Abstract
V1 is a canonical cortical area with clearly delineated architectonic boundaries and a continuous topographic representation of the visual hemifield. It thus serves as a touchstone for understanding what new mapping methods can tell us about cortical organization. By parcellating human cortex using local gradients in functional connectivity,Wig et al. (2013) detected the V1 border with V2. By contrast, previously-published clustering methods that focus on global similarity in connectivity reveal a supra-areal organization that emphasizes eccentricity bands spanning V1 and its neighboring extrastriate areas; i.e. in the latter analysis, the V1 border is not evident. Thus the focus on local connectivity gradients emphasizes qualitatively different features of cortical organization than are captured by global similarity measures. What is intriguing to consider is that each kind of information might be telling us something unique about cortical organization. Global similarity measures may be detecting map clusters and other supra-areal arrangements that reflect a fundamental level of organization.
Keywords: boundary mapping, functional connectivity, resting-state connectivity, fMRI, Brodmann Area, visual cortex
Early anatomists including Alfred Walter Campbell (1905) and Korbinian Brodmann (1909) parcellated regions of the cerebral cortex based on architectonic differences setting the stage for the modern concept of a brain area. Some architectonic transitions are abrupt such as between V1 and its surrounding region, including V1’s dense layer IV known as the line of Gennari. Other transitions are gradual leading to ambiguities in area definition and even debate about whether all cortical zones possess well-define areas with clear histological boundaries (Lashley and Clark, 1946; see Amunts and Zilles, 2012 for interesting discussion). Incorporating information from anatomic connectivity, topographic organization, and neuronal response properties led to clear evidence that certain zones behave as sharply defined cortical areas (e.g., Kaas, 1987).
Primary visual cortex, V1, is an iconic example of a well-defined brain area. In addition to separation from V2 based on histological criteria, V1 also possesses a distinct connectivity pattern (Felleman and Van Essen, 1991; Markov et al., 2012), characteristic response properties (Hubel and Weisel, 1968; Livingstone and Hubel, 1984; see Sincich and Horton, 2005 for review), and a reversal in the visual field map of polar angles at its borders (Cragg and Ainsworth, 1969; Allman and Kaas, 1971; Van Essen and Zeki, 1978; Gattass et al., 1981). The reversal in the retinotopic map at the vertical meridian is the basis for using stimulus-based functional neuroimaging methods to define V1 (Engel et al., 1997; Sereno et al., 1995; see Wandell and Winawer, 2011 for review) and provides gradient information that allows resting-state correlations to map visual areal borders.
The Primary Visual Field Map
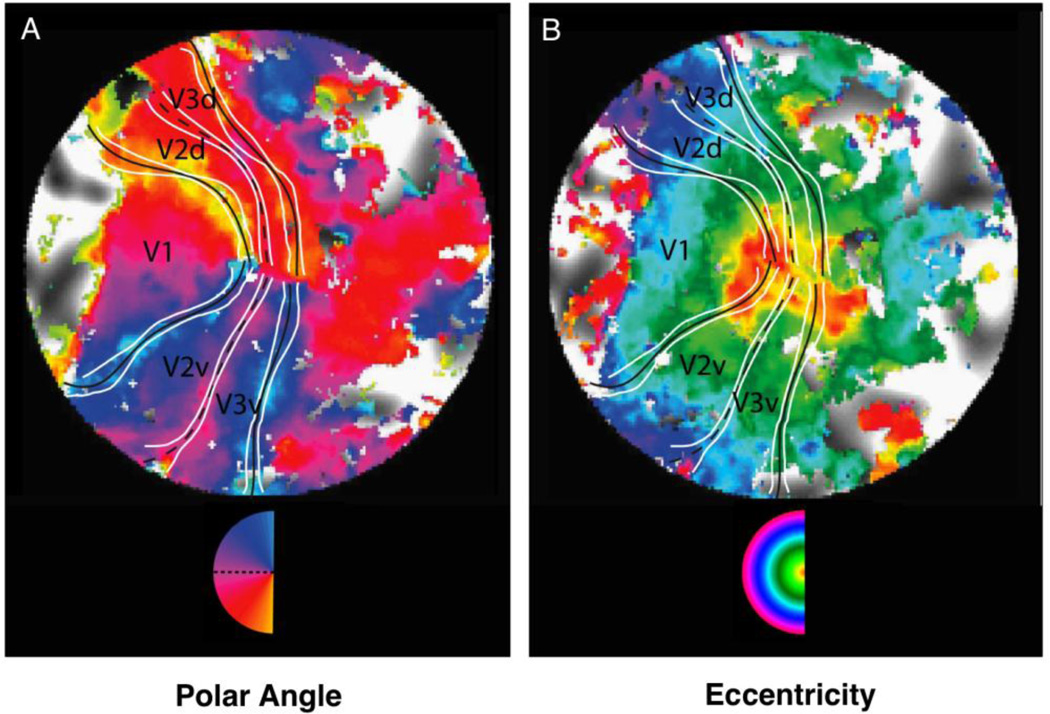
The primary visual area, V1, possesses a complete, continuous map of the visual hemifield (Talbot and Marshall, 1941; Daniel and Whitteridge, 1961). The map is considered a first-order transformation of the visual input because nearby regions of the visual hemifield are always adjacent to each other in the cortical map. Neighboring visual area V2 forms a crescent around V1. The retinotopic representation in V2 is subdivided along the horizontal meridian, so that adjacent visual field locations are split into separate lower and upper quarter-field representations (Van Essen and Zeki, 1978; Gattass et al., 1981). This split makes V2 a second-order map because a field discontinuity is induced beyond that observed in the primary map V11. The representation of polar angle reverses between V1 and V2 such that the border shares a representation of the vertical meridian, but then each map progresses its angular representation in opposite directions (Figure 1 Left). By contrast, along the entire extent of the V1 / V2 border similar eccentricities are mapped forming eccentricity bands that radiate outward from the foveal representation (Figure 1 Right). The border between V1 and V2 can be identified by mapping the reversal in the representation of polar angle at the vertical meridian.
Figure 1. Angular and eccentricity maps of early visual cortex.
The visual hemifield is mapped onto the cortical surface separately for polar angle and eccentricity. Each high-resolution map is from a flattened patch of the same subject’s occipital cortex. The two representations show distinct relations between contiguous visual areas. (A) The polar angle map. The circular color scale shows the polar angle that most effectively drives each cortical location. Polar angle is mapped by displaying a wedge circularly rotating around the visual field like the hand of a clock (see Movie 1 http://www.jneurosci.org/content/29/28/9050/suppl/DC1). The color scale spans the complete left hemifield from yellow representing the lower vertical meridian to blue representing the upper vertical meridian. (B) The eccentricity map. The circular color scale shows the eccentricity that most effectively drives each cortical location. Eccentricity is mapped by displaying an expanding circle that starts near the center of foveal vision and grows larger and larger like the rings of a bullseye (see Movie 2 http://www.jneurosci.org/content/29/28/9050/suppl/DC1). Red and yellow depict the central 0.20○. The outer blue bands are approximately 3○. In each map, vertical meridians are marked with continuous dark lines . The V2/V3 horizontal meridian borders are marked with dotted dark lines. The confidence intervals surrounding the lines are depicted in white. V1, V2d (dorsal), V2v (ventral), V3d, and V3v are labeled. Reprinted with permission from Schira et al. (2009).
Given this background, it is easy to appreciate the major advance that Wig and colleagues (2013) made in using resting-state functional connectivity to map the V1 areal border. By extending the boundary mapping approach initially proposed by Cohen et al. (2008), Wig et al. were able to map the border between V1 and V2 (see their Figure 5). On the one hand, the organization of V1 and the angular map reversal that defines V2 suggest that the V1 border should be readily identifiable. Thus, their results might not come as a surprise. On the other hand, numerous analysis approaches that have explored functional connectivity patterns emphasizing global similarity in connectivity fingerprints, including our own work, were blind to this well accepted feature of cortical organization (e.g., Power et al., 2011; Yeo et al., 2011). Exploring how boundary detection identifies the V1 border and why other methods fail is useful to understand how differences in analysis approaches identify distinct features of cortical organization.
The Essential Distinction Between Local Dissimilarity and Global Similarity
At the core of the issue is the difference between exploring cortical organization based on local dissimilarity versus global similarity in connectivity patterns. The boundary detection approach of Petersen and colleagues (Cohen et al., 2008; Wig et al., 2013) is optimized to detect local transitions or gradients in functional connectivity – places where connectivity patterns change. As they are typically applied, clustering methods (and community detection methods) identify regions of cortex that share similarity in connectivity profiles that are distinct from other profiles. Similarity is defined in relation to global patterns across the brain such that regions are grouped together to maximize their similarity to each other as compared to alternative configurations. Information about local transitions is not considered. As Wig et al. (2013) point out, the “focus on maximizing global separation may come at a cost of more local distinctions.” In some locations, boundary detection and clustering approaches will converge, such as where regions with one similarity profile abut regions with a distinct similarity profile. However in other instances, such as early retinotopic visual cortex, the two methods diverge2. Early visual cortex is thus an illustrative case to understand how emphasis on local dissimilarity versus global similarity uncovers distinct features of cortical organization.
Figure 1 displays retinotopic cortex mapped from high resolution stimulus-based functional MRI (adapted from Schira et al., 2009). Maps of both polar angle and eccentricity are plotted. The black lines show borders between areas. V2 forms a crescent wrapped around V1 on the flattened cortical surface divided into V2d (dorsal) and V2v (ventral). What is striking across the maps is the distinct relationship between V1 and the surrounding areas based on whether polar angle is examined versus eccentricity. Polar angle reveals an abrupt transition throughout the border of V1/V2, corresponding to the shared representation of the vertical meridian. By contrast, the representation of visual field eccentricity reveals a region of high similarity at the foveal representation of V1/V2/V3. Eccentricity gradients smoothly radiate outward from the foveal representation with similar eccentricities mapped near to one another across a V1/V2/V3 supra-areal map3.
These distinct features of polar angle and eccentricity are detected to differing extents by the different analysis approaches. The boundary detection method applied in Wig et al. (2013) identifies the reversal of polar angle representation4. The clustering approach of Yeo et al. (2011) identifies the regions of similar eccentricity that span areas. Clustering approaches are less sensitive to the V1/V2 border because the border is assigned based on local dissimilarity. By contrast, the boundary method is minimally sensitive to the gradual change across the eccentricity gradient, which is detected by global similarity measures (see Figure 15 of Yeo et al., 2011). For these reasons, it is interesting to consider what these distinct features might be telling us about cortical organization.
Implications for Mapping Cortical Areas, Map Clusters, and Association Networks
Wig et al. (2013) demonstrate that resting-state correlations include information about the location of the V1/V2 border. In addition to revealing the boundary using a gradient mapping technique, they also conducted an intuitive analysis to illustrate the correlation structure underlying the detected border (see Figure 5 Wig et al. 2013). They subtracted the correlation for a seed region placed in ventral V2 from a seed region placed in a similar map position of V1. What emerged is a region that follows the contour of the V1 / V2 border. A similar seed region correlation map can illustrate the distinct information captured by clustering approaches. In Figure 2 (right) the correlation structure for a small seed region placed in the periphery of V1 reveals a supra-areal region of correlation that spans multiple visual areas. The clustering analysis of Yeo et al. (2011) divided the peripheral from central representations of V1/V2/V3 without separation of V1 from V2 (see Figure 15 Yeo et al. 2011). The clustering analysis was approximately capturing the correlation structure revealed by the seed region placed in V1 in Figure 2. One might be tempted to dismiss this supra-areal correlation pattern as an incomplete representation of visual cortex organization since it does not find the local transition at the V1/V2d border. However, we suspect that the supra-areal correlation structure may be revealing an important feature of cortical organization that is not captured by analysis of local areal boundaries. To appreciate this perspective it is necessary to revisit the concept of a brain area.
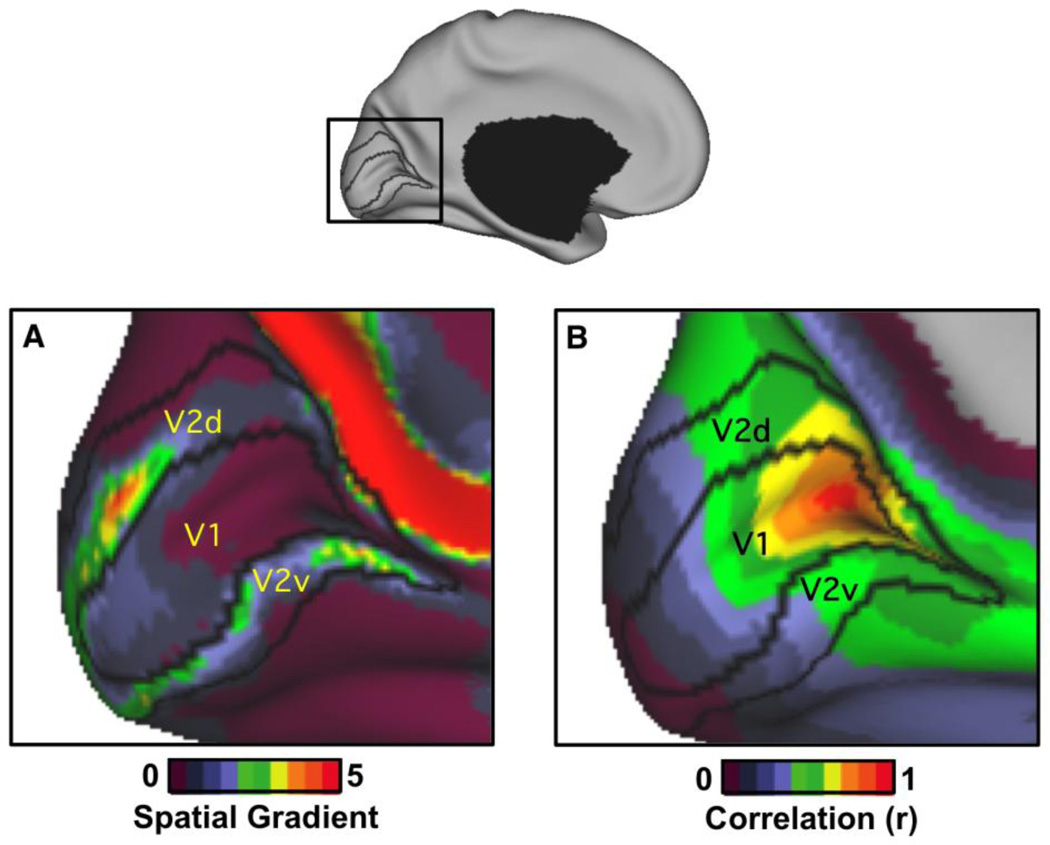
Figure 2. Local spatial gradients and global correlation reveal distinct features of early visual cortex.
Resting-state functional connectivity patterns are plotted in two ways to illustrate distinct features of the correlational structure. In both instances, the exact same data are used from 1000 subjects acquired while subjects rest with their eyes open (Yeo et al., 2011). (A) Highlighting changes in functional connectivity, the spatial gradient of functional connectivity is mapped for visual cortex and overlaid on the V1/V2 architectonic borders. (B) Highlighting global similarity in functional connectivity, the raw correlation for a single seed region placed in peripheral V1 is displayed. The contrast between the two maps illustrates distinct features that boundary mapping and clustering preferentially detect. Boundary mapping is most sensitive to the kind of gradient depicted on the left while clustering is most sensitive to the spatial similarity across space depicted on the right. Specifically, the spatial gradient map displays peak values along the V1/V2 border. In contrast, the peripheral V1 seed region exhibits a supra-areal pattern of correlation spanning V1, V2, and V3. The peripheral V1 seed region is only weakly correlated with central V1 but is strongly correlated to regions of V2 and V3 with similar eccentricity. Quantification of this effect can be found in Yeo et al. (2011) Figures 17 and 18. The gradient map was defined as in Cohen et al. (2008) with the spatial gradient computed via minimally metric-distorted spherical representation as in Yeo et al. (2010). The boundary maps presented in Wig et al. (2013) are processed considerably more than the raw gradient maps illustrated here. Montreal Neurological Institute (MNI) coordinates of the V1 peripheral seed region are -16, - 74, 7. V1/V2 architectonic borders were derived from Fischl et al. (2008).
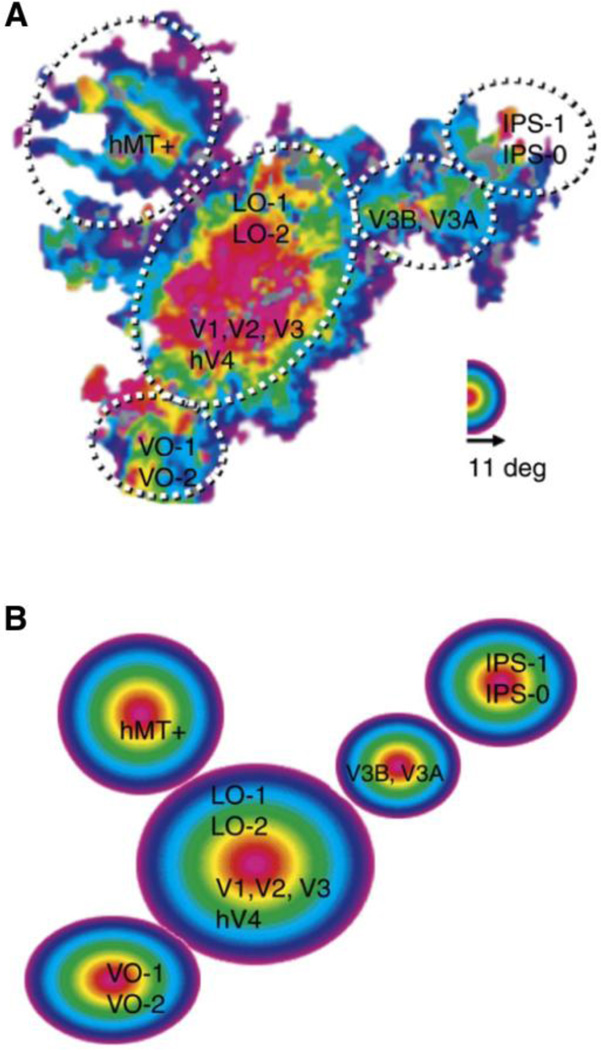
The concept of a brain area captures many features of cortical organization. V1 is a canonical brain area. It possesses well-defined architectonic properties, a complete first-order map of the visual hemifield, and a distinct connectivity profile. However, in focusing on the features that define V1 as a distinct cortical area, a broader organizational arrangement across areas is lost that is emphasized by the eccentricity map. The eccentricity map in Figure 1 (right) illustrates an organization centered on V1 that radiates outward as a coherent V1/V2/V3 cluster. The maps are aligned in a manner that minimizes distances between columns responding to portions of the retinal space. That is, columns with similar response properties tend to map near to one another across the cortical surface. As Wandell et al. (2005) note “Perhaps the only way to bring the various maps into closer alignment would be to do away with the distinct maps altogether and merge the cell mosaics in these three maps into a single map.” Wandell et al. referred to these convergences as “map clusters” and considered the possibility that they represent a coarse scale by which multiple nearby maps are organized. One possibility is that map clusters allow computationally coherent modules to form that juxtapose multiple areas into some form of a larger processing unit. Beyond the V1/V2/V3 cluster, additional candidate map clusters are located near the human homologue of the middle temporal (MT) visual area, V3A/V3B, the ventral occipital (VO) complex, and the intraparietal sulcus (IPS) (Wandell et al., 2007)(Figure 3).
Figure 3. Map clusters.
Empirical data and a schematic illustrate multiple map clusters that are present in human visual cortex. The display is similar to that depicted for polar angle in Figure 1 but spans a larger portion of posterior cortex extending into the parietal and temporal lobes. (A) Eccentricity maps spanning 0○–11○ on a flattened patch of cortex for a single subject. Dotted lines depict clusters that each include multiple field maps labeled by their estimated cortical area or region. hMT is the human homologue of MT; IPS = intra parietal sulcus; VO = ventral occipital. The circular color scale shows the eccentricity that most effectively drives each cortical location. (B) A schematic diagram of the organization of eccentricity representation reveals five distinct map clusters. Each map cluster, depicted by a concentric colored circle, contains multiple field maps. For example the largest cluster includes V1, V2, V3, and hV4 as depicted in Figure 1. Reprinted with permission from Wandell et al. (2007).
In an independent exploration of supra-areal organization based on comparative monkey anatomy, Rosa (2002) also noted map clusters for V1/V2/V3 and MT/MTc. He hypothesized that the supra-areal organization forms because first-order maps like V1 and MT seed the organization of the adjacent maps during early stages of cortical development (see also Rosa and Tweedale, 2005; Bourne and Rosa, 2006; Mashiko et al., 2012). In the adult these developmental constraints are manifest as map clusters that possess radiations of similarly responding columns that span classical areal boundaries (Figure 4). By this view, the supra-areal map clusters reflect key organizing anchors that constrain the topography of the interposed cortical areas during development.
Figure 4. Supra-areal organization.
Marmoset visual areas are displayed on a flattened cortical surface to illustrate a supra-areal organization. The flattened patch of cortex includes a portion of the occipital and temporal lobes. (A) The inset depicts the region being flattened. (B) Supra-areal organization. V1, which possesses a complete and continuous visual field map, is drawn at the left edge. V1 is cut and flattened along the horizontal meridian. The lines demarcate the borders of individual areas. The shading illustrates the organization of eccentricity mapping as shown in the hemifield reference. The darkest region represents the fovea (degree eccentricity noted by labels). The foveal representations of V1, V2, and V3 are aligned with eccentricity bands radiating outwards from V1 through the multiple visual areas. MT also possesses a complete and continuous visual field map with MTc displaying a radiation outward from the MT eccentricity map. Reprinted with permission from Rosa (2002).
Clustering approaches may be detecting these features of supra-areal organization because the approaches are highly sensitive to global similarity. Map clusters, by definition, are regions that show maximal similarity across a range of continuous cortical space. Note that it is not simply the case that the global correlation structure is subdivided by local gradient transitions detected by boundary mapping techniques. Rather, a major feature of the correlational structure of early visual cortex is eccentricity bands that radiate outwards from V1 into adjacent cortical areas. The eccentricity bands are approximately perpendicular to the areal borders within the same map cluster5.
The discussion of these ideas becomes central as the organization of higher-order association regions is considered. The default assumption is that association cortex possesses a mosaic of well-defined cortical areas (Kaas, 1987). But association cortex differs from sensory cortex in several ways that may be important. First, association cortex has disproportionately expanded in evolution and may not be specified to the same degree by strong molecular gradients during development (see Buckner and Krienen, 2013). Second, association cortex is more variable across individuals than sensory cortex (Mueller et al., 2013), a feature that may reflect its protracted development. And finally, association cortex participates in widely distributed networks that contrast to the locally-coupled networks characteristic of sensory systems (Goldman-Rakic, 1988). In this regard, while we assume some form of molecularly-constrained protomap seeds the developing organization of association cortex, it is unclear whether there are equivalent first-order maps like V1 and MT to serve as strong spatially-constrained anchors (Buckner and Krienen, 2013).
When exploring association zones using their boundary mapping technique,Wig et al. (2013) found that large-scale networks commonly observed by resting-state functional connectivity analyses can be subdivided into multiple candidate areas. For example, they discovered that the frontoparietal control network includes at least four candidate prefrontal areas revealed by three transitional borders (see Figure 7 Wig et al. 2013). This observation reflects novel information that has not emerged from clustering or community detection approaches.
However, characterizing the abrupt transitional boundaries as subdividing the larger networks assumes that the large-scale networks are groups of areas. This may be the case in many instances and perhaps often so when large network clusters are considered (e.g., the frontoparietal control network). Analysis of the V1/V2/V3 map cluster above raises an alternative possibility. Just as clustering approaches detect a reflection of the supra-areal organization across the V1/V2/V3 map cluster, they may also detect cortical alignments across distributed association regions that do not follow classical areal boundaries. The large-scale networks commonly observed in the functional connectivity literature may reflect spatially fractured clusters. It is unclear what such an organization might represent but it is fascinating to consider the possibility that multiple levels of cortical organization have been revealed by distinct approaches to cortical mapping.
Highlights.
Boundary mapping detects the border of V1 using only resting-state connectivity data
Boundary mapping can be used to identify candidate areas across all of human cortex
Cluster approaches find networks that do not align to areas detected by boundary mapping
Cluster approaches may be detecting supra-areal arrangements and map clusters
Acknowledgements
We thank Roger Tootell for thoughtful comments on an earlier draft, and Marcello Rosa and Mark Schira for figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The visual map of V1 is divided across the hemispheres but that division begins at the organization of the retina and is carried to the cortex by the thalamocortical inputs. V1 represents a complete first-order topographic representation of the visual hemifield. By contrast, V2 is a second-order representation because it forms a discontinuity that additionally splits the upper and lower visual field into quarter-quadrants. This second-order representation arises for the first time in V2. Rosa (2002) provides an excellent spatial diagram of the relationships between first-order and second-order maps in early visual cortex. The essential point here is that V1 has a continuous map of the portion of visual space it represents while V2 forms a split map that wraps around V1 preserving its relationship to V1 but not the continuity of the visual field.
Clustering techniques may be able to detect the V1/V2 border if just the right spatial resolution was obtained and just the right weighting of local versus distant connectivity was imposed. However, as typically applied clustering methods optimize global similarity of the included regions and maximize the dissimilarity to other regions. In practice it is not simply that clustering techniques miss certain boundaries, but rather that they also cluster broad regions together that cut through borders identified by boundary mapping. For example,Yeo et al. (2011) find a network cluster with a boundary that splits V1/V2/V3 perpendicular to the V1/V2 border (see Wig et al., 2013 Figure 7). A central issue is to understand the distinct features of cortical organization that the two approaches detect.
We are using the definition of a brain area advanced by Kaas (1987). Areas are defined based on multiple criteria including architectonic, connectivity, response property, and topographic differences from neighboring cortical territories. By supra-areal map we refer to organizational properties that extend across the borders of multiple architectonically-distinct brain areas (see Wandell et al., 2005 for further discussion).
It is unclear whether the reversal of the polar angle is the only contributor to the gradient discontinuity detected by the boundary method at the V1/V2 border. Differences in connectivity between V1 and V2 from distant regions of cortex would also accentuate the gradient discontinuity.
Another place where clusters form boundaries that are roughly perpendicular to known architectonic areas is somatomotor cortex. Cluster-based approaches group separate somatosensory and motor areas together, but divide the areas into supra-areal clusters along the face to body representation discontinuity. For discussion see Yeo et al. (2011).
References
- Allman JM, Kaas JH. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Res. 1971;35:89–106. doi: 10.1016/0006-8993(71)90596-8. [DOI] [PubMed] [Google Scholar]
- Amunts K, Zilles K. Architecture and organizational principles of Broca’s region. Trends Cogn. Sci. 2012;16:418–426. doi: 10.1016/j.tics.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Bourne JA, Rosa MG. Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT) Cereb. Cortex. 2006;16:405–414. doi: 10.1093/cercor/bhi119. [DOI] [PubMed] [Google Scholar]
- Brodmann K. In: Localization in the Cerebral Cortex. Garey LJ, translator. New York: Springer; 1909/1994. [Google Scholar]
- Buckner RL, Krienen FM. Evolution and organization of association networks in the human brain. Trends Cogn. Sci. 2013 doi: 10.1016/j.tics.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Campbell AW. Histological Studies on the Localisation of Cerebral Function. Cambridge: University Press; 1905. [Google Scholar]
- Cragg BG, Ainsworth A. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 1969;9:733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NUF, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. Defining functional areas in individual human brains using resting functional connectivity MRI. NeuroImage. 2008;41:45–57. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel PM, Whittteridge D. The representation of the visual field on the cerebral cortex in monkeys. J. Physiol. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb. Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BTT, Mohlberg H, Amunts K, Zilles K. Cortical folding patterns and predicting cytoarchitecture. Cereb. Cortex. 2008;18:1973–1980. doi: 10.1093/cercor/bhm225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Gross CG, Sandell JH. Visual topography of V2 in the macaque. J. Comp. Neurol. 1981;201:519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu. Rev. Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. The organization of neocortex in mammals: implications for theories of brain function. Ann. Rev. Psych. 1987;38:129–151. doi: 10.1146/annurev.ps.38.020187.001021. [DOI] [PubMed] [Google Scholar]
- Lashley KS, Clark G. The cytoarchitecture of the cerebral cortex of Ateles: a critical examination of architectonic studies. J. Comp. Neurol. 1946;85:223–305. doi: 10.1002/cne.900850207. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov NT, Ercsey-Ravasz MM, Ribeiro Gomes AR, Lamy C, Magrou L, Vezoli J, et al. A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb. Cortex. 2012 doi: 10.1093/cercor/bhs270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko H, Yoshida AC, Kikuchi SS, Niimi K, Takahashi E, Aruga J, Okano H, Shimogori T. Comparative anatomy of marmoset and mouse cortex from genomic expression. J. Neurosci. 2012;32:5039–5053. doi: 10.1523/JNEUROSCI.4788-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BTT, Sepulcre J, Sabuncu MR, Shafee R, Lu J, Liu H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77:586–595. doi: 10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MGP. Visual maps in the adult primate cerebral cortex: some implications for brain development and evolution. Braz. J. Med. Biol. Res. 2002;35:1485–1498. doi: 10.1590/s0100-879x2002001200008. [DOI] [PubMed] [Google Scholar]
- Rosa MGP, Tweedale R. Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:665–691. doi: 10.1098/rstb.2005.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schira MM, Tyler CW, Breakspear M, Spehar B. The foveal confluence in human visual cortex. J. Neurosci. 2009;29:9050–9058. doi: 10.1523/JNEUROSCI.1760-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RBH. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Horton JC. The circuitry of V1 and V2: integration of color, form, and motion. Ann. Rev. Neurosci. 2005;28:303–326. doi: 10.1146/annurev.neuro.28.061604.135731. [DOI] [PubMed] [Google Scholar]
- Talbot SA, Marshall WH. Physiological studies of neural mechanisms of visual localization and discrimination. Am. J. Ophthal. 1941;24:1255–1264. [Google Scholar]
- Van Essen DC, Zeki SM. The topographic organization of rhesus monkey prestriate cortex. J. Physiol. 1978;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Brewer AA, Dougherty RF. Visual field map clusters in human cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005;360:693–707. doi: 10.1098/rstb.2005.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vis. Res. 2011;51:718–737. doi: 10.1016/j.visres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Laumann TO, Petersen SE. An approach for parcellating human cortical areas using resting-state correlations. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Sabuncu MR, Vercauteren T, Ayache N, Fischl B, Golland P. Spherical demons: fast diffeomorphic landmark-free surface registration. IEEE Trans. Med. Imaging. 2010;29:650–668. doi: 10.1109/TMI.2009.2030797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]