Abstract
Background
As part of the fight against SARS CoV2 infection, vaccination program for health workers at Giannina Gaslini pediatric hospital (IGG) in Genoa, Italy, started on December 2020. We evaluated the anti-Spike protein response in healthcare workers after a complete vaccination scheme of 2 doses spaced by 3 weeks.
Methods
Immunoglobulin class G (IgG) against SARS-CoV-2 spike RBD were detected by means of a chemiluminescence immunoassay for quantitative IgG antibodies using Maglumi SARS-CoV-2-S-RBD IgG kit during the 3rd week after vaccination completion.
Results
IgG anti SARS-CoV-2 spike protein were detected in 99.88% of 1765 healthcare workers 3 weeks after 2nd dose of BNT162b2. Higher median IgG values were observed in younger subjects (807 UA/mL in under 30 vs 429 UA/mL in over 60; p < 0.001) and those with previous COVID-19 (1284 vs 574 UA/mL; p < 0.001).
Conclusion
BNT162b2 is effective in inducing anti SARS-CoV-2 antibodies even in real-life setting. The higher antibody title observed in workers with a previous documented SARS CoV2 infection confirms the possibility to carry out only one dose of BNT162b2 in a context of vaccines shortage.
Keywords: Vaccines, SARS CoV2, BNT162b2
Introduction
SARS-CoV-2 is the cause of the pandemic COVID-19 observed for the 1st time in December 2019 in China [1]. The mRNA vaccine BNT162b2 directed against the Receptor Binding Domain (RBD) of viral spike protein (S-protein) has been documented effective in reducing COVID-19 [2] and it was authorized for its use in Europe from December 21st, 2020.
On December 31st, 2020, the IRCCS Istituto Giannina Gaslini (IGG) children’s hospital, Genoa-Italy, started a vaccination program involving healthcare workers, residents and volunteers, with 2 doses of BNT162b2 mRNA-vaccine, 21 days apart.
The main purpose of our study was to evaluate the anti-Spike protein antibody titer in healthcare workers after a complete vaccination schedule.
Methods
Healthcare workers who received 2 doses of BNT162b2 mRNA anti SARS-CoV2 vaccine (at IGG signed on a voluntary basis an informed consent for the execution of an anti S-protein serology during the 3rd week after vaccination. Test results were provided to participants for information purposes only and not as a demonstration of effective immunity presence. Study was approved by IGG Internal Review Board. Immunoglobulin class G (IgG) against SARS-CoV-2 spike RBD were detected by means of a chemiluminescence immunoassay for quantitative IgG antibodies using Maglumi SARS-CoV-2-S-RBD IgG kit on a MAGLUMI 800 analyzer (Snibe Diagnostic, Shenzen New Industries Biomedical Engineering Co. Ltd., Shenzen, China). The analyzer automatically calculates the IgG concentration in each sample by means of a calibration curve and the results are expressed in arbitrary units (AU/mL) as follows: a value ≥1.1 AU/mL is considered reactive while <0.9 AU/mL non-reactive. Values between 0.9 and 1.0 AU/mL fall in an undetermined zone. Serums with IgG concentration >100 AU/mL were diluted automatically by analyzer 1:20 and the antibody titer was calculated automatically by analyzer software. It is possible to obtain the binding antibody unit (BAU) value from AU using the conversion factor of 4.33 provided by the company (AU * 4.33 = BAU). No other antibody dosages or B and T cell response tests were performed on sera.
Statistical analysis: categorical variables were reported as numbers and proportions. Mean and 95% confidence intervals (95% CI) were used for normally distributed continuous variables while median and interquartile range (IQR) were used for non-normally distributed data. Non-parametric (Mann–Whitney or Kruskal–Wallis when appropriate) tests were performed to compare variables by means of Jamovi, an open-source R-based software (https://www.jamovi.org/).
Results
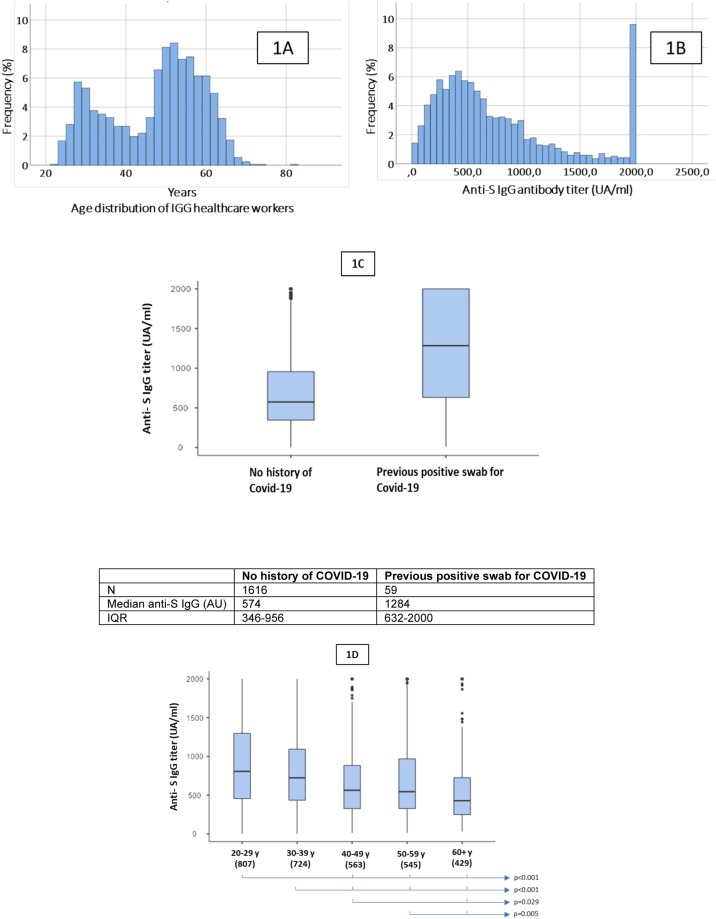
Results are graphically summarized in Fig. 1 . A total of 1675 subjects were tested, 1328 (79.3%) females, with a median age of 50 years (IQR 36, 56), but with a bimodal distribution (peaks at 30 and 55 years), reflecting the composition of healthcare workers, residents and volunteers in IGG (panel 1A). Among them, 59 (3.52%) had COVID-19 as indicated by a positive molecular nasal swab for SARS CoV2 in the previous year.
Fig. 1.
(A) Age distribution; (B) anti Spike protein antibody titer in vaccinated staff (median 585 AU/mL); (C) comparison in antibody titer in subjects with a previous documented SARS-CoV-2 infection vs naïve; (D) antibody titer according to age distribution.
An antibody titer ≥1.1 AU/mL was observed in 1763 (99.88%) with a median titer of 585 AU/mL (IQR 349–989, 95% CI 89.8–2000) (panel 1B); subjects with previous SARS-CoV-2 infection showed a significantly higher antibody titer: median 1284 UA/mL vs. 574 UA/mL, p < 0.001 (panel 1C). Only 42 subjects had a titer below the 2.5th percentile (median 51.5, IQR 31–66.5) and among them 2 (0.12% of total subjects tested) did not produce detectable IgG. Non-responders were 25 and 31 years old and both underwent immunosuppressive therapy (in one case with anti-CD20 monoclonal antibodies) for previous immune disorders.
Finally, significantly differences in median antibody titers were observed according to different age strata, with declining in titer by increasing subjects’ age (panel 1 D).
Discussion
We tested anti SARS-CoV-2 spike-RBD antibodies in a real-life setting during the 3rd week after complete BNT162b2 vaccination in healthcare workers, resident and volunteers of an Italian pediatric hospital. Our data clearly show that vaccination with two doses of BNT162b2 spaced of 21 days determines a detectable antibody production in 99.88% of tested subjects, confirming the effectiveness of this vaccine also in a real-life setting, outside the rules of clinical trials [2]. Moreover, differently from a recent study [3] on 100 patients, our series involving 1675 subjects shown a significantly higher anti-S IgG titer in those who had a previous SARS-CoV-2 infection (1284 vs 574 UA/mL; p < 0.001).
As regards the two enrolled subjects who did not produce antibodies, as evidenced by a recent work by Hagin et al. [6], these subjects could equally develop an active S-peptide T-cell response.
Our results confirm with more extensive numbers those of a previous work by Krammer et al. [4], extending the possibility of carrying out only one dose of BNT162b2 in patients with previous infection, at least in a context of vaccines possible shortage. A recent study showed the efficacy of BNT162b2 in reducing the rate of SARS CoV2 infection in health workers [5], while our study, focusing on antibody levels, showed that antibody titer could be influenced by chronological age and a previous SARS CoV2 infection.
Further studies are needed in order to detect protective antibody titer against SARS-CoV-2 after vaccination and the need and timing of any booster, if needed.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Liu Y.C., Kuo R.L., Shih S.R. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43:328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anichini G., Terrosi C., Gandolfo C., Gori Savellini G., Fabrizi S., Miceli G.B. SARS-CoV-2 antibody response in persons with past natural infection. N Engl J Med. 2021;0:null. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/nejmc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benenson S., Oster Y., Cohen M.J., Nir-Paz R. BNT162b2 mRNA Covid-19 vaccine effectiveness among health care workers. N Engl J Med. 2021:1–2. doi: 10.1056/NEJMc2101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021:19–20. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]