Abstract
Background and Aims
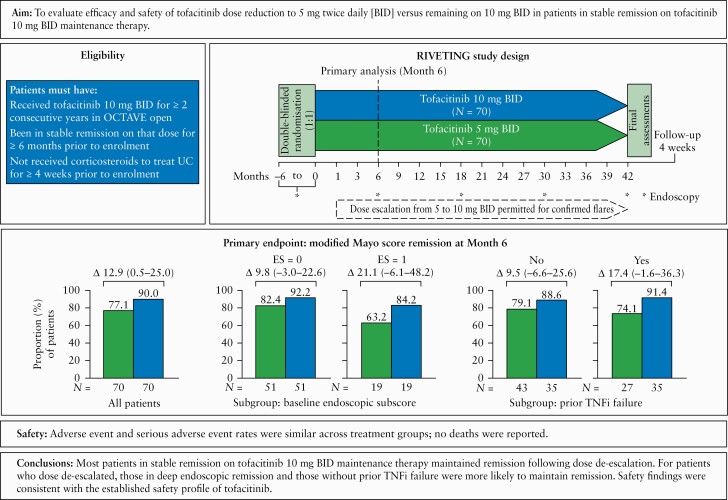
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of ulcerative colitis. We present primary completion analysis from RIVETING, an ongoing, double-blind, randomised, parallel-group trial evaluating efficacy and safety of tofacitinib dose reduction to 5 mg twice daily [BID] versus remaining on 10 mg BID in patients in stable remission on tofacitinib 10 mg BID maintenance therapy.
Methods
Patients had received tofacitinib 10 mg BID for ≥ 2 consecutive years and been in stable remission for ≥ 6 months before enrolment. The primary endpoint was modified Mayo score remission at Month 6. Safety was assessed up to February 20, 2020 [data cut-off].
Results
In all, 140 patients were randomised [1:1] to tofacitinib 5 or 10 mg BID; 77.1% and 90.0% of patients in the 5 and 10 mg BID groups, respectively, were in modified Mayo score remission at Month 6 (adjusted difference 12.9%; 95% confidence interval [CI] 0.5–25.0). Smaller differences between treatment groups were seen in patients with baseline endoscopic subscore of 0 versus 1 [9.8%; –3.0–22.6, and 21.1%; –6.1–48.2, respectively], and in patients without versus with prior tumour necrosis factor inhibitor [TNFi] failure [9.5%; –6.6–25.6, and 17.4%; –1.6–36.3, respectively]. Adverse events [AE] and serious AE rates were similar across treatment groups; no deaths were reported.
Conclusions
Most patients in stable remission on 10 mg BID maintenance therapy maintained remission following dose de-escalation. For patients who dose de-escalated, those in deep endoscopic remission and those without prior TNFi failure were more likely to maintain remission. Efficacy data were limited to the first 6 months; a longer duration of follow-up during RIVETING will further characterise the impact of dose reduction on maintenance of remission. Safety findings were consistent with the established safety profile of tofacitinib.
Keywords: Dose adjustment, maintenance, tofacitinib
Graphical Abstract
Graphical Abstract.
1. Introduction
Ulcerative colitis [UC] is a chronic inflammatory disorder that affects the colon and rectum, and is characterised by relapsing and remitting symptoms that can greatly affect a patient’s quality of life.1–3 The aims of treatment are to achieve sustained steroid-free remission and improve patient quality of life.4,5 However, these must be balanced against the need to reduce medication burden for patients in sustained remission, the potential risks of side effects associated with long-term treatment, and treatment costs.6–8 The flexibility to adjust dosage of a therapy is therefore an important aspect of care.
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC. The efficacy and safety of tofacitinib as induction and maintenance therapy were demonstrated in three global, Phase 3, randomised, double-blind, placebo-controlled trials [OCTAVE Induction 1 and 2, NCT01465763 and NCT01458951; OCTAVE Sustain, NCT01458574] in patients with moderately to severely active UC.9 The tofacitinib Phase 3 clinical programme also includes an ongoing, open-label, long-term extension [OLE] trial [OCTAVE Open, NCT01470612] with tofacitinib 5 and 10 mg twice daily [BID], which enrolled non-responders from OCTAVE Induction 1 and 2, and completers or treatment failures from OCTAVE Sustain.10
Based on the OCTAVE programme results, tofacitinib has been approved for patients with moderately to severely active UC at a dose of 10 mg BID for up to 16 weeks for induction of response, and at a dose of 5 mg BID for maintenance. A dose of 10 mg BID is also permitted during maintenance therapy for patients with loss of response on 5 mg BID maintenance therapy, but should be limited to the shortest duration possible and its use based on the risks and benefits to the individual patient. Currently, tofacitinib labels state that the lowest effective dose needed to maintain response should be used.11,12
While the availability of two tofacitinib maintenance doses provides options for clinicians, it is important to provide further data to assist decisions on optimal maintenance regimens for patients. This question has been partially addressed by data from the OLE trial.8,13 For patients in remission on tofacitinib 10 mg BID at the end of the 52-week OCTAVE Sustain trial, most patients maintained remission following dose de-escalation to 5 mg BID.8 However, those data are limited by their open-label and post-hoc nature, and are not focused on patients in remission for a minimum duration of time, a population more akin to clinical practice for dose reduction.
Here, we present the first data from an ongoing, Phase 3b/4, double-blind, randomised trial [RIVETING, NCT03281304] designed to evaluate the efficacy and safety of tofacitinib in patients with UC in stable remission on 10 mg BID who dose de-escalate to, and remain on, 5 mg BID, compared with patients who remain on 10 mg BID. We report the primary analysis, performed after all patients had either completed their Month 6 trial visit or had dropped out before Month 6. The main objective of this analysis was to estimate the difference in efficacy between tofacitinib 10 and 5 mg BID at Month 6.
2. Materials and Methods
2.1. Trial design and oversight
RIVETING is an ongoing, Phase 3b/4, multicentre, double-blind, randomised, parallel-group trial conducted at 90 sites worldwide [Supplementary Data 1, available as Supplementary data at ECCO-JCC online]. Treatment duration in RIVETING is 42 months; however, this primary analysis was conducted when all enrolled patients completed their Month 6 trial visit and the data were cleaned and locked [February 20, 2020, data cut-off].
The trial protocol was designed by the sponsor [Pfizer Inc] in collaboration with the principal academic investigators. Treatment allocation is described in Supplementary Data 4.1, available as Supplementary data at ECCO-JCC online. Trial investigators and site personnel enrolled patients into the trial. Data were collected by contract research organisations (EPS [Japan]; ICON [all other countries]), analysed by Pfizer Inc, and interpreted by the authors. All authors vouch for the veracity and completeness of the data, and for the fidelity of this report to the protocol. All authors had access to the trial data and reviewed and approved the final manuscript.
2.2. Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
2.3. Patients
Patients were enrolled from the ongoing OLE trial [OCTAVE Open; NCT01470612]. Patients were eligible for RIVETING if they had received tofacitinib 10 mg BID for ≥ 2 consecutive years in the OLE trial and been in stable remission on that dose for ≥ 6 months prior to enrolment. Stable remission was defined as meeting the following criteria: a partial Mayo score of ≤ 2 with no individual subscore of >1, and a rectal bleeding subscore of 0 at each trial visit where data were available during the 6-month period in the OLE trial, prior to and including baseline of the current trial; at least one assessment of remission based on total Mayo score (a confirmed endoscopic subscore of 0 or 1 [locally read] was required in the 6 months prior to randomisation, and all assessments based on Mayo score during this period were required to show remission); and no corticosteroid use to treat UC for at least 4 weeks prior to baseline. Concomitant corticosteroids were prohibited in RIVETING; concomitant oral 5-aminosalicylic acid or sulphasalazine were permitted. Females of childbearing potential were required to use highly effective contraception.
2.4. Randomisation and treatments
Patients were randomised in a 1:1 ratio to receive either tofacitinib 5 or 10 mg BID at baseline, stratified by endoscopic subscore [0 vs 1] from their most recent endoscopy [locally read] prior to or at baseline. Randomisation was performed centrally using a telerandomisation system. Dose adjustments were permitted after the Month 1 trial visit if meeting protocol-specified flare criteria. If a patient experienced an increase in clinical symptoms during the trial, an endoscopy [locally read] was performed to document if the patient was experiencing a flare. Flare was defined as meeting at least one of the following four criteria: an increase in rectal bleeding subscore of ≥ 1 point and an increase in endoscopic subscore of ≥ 1 point; an increase in rectal bleeding subscore of ≥ 2 points and an endoscopic subscore of >0; an increase in stool frequency subscore of ≥ 2 points and an increase in endoscopic subscore of ≥ 1 point; or an increase in endoscopic subscore of ≥ 2 points. When flare was confirmed, the patient’s dose either increased to 10 mg BID or remained on 10 mg BID, depending on their initial treatment assignment which remained blinded.
Following protocol amendment 2 [June 19, 2019], at each trial visit all patients were required to undergo a risk-factor check for newly developed pulmonary embolism [PE] risk factors [Supplementary Data 4.2]. Patients identified as having one or more risk factors were transferred from blinded therapy [tofacitinib 5 or 10 mg BID] to open-label tofacitinib 5 mg BID and were not permitted to dose-escalate to 10 mg BID due to flare.
2.5. Efficacy and safety evaluations
Total Mayo score and modified Mayo score (Mayo score without Physician’s Global Assessment [PGA]) were assessed at baseline and Month 6 by endoscopy [locally read]. Partial Mayo score [all subscores of total Mayo score except endoscopic subscore] was assessed at Months 1, 3 and 6. The following assessments occurred throughout the trial: contraceptive check for females of childbearing potential; adverse event [AE] monitoring; concomitant medication review; and PE risk-factor check [from June 19, 2019].
2.6. Endpoints
Full details of protocol specified endpoints are in Supplementary Table 1, available as Supplementary data at ECCO-JCC online; endpoints included in this primary analysis are indicated. The primary efficacy endpoint was remission based on modified Mayo score [an endoscopic subscore of ≤ 1, stool frequency subscore of ≤ 1, and a rectal bleeding subscore of 0] at Month 6. Secondary efficacy endpoints assessed at Month 6 included: remission based on total Mayo score [a total Mayo score of ≤ 2 with no individual subscore of >1, and a rectal bleeding subscore of 0]; endoscopic improvement [an endoscopic subscore of 0 or 1; defined as ‘mucosal healing’ in the protocol, written prior to new guidance from regulatory bodies]; remission based on modified partial Mayo score [a stool frequency subscore of ≤ 1 and a rectal bleeding subscore of 0]; remission based on partial Mayo score [a partial Mayo score of ≤ 2, with no individual subscore of ≥ 1 and a rectal bleeding subscore of 0]; clinical response based on total Mayo score [a decrease from induction study baseline total Mayo score of ≥ 3 points and 30%, plus a decrease in rectal bleeding subscore of ≥ 1 point or an absolute rectal bleeding subscore of 0 or 1]; change from baseline at all applicable scheduled visits in faecal calprotectin and high sensitivity C-reactive protein [CRP] levels. Safety endpoints included the incidence of AEs and serious infections. Opportunistic infections [OI], malignancies, gastrointestinal perforations, cardiovascular events, hepatic events, and venous thromboembolic events were evaluated by adjudication committees.
2.7. Statistical analysis
The primary objective was to estimate the difference between tofacitinib 10 and 5 mg BID at Month 6. There is no planned hypothesis testing. The sample size was estimated based on the primary endpoint. With 130 [65/group] patients estimated to be enrolled in the trial, the estimated half-width of the 95% confidence interval [CI] for the treatment difference between tofacitinib 10 and 5 mg BID in proportion of remission at Month 6, based on the modified Mayo score, would be about 16%, assuming the remission rates are greater than 70% in both groups.
Statistical analysis was performed using 6-month primary completion data. Secondary objectives will be addressed upon final [42-month] data availability. For analysis of binary efficacy data, the number and proportion of responders were presented by dose group [based on assigned dose at baseline]. The treatment differences were estimated along with 95% CIs, stratified by endoscopic subscore at baseline [0 vs 1]. The Cochran‐Mantel‐Haenszel weight method was used for the stratified estimation of treatment difference. The stratified CI was constructed using the Newcombe method by Yan and Su.14 Patients with missing data were considered non-responders.
For continuous efficacy endpoints based on total Mayo score evaluated for the primary objective at Month 6, change from baseline was analysed using an analysis of covariance model, with dose group and endoscopic subscore at baseline [0 vs 1] as factors, and baseline score as a covariate. For other continuous efficacy endpoints, change from baseline was analysed using a linear mixed-effects model, with baseline value, dose group, endoscopic subscore at baseline [0 vs 1], visit, and dose group by visit interaction as fixed effects, and subject as a random effect. Adjusted estimations and associated 95% CIs for the overall difference between the two dose groups were computed at each visit.
For safety analyses, all safety data up to the data cut-off date [February 20, 2020] were included. Safety data were summarised based on observed-case data by dose group. Missing data were not imputed. Incidence rates [IRs] of AEs of special interest were calculated, with 95% CIs computed using the Exact Poisson method.
For patients who had a dose escalation to tofacitinib 10 mg BID due to flare or a dose de-escalation to tofacitinib 5 mg BID due to PE risk factors, the data after the dose change are handled as follows. For patients in the 10 mg BID group, data collected after dose de-escalation from 10 to 5 mg BID due to PE risk factors were not included in the safety and efficacy analyses, and last observation carried forward was used for binary and continuous efficacy endpoints. Patients were considered as censored at the time of dose de-escalation for the endpoint of time to loss of remission [flare]. For patients in the 5 mg BID group, data collected after dose escalation from 5 to 10 mg BID [with or without subsequent dose reduction due to PE risk factor] were not included in the safety and efficacy analyses. For binary efficacy endpoints, patients were treated as non-responders for any visit after the dose escalation visit. For continuous efficacy endpoints, data collected for any visit after the dose escalation visit were considered as missing. For time to loss of remission [flare], if patients had a dose escalation without meeting the flare criteria, they were considered as censored at the dose escalation visit.
2.8. Ethical consideration
The trial protocol [available with this article] was approved by the institutional review board or independent ethics committee at each participating centre. All patients provided written informed consent.
3. Results
3.1. Patient characteristics
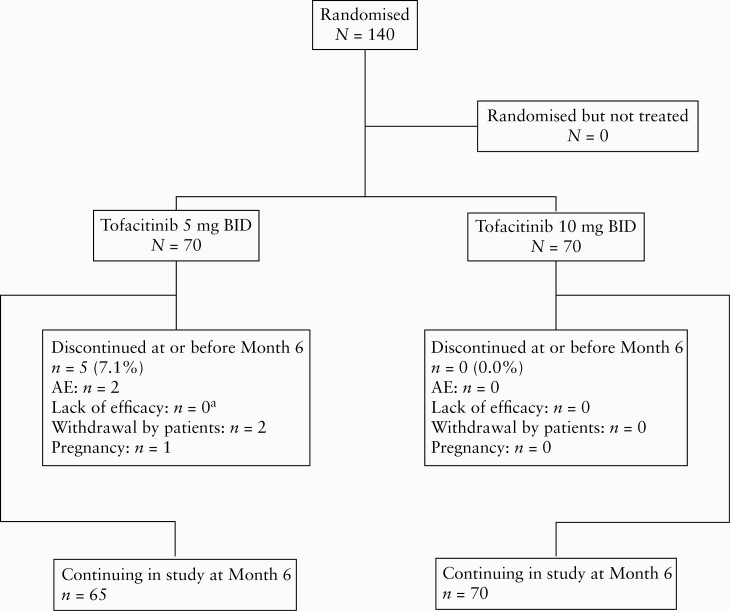
In RIVETING, 140 patients were randomised into two tofacitinib treatment groups: tofacitinib 5 mg BID, n = 70; and tofacitinib 10 mg BID, n = 70. Baseline demographics and clinical characteristics were generally similar across treatment groups, although the proportion of patients with prior TNFi failure was higher in the 10 mg BID group [50.0%] than in the 5 mg BID group [38.6%] [Table 1]. Most patients had an endoscopic subscore of 0 at baseline [72.9% in both treatment groups]. Distributions of Mayo endoscopic subscores between baseline and Month 6, by dose group, are shown in Supplementary Table 2, available as Supplementary data at ECCO-JCC online. Patient dispositions up to Month 6 are shown in Figure 1.
Table 1.
RIVETING baseline demographics and clinical characteristics.
| Tofacitinib 5 mg BID N = 70 | Tofacitinib 10 mg BID N = 70 | Total N = 140 | |
|---|---|---|---|
| Female, n [%] | 26 [37.1] | 22 [31.4] | 48 [34.3] |
| Age [years], mean [SD] | 47.8 [14.1] | 47.8 [13.5] | 47.8 [13.8] |
| Race, n [%] | |||
| White | 50 [71.4] | 50 [71.4] | 100 [71.4] |
| Black | 3 [4.3] | 0 [0.0] | 3 [2.1] |
| Asian | 15 [21.4] | 14 [20.0] | 29 [20.7] |
| Mixed | 0 [0.0] | 1 [1.4] | 1 [0.7] |
| Not reported | 2 [2.9] | 5 [7.1] | 7 [5.0] |
| Region, n [%] | |||
| Europe | 34 [48.6] | 39 [55.7] | 73 [52.1] |
| North America | 14 [20.0] | 12 [17.1] | 26 [18.6] |
| Other | 22 [31.4] | 19 [27.1] | 41 [29.3] |
| Disease duration [years], mean [SD] | 12.5 [7.9] | 13.6 [7.7] | 13.0 [7.8] |
| Endoscopic subscore = 0 at baseline, n [%] | 51 [72.9] | 51 [72.9] | 102 [72.9] |
| Modified Mayo score remission at baseline, n [%]a | 69 [98.6]b | 70 [100] | 139 [99.3] |
| Remission at baseline, n [%]c | 68 [97.1]b | 70 [100] | 138 [98.6] |
| Total Mayo score at baseline, mean [SD] | 0.6 [0.7] | 0.7 [0.8] | 0.6 [0.8] |
| Partial Mayo score at baseline, mean [SD] | 0.4 [0.5] | 0.4 [0.5] | 0.4 [0.5] |
| Prior TNFi failure, n [%] | 27 [38.6] | 35 [50.0] | 62 [44.3] |
| Prior TNFi treatment, n [%] | 30 [42.9] | 37 [52.9] | 67 [47.9] |
| Extent of disease, n [%] | |||
| Proctitis | 0 [0.0] | 0 [0.0] | 0 [0.0] |
| Proctosigmoiditis | 6 [8.6] | 5 [7.1] | 11 [7.9] |
| Left-sided colitis | 23 [32.9] | 26 [37.1] | 49 [35.0] |
| Extensive colitis | 41 [58.6] | 39 [55.7] | 80 [57.1] |
| Smoking status, n [%] | |||
| Current smoker | 1 [1.4] | 3 [4.3] | 4 [2.9] |
| Ex-smoker | 24 [34.3] | 24 [34.3] | 48 [34.3] |
| Never smoked | 45 [64.3] | 43 [61.4] | 88 [62.9] |
BID, twice daily; N, number of patients in the treatment group; n, number of patients within the given category; SD, standard deviation; TNFi, tumour necrosis factor inhibitor.
aModified Mayo score remission was defined as an endoscopic subscore of ≤ 1, a stool frequency subscore of ≤ 1, and a rectal bleeding subscore of 0.
bTwo patients in the 5 mg BID arm were enrolled in error by the trial sites due to a misunderstanding of the enrolment criteria and were subsequently discovered not to be fulfilling all remission criteria by the study monitor [one patient had a total Mayo Score of 3 at baseline of RIVETING as the investigator used the Mayo score prior to baseline for eligibility by mistake; one patient had a stool frequency subscore of 2 at baseline, when the requirement is no subscore ≥ 1 at baseline].
cRemission was defined as a total Mayo score of ≤ 2 with no individual subscore >1, and a rectal bleeding subscore of 0.
Figure 1.
Patient disposition up to Month 6 of the RIVETING trial. AE, adverse event; BID, twice daily; N, number of patients in the analysis population; n, number of patients that withdrew from trial; UC, ulcerative colitis. aPatients who discontinued due to an AE of ‘colitis ulcerative’ [worsening UC] were categorised as lack of efficacy.
3.2. Efficacy
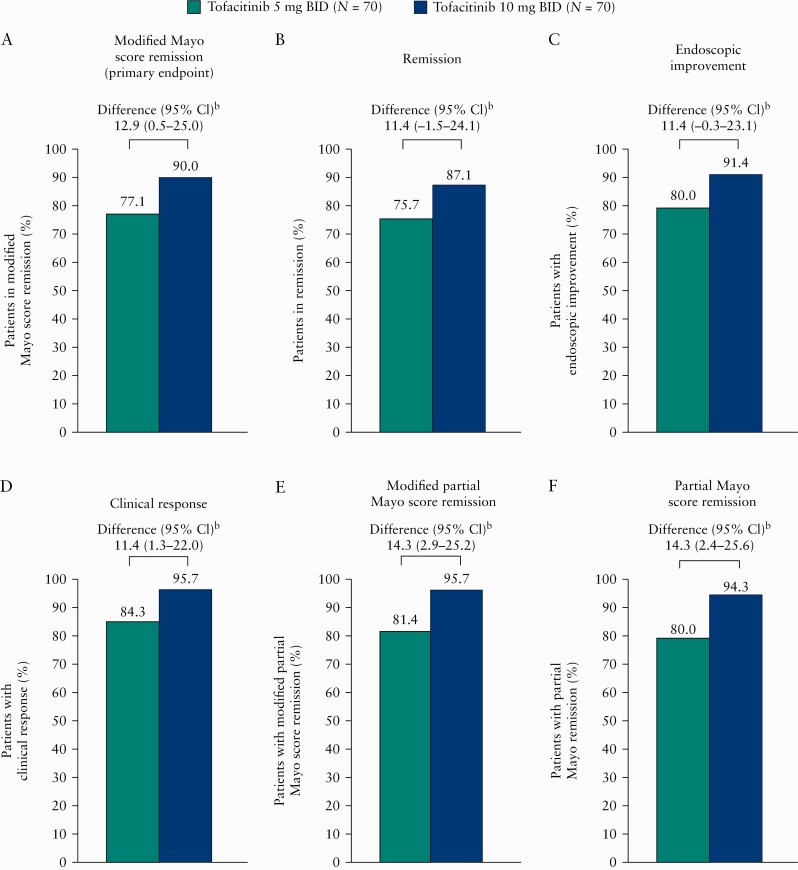
The primary efficacy endpoint [remission based on modified Mayo score at Month 6] was observed in 77.1% and 90.0% of patients in the 5 and 10 mg BID groups, respectively, with an estimated treatment difference of 12.9% [95% CI 0.5–25.0] [Figure 2A]. Similar differences were observed for secondary efficacy endpoints at Month 6 [Figure 2B–F]. Analysis of covariance in change from baseline for both modified Mayo score and total Mayo score at Month 6 showed similar observations between the 5 and 10 mg BID groups [Table 2]. Analysis of changes from baseline in both modified partial Mayo score and partial Mayo score using a linear mixed-effects model also showed similar observations between the two dose groups [Supplementary Table 3, available as Supplementary data at ECCO-JCC online].
Figure 2.
Efficacy endpoints at Month 6 by dose group [FAS, non-respondera]. [A] Proportion of patients in modified Mayo score remission [primary endpoint; defined as an endoscopic subscore of ≤ 1, a stool frequency subscore of ≤ 1, and a rectal bleeding subscore of 0] at Month 6. [B–F] Proportion of patients for secondary efficacy endpoints at Month 6: remission [defined as a total Mayo score of ≤ 2 with no individual subscore >1, and a rectal bleeding subscore of 0]; endoscopic improvement [defined as an endoscopic subscore of 0 or 1]; clinical response based on total Mayo score [defined as a decrease from induction study baseline total Mayo score of ≥ 3 points and 30%, plus a decrease in rectal bleeding subscore of ≥ 1 point or an absolute rectal bleeding subscore of 0 or 1]; remission based on modified partial Mayo score [defined as a stool frequency subscore of ≤ 1 and a rectal bleeding subscore of 0]; remission based on partial Mayo score [defined as a partial Mayo score of ≤ 2, with no individual subscore ≥ 1 and a rectal bleeding subscore of 0]. BID, twice daily; CI, confidence interval; FAS, full analysis set; N, number of patients in the treatment group. aPatients with missing scores were treated as non-responders. Patients in the tofacitinib 5 mg BID group with dose escalation were treated as non-responders for visits after dose escalation. bDifferences are weighted difference based on the Cochran‐Mantel‐Haenszel weight method and 95% CI using the Newcombe method, stratified by baseline endoscopic subscore [0 or 1].
Table 2.
Analysis of covariance in change from baseline for modified Mayo score and total Mayo score at Month 6 [FAS, observed].
| Endpoint | Treatment | N | Least squares mean | SE | Difference from tofacitinib 5 mg BID | |||
|---|---|---|---|---|---|---|---|---|
| Difference | SE difference | 95% CI | ||||||
| Lower | Upper | |||||||
| Modified Mayo score | Tofacitinib 5 mg BID | 67 | 0.6 | 0.2 | ||||
| Tofacitinib 10 mg BID | 68 | 0.3 | 0.2 | –0.3 | 0.2 | –0.8 | 0.1 | |
| Total Mayo score | Tofacitinib 5 mg BID | 67 | 0.9 | 0.2 | ||||
| Tofacitinib 10 mg BID | 68 | 0.4 | 0.3 | –0.6 | 0.3 | –1.2 | 0.1 | |
Change from baseline = treatment + endoscopic subscore at baseline [0 vs 1] + baseline score.
BID, twice daily; CI, confidence interval; FAS, full analysis set; N, number of patients in the analysis set; SE, standard error.
Differences between dose groups for the efficacy endpoints were also evaluated in patients by baseline endoscopic subscore and prior TNFi failure [Table 3]. For the primary efficacy endpoint, observed differences between the 5 and 10 mg BID groups were greater in the subgroup of patients with a baseline endoscopic subscore of 1 [estimated treatment difference: 21.1%; 95% CI –6.1–48.2], than in those with a baseline endoscopic subscore of 0 [estimated treatment difference: 9.8%; 95% CI –3.0–22.6]. Differences were also greater in the subgroup of patients with prior TNFi treatment failure [estimated treatment difference: 17.4%; 95% CI –1.6–36.3], than in those without prior TNFi treatment failure [estimated treatment difference: 9.5%; 95% CI –6.6–25.6]. Similar findings were observed for secondary efficacy endpoints. The CIs overlapped in all subgroup analyses.
Table 3.
Primary and secondary efficacy endpoints at Month 6, by dose group and subgroups [FAS, non-respondera].
| Endpoint, by subgroup | Tofacitinib 5 mg BID n/N [%] | Tofacitinib 10 mg BID n/N [%] | Difference [95% CI]b | |
|---|---|---|---|---|
| Primary efficacy endpoint | ||||
| Modified Mayo score remission | ||||
| Baseline endoscopic subscore | 0 | 42/51 [82.4] | 47/51 [92.2] | 9.8 [–3.0–22.6] |
| 1 | 12/19 [63.2] | 16/19 [84.2] | 21.1 [–6.1–48.2] | |
| Prior TNFi failure | No | 34/43 [79.1] | 31/35 [88.6] | 9.5 [–6.6–25.6] |
| Yes | 20/27 [74.1] | 32/35 [91.4] | 17.4 [–1.6–36.3] | |
| Secondary efficacy endpoints | ||||
| Remission | ||||
| Baseline endoscopic subscore | 0 | 41/51 [80.4] | 46/51 [90.2] | 9.8 [–3.8–23.4] |
| 1 | 12/19 [63.2] | 15/19 [78.9] | 15.8 [–12.6–44.2] | |
| Prior TNFi failure | No | 34/43 [79.1] | 30/35 [85.7] | 6.6 [–10.2–23.4] |
| Yes | 19/27 [70.4] | 31/35 [88.6] | 18.2 [–2.0–38.4] | |
| Endoscopic improvement | ||||
| Baseline endoscopic subscore | 0 | 43/51 [84.3] | 48/51 [94.1] | 9.8 [–2.1–21.7] |
| 1 | 13/19 [68.4] | 16/19 [84.2] | 15.8 [–10.8–42.4] | |
| Prior TNFi failure | No | 36/43 [83.7] | 31/35 [88.6] | 4.9 [–10.4–20.1] |
| Yes | 20/27 [74.1] | 33/35 [94.3] | 20.2 [2.0–38.4] | |
| Modified partial Mayo score remission | ||||
| Baseline endoscopic subscore | 0 | 45/51 [88.2] | 48/51 [94.1] | 5.9 [–5.1–16.8] |
| 1 | 12/19 [63.2] | 19/19 [100.0] | 36.8 [15.2–58.5] | |
| Prior TNFi failure | No | 36/43 [83.7] | 34/35 [97.1] | 13.4 [1.1–25.8] |
| Yes | 21/27 [77.8] | 33/35 [94.3] | 16.5 [–1.0–34.0] | |
| Partial Mayo score remission | ||||
| Baseline endoscopic subscore | 0 | 44/51 [86.3] | 47/51 [92.2] | 5.9 [–6.1–17.9] |
| 1 | 12/19 [63.2] | 19/19 [100.0] | 36.8 [15.2–58.5] | |
| Prior TNFi failure | No | 35/43 [81.4] | 33/35 [94.3] | 12.9 [–1.1–26.8] |
| Yes | 21/27 [77.8] | 33/35 [94.3] | 16.5 [–1.0–34.0] | |
| Clinical response | ||||
| Baseline endoscopic subscore | 0 | 46/51 [90.2] | 49/51 [96.1] | 5.9 [–3.9–15.6] |
| 1 | 13/19 [68.4] | 18/19 [94.7] | 26.3 [3.1–49.5] | |
| Prior TNFi failure | No | 37/43 [86.0] | 33/35 [94.3] | 8.2 [–4.7–21.1] |
| Yes | 22/27 [81.5] | 34/35 [97.1] | 15.7 [0.0–31.3] |
The primary efficacy endpoint was modified Mayo score remission [defined as an endoscopic subscore of ≤ 1, a stool frequency subscore of ≤ 1, and a rectal bleeding subscore of 0] at Month 6. Secondary efficacy endpoints at Month 6 included: remission [defined as a total Mayo score of ≤ 2 with no individual subscore of >1, and a rectal bleeding subscore of 0]; endoscopic improvement [defined as an endoscopic subscore of 0 or 1]; remission based on modified partial Mayo score [defined as a stool frequency subscore of ≤ 1 and a rectal bleeding subscore of 0]; clinical response based on total Mayo score [defined as a decrease from induction trial baseline total Mayo score of ≥ 3 points and 30%, plus a decrease in rectal bleeding subscore of ≥ 1 point or an absolute rectal bleeding subscore of 0 or 1]; remission based on partial Mayo score [defined as a partial Mayo score of ≤ 2, with no individual subscore ≥ 1 and a rectal bleeding subscore of 0].
BID, twice daily; CI, confidence interval; FAS, full analysis set; N, number of patients in the analysis set; n, number of patients meeting the endpoint criteria; TNFi, tumour necrosis factor inhibitor.
aPatients with missing scores were treated as non-responders. Patients in the tofacitinib 5 mg BID group with dose escalation were treated as non-responders for visits after dose escalation.
b95% CI is based on the normal approximation for the difference in binomial proportions.
In the 5 mg BID group, the proportion of patients achieving each efficacy endpoint was consistently higher in the subgroup of patients with a baseline endoscopic subscore of 0, than in the those with a baseline endoscopic subscore of 1 [across efficacy endpoints, differences between these subgroups ranged from 15.9% to 25.0% Table 3]. The proportion of patients achieving each efficacy endpoint was also consistently higher in the subgroup of patients without prior TNFi failure, than in those with prior TNFi failure, although the differences between these subgroups were not as pronounced [across efficacy endpoints, differences between these subgroups ranged from 3.6% to 9.6% Table 3]. In the 10 mg BID group, consistent differences in efficacy endpoints were not observed between the baseline endoscopic subscore or prior TNFi failure subgroups [Table 3].
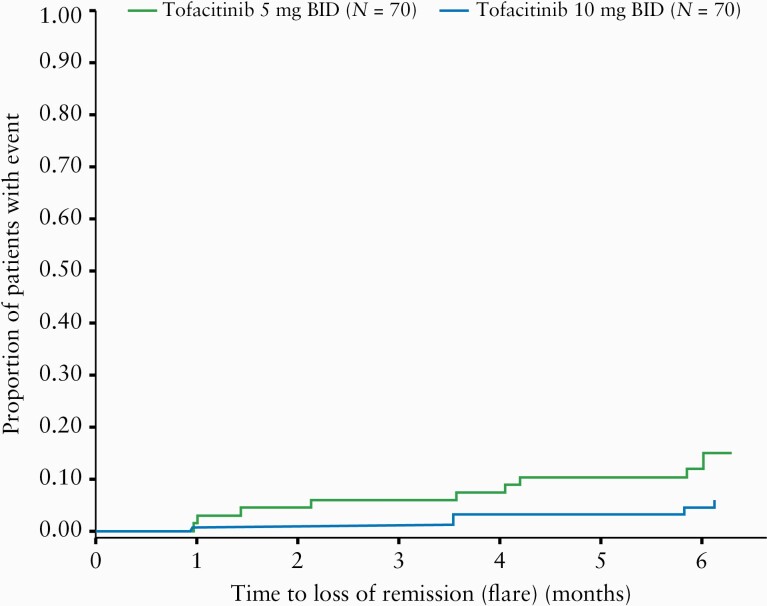
Patients meeting the criteria for flare either had their dose increased to 10 mg BID, or remained on 10 mg BID, depending on initial treatment assignment, which remained blinded [dose adjustments were not permitted before the Month 1 trial visit]. The proportion of patients with loss of remission [flare], based on modified Mayo score up to Month 6, was greater in the 5 mg BID group than in the 10 mg BID group [Figure 3]. The estimated rates of flare at Month 6 were 14.77% and 5.74% in the 5 and 10 mg BID groups, respectively, with a rate difference of –9.03% [95% CI –19.10–1.03] [Supplementary Table 4, available as Supplementary data at ECCO-JCC online]. By the Month 6 trial visit, nine patients in the 5 mg BID group had their dose escalated to 10 mg BID, and three patients in the 10 mg BID group met the criteria for dose escalation [as treatment was blinded, these patients stayed on 10 mg BID].
Figure 3.
Kaplan‐Meier plot of time to loss of remission [flare] based on modified Mayo score [FAS]. BID, twice daily; FAS, full analysis set; N, number of patients in the analysis set.
The biomarker analyses [mean faecal calprotectin, least squares mean change from baseline in log-transformed faecal calprotectin, mean CRP, and least squares mean change from baseline in CRP] were generally consistent between the 5 and 10 mg BID groups at all times up to Month 6 [Supplementary Figures 1 and 2, available as Supplementary data at ECCO-JCC online].
3.3. Safety
Patients had participated in the OCTAVE clinical programme prior to enrolment in RIVETING, which included receiving tofacitinib 10 mg BID for at least 2 years in the OLE trial. The safety data represent all data reported between the RIVETING trial baseline through to the data cut-off date [February 20, 2020]. The median [range] duration of treatment in RIVETING was 538 [29–722] days for patients initially assigned to the 5 mg BID group and 529 [174–804] days for patients initially assigned to the 10 mg BID group [Supplementary Table 5, available as Supplementary data at ECCO-JCC online].
AEs or serious AEs occurred in 67.9% [95 of 140] and 5.7% [8 of 140] of patients, respectively, with similar numbers occurring across the two dose groups [Table 4]. No serious AEs occurred in more than one patient [Supplementary Table 6, available as Supplementary data at ECCO-JCC online]. The proportion of patients who discontinued due to AEs was higher in the 5 mg BID group [10.0%; 7 of 70], than in the 10 mg BID group [2.9%; 2 of 70] [Supplementary Table 7, available as Supplementary data at ECCO-JCC online]. All AEs leading to discontinuation occurred in one patient for each AE, except worsening UC which was reported in two patients receiving 5 mg BID. No patients discontinued the trial due to laboratory values meeting protocol criteria for discontinuation of trial drug [Supplementary Table 8, available as Supplementary data at ECCO-JCC online]. No deaths were reported.
Table 4.
Safety outcomes in the RIVETING trial [SAS].
| 4a | ||||||
|---|---|---|---|---|---|---|
| AEs | Tofacitinib 5 mg BIDaN = 70, n [%] | Tofacitinib 10 mg BID N = 70, n [%] | Total N = 140, n [%] | |||
| Patients with any AEs | 46 [65.7] | 49 [70.0] | 95 [67.9] | |||
| Patients with serious AEs | 4 [5.7] | 4 [5.7] | 8 [5.7] | |||
| Patients with discontinuations due to AEs | 7 [10.0] | 2 [2.9] | 9 [6.4] | |||
| Patients with dose reduction or temporary discontinuation due to AEs | 3 [4.3] | 2 [2.9] | 5 [3.6] | |||
| Deaths | 0 [0.0] | 0 [0.0] | 0 [0.0] | |||
| Most common AEs by system organ class and preferred term | ||||||
| Infections and infestations | 20 [28.6] | 22 [31.4] | 42 [30.0] | |||
| Nasopharyngitis | 6 [8.6] | 7 [10.0] | 13 [9.3] | |||
| Gastrointestinal disorders | 16 [22.9] | 23 [32.9] | 39 [27.9] | |||
| Colitis ulcerative | 10 [14.3] | 9 [12.9] | 19 [13.6] | |||
| 4b | ||||||
| AEs of special interest | n [%] | IR [95% CI]b | n [%] | IR [95% CI]b | n [%] | IR [95% CI]b |
| Herpes zoster [serious and non-serious]c | 1 [1.4] | 1.25 [0.03–6.94] | 3 [4.3] | 3.23 [0.67–9.44] | 4 [2.9] | 2.31 [0.63–5.92] |
| Serious herpes zosterc | 0 [0.0] | 0.00 [0.00–4.56] | 0 [0.0] | 0.00 [0.00–3.83] | 0 [0.0] | 0.00 [0.00–2.08] |
| Opportunistic infectionsc,d | 0 [0.0] | 0.00 [0.00–4.56] | 1 [1.4]e | 1.05 [0.03–5.86] | 1 [0.7] | 0.57 [0.01–3.17] |
| Serious infections | 2 [2.9] | 2.48 [0.30–8.96] | 0 [0.0] | 0.00 [0.00–3.83] | 2 [1.4] | 1.13 [0.14–4.08] |
| MACEc,f | 1 [1.4]g | 1.24 [0.03–6.89] | 0 [0.0] | 0.00 [0.00–3.83] | 1 [0.7] | 0.56 [0.01–3.14] |
| Malignancies [excluding NMSC]c | 1 [1.4]h | 1.24 [0.03–6.93] | 0 [0.0] | 0.00 [0.00–3.83] | 1 [0.7] | 0.57 [0.01–3.15] |
| NMSCc | 1 [1.4] | 1.26 [0.03–7.03] | 0 [0.0] | 0.00 [0.00–3.83] | 1 [0.7] | 0.57 [0.01–3.17] |
| Gastrointestinal perforationsc | 0 [0.0] | 0.00 [0.00–4.56] | 0 [0.0] | 0.00 [0.00–3.83] | 0 [0.0] | 0.00 [0.00–2.08] |
| Deep vein thrombosesc | 0 [0.0] | 0.00 [0.00–4.56] | 0 [0.0] | 0.00 [0.00–3.83] | 0 [0.0] | 0.00 [0.00–2.08] |
| Pulmonary embolismsc | 0 [0.0] | 0.00 [0.00–4.56] | 1 [1.4] | 1.04 [0.03–5.79] | 1 [0.7] | 0.56 [0.01–3.14] |
The safety data presented here represent all safety data reported between RIVETING trial baseline through to the February 20, 2020, data cut-off.
AE, adverse event; BID, twice daily; CI, confidence interval; IR, incidence rate; MACE, major adverse cardiovascular events; N, number of patients in the treatment group; n, number of unique patients with a particular AE; NMSC, non-melanoma skin cancer; SAS, safety analysis set.
aEvents reported after dose escalation were not included in dose group analyses of tofacitinib 5 mg BID.
bIR [number of patients with events per 100 patient-years].
cAdjudicated events.
dExcludes tuberculosis and herpes zoster with two adjacent dermatomes.
eHerpes zoster [non-adjacent or >2 adjacent dermatomes].
fMACE includes fatal MACE, non-fatal MACE, myocardial infarction, cerebrovascular accident, and fatal congestive heart failure.
gMACE [number of events]: cerebrovascular accident [1].
hMalignancies [number of events]: vulvar cancer [1].
The most common AEs by system organ class were infections and infestations, and gastrointestinal disorders, occurring in 30.0% [42 of 140] and 27.9% [39 of 140] of patients, respectively [Table 4]. The proportion of patients with infections and infestations was similar between the 5 and 10 mg BID groups, occurring in 28.6% [20 of 70] and 31.4% [22 of 70] of patients, respectively. Gastrointestinal disorders occurred in a greater proportion of patients in the 10 mg BID group [32.9%; 23 of 70] than in the 5 mg BID group [22.9%; 16 of 70]. However, the proportion of patients with ‘colitis ulcerative’ [worsening UC] was similar, occurring in 14.3% [10 of 70] and 12.9% [9 of 70] of patients in the 5 and 10 mg BID groups, respectively.
Herpes zoster was reported in 2.9% [4 of 140] of patients [Table 4], one case in the 5 mg BID group and three cases in the 10 mg BID group, none of which were classed as serious or severe. Serious infections were reported in 1.4% [2 of 140; cellulitis and urinary tract infection] of patients, both in the 5 mg BID group, and adjudicated OI were reported in 0.7% [1 of 140] of patients [a case of herpes zoster that met the criteria for OI in a patient receiving 10 mg BID]. Major adverse cardiovascular events by adjudication were reported in 0.7% [1 of 140] of patients [a case of cerebrovascular accident in a patient receiving 5 mg BID; the patient was a non-smoker with a history of elevated blood pressure and elevated cholesterol levels throughout the previous OLE trial]. Malignancies (excluding non-melanoma skin cancer [NMSC]) by adjudication were reported in 0.7% [1 of 140] of patients [a case of vulvar cancer in a patient receiving 5 mg BID]. Adjudicated NMSC was reported in 0.7% [1 of 140] of patients [a case of basal cell carcinoma in a patient receiving 5 mg BID]. Adjudicated PE was reported in 0.7% [1 of 140] of patients. There were no cases of gastrointestinal perforations or deep vein thrombosis.
The PE case was reported in a 59-year-old male with a body mass index (BMI) of 29.8 kg/m2 and no known history of PE risk factors. The patient was assigned to receive tofacitinib 10 mg BID in RIVETING, having previously received tofacitinib 10 mg BID during OCTAVE Induction and for 1129 days in the OLE trial, although the patient had received placebo during OCTAVE Sustain. The PE occurred on Day 279 of RIVETING. Available values for the individual components of the Mayo score for this patient were: stool frequency 1, rectal bleeding subscore 0, PGA 0, and endoscopic subscore 0 at baseline and on Day 188; stool frequency 1, rectal bleeding subscore 0, and PGA 0 on Day 273; and PGA and endoscopic subscore 0 on Day 300. Additionally, the patient’s CRP was 0.71 mg/L at baseline, 3.16 on Day 188, 3.10 on Day 273, and 2.95 on Day 300, and the patient’s faecal calprotectin was <30 µg/g at baseline, 278 on Day 188, and 611 on Day 300. Tofacitinib was withdrawn and the patient discontinued the study following the event. The patient recovered from the PE on Day 285.
4. Discussion
The RIVETING trial was designed to provide clinically relevant information regarding the use of tofacitinib 5 and 10 mg BID during maintenance, in particular to address the question of dose reduction in patients with UC in stable remission on tofacitinib 10 mg BID maintenance therapy. The main objective of this primary analysis of the RIVETING trial was to estimate the treatment difference between tofacitinib 10 and 5 mg BID. As this was an estimation study, p-values were not included in the analysis; instead, 95% CIs were reported.
Most patients who dose de-escalated to tofacitinib 5 mg BID remained in remission, although the proportion of patients in remission based on modified Mayo score at Month 6 was greater in the 10 mg BID group than in the 5 mg BID group. Similar findings were observed for secondary efficacy endpoints. These findings show that, for patients in stable remission on 10 mg BID, differences were observed between patients who remained on 10 mg BID versus those who dose de-escalated to 5 mg BID during the short 6-month observation period. These short-term efficacy data may be viewed as providing additional information to incorporate into the totality of evidence for individualised benefit-risk assessment and clinical decision making.
The observed differences in remission based on modified Mayo score or other efficacy endpoints between the 10 and 5 mg BID groups were larger in the subgroup of patients with a baseline endoscopic subscore of 1, compared with those with a baseline endoscopic subscore of 0. When only looking at patients who dose de-escalated to 5 mg BID, more patients with a baseline endoscopic subscore of 0 maintained remission or achieved other efficacy endpoints, than those with a baseline endoscopic subscore of 1. These observations are similar to previous reports of dose reduction with other classes of therapy, including an open-label trial of multimatrix mesalazine where patients who dose-reduced to 2.4 g/day, after achieving complete remission with 4.8 g/day induction dosing, were more likely to remain in remission if they had first achieved endoscopic improvement [mucosal healing, defined as an endoscopy score ≤ 1].15 In addition, our observations are in line with those observed in patients with Crohn’s disease, which showed that for patients taking antimetabolite therapy and infliximab, patients with signs of mild inflammation [by endoscopic scoring or biomarker data] were more likely to relapse after discontinuation of infliximab.16 The implication of these findings is that clinicians should consider directly or indirectly assessing endoscopic improvement prior to consideration of dose reduction.
The observed differences in efficacy endpoints between the 10 and 5 mg BID groups were also larger in the subgroup of patients with prior TNFi treatment failure, compared with those without prior TNFi treatment failure. For patients who dose de-escalated to 5 mg BID, a slightly higher proportion of patients without prior TNFi failure maintained remission or achieved other efficacy endpoints, compared with patients with prior TNFi failure. When interpreting these results, it should be noted that a higher proportion of patients had TNFi failure in the 10 mg BID group [50.0%] than in the 5 mg BID group [38.6%]. Despite this, these findings are consistent with data from the Phase 3 maintenance trial, where the difference in remission between 5 and 10 mg BID at Week 52 of OCTAVE Sustain was higher in patients with prior TNFi treatment failure, than in those without.17 These data further support the assessment of a patient’s response to tofacitinib 10 mg BID and previous UC treatment history before considering a dose reduction.
In a previous post-hoc analysis of the ‘maintenance remission’ subpopulation from OCTAVE Open [patients who were in remission after 52 weeks of tofacitinib 10 mg BID maintenance therapy during OCTAVE Sustain who were assigned to receive 5 mg BID in the OLE trial], 78.9% maintained remission after 2 months and 65.8% after 12 months [non-responder imputation]. In the same analysis, 89.5% of those patients had endoscopic improvement [reported as mucosal healing as per the trial protocol] at Month 2 and 75.0% at Month 12 [non-responder imputation].13 Another post-hoc analysis of this subpopulation showed that duration of remission in OCTAVE Sustain predicted the likelihood of maintaining remission in the OLE trial; maintenance of remission was numerically more likely in patients who had been in remission for ≥ 6 months compared with those who had been in remission for <6 months before dose reduction [proportion of patients in remission at Month 12: 71%, 82%, and 91% for those with <6 months, 6–<12 months, and ≥ 12 months, respectively, in OCTAVE Sustain prior to dose reduction].18 Similar findings have also been reported for sulphasalazine/mesalazine withdrawal in patients with UC; maintenance of remission was more likely to occur in patients who had a longer duration of remission [>2 years] than in those who had a shorter duration of remission [1–2 years] prior to withdrawal.19
Data from the OLE trial are limited by the fact that there was no requirement for patients to be in remission for a minimum duration of time and it was an open-label, non-controlled trial. In contrast, all patients in the RIVETING trial had been in stable remission for ≥ 6 months before enrolment into RIVETING. Additionally, all patients in the ‘maintenance remission’ subpopulation from the OLE had been on tofacitinib for at least 52 weeks in OCTAVE Sustain following successful 8-week induction therapy. In contrast, patients in RIVETING had all been on tofacitinib for longer than 2 years in the OLE, although many of them had also entered the OLE as a treatment failure or non-responder to preceding tofacitinib treatment. Despite these differences, the results previously observed in the OLE trial for dose de-escalation from 10 to 5 mg BID in the ‘maintenance remission’ subpopulation8 are confirmed by this primary completion analysis of RIVETING.
The safety data from this primary completion analysis in patients with UC were consistent with the known safety profile of tofacitinib.20–22 The proportions of patients with AEs and serious AEs were similar between the 5 and 10 mg BID groups, no deaths were reported, and no new or unexpected safety findings were observed.
One case of PE was reported in a 59-year-old male with a BMI of 29.8 kg/m2 in the tofacitinib 10 mg BID group, after having received this dose for 1408 consecutive days across both the OLE and RIVETING trials. Notably, all previously reported cases of venous thromboembolism from the OLE trial occurred in patients with a prior medical history that included risk factors for PE.10,23 Therefore, the case of PE reported in RIVETING is the first case of deep vein thrombosis or PE recorded following long-term treatment with tofacitinib in the UC clinical programme in a patient with no prior medical history that included risk factors for PE, beyond having UC itself, which is a known risk factor for venous thromboembolic events.24–26 It is also worth noting that although the patient’s endoscopic subscore remained at 0 throughout RIVETING, increases in both CRP and faecal calprotectin were recorded, which may indicate increased inflammatory activity contributing to this event. When this is considered with the patient’s age and BMI, the risk of PE is likely to have been elevated.
The RIVETING trial has some limitations; the efficacy data from this primary completion analysis of RIVETING are limited to the first 6 months, and a longer follow-up will be important to further characterise the impact of dose reduction on maintenance of remission. While differences between dose groups were observed for both subgroup analyses [baseline endoscopic subscore and prior TNFi treatment failure status], the relative importance and interaction of these variables in efficacy is unknown, and additional variables could predict the outcome of dose reduction. The ability to address these questions is limited by the small sample size [sample size was limited as the number of patients who were in stable remission in the OLE trial was limited]. Dose escalation data for patients who flare on 5 mg BID in this trial will also be important; there were four dose escalations from 5 to 10 mg BID and one sham escalation in the 10 mg BID group by Month 3, so insufficient data were available to draw conclusions. We acknowledge that given current labelling recommendations, clinical practice has evolved since recruitment began for this trial, so certain subgroups of the study population in RIVETING may be more relevant to these results than the entire trial population. Further post-hoc analyses beyond Month 6 will explore in more detail the dose escalation cases and subgroup stratifications. As the RIVETING trial duration is short and includes a small number of patients, safety data should be interpreted in the context of the well-established, known safety profile of tofacitinib.
In conclusion, most patients in stable remission on 10 mg BID maintenance therapy maintained remission at Month 6 following dose de-escalation to 5 mg BID. For patients who dose de-escalated to 5 mg BID, a higher observed proportion of patients in deep remission [an endoscopic subscore of 0] and a slightly higher observed proportion of patients without prior TNFi failure, maintained remission or achieved other binary efficacy endpoints at Month 6. Although treatment duration in RIVETING is 42 months, per protocol, this primary analysis reported efficacy data that were limited to the first 6 months. A longer duration of follow-up during RIVETING will be important to further characterise the impact of dose reduction on maintenance of remission. The safety profile in this limited dataset was consistent with the established safety profile of tofacitinib across the OCTAVE clinical programme.20,22 These findings may guide clinical management of patients with UC who are prescribed tofacitinib.
Data availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual anonymised participant data. See [https://www.pfizer.com/science/clinical-trials/trial-data-and-results] for more information.
Funding
This study was sponsored by Pfizer Inc.
Conflict of Interest
SV has received grant support from AbbVie, MSD, Pfizer Inc, and Takeda; speaker fees from AbbVie, Dr. Falk Pharma, Ferring Pharmaceuticals, Hospira, MSD, Takeda, and Tillotts; and consulting fees from AbbVie, Celgene, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, Hospira, Janssen, MSD, Mundipharma, Pfizer Inc, Second Genome, Shire, and Takeda. TK has received advisory fees from AbbVie GK, Activaid, Alfresa Pharma, Bristol-Myers Squibb, Celltrion, Covidien, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Janssen, Kissei, Kyorin, Mochida Pharmaceutical, Nippon Kayaku, Pfizer Inc, Takeda, and Thermo Scientific; lecture fees from AbbVie GK, Alfresa Pharma, Astellas, Celltrion, EA Pharma, Gilead Sciences, Janssen, JIMRO, Kyorin, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Takeda, Nippon Kayaku, and ZERIA; and grant support from AbbVie GK, Alfresa Pharma, EA Pharma, Kyorin, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Thermo Scientific, and ZERIA. WJS has received grant support, personal fees, and non-financial support from Pfizer Inc during the conduct of the study; research grants from AbbVie, Amgen, Atlantic Healthcare Limited, Celgene/Receptos, Eli Lilly, Genentech, Gilead Sciences, Janssen, Pfizer Inc, Prometheus Laboratories [now Prometheus Biosciences], and Takeda; consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Eli Lilly, Escalier Biosciences, Ferring Pharmaceuticals, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Oppilan Pharma, Otsuka, Pfizer Inc, Progenity, Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories], Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by the Health Academic Research Trust [HART]), Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, TiGenix, Tillotts, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, and Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Progenity, Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories], Ritter Pharmaceuticals, Ventyx Biosciences, and Vimalan Biosciences. His spouse has received consulting fees from Iveric Bio, Oppilan Pharma, and Progenity; stock options from Escalier Biosciences, Iveric Bio, Oppilan Pharma, Progenity, Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories], Ventyx Biosciences, and Vimalan Biosciences; and is a previous employee of Escalier Biosciences and a current employee of Prometheus Biosciences [merger of Precision IBD and Prometheus Laboratories]. DTR has received grant support from Takeda; and consulting fees from AbbVie, Abgenomics, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene Corp/Syneos, Check-cap, Dizal Pharmaceuticals, Eli Lilly, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, GSK, Ichnos Sciences S.A, Janssen, Narrow River Mgmt, Pfizer Inc, Prometheus Laboratories, Reistone, Shire, Takeda, and Techlab Inc. JP has received personal fees from AbbVie, Arena, Boehringer Ingelheim, Celgene, Celltrion, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, GSK, Immunic, Janssen, Nestlé, Oppilan, Pfizer Inc, Progenity, Takeda, Theravance, and TiGenix. CS, NL, IM, SG, NK, HZ, and WW are all employees and stockholders of Pfizer Inc.
Supplementary Material
Acknowledgments
The authors thank the patients, physicians, and trial team involved in the RIVETING trial. Medical writing support, under the guidance of the authors, was provided by Chris Guise, PhD, CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice [GPP3] guidelines [Ann Intern Med 2015;163:431–4].
Author Contributions
Concept and methodology: CS, NL, HZ, WW. Project administration: CS, NL, IM, SG. Supervision: CS, NL. Data curation: CS, NL, IM, SG, HZ, WW. Investigation: SV, TK, JP. Validation: CS, NL, SG, HZ, WW. Formal analysis, writing, reviewing, and editing the manuscript: SV, CS, NL, TK, WJS, DTR, IM, SG, NK, HZ, WW, JP.
References
- 1. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis 2007;1:10–20. [DOI] [PubMed] [Google Scholar]
- 4. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017;11:769–84. [DOI] [PubMed] [Google Scholar]
- 5. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology . Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501–23; quiz 524. [DOI] [PubMed] [Google Scholar]
- 6. Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2014;40:338–53. [DOI] [PubMed] [Google Scholar]
- 7. Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 2015;149:1716–30. [DOI] [PubMed] [Google Scholar]
- 8. Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther 2020;51:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 10. Lichtenstein GR, Loftus EV Jr, Wei SC, et al. Tofacitinib, an oral, small-molecule Janus kinase inhibitor, in the treatment of ulcerative colitis: analysis of an open-label, long-term extension study with up to 5.9 years of treatment. J Crohns Colitis 2020;14(Suppl 1):S100–1. [Google Scholar]
- 11. European Medicines Agency. Xeljanz [Tofacitinib] ‐ Summary of Product Characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdfAccessed June 12, 2020. [Google Scholar]
- 12. Pfizer Inc. XELJANZ® [Tofacitinib]: Highlights of Prescribing Information.2019. https://labeling.pfizer.com/ShowLabeling.aspx?id=959Accessed August 28, 2020. [Google Scholar]
- 13. Colombel JF, Osterman MT, Ibanez P, et al. Maintenance of remission with tofacitinib in patients with ulcerative colitis: updated results of a subpopulation analysis from an open-label, long-term extension study, OCTAVE Open. J Crohns Colitis 2020;14:S098–9. [Google Scholar]
- 14. Yan X, Su XG. Stratified Wilson and Newcombe confidence intervals for multiple binomial proportions. Stat Biopharm Res 2010;2: 329–35. [Google Scholar]
- 15. Rubin DT, Bradette M, Gabalec L, et al. ; Ulcerative Colitis Remission Study Group. Ulcerative colitis remission status after induction with mesalazine predicts maintenance outcomes: the MOMENTUM trial. J Crohns Colitis 2016;10:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louis E, Mary JY, Vernier-Massouille G, et al. ; Groupe D’etudes Thérapeutiques Des Affections Inflammatoires Digestives . Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012;142:63–70 e65. [DOI] [PubMed] [Google Scholar]
- 17. Dubinsky MC, Peyrin-Biroulet L, Melmed G, et al. Efficacy of tofacitinib in patients with ulcerative colitis by prior tumor necrosis factor inhibitor treatment status: results from OCTAVE induction and maintenance studies. Am J Gastroenterol 2017;112:S354. [Google Scholar]
- 18. Rubin DT, Travis S, Abraham BP, et al. Maintenance of efficacy following tofacitinib dose reduction in patients with ulcerative colitis in stable remission. J Crohns Colitis 2019;13(Suppl 1):S425–6. [Google Scholar]
- 19. Ardizzone S, Petrillo M, Imbesi V, Cerutti R, Bollani S, Bianchi Porro G. Is maintenance therapy always necessary for patients with ulcerative colitis in remission? Aliment Pharmacol Ther 1999;13:373–9. [DOI] [PubMed] [Google Scholar]
- 20. Sandborn WJ, Panés J, Panaccione R, et al. Tofacitinib for the treatment of ulcerative colitis: up to 5.4 years of safety data from global clinical trials. J Crohns Colitis 2019;13:S344. [Google Scholar]
- 21. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17:1541–50. [DOI] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weng MT, Park SH, Matsuoka K, et al. Incidence and risk factor analysis of thromboembolic events in East Asian patients with inflammatory bowel disease, a multinational collaborative study. Inflamm Bowel Dis 2018;24:1791–800. [DOI] [PubMed] [Google Scholar]
- 25. Kappelman MD, Horvath-Puho E, Sandler RS, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut 2011;60:937–43. [DOI] [PubMed] [Google Scholar]
- 26. Bernstein CN, Blanchard JF, Houston DS, Wajda A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost 2001;85:430–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual anonymised participant data. See [https://www.pfizer.com/science/clinical-trials/trial-data-and-results] for more information.