Abstract
This article aims to narrate the various oral complications in individuals suffering from diabetes mellitus. Google search for “diabetes mellitus and oral complications” was done. The search was also carried out for “diabetes mellitus” and its oral complications individually. Diabetes mellitus is a chronic metabolic disorder that is a global epidemic and a common cause of morbidity and mortality in the world today. Currently, there are about 422 million cases of diabetes mellitus worldwide. Diabetic patients can develop different complications in the body such as retinopathy, neuropathy, nephropathy, cardiovascular disease. Complications in the oral cavity have been observed in individuals suffering from diabetes mellitus. A study noted that more than 90% of diabetic patients suffered from oral complications. Another research has shown a greater prevalence of oral mucosal disorders in patients with diabetes mellitus than non-diabetic population: 45–88% in patients with type 2 diabetes compared to 38.3–45% in non-diabetic subjects and 44.7% in type 1 diabetic individuals compared to 25% in the non-diabetic population. Oral complications in people with diabetes are periodontal disease, dental caries, oral infections, salivary dysfunction, taste dysfunction, delayed wound healing, tongue abnormalities, halitosis, and lichen planus. The high glucose level in saliva, poor neutrophil function, neuropathy, and small vessel damage contribute to oral complications in individuals with uncontrolled diabetes. Good oral health is imperative for healthy living. Oral complications cause deterioration to the quality of life in diabetic patients. Complications like periodontal disease having a bidirectional relationship with diabetes mellitus even contribute to increased blood glucose levels in people with diabetes. This article intends to promote awareness regarding the oral health of diabetics and to stress the importance of maintaining proper oral hygiene, taking preventive measures, early detection, and appropriate management of oral complications of these patients through a multidisciplinary approach.
Keywords: diabetes mellitus, hyperglycemia, oral complications, periodontal disease, salivary dysfunction, dental caries, infection, awareness, multidisciplinary approach
Plain Language Summary
Diabetes Mellitus, a global epidemic, is a matter of grave concern worldwide and is a significant cause of morbidity and mortality.
Oral complications are among the many complications suffered by diabetic patients, including periodontal disease, Dental caries, oral infections, salivary dysfunction, taste dysfunction, delayed wound healing, tongue abnormalities, halitosis, and lichen planus.
Diabetes Mellitus and oral complications are interrelated, and in conditions like periodontal diseases, the relationship is bidirectional.
Taste dysfunctions in Diabetes Mellitus often lead to increased sugar consumption, causing further deterioration in glycemic control in diabetic individuals.
Discomfort experienced by diabetic patients when they suffer from oral complications may discourage oral hygiene due to pain or irritation in the oral cavity.
Knowledge regarding oral complications is often inadequate among people with diabetes, and creating awareness is imperative in diabetic individuals about the relationship between diabetes and oral health.
Diabetic patients need to be educated about these complications, encouraged to maintain oral hygiene, and to pay regular visits to diabetic caregivers and dentists.
Oral complication prevention, early detection, and appropriate management can only be achieved through a multidisciplinary approach. Dental surgeons and physicians interact effectively to provide the optimum care needed by individuals with Diabetes Mellitus to maintain good oral health free of complications.
Introduction
Diabetes Mellitus is a non-communicable metabolic disorder that is chronic where there is derangement of insulin action, secretion, or both. A lack of Insulin results in disturbed carbohydrate, protein, and fat metabolism. There are genetic and environmental factors at play for the development of Diabetes Mellitus. The decrease in insulin secretion, reduction in glucose utilization, or increased gluconeogenesis ultimately result in hyperglycemia and harmful changes in different organs.1,2
The general categories into which diabetes is classified are as follows: a) Type 1 diabetes b) Type 2 diabetes (c) Diabetes of specific types resulting from other causes like exocrine pancreas disease, chemical or drug-induced diabetes, monogenic diabetes syndrome (d) Gestational Diabetes Mellitus.3 In the world at present, 422 million people are suffering from Diabetes Mellitus. Every year there are 1.6 million fatalities linked directly to diabetes.4 As per data obtained in studies done throughout the world, an estimation has been made by the International Diabetes Federation that by the year 2045, there will be about 693 million cases of diabetes in the age range of 18–99 years.5
Complications related to diabetes are predicted to significantly impact the economy and society as the prevalence of the disease grows.6–10 The acute complications of diabetes include ketoacidosis or severe hypoglycemia. Examples of chronic disease complications are retinopathy, neuropathy, cardiovascular disease, and nephropathy.11–14
The oral cavity is one of the regions of the body affected by chronic hyperglycemia. Complications arising in the oral cavity due to Diabetes Mellitus result from poor neutrophil function, microangiopathy, neuropathy, collagen synthesis reduction, and collagenase activity reduction.15 A study noted that more than 90% of diabetic patients suffered from oral complications.16 Another systemic review has observed a greater prevalence of oral mucosal disorders in patients with Diabetes Mellitus compared to the non-diabetic population: 45–88% in patients with Type 2 diabetes compared to 38.3–45% in non-Diabetic subjects and 44.7% in type 1 diabetic individuals compared to 25% in the non-Diabetic population.17 Tooth decay, gingivitis, oral candidiasis, altered taste, geographic tongue, fissured tongue, dry mouth, the tendency of infection, oral lichen planus, and poor healing of wound are complications of the oral cavity resulting from Diabetes Mellitus.18–23
Periodontal disease is among the oral complications of Diabetes Mellitus, which worsens due to hyperglycemia. At the same time, systemic inflammation due to periodontitis deteriorates blood glucose levels in diabetic individuals, thus showing a bidirectional relationship.24 Diabetes Mellitus affects the prevalence and incidence of periodontal disease. Formation of deep pockets and loss of attachment is typical in uncontrolled diabetes, and there is a high prevalence rate of periodontitis among diabetic patients of 34%-68%.25,26 Compared to healthy individuals, the risk of losing alveolar bone is 11 times more in uncontrolled diabetes.18
Xerostomia is the dryness of the mouth that has been observed in diabetic patients. A meta-analysis of 32 studies reported that the prevalence of xerostomia was 46.09% in patients with diabetes, while another study found that 92.5% of diabetic patients suffered from the reduced salivary flow.27,28 This complication eventually results in dysgeusia, dental caries, oral pain, dysphagia, which tends to lower the quality of life of diabetic individuals.28–31
Diabetic patients are susceptible to oral infections and delayed wound healing.32,33 A high glucose level in the oral cavity and immunocompromised condition in uncontrolled Diabetes Mellitus facilitate oral bacterial infections.18,32 Delayed wound healing in Diabetes Mellitus may be attributed to damaged small blood vessels and weakened protection against infection and inflammation.33–35
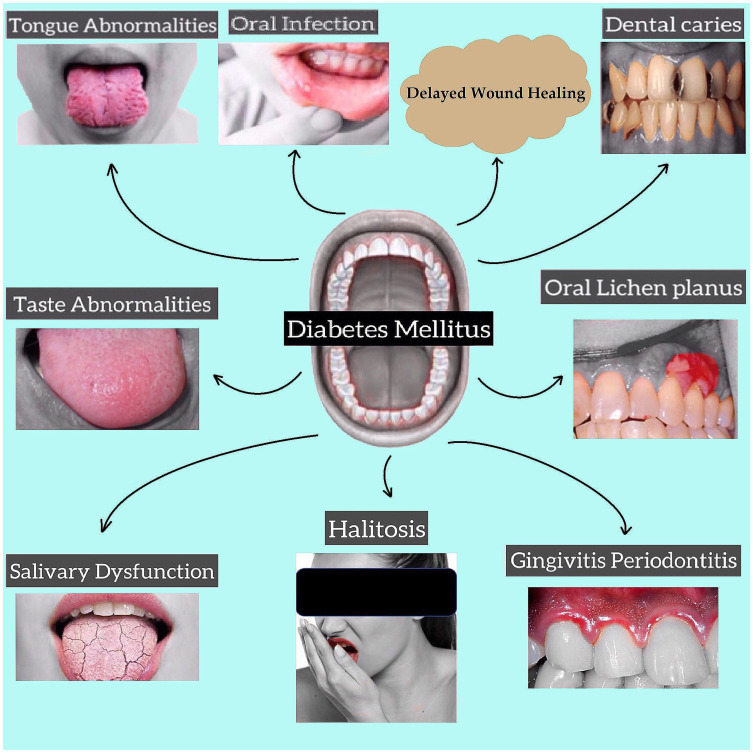
Diabetic patients also suffer from dental caries due to hyposalivation and high salivary glucose level that promotes the growth of bacteria responsible for dental caries.36–38 These patients may develop oral complications like taste dysfunction in which diabetic patients’ ability to distinguish taste sensations diminishes;39–41 Burning Mouth Syndrome is a neuropathic orofacial condition of pain.42 Fissured tongue, atrophic glossitis, rhomboid glossitis, benign migratory glossitis are the abnormalities of the tongue suffered by diabetic patients.23,43,44 diabetic individuals also suffer from Halitosis or bad breath, oral lichen planus, and oral lichenoid reaction (Figure 1). 45–49
Figure 1.
Complications of oral cavity in diabetes mellitus.
Objectives of the Study
Individuals with diabetes suffer from many complications. Oral complications affect the quality of life of diabetic patients negatively.50 However, knowledge, awareness, and practices regarding this issue are often inadequate among diabetic patients and diabetic caregivers.51–53 The objective of this study is to relate the various oral complications of Diabetes Mellitus and highlight the importance of a multidisciplinary approach through effective interaction between dentists and physicians towards developing awareness among diabetic individuals about oral hygiene checkups, prevention, and management of oral complications.
Materials and Methods
This narrative review focuses on identifying oral complications in Diabetes Mellitus and the possible pathology leading to these complications. The study was carried out between March and May 2021. Electronic database search was done using Google search engine, Google Scholar, PubMed, Science Direct. Reference lists in related articles were also searched to obtain more articles on the topics. Keywords used to reach related articles were “Diabetes,” “Oral complications,” “Oral Manifestations in Diabetes Mellitus,” “Dental Caries,” “Periodontitis,” “Taste alteration,” “Xerostomia,” “Burning Mouth Syndrome.” Articles and literature on those dating before 2000 and articles not available in English were excluded from the study. Relevant articles were hand-searched before being included in the study.
Periodontal Disease in Diabetes Mellitus
Lang NP and Bartold PM defined periodontal health in 2017 as
A state free from inflammatory periodontal disease that allows an individual to function normally and not suffer any consequences (mental or physical) as a result of past disease.
There are 4 categories of periodontal health, which include a) pristine periodontal health, defined as the total absence of clinical inflammation and physiological immune surveillance on a periodontium with normal support (no attachment or bone loss) (b) clinical periodontal health in which there is an absence or minimum level of clinical inflammation in periodontium having normal support (c) periodontal disease with reduced stability in a periodontium (d) periodontal disease remission/control in a reduced periodontium.54
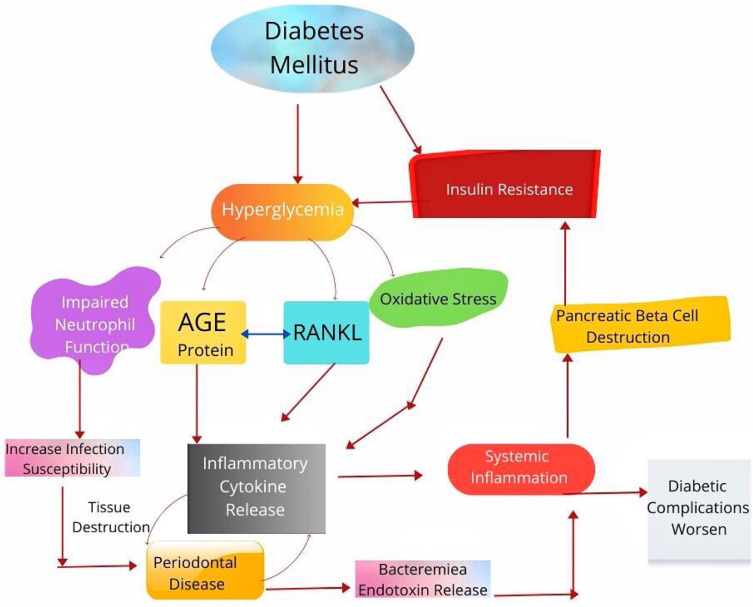
Periodontitis is a chronic inflammatory condition induced by pathogenic biofilm that accumulates on tooth55 Gram-negative bacteria Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola has been suggested to be the primary causative agents for periodontitis.56,57 P-gingivalis can trigger inflammatory loss of periodontal bone.58 DNA and lipopolysaccharides from these bacteria cause inflammatory cytokine production by activating nuclear factor κβ (NFκβ) and activating protein-1 pathways.59,60 Cytokines cause chemoattraction of neutrophils with an enhancement of ROS production, and NFκβ and AP-1 activate osteoclast resulting in tissue damage (Figure 2).61–63
Figure 2.
Bidirectional relationship between periodontal disease and diabetes mellitus.
Increased levels of glucose in crevicular fluid in diabetes may promote the growth of specific microbial species.64 Diabetic patients with periodontitis have been noted to have a significantly higher level of local mediators of inflammation like TNFα, IL-1β, prostaglandin E2, which causes prolonged osteoclast formation and activity.65–67 Interleukin overexpression in diabetes promotes osteoclast genesis and thus prolongs inflammatory response duration.68,69 There is also overexpression of RANKL, which interacts with receptors on osteoclast surface and induces osteoclast formation and activity.56,70
In Diabetes Mellitus, there is enhanced formation of AGE which interacts with RAGE. This, in turn, promotes osteoclast genesis through increased formation of RANKL receptor activator.71 AGE-RAGE interaction also activates NFκβ and increases the production of inflammatory cytokines.72 Neutrophils of diabetic patients release more super-oxides than normal individuals.73 Increased ROS plays a significant role in the destruction of periodontal tissue through oxidative stress.64 TNFα, AGEs, and ROS formation lead to apoptosis of osteoblast in diabetes.74 Periodontal infection causes apoptosis of epithelial cells and fibroblast, facilitated in Diabetes Mellitus through a caspase-3-dependent mechanism.75 The enhancement and apoptosis that occur in Diabetes Mellitus result in loss of epithelial barrier function and inhibition of repair.76,77
People suffering from uncontrolled Diabetes Mellitus are susceptible to periodontal (gum) disease that includes a range of gingiva, ligament, and bone conditions that support teeth.78,79 Bacteria in dental plaque initiate local inflammation of the gingiva that, if remain untreated, progress to chronic periodontitis with gingival, ligament, bone loss that form “pockets” in deeper parts of the periodontium. This may lead to tooth loss.80.
The outcome of periodontal diseases is affected by hyperglycemia, and at the same time, periodontitis affects glucose levels in the blood adversely. This causes diabetic complications to deteriorate.24 An increase in cytokines in saliva and crevicular fluid of gingival, periodontal tissue; oxidative stress with a release of end products of advanced glycation in the hyperglycemic state eventually destroy periodontium due to excessive inflammation.81 Diabetes also causes an increase in RANKL expression and impairment of new bone formation in the periodontium.82
On the other hand, control of blood glucose in Type 2 diabetic patients deteriorates due to periodontal disease. It induces systemic inflammation through pro-inflammatory cytokine release that causes insulin resistance and bacteremia.83 The inflamed periodontium act as a chronic source for bacteria, its products, and mediators of inflammation like TNF α, IL 1, and IL 6 that affect glucose metabolism.84 The systemic inflammatory cytokines released due to periodontal inflammation also induce insulin resistance by destroying pancreatic β cells, insulin action antagonism and alteration of intracellular signaling of insulin through NF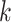 β.85 Improvement of glycemic control was observed in Type2 diabetic patients who received dental treatment when compared to those diabetic patients who did not obtain dental treatment for periodontal disease.86 In a meta-analytic study performed with nine randomized clinical trials, improvement of glycemic control was observed in Diabetes Mellitus patients who received non-surgical treatment of periodontium when compared to those diabetic patients who did not undergo dental treatment for periodontal disease.87
β.85 Improvement of glycemic control was observed in Type2 diabetic patients who received dental treatment when compared to those diabetic patients who did not obtain dental treatment for periodontal disease.86 In a meta-analytic study performed with nine randomized clinical trials, improvement of glycemic control was observed in Diabetes Mellitus patients who received non-surgical treatment of periodontium when compared to those diabetic patients who did not undergo dental treatment for periodontal disease.87
Salivary Dysfunction
The subjective problem of mouth dryness is Xerostomia, and the objective reduction of the flow of saliva is hyposalivation.29 There are several systemic disorders related to Xerostomia, including Diabetes Mellitus, inflammatory conditions (rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome), metabolic conditions (anemia, bulimia, dehydration), infections (HCV, HIV/AIDS), neurological disease (Parkinson’s disease, depression), and other conditions like sarcoidosis.29,88,89 Studies have found a relationship between Diabetes Mellitus (both Type 1 and Type 2) and Xerostomia.28,30,31 In patients with uncontrolled diabetes, complications like autonomic neuropathy, changes in the structure of salivary glands, inflammatory change due to hyperglycemia may be a possible cause of the development of Xerostomia.15,90 This may lead to a decrease in the flow rate and composition of saliva.91 Patients with Xerostomia suffer from glossitis, cervical caries, buccal mucosa dryness, peeled and cracked lips. The individual’s quality of life with Xerostomia deteriorate as the patient eventually develops dysgeusia, dental caries, periodontal disease, oral pain, and dysphagia.29
Infections of the Oral Cavity
Individuals with Diabetes Mellitus are susceptible to oral cavity infections since these patients are immunocompromised (due to defense function impairment).91,92 The bacteria combine with food in teeth forming plaque and result in halitosis, gingivitis, dental caries, and mouth sores.32 In the case of oral infection in diabetic individuals, the commonly found bacteria in the oral cavity are P. gingivalis, Propionibacterium acnes, Actinomyces israelii, Peptostreptococcus prevotii, Fusobacterium nucleatum, Saccharomyces cerevisiae, Streptococcus sanguis, Prevotella intermedia, and Streptococcus intermedius. The high level of glucose in the saliva of diabetic patients encourages bacterial growth.18 Bacterial infections in uncontrolled diabetes may recur and spread from the oral cavity to the rest of the body. Deep neck infections have been observed in diabetic individuals in previous studies.93–97
Poor Wound Healing of the Oral Cavity
There is poor healing of oral wounds in patients having uncontrolled diabetes with associated long-term complications.33 Chronic complications of diabetes result from small blood vessel damage.34 Lack of proper blood supply hinders nutrient supply to cells that perform an inflammatory function and defend against infective agents.92 Inflammation removes dead or damaged tissues, thus allowing healthy tissue to take its place. Temporary spikes in blood sugar cause body defense cells to become paralyzed, resulting in weak protection against infection and inflammatory processes. The tissue healing and regenerative functions are hampered in individuals with uncontrolled diabetes due to hyperglycemia.33,35
Dental Caries
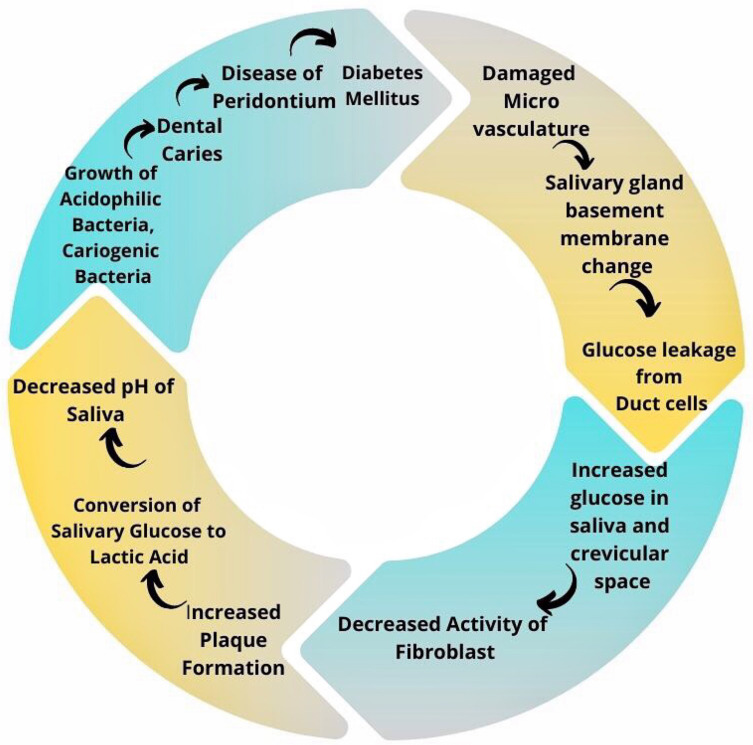
Dental caries is an infectious disease of the teeth in which tooth structure is demineralized due to bacteria, mainly Streptococci mutans, that adheres to the tooth by producing acid from sugar. There are multiple factors like microbial flora: cariogenic, fermentable sugar, environmental factors that trigger dental caries (Figure 3).98 Some studies previously carried out have observed a relationship between Diabetes Mellitus and the formation of dental caries.28,99 Presence of high glucose level in saliva, decreased saliva flow, alteration of biochemical nature of saliva, salivary buffering effect reduction, bad oral hygiene, cariogenic diet, and existing dental plaque have been associated with dental caries formation in Type 1 Diabetes Mellitus.100 Those taking sugar without any restriction are more prone to developing dental caries than those with well-controlled blood glucose levels.43 Dental caries occurring in the cementum of teeth become familiar with increasing age, and caries of the radicular part of the tooth have been noted in older patients with Type 2 Diabetes Mellitus.34 In a study to observe Type 1 diabetes patients’ oral hygiene compared to control, hyposalivation was mentioned as a reason for poor oral hygiene in Type 1 diabetic patients.101 A study done in 2017 observed that sugar-free toothpaste in individuals with Diabetes Mellitus decreased salivary glucose level and increased salivary pH and suggested strict control of blood glucose level to maintain oral hygiene.102
Figure 3.
Vicious cycle of diabetes mellitus and formation of dental caries, periodontal disease.
Patients with Diabetes Mellitus are especially prone to developing dental caries due to hyposalivation and raised glucose levels in saliva, which may be the outcome of Insulin deficiency.36 In people with diabetes, saliva loses protective, buffering as well as cleansing function.37 Damage to microvasculature results in changes in the salivary gland basement membrane. Hence, glucose leakage from cells of the duct escalates, leading to a rise in glucose levels in saliva and crevicular space. As a consequence of this change, the activity of fibroblast decreases, resulting in increased plaque formation. Glucose in the saliva is converted by dental plaque into lactic acid, lowering salivary pH.103,104 Aciduric bacterial growth is enhanced in this low pH, and the proliferation of acidogenic bacteria suppresses the oral protective bacteria. There is a shift in the balance of the natural environment, which favors the bacteria responsible for dental caries. This then further decreases pH, and the cycle continues to repeat.37,38
Taste Dysfunctions
Recognition of food taste plays a vital role in a person’s food choices, nutrition, life quality and may even be responsible for chronic disease development.105 There is a minimum of five modalities of taste sensation that include sweet, sour, bitter, salty, and umami.106 The sensation of taste is sensed by receptor cells of taste present in taste buds and papillae in the oral cavity. Following interaction between taste molecules and taste receptors, signals are transmitted utilizing the cranial nerve to the brain.107 In case of dysfunction of one or more taste receptors that alter taste perception, may lead to unhealthy eating habits.108 Studies have shown that distinction and recognition of taste sensation decrease in Diabetes Mellitus (type 1 and type 2).39–41 Impairment of taste has also been observed in diabetic individuals without neuropathy.109
Burning Mouth Syndrome
Burning mouth syndrome has been recognized by The International Association for the Study of Pain as a neuropathic orofacial condition of pain with oral mucosal burning pain that commonly affects anterior 2/3rd of the tongue lacking visible pathology of the mucosa.42 There is a tingling, burning sensation in the mucosa of the oral cavity without any recognizable cause.110 This syndrome has been observed in 18.8% of type 2 diabetic patients with peripheral neuropathy.111 Another study found uncontrolled diabetes and diabetic peripheral neuropathy as strong predictors of Burning mouth syndrome-like symptoms in type 2 diabetic patients.42 A study carried out in 2019 to compare the Trigeminal nerve nociceptive function of the oral cavity of patients with diabetic peripheral neuropathy, and healthy individuals found increased excitability of trigeminal nerve in diabetic peripheral neuropathy. This can cause hyperesthesia and pain sensation in the oral cavity of diabetic patients with peripheral neuropathy.112 Those individuals suffering from long-term burning pain in the mouth have difficulty maintaining oral hygiene, leading to further deterioration in oral health in diabetic patients.43
Tongue Abnormalities
The tongue is an organ composed of muscles having fungiform, filiform, and vallate papillae. It has several functions like taste function utilizing taste buds present on papillae, facilitating speech, and moving food within the oral cavity with bolus formation for swallowing.113 Patients with Diabetes Mellitus may suffer from tongue abnormalities. A study in 2019 found the presence of blueish tongue with thick yellow fur in type 2 diabetic individuals and suggested screening of the tongue for early detection of type 2 Diabetes Mellitus.114
An abnormality found in diabetic individuals is fissured tongue. This condition is characterized by grooves with depth and size variation on the dorsal tongue surface. Symptoms appear when debris becomes trapped in these fissures.43,115 In the study in 2015, reported fissured tongue was found to have an association with Diabetes Mellitus.115 Fissured tongue formation may result from Xerostomia and decreased salivary flow rate.116
Atrophic glossitis is a condition of the tongue in which there is the absence of fungiform and filiform papillae on the tongue’s dorsal surface, which eventually alters the tongue’s appearance and texture, making it smooth and soft. In diabetes mellitus, candida infection in the oral cavity results in the formation of rhomboid glossitis.44 This glossitis is marked by an erythematous tongue lesion anterior to the circumvallate papillae. The rhomboid-shaped lesion is found on the dorsal surface of the tongue along the midline, which is depapillated, having a shiny, smooth surface, and also referred to as a kissing lesion.117
Benign Migratory Glossitis is also found in patients with Diabetes Mellitus.113 This condition is benign and marked by redness (erythema), filiform papillae atrophy, lined by a serpiginous, white, hyperkeratosis border.23
Halitosis
Halitosis or bad breath is one of the early diabetes symptoms, a typical ketone smell in people with diabetes. Periodontal disease may also lead to sulfide compound odor. Increased levels of fatty acid and methyl nitrate in blood cause oxidative stress that leads to Halitosis.45 A study done in 2015 has found that 23.3% of the diabetic study recruits suffered from halitosis.118
Oral Lichen Planus and Oral Lichenoid Reaction
Lichen Planus is a chronic inflammatory lesion of the skin.119 The lesion is characterized by polygonal, violaceous flat-topped plaques and papules that are pruritic and can appear in different areas of the body, including the oral cavity. The lesion in the oral cavity appears as white raised lines forming a lace-like pattern that is symmetrical and bilateral.120 Studies have found oral lichen planus to be present in patients with diabetes.46,47 Another mucosa-related change that may have an adverse effect on oral hypoglycemic agents prescribed to diabetic patients is an oral lichenoid reaction.48,49 Oral lichen planus is an autoimmune disease in which apoptosis of basal cells of the epithelium of the oral cavity occurs mediated by cytotoxic T cells.121 Those suffering from oral lichen planus may complain of discomfort and burning sensation in the mouth that can cause feeding and swallowing difficulty.48 Oral lichen planus has malignant potential, so it is vital to diagnose and manage it to prevent oral squamous cell carcinoma development.49
Knowledge and Attitude Towards Oral Complications
Previous studies have shown that diabetic patients were not aware of the bidirectional connection between periodontal disease and Diabetes Mellitus and had limited oral health risk knowledge.122–126 In a recent study performed in 2017 on the diabetes care provider’s knowledge and practices in oral health care, several obstacles prevented the caregivers from providing effective management. These included lack of guideline/oral health checking instruments, proper referral system, and inadequate knowledge on bidirectional diabetes – oral health relationship.53 However, it has been observed from past studies that reception of information about oral health from care providers and better education in this area have shown to result in good knowledge of oral health in diabetic patients.126,127 Patients who are informed better regarding diabetes and oral health link take up positive behavior towards oral health.128
Other Major Complication of Diabetes Mellitus
Among the major complications of Diabetes Mellitus, Diabetic neuropathies (a heterogeneous group of disorders) are the most prevailing. Diabetic neuropathies like Diffuse neuropathy include distal symmetric polyneuropathy (the most common form of Diabetic peripheral neuropathy) and Autonomic neuropathy; Mononeuropathy; Radiculopathy.129,130 The most dominant form of Diabetic neuropathy accounting for about 75% of the complication is DSPN.131,132 Some factors that may increase the risk of developing Diabetic peripheral neuropathy include age, duration of diabetes, diabetic retinopathy.133 About half of diabetic patients may suffer from Diabetic neuropathy.134 Oxidative stress, inflammation, damage to small blood vessels supplying nerves (vasa nervorum), and neuronal injury and damage due to metabolic disturbance are the possible cause of pathogenesis of diabetic neuropathy.129,135–137 In diabetic peripheral neuropathy, there is neuropathic pain burning, shooting, tingling, or lancinating in nature, occurring along with paresthesia worsening during the night. There may be an exaggerated response to stimuli of pain and pain upon contact with, for example, footwear and bedclothes. Such pain may disrupt daily functioning, disability, and negative impact on the quality of life.138–140
Cardiovascular complications such as cardiac myopathy and peripheral arterial disease are often diagnosed at the later stage of the disease in diabetic patients.141 Advanced atherosclerotic changes have been observed in coronary arteries in both obstructive and nonobstructive coronary stenosis in Type 2 diabetic patients.142,143 Complications also include loss of function of regeneration in myocardial muscle and produces acute coronary syndrome.143 Response to vasoactive amines is altered that results in adverse cardiac effect.142–144 There is a disturbance in genesis and propagation of action potential in cardiac muscle leading to mechanisms of automaticity and re-entry, therefore causing arrhythmias in atria and ventricle. Congestive heart failure in Diabetes Mellitus occurs at a high rate as there is pump failure due to cardiac muscle abnormalities occurring due to inflammation and cardiac fibrosis.144,145 Adopting diet and lifestyle changes can help prevent or delay the complications of the cardiovascular system in Type 2 Diabetes Mellitus.146
A complication of Diabetes Mellitus that has the probability of leading to blindness is Diabetic Retinopathy.147 Risk factors for developing Diabetic Retinopathy include duration of diabetes, hypertension, and poor control of blood glucose level.148 Production of free radicals and advanced glycation end products (AGE) and inflammation occurring due to hyperglycemia play an essential role in Diabetic Retinopathy development.149 Diabetic Retinopathy is of two types: Non-Proliferative Diabetic Retinopathy (non-threatening to vision) and Proliferative Diabetic Retinopathy (Threatening to vision).147 Complications of Proliferative Diabetic Retinopathy like tractional retinal detachment, vitreous hemorrhage, neovascular glaucoma as well as diabetic maculopathy are the causes for vision loss in Diabetic Retinopathy.150 Vascular leakage resulting from microangiopathy in diabetic patients causes macular edema and capillary occlusion leading to retinal ischemia and vascular endothelial growth factor elevation. Neovascularization and Proliferative Diabetic Retinopathy thus result from these changes.151,152 Non-Proliferative Diabetic Retinopathy eventually progresses to Proliferative Diabetic Retinopathy, but this progress may be delayed through strict glycemic control in diabetic patients.153
One of the major complications of Diabetes Mellitus is Diabetic nephropathy that is found to develop in both Type 1 and Type 2 Diabetes Mellitus.154,155 Diabetic nephropathy progresses to chronic kidney disease and eventually leads to end-stage kidney disease.156 Upon chronic exposure to high blood glucose levels, podocytes of the glomerular filtration membrane become abnormal. Podocyte loss is one of the earliest morphological changes in the glomerulus, playing an essential role in diabetic nephropathy development. Proteinuria and impairment of renal function are the characteristics of Diabetic Nephropathy.157–159 This complication is diagnosed pathologically by findings of renal hypertrophy, thickening of the basement membrane, mesangial substrate increase, nodular lesion, interstitial fibrosis.160–163 Changes in renal hemodynamics, ischemia, renin-angiotensin-aldosterone system overactivity, and increased oxidative stress resulting from glucose metabolism and inflammation lead to renal fibrosis in diabetic nephropathy. Control of blood glucose, blood pressure, and lipid; use of Renin-angiotensin-aldosterone system blocker and cessation of smoking can help improve the condition of patients with Diabetic Nephropathy in type 2 Diabetes Mellitus.164
Limitation of the Study
The following were some limitations of the study
This study is a narrative review, and no systematic review or meta-analysis was not carried out.
Search engines that require to be accessed through institutions could not be used.
Articles that require to be purchased to be accessed could not be included in the study.
Conclusion
Diabetes Mellitus has become a significant epidemic in the present world. This metabolic disorder leads to complications, including that of the oral cavity. Oral complications are very much likely to harm the quality of life of the diabetic individual. The individual would suffer hindrance in speech, chewing, swallowing, and have painful sensation in the mouth resulting from these oral complications. In addition, they are prone to oral infections, and taste abnormalities lead them to increase sugar and salt consumption, which further deteriorates their glycemic control and, in turn, degrades the health of the oral cavity. In diabetic patients, mainly when there is insufficient control of blood glucose level, hyperglycemia contributes to several oral complications. At the same time, complications like periodontitis lead to an increase in blood glucose levels and the progress of other complications in the body. Periodontal health is a condition of the inflammation-free periodontium, which allows normal functioning of an individual without any physical or mental consequences due to past disease. The periodontal health is compromised in Diabetes Mellitus, that is, inflammation of periodontium is prolonged and worsened in diabetic individuals suffering from periodontitis. The bidirectional relationship between Periodontitis and Diabetes Mellitus results from the release of inflammatory cytokines like TNF α and interleukins that cause worsening of periodontal disease condition and develop insulin resistance. Systemic inflammatory cytokines and bacterial endotoxins from infected periodontium cause resistance to insulin through pancreatic β cell destruction, leading to hyperglycemia. Awareness needs to be built regarding these oral complications since maintaining proper oral hygiene can reduce the incidence and severity of such complications. A multidisciplinary approach that includes dental professionals and physicians is necessary to tackle these complications of the oral cavity in diabetic patients. With regular visits to the dentist and physicians, the oral cavity’s blood glucose level and health can remain in check through prevention, early detection, and proper management.
Recommendation
The interrelated nature of the Diabetes Mellitus and complications of the oral cavity have severe implications for the human body. Awareness needs to be developed regarding the oral complications arising in people with diabetes. Diabetic individuals should be given education about the increased oral health risks, encouraging regular visits to the dentist, and paying attention to oral hygiene. Periodontal screening should be done by dentists and physicians each time a diabetic individual comes for a visit. To halt the appearance of such oral complications, strategies should be in place to assess and manage diabetic individuals at risk. Dentists need to interact effectively with physicians in concerned fields of expertise to provide effective oral care for these diabetic individuals. Guidebooks can be developed by the Diabetic Association, which outlines issues of diabetic care to help dentists recognize better signs and symptoms that require a referral, recommendation for annual screening. This will thus allow for an approach to diabetic care that is proactive beyond their discipline’s scope. Also, booklets may be made available for the general population to maintain oral hygiene for diabetic individuals. Physicians and dentists should continue to stress the importance of glycemic control to diabetic patients to maintain a good quality of life.
Acknowledgment
The authors express their gratitude to Naufela Nafisa Ahmad, Master of Arts in English Language (Linguistics), Jalan Wangsa Delima 7, Wangsa Maju, 53300 Kuala Lumpur, Malaysia, for revising and providing her expert opinion about the quality of the English language of this article. The authors also show gratitude to Faiza Binte Mozammel, Photographer and Editor, 7/16/1 South Mugdapara Dhaka, Bangladesh, for her kind effort and time regarding image development and editing.
Funding Statement
This paper was not funded.
Consent for Publication
The author reviewed and approved the final version and has agreed to be accountable for all aspects of the work, including any accuracy or integrity issues.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they do not have any financial involvement or affiliations with any organization, association, or entity directly or indirectly with the subject matter or materials presented in this article. This also includes honoraria, expert testimony, employment, ownership of stocks or options, patents or grants received or pending, or royalties.
References
- 1.Blaslov K, Naranđa FS, Kruljac I, Renar IP. Treatment approach to type 2 diabetes: past, present, and future. World J Diabetes. 2018;9(12):209–219. doi: 10.4239/wjd.v9.i12.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aynalem SB, Zeleke AJ. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan-Aman Town, Southwest Ethiopia, 2016: a Cross-Sectional Study. Int J Endocrinol. 2018;2018:9317987. doi: 10.1155/2018/9317987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–S33. doi: 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Health topics/diabetes. 20 Avenue Appia, 1211 Geneva 27, Switzerland: WHO Press, World Health Organization; 2021. Available from: https://www.who.int/health-topics/diabetes#tab=tab_1. Accessed April17, 2021. [Google Scholar]
- 5.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Expert Committee. Global report on diabetes. 20 Avenue Appia, 1211 Geneva 27, Switzerland: WHO Press, World Health Organization; 2016. Available from: https://www.who.int/publications/i/item/9789241565257. Accessed April17, 2021. [Google Scholar]
- 7.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dall TM, Yang W, Halder P, et al. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37(12):3172–3179. doi: 10.2337/dc14-1036 [DOI] [PubMed] [Google Scholar]
- 9.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–1735. doi: 10.1161/CIRCRESAHA.115.306825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seng JJB, Kwan YH, Lee VSY, et al. Differential health care use, diabetes-related complications, and mortality among five unique classes of patients with type 2 diabetes in Singapore: a latent class analysis of 71,125 patients. Diabetes Care. 2020;43(5):1048–1056. doi: 10.2337/dc19-2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickerson HD, Dutta S. Diabetic complications: current challenges and opportunities. J Cardiovasc Transl Res. 2012;5(4):375–379. doi: 10.1007/s12265-012-9388-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maugeri G, Bucolo C, Drago F, et al. Attenuation of high glucose-induced damage in RPE cells through p38 MAPK signaling pathway inhibition. Front Pharmacol. 2021;12:684680. doi: 10.3389/fphar.2021.684680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hap K, Biernat K, Konieczny G. Patients with diabetes complicated by peripheral artery disease: the current state of knowledge on physiotherapy interventions. J Diabetes Res. 2021;2021:5122494. doi: 10.1155/2021/5122494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a Real-World Study. Diabetes Care. 2019;42(12):2220–2227. doi: 10.2337/dc19-0830 [DOI] [PubMed] [Google Scholar]
- 15.Cicmil A, Govedarica O, Lečić J, Mališ S, Cicmil S, Čakić S. Oral symptoms and mucosal lesions in patients with diabetes mellitus type 2. Balk J Dent Med. 2017;21(1):50–54. doi: 10.1515/bjdm-2017-0007 [DOI] [Google Scholar]
- 16.Nazir MA, AlGhamdi L, AlKadi M, AlBeajan N, AlRashoudi L, AlHussan M. The burden of diabetes, its oral complications and their prevention and management. Open Access Maced J Med Sci. 2018;6(8):1545–1553. doi: 10.3889/oamjms.2018.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-Serrano J, Serrano J, López-Pintor RM, Paredes VM, Casañas E, Hernández G. Prevalence of oral mucosal disorders in diabetes mellitus patients compared with a control group. J Diabetes Res. 2016;2016:5048967. doi: 10.1155/2016/5048967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Indurkar MS, Maurya AS, Indurkar S. Oral manifestations of diabetes. Clin Diabetes. 2016;34(1):54–57. doi: 10.2337/diaclin.34.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Maskari AY, Al-Maskari MY, Al-Sudairy S. Oral manifestations and complications of diabetes mellitus: a review. Sultan Qaboos Univ Med J. 2011;11(2):179–186. [PMC free article] [PubMed] [Google Scholar]
- 20.Mauri-Obradors E, Estrugo-Devesa A, Jané-Salas E, Viñas M, López-López J. Oral manifestations of diabetes mellitus. A systematic review. Med Oral Patol Oral Cir Bucal. 2017;22(5):e586–e594. doi: 10.4317/medoral.21655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ship JA. Diabetes and oral health: an overview. J Am Dent Assoc. 2003;134 Spec No:4S–10S. doi: 10.14219/jada.archive.2003.0367 [DOI] [PubMed] [Google Scholar]
- 22.Trentin MS, Verardi G, Verardi G, Ferreira MDC, da Silva SO, Paranhos LR. Most frequent oral lesions in patients with type 2 diabetes mellitus. J Contemp Dent Pract. 2017;18(2):107–111. doi: 10.5005/jp-journals-10024-1999 [DOI] [PubMed] [Google Scholar]
- 23.Khan T. Oral manifestations and complications of diabetes mellitus: a review. Int J Med Health Res. 2018;4:50–52. doi: 10.22271/ijmhr [DOI] [Google Scholar]
- 24.Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40(Suppl 14):S113–34. doi: 10.1111/jcpe.12059 [DOI] [PubMed] [Google Scholar]
- 25.Llambés F, Arias-Herrera S, Caffesse R. Relationship between diabetes and periodontal infection. World J Diabetes. 2015;6(7):927–935. doi: 10.4239/wjd.v6.i7.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajaj S, Prasad S, Gupta A, Singh VB. Oral manifestations in type-2 diabetes and related complications. Indian J Endocrinol Metab. 2012;16(5):777–779. doi: 10.4103/2230-8210.100673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lessa LS, Pires PDS, Ceretta RA, et al. Meta-analysis of prevalence of xerostomia in diabetes mellitus. Int Arch Med. 2015;8(224):1–13. doi: 10.3823/1823 [DOI] [Google Scholar]
- 28.Lima DLF, Carneiro SDRM, Barbosa FTS, Saintrain MVL, Moizan JAH, Doucet J. Salivary flow and xerostomia in older patients with type 2 diabetes mellitus. PLoS One. 2017;12(8):e0180891. doi: 10.1371/journal.pone.0180891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortazavi H, Baharvand M, Movahhedian A, Mohammadi M, Khodadoustan A. Xerostomia due to systemic disease: a review of 20 conditions and mechanisms. Ann Med Health Sci Res. 2014;4(4):503–510. doi: 10.4103/2141-9248.139284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navea Aguilera C. The relationship between xerostomia and diabetes mellitus: a little-known complication. Endocrinol Nutr. 2015;62(1):45–46. English, Spanish. doi: 10.1016/j.endonu.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 31.Hoseini A, Mirzapour A, Bijani A, Shirzad A. Salivary flow rate and xerostomia in patients with type I and II diabetes mellitus. Electron Physician. 2017;9(9):5244–5249. doi: 10.19082/5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohanty S, Mohanty N, Rath S. Analysis of oral health complications in diabetic patients – a diagnostic perspective. J Oral Res. 2018;7(8):278–281. doi: 10.17126/joralres.2018.072 [DOI] [Google Scholar]
- 33.Jha R, Kalyani P, Bavishi R. Oral manifestations of diabetes. J Res Med Dent Sci. 2017;2(3):6–8. doi: 10.5455/jrmds.2014232 [DOI] [Google Scholar]
- 34.Sasaki H, Hirai K, Martins CM, Furusho H, Battaglino R, Hashimoto K. Interrelationship between periapical lesion and systemic metabolic disorders. Curr Pharm Des. 2016;22(15):2204–2215. doi: 10.2174/1381612822666160216145107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharmaceuticals (Basel). 2020;13(4):60. doi: 10.3390/ph13040060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moin M, Malik A. Frequency of dental caries and level of risk among type II diabetic. Dentistry. 2015;5:334–338. doi: 10.4172/2161-1122.1000334 [DOI] [Google Scholar]
- 37.Seetha Lakshmi C, Reddy RC, Asifa N, Prabhu S. Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: a Cross-Sectional Study. J Clin Diagn Res. 2016;10(3):ZC12–4. doi: 10.7860/JCDR/2016/16310.7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latti BR, Kalburge JV, Birajdar SB, Latti RG. Evaluation of relationship between dental caries, diabetes mellitus and oral microbiota in diabetics. J Oral Maxillofac Pathol. 2018;22(2):282. doi: 10.4103/jomfp.JOMFP_163_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su N, Ching V, Grushka M. Taste disorders: a review. J Can Dent Assoc. 2013;79:d86. [PubMed] [Google Scholar]
- 40.Khera S, Saigal A. Assessment and evaluation of gustatory functions in patients with diabetes mellitus type II: a study. Indian J Endocrinol Metab. 2018;22(2):204–207. doi: 10.4103/ijem.IJEM_555_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wasalathanthri S, Hettiarachchi P, Prathapan S. Sweet taste sensitivity in pre-diabetics, diabetics and normoglycemic controls: a comparative cross-sectional study. BMC Endocr Disord. 2014;14(1):67. doi: 10.1186/1472-6823-14-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renton T. Burning mouth syndrome. Rev Pain. 2011;5(4):127. doi: 10.1177/204946371100500403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad P, Akhtar U, Chaudhry A, Rahid U, Saif S, Asif JA. Repercussions of diabetes mellitus on the oral cavity. Eur J Gen Dent. 2019;8(3):55–62. doi: 10.4103/ejgd.ejgd_28_19 [DOI] [Google Scholar]
- 44.Erriu M, Pili FM, Cadoni S, Garau V. Diagnosis of lingual atrophic conditions: associations with local and systemic factors. a descriptive review. Open Dent J. 2016;10(1):619–635. doi: 10.2174/1874210601610010619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aylıkcı BU, Colak H. Halitosis: from diagnosis to management. J Nat Sci Biol Med. 2013;4(1):14–23. doi: 10.4103/0976-9668.107255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atefi N, Majedi M, Peyghambari S, Ghourchian S. Prevalence of diabetes mellitus and impaired fasting blood glucose in patients with lichen planus. Med J Islam Repub Iran. 2012;26(1):22–26. [PMC free article] [PubMed] [Google Scholar]
- 47.Mozaffari HR, Sharifi R, Sadeghi M. Prevalence of oral lichen planus in diabetes mellitus: a Meta-Analysis Study. Acta Inform Med. 2016;24(6):390–393. doi: 10.5455/aim.2016.24.390-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iqbal MA, Yesmin S, Maaisha F, Ibrahim S, Gotame P. Oral lichen planus and its recent management: a review update. Dent Coll J. 2020;10(2):29–34. doi: 10.3329/updcj.v10i2.50179 [DOI] [Google Scholar]
- 49.Uma Maheswari T, Chaudhary M. Management of oral lichen planus based on the existing clinical practice guidelines. J Indian Acad Oral Med Radiol. 2020;32(3):284. doi: 10.4103/jiaomr.jiaomr_55_20 [DOI] [Google Scholar]
- 50.Davis TME, Bruce DG, Curtis BH, Barraclough H, Davis WA. The relationship between intensification of blood glucose-lowering therapies, health status and quality of life in type 2 diabetes: the Fremantle Diabetes Study Phase II. Diabetes Res Clin Pract. 2018;142:294–302. doi: 10.1016/j.diabres.2018.05.047 [DOI] [PubMed] [Google Scholar]
- 51.Bowyer V, Sutcliffe P, Ireland R, et al. Oral health awareness in adult patients with diabetes: a Questionnaire Study. Br Dent J. 2011;211(6):E12. doi: 10.1038/sj.bdj.2011.769 [DOI] [PubMed] [Google Scholar]
- 52.Shanmukappa SM, Nadig P, Puttannavar R, Ambareen Z, Gowda TM, Mehta DS. Knowledge, attitude, and awareness among diabetic patients in davangere about the association between diabetes and periodontal disease. J Int Soc Prev Community Dent. 2017;7(6):381–388. doi: 10.4103/jispcd.JISPCD_390_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poudel P, Griffiths R, Wong VW, et al. Perceptions and practices of diabetes educators in providing oral health care: a Qualitative Study. Diabetes Educ. 2018;44(5):454–464. doi: 10.1177/0145721718796055 [DOI] [PubMed] [Google Scholar]
- 54.Lang NP, Bartold PM. Periodontal health. J Periodontol. 2018;89(Suppl1):S9–S16. doi: 10.1002/JPER.16-0517 [DOI] [PubMed] [Google Scholar]
- 55.Guthmiller JM, Novak KF. Periodontal diseases. In: Brogden KA, Guthmiller JM, editors. Polymicrobial Diseases. Washington (DC): ASM Press; 2002. Chapter 8. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2496/. Accessed April17, 2021. [PubMed] [Google Scholar]
- 56.Tsaousoglou P, Nietzsche S, Cachovan G, Sculean A, Eick S. Antibacterial activity of moxifloxacin on bacteria associated with periodontitis within a biofilm. J Med Microbiol. 2014;63(Pt 2):284–292. doi: 10.1099/jmm.0.065441-0 [DOI] [PubMed] [Google Scholar]
- 57.Jünemann S, Prior K, Szczepanowski R, et al. Bacterial community shift in treated periodontitis patients revealed by ion torrent 16S rRNA gene amplicon sequencing. PLoS One. 2012;7(8):e41606. doi: 10.1371/journal.pone.0041606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10(5):497–506. doi: 10.1016/j.chom.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. doi: 10.3389/fimmu.2019.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–241. doi: 10.1111/jth.13911 [DOI] [PubMed] [Google Scholar]
- 61.Dahiya P, Kamal R, Gupta R, Bhardwaj R, Chaudhary K, Kaur S. Reactive oxygen species in periodontitis. J Indian Soc Periodontol. 2013;17(4):411–416. doi: 10.4103/0972-124X.118306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yasunari K, Watanabe T, Nakamura M. Reactive oxygen species formation by polymorphonuclear cells and mononuclear cells as a risk factor of cardiovascular diseases. Curr Pharm Biotechnol. 2006;7(2):73–80. doi: 10.2174/138920106776597612 [DOI] [PubMed] [Google Scholar]
- 64.Daniel R, Gokulanathan S, Shanmugasundaram N, Lakshmigandhan M, Kavin T. Diabetes and periodontal disease. J Pharm Bioallied Sci. 2012;4(Suppl 2):S280–2. doi: 10.4103/0975-7406.100251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tavares RDCR, Ortigara GB, Tatsch KF, Ferreira CM, Boligon J, Moreira CHC. Association between periodontitis and glycated hemoglobin levels in individuals living in rural Southern Brazil. Clin Oral Investig. 2021. doi: 10.1007/s00784-021-03980-y [DOI] [PubMed] [Google Scholar]
- 66.Sima C, Van Dyke TE. Therapeutic targets for management of periodontitis and diabetes. Curr Pharm Des. 2016;22(15):2216–2237. doi: 10.2174/1381612822666160216150338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kayal RA. The role of osteoimmunology in periodontal disease. Biomed Res Int. 2013;2013:639368. doi: 10.1155/2013/639368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva JA, Ferrucci DL, Peroni LA, et al. Sequential IL-23 and IL-17 and increased Mmp8 and Mmp14 expression characterize the progression of an experimental model of periodontal disease in type 1 diabetes. J Cell Physiol. 2012;227(6):2441–2450. doi: 10.1002/jcp.22979 [DOI] [PubMed] [Google Scholar]
- 69.Duarte PM, de Oliveira MC, Tambeli CH, Parada CA, Casati MZ, Nociti FH Jr. Overexpression of interleukin-1beta and interleukin-6 may play an important role in periodontal breakdown in type 2 diabetic patients. J Periodontal Res. 2007;42(4):377–381. doi: 10.1111/j.1600-0765.2006.00961.x [DOI] [PubMed] [Google Scholar]
- 70.Lappin DF, Eapen B, Robertson D, Young J, Hodge PJ. Markers of bone destruction and formation and periodontitis in type 1 diabetes mellitus. J Clin Periodontol. 2009;36(8):634–641. doi: 10.1111/j.1600-051X.2009.01440.x [DOI] [PubMed] [Google Scholar]
- 71.Taylor GW, Graves DT, Lamster IB. Periodontal disease as a complication of diabetes mellitus. In: Lamster IB, editor. Diabetes Mellitus and Oral Health: An Interprofessional Approach. Ames: John Wiley & Sons; 2014:121–142. 9600 Garsington Road, 29 Dzerzhinskogo St. Oxford, OX4 2DQ UK. [Google Scholar]
- 72.Kocher T, König J, Borgnakke WS, Pink C, Meisel P. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol 2000. 2018;78(1):59–97. doi: 10.1111/prd.12235 [DOI] [PubMed] [Google Scholar]
- 73.Shetty N, Thomas B, Ramesh A. Comparison of neutrophil functions in diabetic and healthy subjects with chronic generalized periodontitis. J Indian Soc Periodontol. 2008;12(2):41–44. doi: 10.4103/0972-124X.44089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pacios S, Andriankaja O, Kang J, et al. Bacterial infection increases periodontal bone loss in diabetic rats through enhanced apoptosis. Am J Pathol. 2013;183(6):1928–1935. doi: 10.1016/j.ajpath.2013.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang J, de Brito Bezerra B, Pacios S, et al. Aggregatibacter actinomycetemcomitans infection enhances apoptosis in vivo through a caspase-3-dependent mechanism in experimental periodontitis. Infect Immun. 2012;80(6):2247–2256. doi: 10.1128/IAI.06371-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Graves DT, Liu R, Oates TW. Diabetes-enhanced inflammation and apoptosis: impact on periodontal pathosis. Periodontol 2000. 2007;45(1):128–137. doi: 10.1111/j.1600-0757.2007.00219.x [DOI] [PubMed] [Google Scholar]
- 77.Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;2012:939751. doi: 10.1155/2012/939751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng M, Wang C, Ali A, Shih YA, Xie Q, Guo C. Prevalence of periodontitis in people clinically diagnosed with diabetes mellitus: a meta-analysis of epidemiologic studies. Acta Diabetol. 2021. doi: 10.1007/s00592-021-01738-2 [DOI] [PubMed] [Google Scholar]
- 79.Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. doi: 10.1007/s00125-011-2342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Könönen E, Gursoy M, Gursoy UK. Periodontitis: a multifaceted disease of tooth-supporting tissues. J Clin Med. 2019;8(8):1135. doi: 10.3390/jcm8081135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapple IL, Genco R; working group 2 of the joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol. 2013;84(4Suppl):S106–12. doi: 10.1902/jop.2013.1340011 [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Wang H, Zhang T, Cai L, Kong C, He J. Current knowledge regarding the interaction between oral bone metabolic disorders and diabetes mellitus. Front Endocrinol (Lausanne). 2020;11:536. doi: 10.3389/fendo.2020.00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain A, Chawla M, Kumar A, et al. Management of periodontal disease in patients with diabetes- good clinical practice guidelines: a joint statement by Indian society of periodontology and research society for the study of diabetes in India. J Indian Soc Periodontol. 2020;24(6):498–524. doi: 10.4103/jisp.jisp_688_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Negrato CA, Tarzia O, Jovanovič L, Chinellato LE. Periodontal disease and diabetes mellitus. J Appl Oral Sci. 2013;21(1):1–12. doi: 10.1590/1678-7757201302106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang P-C, Lim LP. Interrelationships of periodontitis and diabetes: a review of the current literature. J Dent Sci. 2012;7(3):272–282. doi: 10.1016/j.jds.2012.02.002 [DOI] [Google Scholar]
- 86.Sundar C, Ramalingam S, Mohan V, Pradeepa R, Ramakrishnan MJ. Periodontal therapy as an adjunctive modality for HbA1c reduction in type-2 diabetic patients. J Edu Health Promot. 2018;7:152. doi: 10.4103/jehp.jehp_66_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials. 2015;16(1):291. doi: 10.1186/s13063-015-0810-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.López-Pintor RM, Casañas E, González-Serrano J, et al. Xerostomia, hyposalivation, and salivary flow in diabetes patients. J Diabetes Res. 2016;2016:4372852. doi: 10.1155/2016/4372852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roi A, Rusu LC, Roi CI, et al. Approach for the diagnosis of systemic and oral diseases based on salivary biomolecules. Dis Markers. 2019;2019:8761860. doi: 10.1155/2019/8761860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verhulst MJL, Loos BG, Gerdes VEA, Teeuw WJ. Evaluating all potential oral complications of diabetes mellitus. Front Endocrinol (Lausanne). 2019;10:56. doi: 10.3389/fendo.2019.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al Mubarak S, Robert AA, Baskaradoss JK, et al. The prevalence of oral Candida infections in periodontitis patients with type 2 diabetes mellitus. J Infect Public Health. 2013;6(4):296–301. doi: 10.1016/j.jiph.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 92.Buranasin P, Mizutani K, Iwasaki K, Pawaputanon Na Mahasarakham C, Kido D, Takeda K. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS One. 2018;13(8):e0201855. doi: 10.1371/journal.pone.0201855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl1):S27–S36. doi: 10.4103/2230-8210.94253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mustăţea P, Bugă C, Doran H, et al. Soft tissue infections in diabetic patients. Chirurgia (Bucur). 2018;113(5):651–667. doi: 10.21614/chirurgia.113.5.651 [DOI] [PubMed] [Google Scholar]
- 95.Sharma K, Das D, Joshi M, Barman D, Sarma AJ. Deep neck space infections-A study in diabetic population in a tertiary care centre. Indian J Otolaryngol Head Neck Surg. 2018;70(1):22–27. doi: 10.1007/s12070-017-1196-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tibau AV, Grube D. Biological dentistry-whole body health shifting the paradigm in the 21st century. Glob J Otolaryngol. 2020;22(1):556079. doi: 10.19080/GJO.2020.22.556079 [DOI] [Google Scholar]
- 97.Akhtar N, Saleem M, Mian FA, Shareef MJ, Hussain F. Head and neck infections; secondary to dental causes; diagnosis and treatment. Prof Med J. 2015;22:787–792. [Google Scholar]
- 98.Rathee M, Sapra A. Dental caries. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [Updated 2021 March12]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551699/. Accessed April17, 2021. [Google Scholar]
- 99.Jeong I‑H, Park M‑J. The association between diabetes mellitus and community periodontal index. Int J Appl Eng Res. 2017;12(10):2277‑81. [Google Scholar]
- 100.Sampaio N, Mello S, Alves C. Dental caries-associated risk factors and type 1 diabetes mellitus. Pediatr Endocrinol Diabetes Metab. 2011;17(3):152–157. [PubMed] [Google Scholar]
- 101.Ferizi L, Dragidella F, Spahiu L, Begzati A, Kotori V. The influence of type 1 diabetes mellitus on dental caries and salivary composition. Int J Dent. 2018;2018:5780916. doi: 10.1155/2018/5780916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kapadia J, Dodamani A, Baviskar P, Karibasappa GN, Pathak P, Bezalwar A. Effect of sugar-free and regular toothpaste on salivary glucose and ph among type 2 diabetes- a randomized crossover trial. J Clin Diagn Res. 2017;11(7):ZC71–ZC75. doi: 10.7860/JCDR/2017/25580.10250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abikshyeet P, Ramesh V, Oza N. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab Syndr Obes. 2012;5:149–154. doi: 10.2147/DMSO.S32112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hariharavel V, Rao AP, Venugopal RN, Peter J. Diabetes, diet, and dental caries. Int J Diabetes Dev Ctries. 2017;37(1):94. doi: 10.1007/s13410-015-0400-6 [DOI] [Google Scholar]
- 105.Puputti S, Hoppu U, Sandell M. Taste sensitivity is associated with food consumption behavior but not with recalled pleasantness. Foods. 2019;8(10):444. doi: 10.3390/foods8100444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kinnamon SC. Taste receptor signaling - from tongues to lungs. Acta Physiol (Oxf). 2012;204(2):158–168. doi: 10.1111/j.1748-1716.2011.02308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Puputti S, Hoppu U, Sandell M, Aisala H. Factors explaining individual differences in taste sensitivity and taste modality recognition among Finnish adults. J Sens Stud. 2019;34(4):e12506. doi: 10.1111/joss.12506 [DOI] [Google Scholar]
- 108.Pugnaloni S, Alia S, Mancini M, et al. A study on the relationship between type 2 diabetes and taste function in patients with good glycemic control. Nutrients. 2020;12(4):1112. doi: 10.3390/nu12041112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhandare NN, Keny MS, Nevrekar RP, Bhandare PN. Diabetic tongue - could it be a diagnostic criterion? J Fam Med Prim Care. 2014;3(3):290–291. doi: 10.4103/2249-4863.141654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamala KA, Sankethguddad S, Sujith SG, Tantradi P. Burning mouth syndrome. Indian J Palliat Care. 2016;22(1):74–79. doi: 10.4103/0973-1075.173942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nada A, Abdel Moneim W, Fakhr M, El Sawy S. Prevalence of burning mouth syndrome in a sample of Egyptian patients with diabetic neuropathy: a Cross-Sectional Hospital-Based Study. Adv Dent J. 2020;2(2):34–42. doi: 10.21608/adjc.2020.23213.1050 [DOI] [Google Scholar]
- 112.Costa YM, Karlsson P, Bonjardim LR, et al. Trigeminal nociceptive function and oral somatosensory functional and structural assessment in patients with diabetic peripheral neuropathy. Sci Rep. 2019;9(1):169. doi: 10.1038/s41598-018-37041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dotiwala AK, Samra NS. Anatomy, head and neck, tongue. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [Updated 2020 August22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507782/. Accessed April17, 2021. [Google Scholar]
- 114.Hsu PC, Wu HK, Huang YC, et al. The tongue features associated with type 2 diabetes mellitus. Medicine (Baltimore). 2019;98(19):e15567. doi: 10.1097/MD.0000000000015567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sudarshan R, Sree Vijayabala G, Samata Y, Ravikiran A. Newer classification system for fissured tongue: an epidemiological approach. J Trop Med. 2015;2015:262079. doi: 10.1155/2015/262079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2014;11:45–51. doi: 10.2147/TCRM.S76282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bihari M, Srivastava R, Jyoti B, Mehrota V, Gupta M, Pradhan S. Median rhomboid glossitis with palatal kissing lesion-a case report. Bang J Dent Res Edu. 2014;4(2):94–97. doi: 10.3329/bjdre.v4i2.20273 [DOI] [Google Scholar]
- 118.Ravindran R, Deepa MG, Sruthi AK, et al. Evaluation of oral health in type II diabetes mellitus patients. Oral Maxillofac Path J. 2015;6(1):525–531. doi: 10.5005/10037-1030 [DOI] [Google Scholar]
- 119.Khandelwal V, Nayak PA, Nayak UA, Gupta A. Oral lichen planus in a young Indian child. BMJ Case Rep. 2013;2013(aug13 2):bcr2013010516. doi: 10.1136/bcr-2013-010516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1(3):140–149. doi: 10.1016/j.ijwd.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis, and management. Indian J Dermatol. 2015;60(3):222–229. doi: 10.4103/0019-5154.156315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sahril N, Aris T, Mohd Asari AS, et al. Oral health-seeking behavior among Malaysians with type II diabetes. J Public Health Aspects. 2014;1(1):1. doi: 10.7243/2055-7205-1-1 [DOI] [Google Scholar]
- 123.Arunkumar S, Amur S, Sambrani U, Burde KM. Survey on awareness and knowledge about the effect of diabetes mellitus on systemic and oral health in patients visiting general medicine outpatient department in dental hospital. J Krishna Inst Med Sci. 2015;4(2):100–106. [Google Scholar]
- 124.Badiah B. A preliminary survey on awareness of periodontal risk and oral health practices among diabetic patients in hospital Kuala Lumpur. Malaysian Dent J. 2012;34(1):1–7. [Google Scholar]
- 125.Aggarwal A, Panat SR. Oral health behavior and HbA1c in Indian adults with type 2 diabetes. J Oral Sci. 2012;54(4):293–301. doi: 10.2334/josnusd.54.293 [DOI] [PubMed] [Google Scholar]
- 126.Bahammam MA. Periodontal health and diabetes awareness among Saudi diabetes patients. Patient Prefer Adherence. 2015;9:225–233. doi: 10.2147/PPA.S79543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Al Amassi BY, Al Dakheel RS. Oral hygiene practice of adult diabetic patients and their awareness about oral health problems related to diabetes. J Dent Oral Hyg. 2017;9(2):8–14. doi: 10.5897/JDOH2017.0219 [DOI] [Google Scholar]
- 128.Poudel P, Griffiths R, Wong VW, et al. Oral health knowledge, attitudes and care practices of people with diabetes: a systematic review. BMC Public Health. 2018;18(1):577. doi: 10.1186/s12889-018-5485-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Busui RP, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi: 10.2337/dc16-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weng YC, Tsai SS, Lyu RK, et al. Diabetic distal symmetrical polyneuropathy: correlation of clinical, laboratory, and electrophysiologic studies in patients with type 2 diabetes mellitus. J Diabetes Res. 2020;2020:6356459. doi: 10.1155/2020/6356459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14(8):473. doi: 10.1007/s11910-014-0473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dyck PJ, Albers JW, Andersen H, et al.; Toronto Expert Panel on Diabetic Neuropathy. Diabetic polyneuropathies: update on research definition, diagnostic criteria, and estimation of severity. Diabetes Metab Res Rev. 2011;27(7):620–628. doi: 10.1002/dmrr.1226 [DOI] [PubMed] [Google Scholar]
- 133.Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS One. 2019;14(2):e0212574. doi: 10.1371/journal.pone.0212574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alam U, Riley DR, Jugdey RS, et al. Diabetic neuropathy and gait: a review. Diabetes Ther. 2017;8(6):1253–1264. doi: 10.1007/s13300-017-0295-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Islam MR, Rahman T, Habib R, et al. Electrophysiological patterns of diabetic polyneuropathy: experience from a tertiary care hospital of Bangladesh. BIRDEM Med J. 2017;7(2):114–120. doi: 10.3329/birdem.v7i2.32448 [DOI] [Google Scholar]
- 136.Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis. 2015;6(1):15–28. doi: 10.1177/2040622314552071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arpith MN, Narayan V. Prevalence and determinants of peripheral neuropathy in patients with type 2 diabetes mellitus. Indian J Clin Anat Physiol. 2021;8(1):57–59. doi: 10.1823/j.ijcap.2021.013 [DOI] [Google Scholar]
- 138.Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–2383. doi: 10.2337/diacare.28.10.2378 [DOI] [PubMed] [Google Scholar]
- 139.Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes. 2015;6(3):432–444. doi: 10.4239/wjd.v6.i3.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vinik A, Casellini C, Nevoret ML. Diabetic neuropathies. In: Feingold KR, Anawalt B, Boyce A, et al. editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279175/. Accessed April17, 2021. [Google Scholar]
- 141.Zhou B, Lu Y, Hajifathalian K; NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sardu C, Paolisso P, Sacra C, et al. Cardiac resynchronization therapy with a defibrillator (CRTd) in failing heart patients with type 2 diabetes mellitus and treated by glucagon-like peptide 1 receptor agonists (GLP-1 RA) therapy vs. conventional hypoglycemic drugs: arrhythmic burden, hospitalizations for heart failure, and CRTd responders rate. Cardiovasc Diabetol. 2018;17(1):137. doi: 10.1186/s12933-018-0778-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sardu C, Barbieri M, Santamaria M, et al. Multipolar pacing by cardiac resynchronization therapy with a defibrillators treatment in type 2 diabetes mellitus failing heart patients: impact on responders rate, and clinical outcomes. Cardiovasc Diabetol. 2017;16(1):75. doi: 10.1186/s12933-017-0554-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sardu C, Barbieri M, Balestrieri ML, et al. Thrombus aspiration in hyperglycemic ST-elevation myocardial infarction (STEMI) patients: clinical outcomes at 1-year follow-up. Cardiovasc Diabetol. 2018;17(1):152. doi: 10.1186/s12933-018-0795-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sardu C, D’Onofrio N, Mauro C, Balestrieri ML, Marfella R. Thrombus aspiration in hyperglycemic patients with high inflammation levels in coronary thrombus. J Am Coll Cardiol. 2019;73(4):530–531. doi: 10.1016/j.jacc.2018.10.074 [DOI] [PubMed] [Google Scholar]
- 146.Galaviz KI, Narayan KMV, Lobelo F, Weber MB. Lifestyle and the prevention of type 2 diabetes: a status report. Am J Lifestyle Med. 2015;12(1):4–20. doi: 10.1177/1559827615619159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular complications of diabetes and therapeutic approaches. Biomed Res Int. 2016;2016:3801570. doi: 10.1155/2016/3801570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Simó-Servat O, Hernández C, Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. 2019;62(4):211–217. doi: 10.1159/000499541 [DOI] [PubMed] [Google Scholar]
- 149.Simó R, Hernández C; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014;25(1):23–33. doi: 10.1016/j.tem.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 150.Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015;6(3):489–499. doi: 10.4239/wjd.v6.i3.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barot M, Gokulgandhi MR, Patel S, Mitra AK. Microvascular complications and diabetic retinopathy: recent advances and future implications. Future Med Chem. 2013;5(3):301–314. doi: 10.4155/fmc.12.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fong DS, Aiello LP, Ferris FL 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27(10):2540–2553. doi: 10.2337/diacare.27.10.2540 [DOI] [PubMed] [Google Scholar]
- 153.Simó R, Hernández C. Advances in the medical treatment of diabetic retinopathy. Diabetes Care. 2009;32(8):1556–1562. doi: 10.2337/dc09-0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim K-S, Lee J-S, Park J-H, et al. Identification of novel biomarker for early detection of diabetic nephropathy. Biomedicines. 2021;9(5):457. doi: 10.3390/biomedicines9050457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rigalleau V, Lasseur C, Raffaitin C, et al. Normoalbuminuric renal-insufficient diabetic patients: a lower-risk group. Diabetes Care. 2007;30(8):2034–2039. doi: 10.2337/dc07-0140 [DOI] [PubMed] [Google Scholar]
- 156.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fakhruddin S, Alanazi W, Jackson KE. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. J Diabetes Res. 2017;2017:8379327. doi: 10.1155/2017/8379327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cao Z, Cooper ME. Pathogenesis of diabetic nephropathy. J Diabetes Investig. 2011;2(4):243–247. doi: 10.1111/j.2040-1124.2011.00131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park TS. How much glycemic control is needed to prevent progression of diabetic nephropathy? J Diabetes Investig. 2012;3(5):411–412. doi: 10.1111/j.2040-1124.2012.00225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Williams ME, Garg R. Glycemic management in ESRD and earlier stages of CKD. Am J Kidney Dis. 2014;63(2 Suppl 2):S22–38. doi: 10.1053/j.ajkd.2013.10.049 [DOI] [PubMed] [Google Scholar]
- 161.DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17(5):319–334. doi: 10.1038/s41581-021-00393-8 [DOI] [PubMed] [Google Scholar]
- 162.Phillips AO, Baboolal K, Riley S, et al. Association of prolonged hyperglycemia with glomerular hypertrophy and renal basement membrane thickening in the Goto Kakizaki model of non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 2001;37(2):400–410. doi: 10.1053/ajkd.2001.21322 [DOI] [PubMed] [Google Scholar]
- 163.Tervaert TW, Mooyaart AL, Amann K, et al.; Renal Pathology Society. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–563. doi: 10.1681/ASN.2010010010 [DOI] [PubMed] [Google Scholar]
- 164.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117(8):662–675. doi: 10.1016/j.jfma.2018.02.007 [DOI] [PubMed] [Google Scholar]