Abstract
We describe the step-by-step procedure for culturing and differentiating mouse embryonic stem cells into neuronal lineages, followed by a series of assays to characterize the differentiated cells. The E14 mouse embryonic stem cells were used to form embryoid bodies through the hanging drop method, and then induced to differentiate into neural progenitor cells by retinoic acid, and finally differentiated into neurons. RT-qPCR and Immunofluorescence experiments revealed that the neural progenitors and neurons exhibit corresponding markers at day 8 and 12 post-differentiation, respectively. Flow cytometry experiment on an E14 line expressing a Sox1 promoter-driven GFP reporter showed that about 60% of cells at day 8 are GFP positive, indicating the successful differentiation of neural progenitor cells at this stage. Finally, RNA-seq analysis was used to profile the global transcriptomic changes. These methods are useful for analyzing the involvement of specific genes and pathways in regulating the cell identity transition during neuronal differentiation.
Keywords: Mouse embryonic stem cells, embryoid bodies, neural progenitor cell, neurons, differentiation, hanging drop, E14
SUMMARY:
Here we describe the procedure for the in vitro differentiation of mouse embryonic stem cells into neuronal cells using the hanging drop method. Furthermore, we perform a comprehensive phenotypic analysis through RT-qPCR, immunofluorescence, RNA-seq, and flow cytometry.
INTRODUCTION:
Since their first derivation from the inner cell mass of the developing mouse blastocysts1, 2, mouse embryonic stem cells (mESC) have been used as powerful tools to study stem cell self-renewal and differentiation3. Furthermore, studying mESC differentiation leads to tremendous understanding of molecular mechanisms that may improve efficiency and safety in stem cell-based therapy in treating diseases such as neurodegenerative disorders4. Compared to animal models, this in vitro system provides many advantages including simplicity in practice and assessment, low cost in maintaining cell lines in contrast to animals, and relative ease in genetic manipulations. However, the efficiency and quality of differentiated cell types are often affected by different lines of mESCs as well as the differentiation methods5, 6. Also, the traditional assays to evaluate differentiation efficiency rely on qualitative examination on selected marker genes therefore lack robustness and fail to grasp global changes in gene expression.
Here we aim to use a battery of assays for systematic assessment of the neuronal differentiation. Using both traditional in vitro analyses on selected markers and RNA-seq, we establish a platform for measurement of the differentiation efficiency as well as the transcriptomic changes during this process. Based on a previously established protocol7, we generated embryoid bodies (EB) through the hanging drop technique, followed by induction of supraphysiologic amount of retinoic acid (RA) to generate neural progenitor cells (NPCs), which was subsequently differentiated to neurons with neural induction medium. To examine the efficiency of the differentiation, in addition to traditional RT-qPCR and immunofluorescence (IF) assays, we performed RNA-seq and flow cytometry. These analyses provide comprehensive measurement of the progression of the stage-specific differentiation.
PROTOCOL:
1. mESC culture
1.1. Coat a 10 cm tissue-culture-treated plate with 0.1% gelatin and allow the gelatin to set for at least 15 – 30 minutes before aspirating it out.
1.2. Seed γ-irradiated MEFs in the pre-warmed mESC medium (DMEM with 15% FBS, non-essential amino acids, β-mercaptoethanol, L-glutamine, penicillin/streptomycin, sodium pyruvate, LIF, PD0325901 (PD), and Chir99021 (CH)).
1.3. Allow for MEFs to settle and attach to the plate surface before culturing E14 cells.
NOTE: MEFs can be plated a day before culturing the ES cells.
1.4. Thaw E14 ESCs in 37°C water bath and quickly transfer the cells in a 15 cm conical tube with warm mESC medium. Pellet the cells at 200 × g for 3 minutes and remove the supernatant.
1.5. Resuspend the cells in 10 mL mESC medium and plate the cells on the culture plate containing the MEFs seeded earlier.
1.6. Incubate the cell culture in a 37°C incubator with 5% CO2 content.
1.7. For culture passaging, aspirate medium and wash the plate with sterile 1 × PBS. Add enough 0.05% trypsin to cover the plate surface and incubate at 37°C for 3 minutes.
1.8. Neutralize the trypsin with mESC medium and pipette to generate single-cell suspension. Centrifuge the cells at 200 × g for 3 minutes and remove the supernatant.
1.9. Count the cells with a hemocytometer or cell counter and seed about 5.0 × 105 cells in a 10 cm culture plate.
1.10. Resuspend the cells in 10 mL mESC medium and plate the cells on the gelatin-coated tissue culture plate and incubate cultures described earlier.
NOTE: It is recommended that mESCs be passaged every 2 days to prevent the cells from differentiating in their colonies. Depending on the cellular density, the phenol red indicator may turn yellowish sooner than 2 days thus it may be necessary to change the medium every day. The MEFs will eventually die off after a couple of passages.
2. EB, NPC, and neuron differentiation
2.1. Perform culture passaging protocol mentioned earlier and count the cells (steps 1.7. – 1.10).
2.2. Hanging drop method (Day 0) : For a 10 cm cell culture plate, count roughly 25,000 cells where 500 cells will be suspended in 20 μL differentiation medium (DMEM with 15% FBS, non-essential amino acids, β-mercaptoethanol, L-glutamine, penicillin/streptomycin, and sodium pyruvate). Roughly 50 20 μL droplets containing the cells can be plated for one 10 cm plate.
2.3. Aliquot the appropriate number of cells before centrifuging the cells at 200 × g for 3 minutes and removing the supernatant.
2.4. Resuspend the cells in the appropriate volume of differentiation medium to acquire 500 cells per 20 μL cell density (e.g. 25,000 cells in 1 mL differentiation medium).
2.5. Using a micropipette or a repeater pipette, place 20 μL droplets of the cell suspension onto the lid of the tissue culture plate. Make sure that the droplets are not too close to one another to prevent them from merging.
NOTE: The droplets can be plated on either a tissue-culture-treated attachment plate or suspension plate as they will be done on the lid and not the plate itself. For a more feasible and sterile approach, plate the droplets on an attachment tissue-culture plate and transfer them to a suspension plate as described below.
2.6. Fill up the plate with 5 – 10 mL 1 × PBS and carefully put the lid back on the plate.
2.7. Incubate the culture in the 37°C incubator.
2.8. Day 2: Use a micropipette to collect the droplets on the lid and place them in a 10 cm cell culture suspension plate filled with 10 mL differentiation medium.
2.9. Incubate the culture on an orbital shaker shaking at low speed in the incubator.
2.10. Day 4: To harvest the EBs, collect the cells, centrifuge at 200 × g for 3 minutes, and remove the supernatant.
NOTE: The EBs can also be washed with 1 × PBS according to the needs of subsequent experiments.
2.11. To continue the procedure and induce the EBs’ differentiation into NPCs, prepare the differentiation medium with 5 μM retinoic acid (RA).
2.12. Remove the old medium by pelleting the EBs at 100 × g for 3 minutes or allow the EBs to settle before aspirating out the old medium.
2.13. Add 10 mL of the differentiation medium with 5 μM RA to the culture plate.
2.14. Day 6: Replace at least half of the medium with new medium with 5 μM RA by slanting the plate and pipetting out the medium as described above.
NOTE: It is recommended that at least half of the medium be replaced with new medium with RA at days 5 and 7. If the phenol red indicator in the medium turns yellowish, it is best to replace all the medium.
2.15. Day 8: To harvest the NPCs, collect the cells, centrifuge at 200 × g for 3 minutes, and remove the supernatant.
NOTE: The NPCs can also be washed with 1 × PBS according to the needs of subsequent experiments.
2.16. To continue the procedure and differentiate NPCs into neurons, collect NPCs in 15 mL conical tube by centrifugation and dissociate them with trypsin and incubating them at 37°C for 3 minutes. Pipette the NPCs to ensure that all NPC aggregates are dissociated and neutralize the trypsin with medium.
2.17. Filter the cells with 40-μm nylon cell strainer and count the cells before plating them at a density of 1.5 × 105/cm2 in N2 medium (DMEM/F12 medium with 3 mg/mL glucose, 3 mg/mL Albumax, 1/100 | supplement, 10 ng/mL bFGF, 50 U/mL pen/strep, and 1 mM L-glutamine) on a tissue-culture-treated plate for subsequent PCR and Western blot experiments; or on tissue culture chamber for immunofluorescent experiments.
2.18. Day 9: Replace the old medium with a new N2 medium.
2.19. Day 10: Switch the N2 medium with N2/B27 medium (50% DMEM/F12 1 and 50% Neural Basal, 3 mg/mL Albumax, 1/200 N2 supplement, 1/100 B27 supplement, 50 U/mL pen/strep, and 1 mM L-glutamine).
2.20. Day 11 – 12: To harvest the neurons, wash the cells with 1 × PBS, add trypsin, and incubate the culture in the 37°C incubator.
2.21. Neutralize the trypsin with medium and centrifuge at 200 × g for 3 minutes.
3. Characterization of mESCs and differentiated cells
3.1. Alkaline phosphatase (AP) assay: Use the Alkaline Phosphatase Staining Kit II (Stemgent, Cat #00–005).
3.2. Remove the medium from the culture plate and wash the ESCs with 1× PBS.
3.3. Add 1 mL of the Fix solution provided with the kit to the plate and incubate it at room temperature for 2 – 5 minutes.
NOTE: Over-incubation in the Fix solution can compromise the AP activity.
3.4. Remove the Fix solution and wash the ESCs with 1× PBS and leave some amount of PBS in the plate.
NOTE: Keep the ESCs moist in PBS to not compromise the AP activity.
3.5. Prepare the AP solution by mixing the A, B, and C Substrate Solutions at 1:1:1 ratio. Mix A and B solutions first and incubate the mixture at room temperature for 2 minutes before adding the C solution.
3.6. Remove the 1 × PBS and add the AP solution prepared earlier.
3.7. Incubate the ESCs for about 15 minutes in the dark by wrapping the culture plate with aluminum foil or performing step 3.5. in a dark room.
3.8. Monitor the reaction and remove the reaction solution when the solution turns bright to avoid non-specific staining.
3.9. Wash the ESCs twice with 1 × PBS
3.10. Prevent the sample from drying by covering the ESCs with 1 × PBS or mounting medium.
NOTE: A red or purple stain will appear for AP expression. The plate can be stored in a 4°C fridge.
3.11. RT-qPCR: Collect cells at various stages by following steps 1.7. – 1.8. and 2.10. for ESCs and EBs/NPCs, respectively.
3.12. Transfer the cell pellet into an Eppendorf tube.
NOTE: RNA isolation protocol is adapted from the recommended protocol for TRIzol™ Reagent (Invitrogen, Cat. # 15596026)
3.13. Add 0.5 mL TriPure Isolation Reagent per 2 million cells collected and homogenize the suspension by pipetting it.
NOTE: The samples can be stored in the TriPure solution at 4°C overnight or at − 20°C for up to a year.
3.14. Incubate the samples at room temperature for at least 5 minutes.
3.15. To each sample, add 0.2 mL of chloroform per 1 mL of TriPure solution used.
3.16. Shake the tube vigorously for 15 seconds.
3.17. Incubate the sample at room temperature for 2 – 3 minutes.
3.18. Centrifuge the sample at 12,000 × g at 4°C for 15 minutes.
3.19. Transfer only the clear aqueous phase at the upper portion to a new Eppendorf tube.
NOTE: Slant the tube at a 45° angle and slowly pipette out the upper aqueous solution without taking in the phenol-chloroform phase.
3.20. To each transferred aqueous solution, add 0.5 mL of 100% isopropanol per 1 mL of TriPure solution used.
3.21. Incubate the sample at room temperature for 10 minutes.
3.22. Centrifuge the sample at 12,000 × g at 4°C for 10 minutes.
3.23. Discard the supernatant with a micropipettor or an aspirator with a needle tip.
NOTE: RNA is pelleted in a clear gel-like form, which is not always visible.
3.24. Resuspend the pellet in 1 mL of 75% ethanol per 1 mL of TriPure solution used.
NOTE: The RNA-ethanol mixture can be stored at – 4°C or – 20°C for a least 1 week or 1 year, respectively.
3.25. Vortex the sample briefly then centrifuge at 7,500 × g at 4°C for 5 minutes.
3.26. Discard the supernatant with a micropipettor or an aspirator with a needle tip.
3.27. Air dry the RNA pellet for 5 – 10 minutes.
3.28. Resuspend the RNA pellet in 20 μL de-ionized water.
3.29. Measure the RNA concentration with a spectrophotometer.
3.30. Generate cDNA using the SuperScript™ III Reverse Transcriptase and follow the manufacturer’s manual.
3.31. For the first reaction, mix 0.25 μL of random hexamer primer (0.2 ng/μL), 500 ng of RNA sample, 1 μL of dNTP mix (10 mM), and dH2O up to 14 μL.
3.32. Incubate the samples in the thermocycler according to the following temperature steps: 65°C for 5 minutes and hold at 12°C.
3.33. For the second reaction, mix 4μL of 5 × First-Strand Buffer, 1 μL of DTT (0.1 M), 0.3 μL SuperScript III RT, and dH2O up to 6 μL.
3.34. Incubate the samples in the thermocycler according to the following temperature steps: 25°C for 5 minutes, 50°C for 1 hour, 70°C for 15 minutes, and hold at 12°C.
NOTE: The cDNA samples were diluted 5 × before proceeding to the next step.
3.35. Quantify transcript abundance using the AzuraQuant™ Green Fast qPCR Mix LoRox system.
3.36. For each primer set, prepare the reaction mix by mixing 5 μL of 2 × AzuraQuant Green Fast qPCR LoRox, 0.5 μL of the forward primer (10 μM), 0.5 μL of the reverse primer (10 μM), and 4 μL of the 5 × diluted cDNA sample.
3.37. Incubate the samples in the thermocycler according to the following temperature cycle: initial denaturation at 95°C for 3 minutes, denaturation at 95°C for 5 seconds, annealing at 60°C for 10 seconds, extension at 72°C for 20 seconds, and repeat the denaturation to the extension steps 39 times. Final extension at 65°C for 5 seconds and denaturation at 95°C for 50 seconds.
3.38. Fixation and embedding: Harvest EBs/NPCs as described above (step 2.10.) and fix them with 4% paraformaldehyde (PFA) solution in 1 × PBS for 30 minutes at room temperature.
3.39. Remove the PFA and wash the sample with 1 × PBS for 5 minutes.
3.40. Place the sample in a serial dilution of 1 × PBS, 10%-, 20%-, and 30%-sucrose solutions at 25 – 28°C where the sample is transferred to the next solution after 30-minute incubation.
NOTE: The sample can be stored in the 30% sucrose solution at 4°C before continuing with the embedding step.
3.41. Wet the pipette tip with sucrose solution before placing the sample (without stacking them) at the center of the cryo-mold and pipette out the excess liquid.
NOTE: Filter paper can also be used to remove the excess solution. Wetting the pipette tip with sucrose solution is important to prevent the EBs/NPCs from sticking to the walls of the tip.
3.42. Carefully add OCT solution to the mold without resuspending the samples and remove excess bubbles with a pipette.
3.43. Place the mold with the sample on a laboratory mixer at low speed to mildly agitate the sample for 15 minutes. This helps to settle the EBs/NPCs to the bottom if they are resuspended in the OCT solution.
3.44. Quick freeze the sample by placing the mold in liquid nitrogen or on dry ice.
NOTE: Samples can be stored in a −70°C freezer before continuing to the next step.
3.45. Cryosectioning: Set the cryostat to cool down to −20 – −18°C before transferring the sample to the instrument.
3.46. Detach the frozen OCT block from the mold and secure it on the holder with a little OCT solution placed at the surface of the holder.
3.47. Align the OCT block so that the EBs/NPCs are closest to the blade to ensure that the sample is not lost during sectioning.
3.48. Carefully section 10 μm of the block and pay close attention to the slices that contain the sample.
3.49. Quickly place the OCT slice containing the sample onto the tissue-embedding glass slide and allow the OCT slices to air-dry for 1 hour at room temperature.
NOTE: The samples can be stored at −70°C for later use.
3.50. Immunofluorescence (IF): Block OCT sections or culture chambers containing neurons in 10% normal donkey serum/0.1% Triton X-1000 in 1 × PBS for 1 hour at room temperature.
3.51. Incubate the samples in primary antibody diluted in 5% normal donkey serum/0.05% Triton X-1000 in 1 × PBS overnight at 4°C.
3.52. Wash samples in 1 × PBS/0.1% Triton X-1000 thrice for 5 minutes each wash.
3.53. Incubate the samples with secondary antibody diluted in 5% normal donkey serum/0.05% Triton X-1000 in 1 × PBS for 1 hour at room temperature.
3.54. Wash samples in 1 × PBS/0.1% Triton X-1000 thrice for 5 minutes each wash.
3.55. Incubate the samples in 1 μg/mL DAPI.
3.56. Mount the samples with a coverslip and some mounting medium and allow it to dry.
3.57. Observe the samples under a fluorescent microscope.
3.58. RNA-seq analysis: Collect the cells at various stages and perform RNA extraction according to the 3.11. – 3.29. steps described earlier.
3.59. Prepare the cDNA libraries and perform deep sequencing according to the protocol described in Wang et al. (Stem Cell Res., 2018)8.
3.60. Align the RNA-sequencing reads to the M. musculus genome (UCSC mm9, July. 2007) using TopHat (version 2.1.1) with the default parameters.
3.61. Estimate the relative abundances (FPKM) of the transcripts with Cufflinks (version 2.2.1).
3.62. Filter the FPKM values for all genes to eliminate lowly expressed genes.
3.63. Calculate the z-score and cluster the filtered genes using k means, then plot the heatmap, and perform the Gene Ontology (GO) analysis using the R package, clusterProfiler.
3.64. Flow cytometry: Collect ESCs by following the 1.7. – 1.8. steps and resuspend the cells in medium. Collect the EBs/NPCs by following the 2.15. – 2.16. steps and resuspend the cells in medium.
3.65. Filter the cell suspension using 40-μm nylon cell strainer into a new 15 mL conical tube.
3.66. Measure the GFP signal of the samples using the flow cytometer (performed by the institution’s core facility).
REPRESENTATIVE RESULTS:
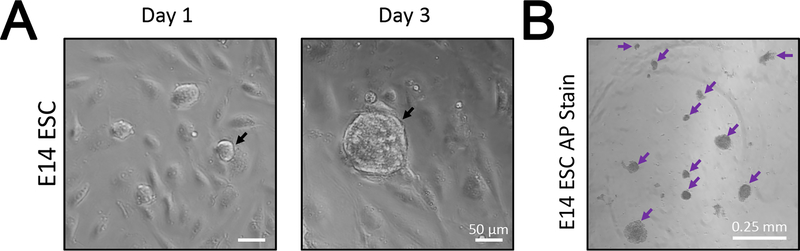
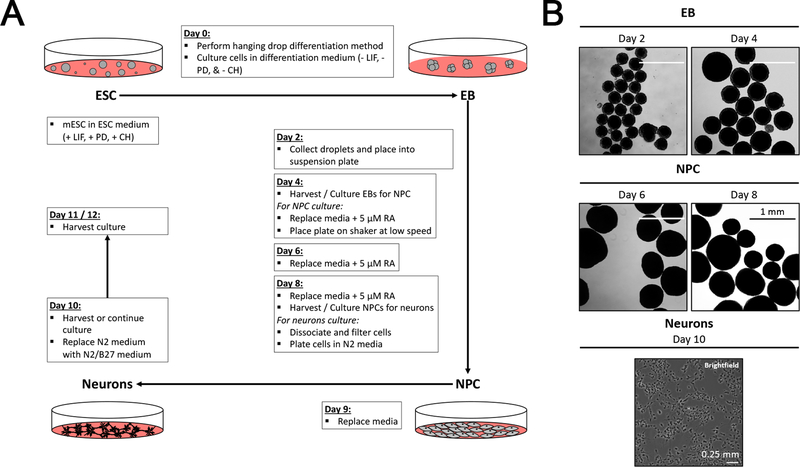
As a representation of our method, we performed an EB, NPC, and neuron differentiation experiment on E14 cells. E14 cells were cultured on MEFs (Figure 1A) until MEF population is diluted out. We confirmed the pluripotency of the E14 cells by performing Alkaline Phosphatase (AP) staining (Figure 1B) and later RT-qPCR (Figure 3B) for Nanog and Oct4 markers. The MEF-free E14 cells were then induced for differentiation using the protocol outlined in Figure 2A. Briefly, differentiation media droplets of 20 μl containing 500 cells were seeded on the lid of the culture plate (See Protocol 2 for details). The EBs formed were then collected and placed in suspension in fresh differentiation media. From day 4 to day 8 of differentiation, 5 μM RA was added to the culture plates to induce NPCs. Differentiated EBs showed round shape and their size continued to increase during differentiation (Figure 2B). At day 8, the NPCs were harvested and trypsinized, and then the resulting single cell suspension was plated in a tissue culture chamber in DMEM/F12 medium with N2 supplement and later in B27 supplement. By day 10, NPCs differentiating into neurons appear to have elongated-cell shape (Figure 2B).
Figure 1: E14 ESCs in culture.
(A) The light microscope images show E14 cells (black arrow) growing in colonies atop the MEFs. E14 colonies continue to proliferate as seen in the colony size difference between day 1 and day 3 cultures. (B) Confirmation of the pluripotency of E14 ESCs by the alkaline phosphatase (AP) stain.
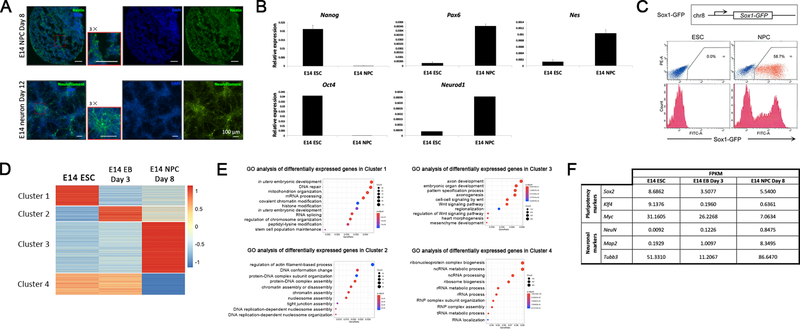
Figure 3: Characterization of differentiated cells.
(A) Immunofluorescent images in the top panel show NPCs at day 8 probed for Nestin (green) and nuclei (DAPI, blue). The bottom panel shows immunofluorescent images for neurons at day 12 probed for NF (green). The red box in the merged images are zoomed in 3 × for better view. (B) RT-qPCR analysis showing the pluripotency markers (Nanog and Oct4) and NPC markers (Pax6, NeuroD1, and Nes) of E14 ESCs and NPCs. All mean values and standard deviations were calculated from three independent measurements. (C) E14 cells expressing a Sox1 promoter-driven GFP reporter were differentiated into NPCs. Both the ESCs and NPCs at day 8 were quantified for positive GFP fluorescent signal with flow cytometry. (D) The heatmap shows the z-scores for the determined FPKM of the genes expressed in the ESC, EB, and NPC stages. Four distinct gene clusters were identified signifying groups of genes that are differentially expressed in either the ESC, EB, or NPC stage. (E) GO analysis was performed using the R package, clusterProfiler, on the four clusters identified in the RNA-seq.
Figure 2: E14 differentiation into EBs, NPCs, and neurons.
(A) The schematic depicts the major steps for differentiating E14 cells into EBs, NPCs, and neurons. (B) E14 ESCs cultured in medium without LIF and 2i in a suspension plate form individual spheres of differentiated stem cells visible at day 2. EBs continue to grow and expand in size. RA is added at day 4 of differentiation to induce the differentiation into NPCs. After 4 days of induction, these NPCs are plated for differentiation into neurons, which are shown in the bottom panel.
To further evaluate our differentiation experiment, we performed immunofluorescent (IF) experiments on E14 NPCs at day 8 and E14 neurons at day 12. We observed positive staining for Nestin in NPCs and Neurofilament (NF) signal for neurons (Figure 3A). Alternatively, RT-qPCR confirmed the induction of NPC marker genes and loss of pluripotency genes in NPCs (Figure 3B). As a quantitative method to test the success of ESC differentiation, we differentiated a mouse ESC line expressing a Sox1 promoter-driven GFP reporter9, followed by flow cytometry analysis on ESCs and NPCs. We found that 58.7% of total cells at the NPC stage are GFP-positive while the GFP signal is 0.0% at the ESC stage (Figure 3C). To profile the transcriptomic changes during differentiation, RNA-seq experiments for E14 ESCs, EB day 3, and NPC day 8 were performed and revealed gene clusters associated with the respective stages (Figure 3D). Gene Ontology (GO) analysis for the four gene clusters showed that these clusters correspond to distinct cellular functions or pathways (Figure 3E).
DISCUSSION:
The method for neural differentiation of mouse embryonic stem cells has been established for decades and researchers have continued to modify the previous protocols or create new ones for various purposes 7, 10, 11. We utilized a series of assays to analyze the differentiation stage and efficiency, which may be used in analysis of other lineage differentiation of mouse or human ESCs. Furthermore, our approaches have proved to be useful tools to evaluate the impact of specific genes or pathways on neuronal differentiation in vitro8.
With our methods, pluripotent un-committed ESCs treated with retinoic acid (RA) commit to the neural lineage with a high efficiency and are further induced to generate neurons 7. To improve the successful differentiation of ES cells into neural cells and reduce the heterogeneity, it is important to keep the ES cells in an undifferentiated state12. Non-proliferative MEFs, treated with γ-irradiation or mitomycin C, function to maintain the pluripotency of the ESs and provide a scaffold for their growth13, 14. To obtain consistent results, we start each differentiation experiment with mESCs cultured on MEFs. After a few passages, the MEFs die out and the culture eventually becomes homogenous for mESC cells. Leukemia inhibitory factor (LIF) has long been used to maintain the pluripotency state of cultured mouse ES cells by activating the JAK/STAT pathway15–17. More recently, PD0325901 (PD, a MEK inhibitor) and CHIR99021 (CH, a GSK inhibitor) were found to provide additional pluripotency maintenance of the ES cells3, 18. In our protocol, we culture mESCs with these inhibitors together with LIF to maintain high pluripotency of mESCs.
Another critical factor to achieve successful differentiation is the quality of EBs. We perform the differentiation of E14 cells by the hanging drop method, which has been applied by other investigators5, 19, 20. With this method, single ES cells are allowed to suspend in the differentiation medium droplet for 2 days where they spontaneously aggregate and form EBs. The resulting EBs are typically more well-defined in terms of their morphology (Figure 2B) compared to the method of suspending isolated mESCs in medium, which results in EB sizes in a much wider range in our experience (data not shown). To prevent EBs from attaching to the plates, it is important to do a low speed rotation starting from day 3 continuing through the differentiation process.
The samples can be collected across the differentiation period e.g. ESC stage, EB day 2, EB day 4, NPC day 6, NPC day 8, neurons day 10, and neurons day 12 to track the differentiation process by performing RT-qPCR for pluripotency and ectoderm markers. Comparing the pluripotency markers such as Oct4, Sox2, and Nanog between the different cell stages will verify the pluripotency of the mESCs (Figure 3B). To investigate the efficiency of the neural differentiation, markers for the mesoderm and endoderm layers can also be probed for. Further verification can be performed with immunofluorescence (IF) probing for NPC or neuronal markers (Figure 3A). However, these methods are not quantitative and biased toward the selected markers. To overcome these limitations, we incorporate flow cytometry and RNA-seq analyses (Figure 3C–E). These analyses are particularly beneficial to investigate the differentiation defect caused by gene manipulation or chemical treatment.
Table 1:
JoVE materials
| Name of Material/Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| 0.05% Trypsin + 0.53mM EDTA 1X | Corning | 25–052-CV | |
| 0.1% Gelatin | Sigma | G1890–100G | Prepared in de-ionized water |
| 16% Paraformaldehyde | Thermo Scientific | 28908 | Diluted in 1X PBS |
| 40-μm cell strainer | Falcon | 352340 | |
| Albumax | Thermo Fisher Scientific | 11020021 | |
| AlexaFluor 488 goat anti-mouse IgG (H+L) | Invitrogen | A11001 | Antibody was diluted at 1:500 for IF |
| Alkaline Phosphatase Staining Kit II | Stemgent | 00–0055 | |
| AzuraQuant Green Fast qPCR Mix LoRox | Azura Genomics | AZ-2105 | |
| B27 supplement | Thermo Fisher Scientific | 17504044 | |
| BD FACSCanto | BD | 657338 | |
| bFGF | Sigma | 11123149001 | |
| BioAnalyzer High Sensitivity DNA Kit | Agilent | 5067–4626 | |
| Chir99021 | Cayman Chemicals | 13122 | |
| Chloroform | C298–500 | Fisher Chemical | |
| DAPI | Invitrogen | R37606 | |
| DMEM | Corning | 10–017-CM | |
| DMEM/F12 medium | Thermo Fisher Scientific | 11320033 | |
| EB buffer | Qiagen | 19086 | |
| Ethanol | 111000200 | Pharmco | Diluted in de-ionized water |
| Fetal bovine serum | Atlanta Biologicals | S10250 | |
| Fisherbrand Superfrost Plus Microscope Slides | Fisher Scientific | 12–550–15 | |
| HiSeq 2500 Sequencing System | Illumina | SY-401–2501 | |
| Isopropanol | BDH1133–4LG | BDH VWR Analytical | Diluted in de-ionized water |
| L-glutamine | Thermo Fisher Scientific | 25030024 | |
| LIF | N/A | N/A | Collected from MEF supernatant |
| m18srRNA primers | IDTDNA | N/A | 5’-GCAATTATTCCCCATGAACG-3’ 5’-GGCCTCACTAAACCATCCAA-3’ |
| MEM Non-essential amino acids | Corning | 25–025-Cl | |
| mNanog primers | IDTDNA | N/A | 5’-AGGCTTTGGAGACAGTGAGGTG-3’ 5’-TGGGTAAGGGTGTTCAAGCACT-3’ |
| mNes primers | IDTDNA | N/A | 5’-AGTGCCCAGTTCTAGTGGTGTCC-3’ 5’-CCTCTAAAATAGAGTGGTGAGGGTTG-3’ |
| mNeuroD1 primers | IDTDNA | N/A | 5’-CGAGTCATGAGTGCCCAGCTTA-3’ 5’-CCGGGAATAGTGAAACTGACGTG-3’ |
| mOct4 primers | IDTDNA | N/A | 5’-AGATCACTCACATCGCCAATCA-3’ 5’-CGCCGGTTACAGAACCATACTC-3’ |
| mPax6 primers | IDTDNA | N/A | 5’-CTTGGGAAATCCGAGACAGA-3’ 5’-CTAGCCAGGTTGCGAAGAAC-3’ |
| N2 supplement | Thermo Fisher Scientific | 17502048 | |
| Nestin primary antibody | Millipore | MAB5326 | Antibody was diluted at 1:200 for IF |
| Neural basal | Thermo Fisher Scientific | 21103049 | |
| Neurofilament primary antibody | DSHB | 2H3 | |
| NEXTflex Illumina Rapid Directional RNA-Seq Library Prep Kit | BioO Scientific | NOVA-5138–07 | |
| PD0325901 | Cayman Chemicals | 13034 | |
| Penicillin/streptomycin | Corning | 30–002-Cl | |
| Phosphate-buffered saline (PBS) | N/A | N/A | Prepared in de-ionized water |
| - Potassium chloride | P217–500G | VWR | |
| - Potassium phosphate monobasic anhydrous | 0781–500G | VWR | |
| - Sodium chloride | BP358–10 | Fisher Bioreagents | |
| - Sodium phosphate, dibasic, heptahydrate | SX0715–1 | Milipore | |
| Random hexamer primer | Thermo Scientific | SO142 | |
| Retinoic acid | Sigma | R2625 | Prepared in DMSO |
| Sodium pyruvate | Corning | 25–000-Cl | |
| Sucrose | Sigma | 84097 | Diluted in 1X PBS |
| SuperScript III Reverse Transcriptase | Invitrogen | 18064022 | |
| Tissue-Tek O.C.T. compound | Sakura | 4583 | |
| TriPure Isolation Reagent | Sigma-Aldrich | 11667165001 | |
| TruSeq Rapid | Illumina | 20020616 | |
| β-mercaptoethanol | Fisher BioReagents | BP176–100 |
ACKNOWLEDGMENTS:
This work was supported by a grant from the NIH (1R35GM133496–01) to Z. Gao. We would like to thank Dr. Ryan Hobbs for the assistance in sectioning. We thank Penn State College of Medicine’s genome sciences facility and Dr. Yuka Imamura for the assistance in RNA-seq analysis.
Footnotes
DISCLOSURES:
Authors declare that there are no competing financial interests.
REFERENCES:
- 1.Kaufman MH, Evans MJ Establishment in culture of pluripotential cells from mouse embryos. Nature. 292 (July), 154–156 (1981). [DOI] [PubMed] [Google Scholar]
- 2.Martin GR Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America. 78 (12 II), 7634–7638, doi: 10.1073/pnas.78.12.7634 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czechanski A et al. Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nature Protocols. 9 (3), 559–574, doi: 10.1038/nprot.2014.030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugaya K, Vaidya M Stem Cell Therapies for Neurodegenerative Diseases. Exosomes, Stem Cells and MicroRNA: Aging, Cancer and Age Related Disorders. 61–84, doi: 10.1007/978-3-319-74470-4_5 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnology and Bioengineering. 78 (4), 442–453, doi: 10.1002/bit.10220 (2002). [DOI] [PubMed] [Google Scholar]
- 6.McKee C, Chaudhry GR Advances and challenges in stem cell culture. Colloids and Surfaces B: Biointerfaces. 159, 62–77, doi: 10.1016/j.colsurfb.2017.07.051 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Bibel M et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nature Neuroscience. 7 (9), 1003–1009, doi: 10.1038/nn1301 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Wang Q et al. WDR68 is essential for the transcriptional activation of the PRC1-AUTS2 complex and neuronal differentiation of mouse embryonic stem cells. Stem Cell Research. 33 (November), 206–214, doi: 10.1016/j.scr.2018.10.023 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Ying QL, Stavridis M, Griffiths D, Li M, Smith A Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nature Biotechnology. 21 (2), 183–186, doi: 10.1038/nbt780 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Visan A et al. Neural differentiation of mouse embryonic stem cells as a tool to assess developmental neurotoxicity in vitro. NeuroToxicology. 33 (5), 1135–1146, doi: 10.1016/j.neuro.2012.06.006 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J In vitro differentiation of embryonic stem cells into glial cells and functional neurons. Journal of Cell Science. 108 (10), 3181–3188 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Stavridis MP, Smith AG Neural differentiation of mouse embryonic stem cells. Biochemical So. 31, 45–49, doi: 10.3791/52823 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Park Y-G et al. Effects of Feeder Cell Types on Culture of Mouse Embryonic Stem Cell In Vitro. Development & Reproduction. 19 (3), 119–126, doi: 10.12717/dr.2015.19.3.119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Lee EJ, Lee CH, Park JH, Han JY, Lim JM Requirement of leukemia inhibitory factor for establishing and maintaining embryonic stem cells in mice. Fertility and Sterility. 92 (3), 1133–1140, doi: 10.1016/j.fertnstert.2008.07.1733 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Onishi K, Zandstra PW LIF signaling in stem cells and development. Development (Cambridge). 142 (13), 2230–2236, doi: 10.1242/dev.117598 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Smith AG et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 336, 688–90, doi: 10.1038/336688a0 (1988). [DOI] [PubMed] [Google Scholar]
- 17.Williams RL et al. Myeloid leukemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 336, 684–687, doi: 10.1038/336684a0 (1988). [DOI] [PubMed] [Google Scholar]
- 18.Ghimire S et al. Comparative analysis of naive, primed and ground state pluripotency in mouse embryonic stem cells originating from the same genetic background. Scientific Reports. 8 (1), 1–11, doi: 10.1038/s41598-018-24051-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurosawa H, Imamura T, Koike M, Sasaki K, Amano Y A Simple Method for Forming Embryoid Body from Mouse Embryonic Stem Cells. Journal of Bioscience and Bioengineering. 96 (4), 409–411, doi: 10.1263/jbb.96.409 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Yang P In vitro differentiation of mouse embryonic stem (mES) cells using the hanging drop method. Journal of Visualized Experiments. (17), 2–3, doi: 10.3791/825 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]