past few years, which refers to the timed dosing of drugs to enhance treatment efficacy and patient tolerance (123). However, the application of this field is still relatively unknown with regard to the female reproductive system and will be a direction of future research.
Remaining Questions and Future Direction
The normal routine of working at sunrise and resting at sunset has been gradually eroded in the social development process. Instead, high-intensity working patterns along with consequent eating and sleeping disorders and other “modern diseases” are becoming the main theme of daily life. Therefore, larger population studies are needed to characterize this remolding of the circadian rhythm. On this basis, chronobiology can help reverse poor physical conditions and improve existing treatment methods.
As early as 1994, the United Nations highlighted the importance of reproductive health in human development. The decline in female fertility resulting from abnormal circadian rhythms caused by unhealthy diet and sleep habits in modern life has become a global health concern (124, 125). Therefore, breaking the “time code” of the female reproductive system is an urgent task. Despite a large number of studies at present, most studies confine the role of a certain clock gene to a specific cell. As the female reproductive system is by no means a simple superposition of cells and tissues, future chronobiology studies are encouraged from a holistic perspective. Furthermore, constructing and improving the circadian clock network of the female reproductive system is needed to help determine biomarkers that could detect and diagnose circadian rhythm disturbances.
Acknowledgments
Author Contributions: S.Y.S. and Y.Z. designed the study. S.Y.S. wrote the manuscript. S.Y.S. and H.Q.Z. generated the figures and table. H.Q.Z., X.H.L., and Z.Y.L. supervised the study. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare no conflict of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Glossary
Abbreviations
- AVP
arginine vasopressin
- AVPV
anteroventral periventricular nucleus
- CCG
clock-controlled gene
- DHT
dihydrotestosterone
- E2
estradiol
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- GV
germinal vesicle
- HPG
hypothalamic–pituitary–gonadal
- LH
luteinizing hormone
- OSA
obstructive sleep apnea
- PCOS
polycystic ovarian syndrome
- PMS
premenstrual syndrome
- POI
premature ovarian insufficiency
- SCN
suprachiasmatic nucleus
- SHBG
sex hormone–binding globulin
- StAR
steroid hormone–related enzyme
- TTFL
translational feedback loop
- VIP
vasoactive intestinal peptide
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Lemarchand-Béraud T, Zufferey MM, Reymond M, Rey I. Maturation of the hypothalamo-pituitary-ovarian axis in adolescent girls. J Clin Endocrinol Metab. 1982;54(2):241-246. [DOI] [PubMed] [Google Scholar]
- 2. Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57(4):792-796. [DOI] [PubMed] [Google Scholar]
- 3. Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020;21(2):67-84. [DOI] [PubMed] [Google Scholar]
- 4. Silva CC, Domínguez R. Clock control of mammalian reproductive cycles: Looking beyond the pre-ovulatory surge of gonadotropins. Rev Endocr Metab Disord. 2020;21(1):149-163. [DOI] [PubMed] [Google Scholar]
- 5. Wiggins G, Legge M. Cyclic variation of cellular clock proteins in the mouse estrous ovary. J Reprod Infertil. 2016;17(4):192-198. [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng Y, Liu C, Li Y, et al. Loss-of-function mutations with circadian rhythm regulator Per1/Per2 lead to premature ovarian insufficiency†. Biol Reprod. 2019;100(4):1066-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahman SA, Grant LK, Gooley JJ, Rajaratnam SMW, Czeisler CA, Lockley SW. Endogenous circadian regulation of female reproductive hormones. J Clin Endocrinol Metab. 2019;104(12):6049-6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Johnson BP, Shen AL, et al. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc Natl Acad Sci U S A. 2014;111(39):14295-14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1-/- mice. Endocrinology. 2009;150(4):1879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yaw AM, Duong TV, Nguyen D, Hoffmann HM. Circadian rhythms in the mouse reproductive axis during the estrous cycle and pregnancy. J Neurosci Res. 2021;99(1):294-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cederroth CR, Albrecht U, Bass J, et al. Medicine in the fourth dimension. Cell Metab. 2019;30(2):238-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka S, Ueno T, Tsunemi A, et al. The adrenal gland circadian clock exhibits a distinct phase advance in spontaneously hypertensive rats. Hypertens Res. 2019;42(2):165-173. [DOI] [PubMed] [Google Scholar]
- 13. Kloehn I, Pillai SB, Officer L, Klement C, Gasser PJ, Evans JA. Sexual differentiation of circadian clock function in the Adrenal Gland. Endocrinology. 2016;157(5):1895-1904. [DOI] [PubMed] [Google Scholar]
- 14. Cai C, Cai P, Chu G. Selection of suitable reference genes for core clock gene expression analysis by real-time qPCR in rat ovary granulosa cells. Mol Biol Rep. 2019;46(3):2941-2946. [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi M, Watanabe K, Matsumura R, et al. Involvement of the luteinizing hormone surge in the regulation of ovary and oviduct clock gene expression in mice. Genes Cells: Devoted Mol Cell Mechanisms. Published online June 19, 2018. Doi: 10.1111/gtc.12605 [DOI] [PubMed] [Google Scholar]
- 16. Bur IM, Zouaoui S, Fontanaud P, et al. The comparison between circadian oscillators in mouse liver and pituitary gland reveals different integration of feeding and light schedules. PLoS One. 2010;5(12):e15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang F, Xie N, Wu Y, et al. Association between circadian rhythm disruption and polycystic ovary syndrome. Fertil Steril. 2021;115(3):771-781. [DOI] [PubMed] [Google Scholar]
- 19. Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13(5):430-436. [DOI] [PubMed] [Google Scholar]
- 20. Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1675-R1683. [DOI] [PubMed] [Google Scholar]
- 21. Pittendrigh CS, Minis DH. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1972;69(6):1537-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol. 2004;14(16):1481-1486. [DOI] [PubMed] [Google Scholar]
- 23. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Preitner N, Damiola F, Lopez-Molina L, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251-260. [DOI] [PubMed] [Google Scholar]
- 25. Sato TK, Panda S, Miraglia LJ, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43(4):527-537. [DOI] [PubMed] [Google Scholar]
- 26. Ueda HR, Chen W, Adachi A, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418(6897):534-539. [DOI] [PubMed] [Google Scholar]
- 27. Sciarra F, Franceschini E, Campolo F, et al. Disruption of circadian rhythms: a crucial factor in the etiology of infertility. Int J Mol Sci. Published online May 30, 2020. Doi: 10.3390/ijms21113943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14(15):1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev. 2008;57(2):288-298. [DOI] [PubMed] [Google Scholar]
- 31. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264-272. [DOI] [PubMed] [Google Scholar]
- 33. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A. 2015;112(42):13109-13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hickok JR, Tischkau SA. In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology. 2010;91(1):110-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chassard D, Bur I, Poirel VJ, Mendoza J, Simonneaux V. Evidence for a putative circadian kiss-clock in the hypothalamic AVPV in female mice. Endocrinology. 2015;156(8):2999-3011. [DOI] [PubMed] [Google Scholar]
- 37. Smarr BL, Gile JJ, de la Iglesia HO. Oestrogen-independent circadian clock gene expression in the anteroventral periventricular nucleus in female rats: possible role as an integrator for circadian and ovarian signals timing the luteinising hormone surge. J Neuroendocrinol. 2013;25(12):1273-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao S, Kriegsfeld LJ. Daily changes in GT1-7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009;89(4):448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci. 2003;23(35):11202-11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choe HK, Kim HD, Park SH, et al. Synchronous activation of gonadotropin-releasing hormone gene transcription and secretion by pulsatile kisspeptin stimulation. Proc Natl Acad Sci U S A. 2013;110(14):5677-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bittman EL. Circadian function in multiple cell types is necessary for proper timing of the preovulatory LH surge. J Biol Rhythms. 2019;34(6):622-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alvarez JD, Hansen A, Ord T, et al. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23(1):26-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olcese J, Sikes HE, Resuehr D. Induction of PER1 mRNA expression in immortalized gonadotropes by gonadotropin-releasing hormone (GnRH): involvement of protein kinase C and MAP kinase signaling. Chronobiol Int. 2006;23(1-2):143-150. [DOI] [PubMed] [Google Scholar]
- 44. Resuehr D, Wildemann U, Sikes H, Olcese J. E-box regulation of gonadotropin-releasing hormone (GnRH) receptor expression in immortalized gonadotrope cells. Mol Cell Endocrinol. 2007;278(1-2):36-43. [DOI] [PubMed] [Google Scholar]
- 45. Chu A, Zhu L, Blum ID, et al. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology. 2013;154(8):2924-2935. [DOI] [PubMed] [Google Scholar]
- 46. Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod. 2006;75(4):624-632. [DOI] [PubMed] [Google Scholar]
- 47. Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gräs S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology. 2006;147(8):3769-3776. [DOI] [PubMed] [Google Scholar]
- 48. Gräs S, Georg B, Jørgensen HL, Fahrenkrug J. Expression of the clock genes Per1 and Bmal1 during follicle development in the rat ovary. Effects of gonadotropin stimulation and hypophysectomy. Cell Tissue Res. 2012;350(3):539-548. [DOI] [PubMed] [Google Scholar]
- 49. Sellix MT. Circadian clock function in the mammalian ovary. J Biol Rhythms. 2015;30(1):7-19. [DOI] [PubMed] [Google Scholar]
- 50. Chen H, Zhao L, Kumazawa M, et al. Downregulation of core clock gene Bmal1 attenuates expression of progesterone and prostaglandin biosynthesis-related genes in rat luteinizing granulosa cells. Am J Physiol Cell Physiol. 2013;304(12):C1131-C1140. [DOI] [PubMed] [Google Scholar]
- 51. Vanselow J, Fürbass R, Zsolnai A, Kalbe C, Said HM, Schwerin M.. Expression of the aromatase cytochrome P450 encoding gene in cattle and sheep. J Steroid Biochem Mol Biol. 2001;79(1-5):279-288. [DOI] [PubMed] [Google Scholar]
- 52. Chu G, Ma G, Sun J, et al. Leptin receptor mediates bmal1 regulation of estrogen synthesis in granulosa cells. Animals (Basel). Published online November 1, 2019. Doi: 10.3390/ani9110899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shimizu T, Hirai Y, Murayama C, Miyamoto A, Miyazaki H, Miyazaki K. Circadian Clock genes Per2 and clock regulate steroid production, cell proliferation, and luteinizing hormone receptor transcription in ovarian granulosa cells. Biochem Biophys Res Commun. 2011;412(1):132-135. [DOI] [PubMed] [Google Scholar]
- 54. Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction. 2008;135(4):559-568. [DOI] [PubMed] [Google Scholar]
- 55. Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ. Reproductive biology of female Bmal1 null mice. Reproduction. 2010;139(6):1077-1090. [DOI] [PubMed] [Google Scholar]
- 56. Chu G, Yoshida K, Narahara S, et al. Alterations of circadian clockworks during differentiation and apoptosis of rat ovarian cells. Chronobiol Int. 2011;28(6):477-487. [DOI] [PubMed] [Google Scholar]
- 57. Mereness AL, Murphy ZC, Forrestel AC, et al. Conditional deletion of Bmal1 in ovarian theca cells disrupts ovulation in female mice. Endocrinology. 2016;157(2):913-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Y, Chen M, Xu J, et al. Core clock gene Bmal1 deprivation impairs steroidogenesis in mice luteinized follicle cells. Reproduction. 2020;160(6):955-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sasano H, Okamoto M, Mason JI, et al. Immunolocalization of aromatase, 17 alpha-hydroxylase and side-chain-cleavage cytochromes P-450 in the human ovary. J Reprod Fertil. 1989;85(1):163-169. [DOI] [PubMed] [Google Scholar]
- 60. Johnson MH, Lim A, Fernando D, Day ML. Circadian clockwork genes are expressed in the reproductive tract and conceptus of the early pregnant mouse. Reprod Biomed Online. 2002;4(2):140-145. [DOI] [PubMed] [Google Scholar]
- 61. Amano T, Tokunaga K, Kakegawa R, et al. Expression analysis of circadian genes in oocytes and preimplantation embryos of cattle and rabbits. Anim Reprod Sci. 2010;121(3-4):225-235. [DOI] [PubMed] [Google Scholar]
- 62. Amano T, Matsushita A, Hatanaka Y, et al. Expression and functional analyses of circadian genes in mouse oocytes and preimplantation embryos: Cry1 is involved in the meiotic process independently of circadian clock regulation. Biol Reprod. 2009;80(3):473-483. [DOI] [PubMed] [Google Scholar]
- 63. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stener-Victorin E, Padmanabhan V, Walters KA, et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):538-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li S, Zhai J, Chu W, Geng X, Chen ZJ, Du Y. Altered circadian clock as a novel therapeutic target for constant darkness-induced insulin resistance and hyperandrogenism of polycystic ovary syndrome. Transl Res. 2020;219:13-29. [DOI] [PubMed] [Google Scholar]
- 66. Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM. Sleep disturbances in a community-based sample of women with polycystic ovary syndrome. Hum Reprod. 2015;30(2):466-472. [DOI] [PubMed] [Google Scholar]
- 67. Eckel RH, Depner CM, Perreault L, et al. Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr Biol. 2015;25(22):3004-3010. [DOI] [PubMed] [Google Scholar]
- 68. Simon SL, McWhirter L, Diniz Behn C, et al. Morning circadian misalignment is associated with insulin resistance in girls with obesity and polycystic ovarian syndrome. J Clin Endocrinol Metab. 2019;104(8):3525-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chu W, Zhai J, Xu J, et al. Continuous light-induced PCOS-like changes in reproduction, metabolism, and gut microbiota in Sprague-Dawley rats. Front Microbiol. 2019;10:3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kahal H, Kyrou I, Uthman OA, et al. The prevalence of obstructive sleep apnoea in women with polycystic ovary syndrome: a systematic review and meta-analysis. Sleep Breath. 2020;24(1):339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(10):3878-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165(4):447-452. [DOI] [PubMed] [Google Scholar]
- 73. Fang MZ, Ohman-Strickland P, Kelly-McNeil K, et al. Sleep interruption associated with house staff work schedules alters circadian gene expression. Sleep Med. 2015;16(11):1388-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Davison SL, Bell R. Androgen physiology. Semin Reprod Med. 2006;24(2):71-77. [DOI] [PubMed] [Google Scholar]
- 75. Sellix MT, Murphy ZC, Menaker M. Excess androgen during puberty disrupts circadian organization in female rats. Endocrinology. 2013;154(4):1636-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen M, Xu Y, Miao B, et al. Expression pattern of circadian genes and steroidogenesis-related genes after testosterone stimulation in the human ovary. J Ovarian Res. 2016;9(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wilson JL, Chen W, Dissen GA, et al. Excess of nerve growth factor in the ovary causes a polycystic ovary-like syndrome in mice, which closely resembles both reproductive and metabolic aspects of the human syndrome. Endocrinology. 2014;155(11):4494-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dissen GA, Garcia-Rudaz C, Paredes A, Mayer C, Mayerhofer A, Ojeda SR. Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinology. 2009;150(6):2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hughes FM Jr, Gorospe WC. Biochemical identification of apoptosis (programmed cell death) in granulosa cells: evidence for a potential mechanism underlying follicular atresia. Endocrinology. 1991;129(5):2415-2422. [DOI] [PubMed] [Google Scholar]
- 80. Rajakoski E. The ovarian follicular system in sexually mature heifers with special reference to seasonal, cyclical, end left-right variations. Acta Endocrinol Suppl (Copenh). 1960;34(Suppl 52):1-68. [PubMed] [Google Scholar]
- 81. Sun Y, Wang P, Li H, Dai J. BMAL1 and CLOCK proteins in regulating UVB-induced apoptosis and DNA damage responses in human keratinocytes. J Cell Physiol. 2018;233(12):9563-9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Qin T, Lu XT, Li YG, et al. Effect of Period 2 on the proliferation, apoptosis and migration of osteosarcoma cells, and the corresponding mechanisms. Oncol Lett. 2018;16(2):2668-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic spectrum. Endocr Rev. 2016;37(6):609-635. [DOI] [PubMed] [Google Scholar]
- 84. Takasu NN, Nakamura TJ, Tokuda IT, Todo T, Block GD, Nakamura W. Recovery from age-related infertility under environmental light-dark cycles adjusted to the intrinsic circadian period. Cell Rep. 2015;12(9):1407-1413. [DOI] [PubMed] [Google Scholar]
- 85. Brzezinski A, Saada A, Miller H, Brzezinski-Sinai NA, Ben-Meir A. Is the aging human ovary still ticking?: Expression of clock-genes in luteinized granulosa cells of young and older women. J Ovarian Res. 2018;11(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sen A, Hoffmann HM. Role of core circadian clock genes in hormone release and target tissue sensitivity in the reproductive axis. Mol Cell Endocrinol. 2020;501:110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod. 2006;75(5):778-784. [DOI] [PubMed] [Google Scholar]
- 88. Pan X, Taylor MJ, Cohen E, Hanna N, Mota S. Circadian clock, time-restricted feeding and reproduction. Int J Mol Sci. 2020;21(3). doi: 10.3390/ijms21030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hasegawa T, Zhao L, Caron KM, et al. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. 2000;14(9):1462-1471. [DOI] [PubMed] [Google Scholar]
- 90. Bose HS, Pescovitz OH, Miller WL. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia due to a homozygous frameshift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol METAB. 1997;82(5):1511-1515. [DOI] [PubMed] [Google Scholar]
- 91. Fujieda K, Tajima T, Nakae J, et al. Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest. 1997;99(6):1265-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shechter A, Lespérance P, Ng Ying Kin NM, Boivin DB. Pilot investigation of the circadian plasma melatonin rhythm across the menstrual cycle in a small group of women with premenstrual dysphoric disorder. PLoS One. 2012;7(12):e51929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rahman SA, Marcu S, Kayumov L, Shapiro CM. Altered sleep architecture and higher incidence of subsyndromal depression in low endogenous melatonin secretors. Eur Arch Psychiatry Clin Neurosci. 2010;260(4):327-335. [DOI] [PubMed] [Google Scholar]
- 95. Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997;12(1):47-64. [DOI] [PubMed] [Google Scholar]
- 96. Bajalan Z, Alimoradi Z, Moafi F. Nutrition as a potential factor of primary dysmenorrhea: a systematic review of observational studies. Gynecol Obstet Invest. 2019;84(3):209-224. [DOI] [PubMed] [Google Scholar]
- 97. Abu Helwa HA, Mitaeb AA, Al-Hamshri S, Sweileh WM. Prevalence of dysmenorrhea and predictors of its pain intensity among Palestinian female university students. BMC Womens Health. 2018;18(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hu Z, Tang L, Chen L, Kaminga AC, Xu H. Prevalence and risk factors associated with primary dysmenorrhea among Chinese female university students: a cross-sectional study. J Pediatr Adolescent Gynecol. 2020;33(1):15-22. [DOI] [PubMed] [Google Scholar]
- 99. Fujiwara T, Ono M, Mieda M, et al. Adolescent dietary habit-induced obstetric and gynecologic disease (ADHOGD) as a new hypothesis-possible involvement of clock system. Nutrients. Published online May 2, 2020. Doi: 10.3390/nu12051294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016;4(3):265-274. [DOI] [PubMed] [Google Scholar]
- 101. Hance MW, Mason JI, Mendis-Handagama SM. Effects of photo stimulation and nonstimulation of golden hamsters (Mesocricetus auratus) from birth to early puberty on testes structure and function. Histol Histopathol. 2009;24(11):1417-1424. [DOI] [PubMed] [Google Scholar]
- 102. Attanasio A, Borrelli P, Gupta D. Circadian rhythms in serum melatonin from infancy to adolescence. J Clin Endocrinol Metab. 1985;61(2):388-390. [DOI] [PubMed] [Google Scholar]
- 103. de Holanda FS, Tufik S, Bignotto M, et al. Evaluation of melatonin on the precocious puberty: a pilot study. Gynecol Endocrinol. 2011;27(8):519-523. [DOI] [PubMed] [Google Scholar]
- 104. Ehrenkranz JR, Tamarkin L, Comite F, et al. Daily rhythm of plasma melatonin in normal and precocious puberty. J Clin Endocrinol Metab. 1982;55(2):307-310. [DOI] [PubMed] [Google Scholar]
- 105. Waldhauser F, Boepple PA, Schemper M, Mansfield MJ, Crowley WF Jr. Serum melatonin in central precocious puberty is lower than in age-matched prepubertal children. J Clin Endocrinol Metab. 1991;73(4):793-796. [DOI] [PubMed] [Google Scholar]
- 106. Shaaban Z, Jafarzadeh Shirazi MR, Nooranizadeh MH, et al. Decreased expression of arginine-phenylalanine-amide-related peptide-3 gene in dorsomedial hypothalamic nucleus of constant light exposure model of polycystic ovarian syndrome. Int J Fertil Steril. 2018;12(1):43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Park YM, White AJ, Jackson CL, Weinberg CR, Sandler DP. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern Med. 2019;179(8):1061-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wright KP Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23(16):1554-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mo L, Mansfield DR, Joham A, et al. Sleep disturbances in women with and without polycystic ovary syndrome in an Australian national cohort. Clin Endocrinol (Oxf). 2019;90(4):570-578. [DOI] [PubMed] [Google Scholar]
- 110. Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shang Y, Zhou H, Hu M, Feng H. Effect of diet on insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. Published online July 4, 2020. Doi: 10.1210/clinem/dgaa425 [DOI] [PubMed] [Google Scholar]
- 112. Porchia LM, Hernandez-Garcia SC, Gonzalez-Mejia ME, López-Bayghen E. Diets with lower carbohydrate concentrations improve insulin sensitivity in women with polycystic ovary syndrome: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;248:110-117. [DOI] [PubMed] [Google Scholar]
- 113. Zhang X, Zheng Y, Guo Y, Lai Z. The effect of low carbohydrate diet on polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2019;2019:4386401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. 2021;19(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2016;32(1):69-73. [DOI] [PubMed] [Google Scholar]
- 116. Kim MK, Park EA, Kim HJ, et al. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod Biomed Online. 2013;26(1):22-29. [DOI] [PubMed] [Google Scholar]
- 117. Mousavi R, Alizadeh M, Asghari Jafarabadi M, et al. Effects of melatonin and/or magnesium supplementation on biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. Published online May 19, 2021. Doi: 10.1007/s12011-021-02725-y [DOI] [PubMed] [Google Scholar]
- 118. Stanosz S, von Mach-Szczypiński J, Sieja K, Koœciuszkiewicz J. Micronized estradiol and progesterone therapy in primary, preinvasive endometrial cancer (1A/G1) in young women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2014;99(12):E2472-E2476. [DOI] [PubMed] [Google Scholar]
- 119. Sen A, Sellix MT. The circadian timing system and environmental circadian disruption: from follicles to fertility. Endocrinology. 2016;157(9):3366-3373. [DOI] [PubMed] [Google Scholar]
- 120. Yang Y, Yang L, Qi C, et al. Cryptotanshinone alleviates polycystic ovary syndrome in rats by regulating the HMGB1/TLR4/NFkappaB signaling pathway. Mol Med Rep. 2020;22(5):3851-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Huang Y, Li W, Wang CC, Wu X, Zheng J. Cryptotanshinone reverses ovarian insulin resistance in mice through activation of insulin signaling and the regulation of glucose transporters and hormone synthesizing enzymes. Fertil Steril. 2014;102(2): 589-596.e4. [DOI] [PubMed] [Google Scholar]
- 122. Xia Y, Zhao P, Huang H, Xie Y, Lu R, Dong L. Cryptotanshinone reverses reproductive disturbances in rats with dehydroepiandrosterone-induced polycystic ovary syndrome. Am J Transl Res. 2017;9(5):2447-2456. [PMC free article] [PubMed] [Google Scholar]
- 123. Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593-628. [DOI] [PubMed] [Google Scholar]
- 124. Mills J, Kuohung W. Impact of circadian rhythms on female reproduction and infertility treatment success. Curr Opin Endocrinol Diabetes Obes. 2019;26(6):317-321. [DOI] [PubMed] [Google Scholar]
- 125. Harter CJL, Kavanagh GS, Smith JT. The role of kisspeptin neurons in reproduction and metabolism. J Endocrinol. 2018;238(3):R173-R183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.