Abstract
Introduction
Remdesivir has demonstrated antiviral activity against coronavirus, shortening the time to recovery in adults hospitalized with moderate/severe COVID-19. Severe adverse events such as acute kidney injury have been reported. Scant data are available on the use and safety of remdesivir in kidney transplant recipients.
Methods
We present a multicenter cohort study of 51 kidney transplant recipients with COVID-19 treated with remdesivir. Outcomes and safety were assessed.
Results
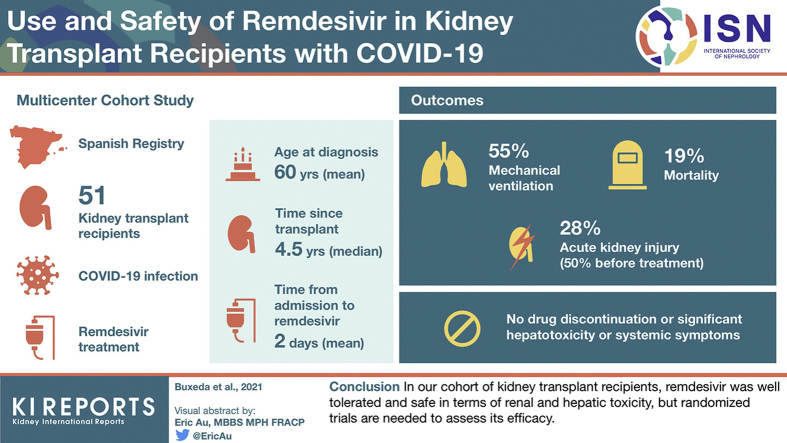
Mean age at diagnosis was 60 years, with a median time since kidney transplant of 4.5 years. Mean time since admission to remdesivir was 2 days. Twenty-eight patients (54.9%) required mechanical ventilation (19 noninvasive). Mortality was 18.9% and markedly higher if aged ≥65 years (45% vs. 3.2% in younger patients). Acute kidney injury was present in 27.7% of patients, but was diagnosed in 50% before treatment. No patients required remdesivir discontinuation because of adverse events. We did not find significant hepatoxicity or systemic symptoms resulting from the drug.
Conclusion
In our cohort of kidney transplant recipients, remdesivir was well tolerated and safe in renal and hepatic toxicity, but randomized trials are needed to assess its efficacy.
Keywords: acute kidney injury, immunosuppression, kidney transplantation, remdesivir, SARS-CoV2l
Graphical abstract
Since March 11, 2020, Spain and many other countries have seen a 3-wave pattern in reported cases of SARS-CoV2 COVID-19.1,2 As of February 17, 2021, more than 3,105,000 cases of COVID-19 and 55,000 deaths due to the infection have been reported in Spain, being the seventh most affected country.1,3
Kidney transplant (KT) recipients appear to be at notable high risk for critical COVID-19 illness due to long-term immunosuppression, age, coexisting comorbidities, and frequent contact with health care facilities.4, 5, 6, 7, 8 Among 35,183 prevalent KT recipients in Spain,9 almost 1700 cases of COVID-19 have been reported by the Spanish Society of Nephrology (S.E.N.) registry. This results in a global incidence of 4.8%,10 similar to 6.1% reported in the general population.1
Treatment for COVID-19 has been a matter of debate during the pandemic spread, including both antiviral and anti-inflammatory drugs.11, 12, 13, 14, 15, 16, 17 Remdesivir is a prodrug of an adenosine analog that has demonstrated antiviral activity against a broad range of RNA virus families, including coronaviruses. This drug has shown nanomolar in vitro activity against SARS-CoV2 in human airway epithelial cells and clinical and virologic efficacy in a primate model.18 Some clinical trials showed that remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with COVID-19.16,17
However, all remdesivir trials have excluded patients with severe acute kidney injury (AKI), chronic kidney disease (CKD) with an estimated glomerular filtrate rate (eGFR) <30 ml/min per 1.73 m2, or those on renal replacement therapy (RRT). There are concerns about the potential toxicity of remdesivir in patients with renal impairment related both to dose-dependent direct tubular damage produced by remdesivir in experimental studies and the potential accumulation of its sulfobutylether-β-cyclodextrin (SBECD) carrier.19
The most common adverse events described in patients treated with remdesivir are increased hepatic enzymes, diarrhea, rash, renal impairment, and hypotension.20,21 Particularly, the development of AKI has been described in up to 22.8% of patients treated with remdesivir.21,22 Nevertheless, randomized clinical trials presented a comparable incidence of AKI between remdesivir and placebo.16,23 Moreover, recent studies reported remdesivir was well tolerated in patients with AKI and CKD, including those on RRT.24,25 Assessment of drug-induced nephrotoxicity is particularly challenging because COVID-19 also bears frequent renal complications.26, 27, 28
Remdesivir has scarcely been used in KT patients with COVID-19.5,7,24,29 KT recipients have only 1 functioning kidney most of the time, with reduced eGFR compared with the general population, and frequently receive calcineurin inhibitors, which compromise the glomerular blood flow. Minimal data are currently available on the use and safety of remdesivir in this at-risk population.24 Here, we present the results of a Spanish multicenter study gathering data of 51 KT recipients treated with remdesivir for COVID-19. Their outcomes, safety profile, renal impairment, and tacrolimus interaction were assessed.
Methods
Patients
A Spanish registry comprising dialysis and KT patients with a confirmed diagnosis of COVID-19 started on March 18, 2020 (S.E.N. COVID-19 registry), as previously described.10 Only patients diagnosed with positive reverse-transcriptase polymerase chain reaction assay of a specimen collected on a nasopharyngeal swab or bronchoalveolar lavage were included. Of these, KT patients who received remdesivir treatment were included in this study. Until February 1, 2021, 48 were identified to have received remdesivir in 26 different hospitals. Each center was contacted and invited to participate in the study by completing an expanded retrospective database with more detailed data from this cohort. Twenty-four centers completed the database for 43 patients, and 8 additional patients were included, resulting in a final number of 51 patients. The median time of follow-up since the onset of symptoms to recovery/death was 25 days (interquartile range [IQR], 16–35 days) and to the end of follow-up was 49 days (IQR, 34–68 days).
Data Collection and Definitions
The characteristics and variables included in the COVID-19 registry have been previously reported.8,30 Added in the expanded database were more specific and detailed data regarding demographics (recipient age, sex, race, and comorbidity), time after KT, time between onset of symptoms and admission, epidemic waves, respiratory situation, including arterial partial pressure of arterial oxygen/fraction of inspired oxygen ratio (Pao2/Fio2) index, acute respiratory distress syndrome, oxygen saturation by pulse oximetry, or pneumonia demonstrated by X-ray imaging; remdesivir treatment (days of remdesivir treatment, remdesivir dose), inflammatory markers, maintenance immunosuppressive treatment at admission, tacrolimus blood levels, kidney function, liver parameters, and adverse events. These variables were chosen based on their clinical relevance and previous results obtained from our group.
Criteria for remdesivir treatment were based on the protocol of each hospital, although all patients presented with one of the following: requirement of supplemental oxygen, rapidly progressive acute respiratory distress syndrome (defined as Pao2/Fio2 index <300) and/or bilateral pneumonia with rapidly increased levels of inflammatory markers, such as C-reactive protein (CRP), interleukin-6, D-dimer, ferritin, or lactic acid dehydrogenase. Most patients had given oral informed consent before remdesivir administration. Remdesivir dose regimens were based on the protocol of each hospital. The most common dosing regimen was 200 mg on day 1, followed by 100 mg on days 2 through 5.
Obesity was defined as body mass index >30 kg/m2. The immunosuppression regimen corresponds to that received at the time of COVID-19 diagnosis. AKI was defined following Kidney Disease: Improving Global Outcomes criteria for stage 1, stage 2, and stage 3.31 The last serum creatinine (SCr) value before admission was considered as the baseline to assess the incidence of AKI. Drug-induced liver injury was defined according to a grading system developed by the Drug-Induced Liver Injury Network. Grade 1 or mild was defined as raised serum alanine aminotransferase (ALT) or alkaline phosphatase levels, or both, but total serum bilirubin <2.5 mg/dl and no coagulopathy (international normalized ratio <1.5). No patients presented severity grades 2 to 5.32 Respiratory symptoms included cough, sneezing, and rhinorrhea but excluded dyspnea, which was collected separately. Respiratory status was recorded through Pao2/Fio2 when available (n = 21). If data to calculate the Pao2/Fio2 index were missing, oxygen saturation by pulse oximetry was considered (n = 28).
Clinical follow-up and laboratory tests were collected at 5 points: admission, when remdesivir was administered, early after remdesivir infusion (median, 2 days; IQR, 1–4 days), at discharge, and at early follow-up after discharge (median, 28 days; IQR, 17–57 days). Additionally, we collected the last SCr and tacrolimus levels before admission to assess the incidence and outcomes of AKI and tacrolimus levels variability.
Outcomes were assessed as COVID-19–related mortality or recovery until February 10, 2021. Recovery was defined as hospital discharge or clinical judgment based on 1 negative reverse-transcriptase polymerase chain reaction result and symptom resolution. Time to events was defined as days from COVID-19 diagnosis to death or recovery.
The study was approved by the local Ethical Committee at Hospital del Mar, Barcelona, Spain, and conducted according to the guidelines as dictated by the Declaration of Helsinki. All data were recorded anonymously.
Statistical Analysis
Categorical data are summarized as counts and percentages. According to their distribution, continuous data are expressed as mean and SD or median and IQR. Univariate analyses were performed according to variables normality, using χ2 or Fisher exact tests to analyze categorical variables, the Student t test for continuous variables with normal distribution, and the Mann-Whitney test U test for abnormal distribution data. Patient survival analysis according to age was performed using Kaplan-Meier survival curves, applying the log-rank test. Statistical analysis was performed using SPSS 22.0 software (IBM, Armonk, NY). A P value of <.05 was considered statistically significant. We used GraphPad Prism 7 (GraphPad Software, San Diego, CA) for data presentation.
Results
Patient Characteristics
During the observation period (March 12, 2020–February 1, 2021), 51 fully documented patients with COVID-19 had been treated with remdesivir and were included in the study. Baseline characteristics of the study population are summarized in Table 1. The mean recipient age at the time of the COVID-19 diagnosis was 60 ± 13 years. Most recipients were men (65.7%), Caucasian (70.6%), and with a high prevalence of hypertension (82.4%). Diabetes mellitus was present in 31.4% of the cohort, ischemic heart disease in 13.7%, and 11.8% had previous pulmonary disease. Tacrolimus plus mycophenolate-based immunosuppression was the most common therapy at admission (68.6% of recipients). Median time from KT to COVID-19 infection was 4.5 years, and 17.6% had received their transplant within the last 6 months. Before admission, the mean SCr levels were 1.4 ± 0.4 mg/dl and a mean eGFR of 53.8 ± 18.7 ml/min per 1.732.
Table 1.
Baseline characteristics among kidney transplant recipients with COVID-19 infection who received remdesivir
| Variablesa | All (N = 51) |
|---|---|
| Baseline characteristics | |
| Recipient age, y | 60 ± 13 |
| Recipient age ≥65 y | 20 (39.2) |
| Male sex | 33 (65.7) |
| White race | 36 (70.6) |
| Underlying diabetic kidney disease | 8 (15.7) |
| First kidney transplant | 43 (84.3) |
| History of smoking | 5 (9.8) |
| Comorbidities | |
| Lung disease | 6 (11.8) |
| Ischemic heart disease | 7 (13.7) |
| Arterial hypertension | 42 (82.4) |
| Diabetes mellitus | 16 (31.4) |
| History of cancer | 7 (13.7) |
| Obesity (body mass index >30 kg/m2) | 12 (23.5) |
| ACEi or ARB treatment | 17 (33.3) |
| Immunosuppressive therapy | |
| Thymoglobulin induction | 15 (29.4) |
| Calcineurin inhibitor | 48 (94.1) |
| Tacrolimus | 44 (86.3) |
| Prednisone | 43 (84.3) |
| Mycophenolate | 42 (82.4) |
| mTOR inhibitor | 7 (13.7) |
| Baseline kidney function (before admission) | |
| Serum creatinine, mg/dl | 1.4 ± 0.4 |
| eGFR CKD-EPI, ml/min per 1.73 m2 | 53.8 ± 18.7 |
| eGFR <30 ml/min per 1.73 m2 | 2 (3.9) |
| At admission | |
| Time from KT to COVID-19 diagnosis, y | 4.5 (1–10.5) |
| Time from KT ≤6 months | 9 (17.6) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR CKD-EPI, estimated glomerular filtrate rate calculated using Chronic Kidney Disease Epidemiology Collaboration equation; KT, kidney transplantation, mTOR, mammalian target of rapamycin.
Continuous data are presented as mean ± SD or median (interquartile range) and categorical data as n (%).
Data correspond to clinical characteristics before hospital admission.
Clinical Characteristics and Laboratory Findings at COVID-19 Presentation
Table 2 summarizes clinical characteristics and laboratory data. The COVID-19 diagnosis in 90% of patients occurred during the second wave. The median time between the onset of symptoms and hospital admission was 3 days (IQR, 2–5 days). There was just 1 nosocomial SARS-CoV2 infection. The most common clinical manifestations at admission were fever and respiratory symptoms (78.4%), followed by dyspnea (70.6%) and gastrointestinal symptoms (37.3%). Chest X-ray imaging showed pneumonia in 90% of patients. Twenty-six patients (51%) presented acute respiratory distress syndrome or oxygen saturation ≤94% at admission. Analytical parameters at admission showed lymphopenia and elevated inflammatory markers.
Table 2.
Clinical and analytical features at admission for the COVID-19 episode
| Variable | All |
|---|---|
| (N = 51) | |
| General data | |
| Wave | |
| 1st wave (March–June, 2020) | 3 (5.9) |
| 2nd wave (July–December, 2020) | 46 (90.2) |
| 3rd wave (since January, 2021) | 2 (3.9) |
| Time between symptoms onset and admission, d | 3 (2–5) |
| Clinical features at COVID-19 diagnosis | |
| Fever | 40 (78.4) |
| Dyspnea | 36 (70.6) |
| Cough, expectoration, and/or rhinorrhea | 40 (78.4) |
| Gastrointestinal symptoms | 19 (37.3) |
| Respiratory situation | |
| PaFi, mm Hg (n = 21) | 300 (249–360) |
| ARDS (PaFi <300) | 10 (19.6) |
| Oxygen saturation, % (n = 28) | 93 (88.3–96.8) |
| PaFi <300 or oxygen saturation ≤94% (n = 45) | 26 (51) |
| Pneumonia demonstrated by X-ray imaging | 46 (90.2) |
| Blood test at admission | |
| Lymphocytes, ×103/μl (n = 51) | 0.9 ± 1.2 |
| C-reactive protein, mg/L (n = 50) | 50.5 (7.6–109.5) |
| Procalcitonin, ng/ml (n = 38) | 0.3 (0.1–0.8) |
| Interlrukin-6, pg/ml (n = 34) | 42.8 (17.6–106.7) |
| Lactate dehydrogenase, IU/l (n = 46) | 295 (238.5–361.5) |
| Ferritin, ng/ml (n = 45) | 520 (295.5–1,134.5) |
| D-dimer, μg/l (n=45) | 667 (225–1,261) |
| Serum creatinine, mg/dl (n = 51) | 1.68 ± 0.7 |
| Bilirubin, mg/dl (n = 45) | 0.63 (0.4–0.88) |
| Alanine aminotransferase, IU/l (n = 49) | 22 (14–31.5) |
| Aspartate aminotransferase, IU/l (n = 45) | 28 (18–33.5) |
ARDS, acute respiratory distress syndrome, PaFi, partial pressure of arterial oxygen/fraction of inspired oxygen.
Categorical data are presented as n (%) and continuous date as mean ± SD or median (interquartile range).
Treatment of COVID-19
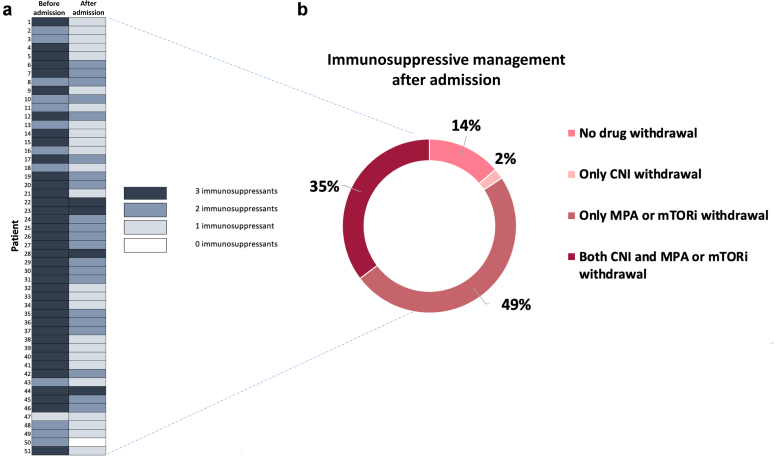
Changes in immunosuppression strategy are displayed in Figure 1. Up to 86.3% of patients had at least 1 of their immunosuppressants withdrawn at admission. Mycophenolate or mammalian target of rapamycin inhibitor withdrawal was the most frequent immunosuppression reduction strategy (49%), and only 4 patients maintained triple immunosuppressant therapy during admission. Calcineurin inhibitor was withdrawn in 39.6% of patients treated with this therapy.
Figure 1.
Changes in immunosuppressive treatment after admission with SARS-CoV2 infection. (a) Changes in the number of immunosuppressant drugs before hospital admission (left) and after admission (right). The number of immunosuppressant agents is color-coded. (b) Immunosuppressive withdrawal management after admission. CNI, calcineurin inhibitor; MPA, mycophenolate acid; mTORi, mammalian target of rapamycin inhibitor.
Regarding anti–COVID-19 therapies, remdesivir was administered at a median time of 2 days after admission. Of the 15 patients who required intensive care unit management, remdesivir was generally administered before admission, because the median time since hospital admission to intensive care unit was 4.5 days. A trend toward a decrease in the mean duration of hospitalization was seen in the 27 patients in whom the drug was initiated within 48 hours of admission (12.5 days; IQR, 9–30 days) compared with 20 patients who received remdesivir beyond the first 48 hours after admission (22.5 days; IQR, 15–34; P = .07). Similarly, the duration of oxygen therapy in the first group was slightly shorter (10 days [IQR, 5–23 days] vs. 16 days [IQR, 10–25 days], P = .21). Nevertheless, we did not observe a lower mortality rate in patients who initiated the drug earlier (11.1% vs. 24%, P = .21). The most frequent remdesivir regimen was 200 mg on day 1 and 100 mg on days 2 through 5 (56%) (Figure 2).
Figure 2.
Remdesivir regimens. Remdesivir dose regimens based on the protocol of each hospital. The most frequent remdesivir regimen was 200 mg (MG) on day 1 and 100 mg on days 2 through 5.
Most patients received combined treatment with steroid pulses, and up to 70.6% received antibiotics (other than azithromycin) due to confirmed or suspected bacterial coinfections. Tocilizumab was administered in 14 patients, and most of them received the drug after remdesivir treatment, with a median time from admission to the administration of tocilizumab of 5 days. Azithromycin, hydroxychloroquine, and ritonavir/lopinavir were less frequently used (Table 3).
Table 3.
Management and outcomes of kidney transplant recipients with COVID-19 infection who received remdesivir
| Variable | All |
|---|---|
| (N = 51) | |
| COVID-19 treatment | |
| Time since symptoms onset to remdesivir treatment, d | 7 (5–9) |
| Time since admission to remdesivir treatment, d | 2 (1–4) |
| Azithromycin | 6 (11.8) |
| Other antibiotics | 36 (70.6) |
| Steroids (methylprednisolone bolus or dexamethasone) | 49 (96.1) |
| Time since admission to steroids treatment, d | 1 (0–4) |
| Hydroxychloroquine | 2 (3.9) |
| Ritonavir/lopinavir | 4 (7.8) |
| Anakinra | 6 (11.8) |
| Tocilizumab | 14 (27.5) |
| Time since admission to tocilizumab treatment, d | 5 (4–7) |
| Kidney function at remdesivir initiation | |
| Serum creatinine, mg/dl | 1.4 ± 0.5 |
| eGFR CKD-EPI, ml/min per 1.73 m2 | 57.5 ± 18.8 |
| eGFR <30 ml/min per 1.73 m2 | 4 (8.5) |
| Outcomes and follow-up | |
| Supplemental oxygen | 46 (90.2) |
| ICU admission | 15 (29.4) |
| Time since hospital admission to ICU admission, d | 4.5 (2.8–14.3) |
| Noninvasive mechanical ventilation | 19 (37.3) |
| Endotracheal intubation | 9 (17.6) |
| AKIa | 14 (27.5) |
| AKI stage 1 | 8 (57.1) |
| AKI stage 2 | 4 (28.6) |
| AKI stage 3 | 2 (14.3) |
| AKI with renal replacement therapy need | 1 (2) |
| Time since hospital admission to SCr peak in patients with AKI, d | 5 (0–8.5) |
| Time since remdesivir to SCr peak in patients with AKI, d | −1 (−4.5 to 8) |
| Acute rejection | 1 (2) |
| X-ray improvement after remdesivir (n = 32) | 13 (25.5) |
AKI, acute kidney injury, eGFR CKD-EPI, estimated glomerular filtrate rate calculated using Chronic Kidney Disease Epidemiology Collaboration equation; ICU, intensive care unit; SCr, serum creatinine.
Continuous data are presented as mean ± SD or median (interquartile range) and categorical data as n (%).
AKI stages 1, 2 and 3 outline the most severe AKI stage during hospital admission.
General Outcomes and Safety End Points
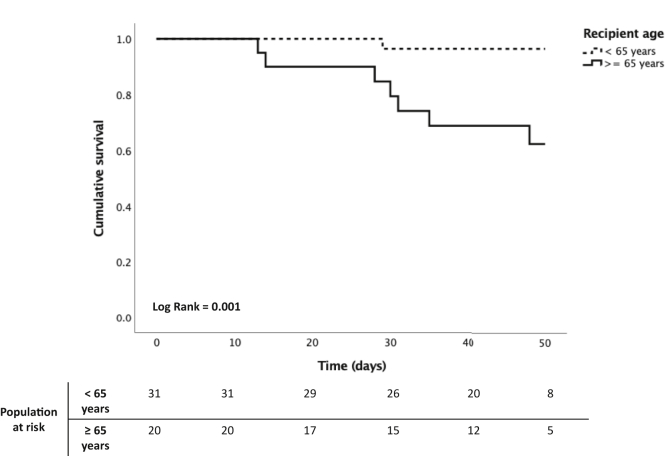
Ninety percent of patients required supplemental oxygen. The respiratory failure led to noninvasive mechanical ventilation support in 19 patients (8 of them outside an intensive care unit facility), and 9 required endotracheal intubation (Table 3). The fatality rate was 18.9%, although markedly higher in recipients aged ≥65 years (45% vs. 3.2%, P < .001) (Figure 3).
Figure 3.
Patient survival according to age in kidney transplant recipient with COVID-19 treated with remdesivir. Kaplan-Meier curve shows mortality rates of kidney transplantation patients with COVID-19 according to recipient age (<65 years vs. ≥65 years). The median time between the onset of symptoms and the end of follow-up was 49 days (interquartile range, 34–68 days).
AKI was present in 27.7% of the cohort. Most patients presented with AKI stage 1 (57.1%), and only 1 patient required RRT, with complete recovery of renal function at discharge. Of 14 recipients with AKI, 8 presented the peak SCr before the initiation of remdesivir, and therefore, renal function abnormalities could not be attributable to the drug. The mean SCr levels at remdesivir administration were 1.4 ± 0.5 mg/dl, with a mean eGFR of 57.5 ± 18.8 ml/min per 1.73 m2. Only 8.5% of patients had eGFR <30 ml/min per 1.73 m2 when remdesivir was initiated. AKI after remdesivir initiation happened in 11.7%. Of note, T-cell mediated rejection developed in 1 patient, and another patient presented thrombotic microangiopathy.
No patients required discontinuation of remdesivir therapy because of renal impairment. The dynamic profile of SCr after remdesivir treatment is detailed in Figure 4a. Tacrolimus trough levels were analyzed before and after remdesivir use in 22 patients who maintained the treatment during admission. We found a significant decrease in tacrolimus levels after remdesivir treatment (6.9 ng/ml [IQR, 5.7–8.8 ng/ml] vs. 5.2 ng/ml [IQR, 4.2–8.2 ng/ml], P = .04), although the tacrolimus dose was reduced in 11 of 22 patients at admission.
Figure 4.
Dynamic profile of kidney and liver function regarding COVID-19 infection evolution and remdesivir use. (a) Serum creatinine (Cr) levels at different time points: basal (preadmission), hospital admission (Adm), remdesivir administration (RDV), 72 hours after remdesivir, and early follow-up after discharge. Laboratory findings regarding liver function for (b) bilirubin, (c) aspartate aminotransferase (AST), and (d) alanine aminotransferase (ALT) between different time points: admission, remdesivir administration, 72 hours after remdesivir, and early follow-up after discharge. NS, not significant.
Evolution of liver function is depicted in Figure 4b–d. Baseline liver function abnormalities (elevated aspartate aminotransferase [AST] and ALT levels) were observed in 4 patients before starting remdesivir. Only 2 patients were found to have newly occurring grade 1 elevation of AST/ALT, and liver function remained stable in 95.7% of patients after the drug administration. In the 4 patients with raised baseline AST and ALT levels, remdesivir therapy was not associated with worsening transaminitis.
We observed a significant decrease in CRP levels after treatment (53.6 mg/l [IQR, 9.1–114.7 mg/l] vs. 7.2 mg/l [IQR, 1.4–31.5 mg/l]), whereas other inflammatory markers remained stable (Supplementary Table S1). Finally, no rash or hypotension episodes were described after remdesivir administration in our cohort.
Discussion
Our study reports the largest cohort of KT recipients with COVID-19 treated with remdesivir. Outcomes of 51 patients were analyzed, with an overall mortality of 18.9%, being markedly higher in recipients aged ≥65 years. Most patients were diagnosed during the second wave. AKI developed in 27% of patients during the study period, but only 11.7% was potentially attributed to the drug. Remdesivir therapy was not withdrawn because of renal impairment. We observed no clinically significant ALT/AST increase after patients received the drug.
The evolution of the pandemic in Spain has occurred a 3-wave pattern in reported cases of COVID-19, with some differences in clinical features.1,2 Anti–COVID-19 therapies and epidemiologic characteristics of KT with SARS-CoV2 infection have changed between waves due to a better knowledge of the disease.10 Anti–COVID-19 therapies, such as hydroxychloroquine or ritonavir/lopinavir, are no longer used, whereas pulse steroid therapy has increased in consonance with the current scientific data10, 11, 12, 13, 14 as well as remdesivir.
Developed initially as a possible treatment for the Ebola virus disease, remdesivir has shown promising results against SARS-CoV2 in reducing hospital stay and mortality.15, 16, 17 However, a later study reported no effect on hospitalized patients with COVID-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay.33 The United States Food and Drug Administration and the European Medicines Agency granted remdesivir emergency use during the first wave,34,35 but our study shows that remdesivir treatment in KT has been predominantly administered during the second wave.
The most common adverse events described in patients treated with remdesivir are increased hepatic enzymes, diarrhea, rash, AKI, and hypotension.20,21 Serious adverse events were reported in 24.6% of 532 remdesivir-treated patients versus 31.6% of 516 placebo recipients in a randomized trial.16 The drug was withdrawn early because of adverse events in 9.8% of patients. No KT patient in our experience required remdesivir interruption due to adverse events.
The presumed toxicity in patients with renal impairment is attributed to both remdesivir direct tubular damage in experimental studies and SBECD carrier accumulation. SBECD is a solubility-enhancing agent predominantly excreted through glomerular filtration. Studies in animals have associated SBECD accumulation with renal tubule obstruction due to vacuolation at doses 50-fold higher than those typically administered in humans. However, the short duration of treatment (5–10 days) and relatively low concentration of SBECD carrier used in COVID-19 treatment (100 mg of lyophilized powder of remdesivir contains 3 g of SBECD, and 100 mg solution of remdesivir contains 6 g of the carrier) would be far below SBECD recommended safety thresholds of 250 mg/kg per day.19 Of note, most patients in our cohort received lyophilized remdesivir powder.
Probably due to this potential nephrotoxicity, patients with CKD, including KT recipients, are underrepresented in the randomized studies with remdesivir. For this reason, we aimed to evaluate the potential toxicity related to this drug in these patients. Renal impairment developed in 27% of our KT cohort, in line with previous reported evidence.5,7,30 However, nearly half of the patients in our cohort cases presented the peak SCr before remdesivir was initiated, including a case of acute rejection, and therefore, renal function abnormalities could not be attributable to the drug.
Of interest, all patients with AKI who survived recovered baseline graft function at the end of follow-up. Antinori et al.21 described up to 22.8% of AKI in a cohort of patients treated with remdesivir. Nonetheless, as the authors postulated, the assessment of drug-induced nephrotoxicity is particularly challenging because COVID-19 also displays frequent renal complications, and randomized clinical trials have presented a comparable incidence of AKI between remdesivir and placebo.16,23,26, 27, 28
When we evaluated the potential hepatic toxicity, only 2 patients were found to have newly occurring grade 1 elevations of AST/ALT. Moreover, remdesivir therapy was not associated with worsening transaminitis in those patients with raised baseline AST and ALT levels. Similar results have been previously described in patients with AKI, CKD, or RRT.24,25
The use and safety of remdesivir as part of COVID-19 management was previously reported in 16 patients from 2 KT cohorts.24,29 Elec et al.29 analyzed 8 patients, with 6 of them presenting slightly decreased eGFR upon discharge, which normalized within 1 month. Thakare et al.24 also included 8 KT patients with COVID-19 treated with remdesivir. No renal function abnormalities attributable to the drug were observed.24
More evidence is available from the use of remdesivir in non-KT patients with AKI, CKD, or RRT, suggesting generally good tolerance.19,24,25,36,37 Aiswarya et al.25 analyzed 48 hemodialysis patients and showed no association with severe adverse events after 2 to 6 doses of remdesivir were administered. They did not administer a loading dose of the drug because no evidence of safety in patients with end-stage renal disease was available.25
Other adverse effects reported after remdesivir use, such as hypotension or rash,16,17 were not observed in our cohort.
As for immunosuppression, we found it of utmost importance to assess tacrolimus blood trough levels variability in those patients treated with remdesivir who maintained the drug during admission. As an inhibitor of cytochrome P450 3A4 in vitro, remdesivir could potentially interact with tacrolimus. However, its capacity to be a perpetrator to clinically significant drug-drug interactions is limited by its rapid clearance if kidney function is stable.38 Coadministration of remdesivir with this nephrotoxic agent may increase the plasma concentration of remdesivir metabolite, SBECD carrier, or tacrolimus levels. In our cohort, tacrolimus blood trough levels were significantly lower after remdesivir treatment (6.9 vs. 5.2 ng/ml), but most patients underwent dose reduction at admission. More importantly, we found no tacrolimus accumulation in the plasma due to remdesivir.
Those patients who initiated remdesivir more than 48 hours from admission showed a delayed discharge (12.5 vs. 22.5 days since admission), which suggests that early treatment onset could be associated with a better clinical response. Aiswarya et al.25 found early initiation of remdesivir (within 48 hours of hospital admission) was associated with a decrease of 5.5 days in the mean duration of hospitalization.25 These results are consonant with what has been reported in the general population.15,16,39
The mortality rate due to SARS-CoV2 infection in Spain has progressively decreased throughout the different waves. As of February 2020, the fatality rate was 1.77% in the general population and 17% in the KT population.1,3,10 Impaired kidney function, immunosuppression, advanced age, pneumonia, and early post-KT period have been identified as risk factors for death.4,30,40, 41, 42 Our study cohort showed similar COVID-19–related mortality to that previously described in the overall cohort of KT by the S.E.N. registry, including both hospital and nonhospitalized infected patients. However, previously published experience with remdesivir in COVID-19 patients with AKI, CKD, or RRT displayed higher mortality rates of 20% to 44%.24,25,36 Univariate analysis comparing survivors and nonsurvivors in our cohort identified recipient age >65 years was associated with an increased risk of death. In this group, the fatality rate reached 45%. Multivariate analysis could not be performed due to the inherent limitations of the sample size.
Our study has several limitations. Owing to its retrospective nature, some relevant clinical information might be underreported, and residual confounders may be present. The small sample size and the lack of a comparison group preclude establishing definitive associations about safety or efficacy. Nonetheless, the administration criteria were disease severity, and patients who received the drug presented a worse clinical status than an eventual control group not receiving the drug. Therefore, to avoid selection bias, we did not include a contemporary or historical control group because only a placebo-controlled trial can achieve this aim and very few KT patients with COVID-19 were left untreated when center protocols indicated remdesivir. Some patients received other anti–COVID-19 treatments (i.e., tocilizumab), and therefore, we cannot discard the potential influence of these therapies in outcomes. Finally, the patients included received variable dosing of remdesivir.
Notwithstanding, to date, this is the largest series focused on KT with COVID-19 treated with remdesivir, and the extensive clinical and analytical workup contributes to the understanding of the use and safety of the drug in this at-risk population.
Conclusion
We present the largest cohort so far of KT recipients treated with remdesivir for COVID-19 management. Remdesivir was well tolerated, and there were no significant safety concerns related to the drug administration. More extensive, randomized controlled trials evaluating the efficacy and safety of remdesivir in KT patients with COVID-19 who develop acute respiratory distress syndrome need to be further assessed.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors are indebted to the many physicians and nurses who take care of these patients and are facing the COVID-19 pandemic in our country. This research was supported by Rio Hortega contract CM19/00004 (ISCIII) (AB), and RD16/0009/0013 (ISCIII FEDER RedinRen). MJP-S is supported by a Spanish Society of Transplant scholarship.
Author Contributions
AB, CA-C, MJP-S, JP, and MC designed the study, analyzed the data, and drafted the manuscript. AB was the major contributor in writing the manuscript. (AB, CA-C, MJP-S, JC, SC-P, EM, MJA, CG, IL, AM, IMS, AF, MCR-F, LAS-C, OS, MLM, EG-G, VL, PLM, IM, MEM, FM, JMP, RS-E, SZ, CC, ES-A, JP, and MC revised the article, made substantial contributions, and approved the final version of the manuscript.
Footnotes
Table S1. Dynamics of inflammatory markers regarding remdesivir administration in KT recipients with COVID-19
STROBE statement
Supplementary Information
Contributor Information
Julio Pascual, Email: julpascual@gmail.com.
Marta Crespo, Email: mcrespo@psmar.cat.
Supplementary Material
Table S1. Dynamics of inflammatory markers regarding remdesivir administration in KT recipients with COVID-19
STROBE statement
Supplementary Information
References
- 1.Informe no 66. Situación de COVID-19 en España a 17 de febrero de 2021. Equipo COVID-19. RENAVE. CNE. CNM (ISCIII) https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/INFORMES%20COVID-19%202021/Informe%20COVID-19.%20N%C2%BA%2066_17%20de%20febrero%20de%202021.pdf Accessed February 26, 2021.
- 2.Villanego F., Mazuecos A., Pérez-Flores I.M. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry. Am J Transplant. 2021;21:2573–2582. doi: 10.1111/ajt.16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Informe no 30. Situación de COVID-19 en España a 11 de mayo de 2020. Equipo COVID-19. RENAVE. CNE. CNM (ISCIII) https://mateomaticas.files.wordpress.com/2021/01/espana-11-mayo.pdf Accessed February 5, 2021.
- 4.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahalingasivam V., Craik A., Tomlinson L.A. A systematic review of COVID-19 and kidney transplantation. Kidney Int Reports. 2020;6:24–45. doi: 10.1016/j.ekir.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilbrands L.B., Duivenvoorden R., Vart P. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cravedi P., Mothi S.S., Azzi Y. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crespo M., Mazuecos A., Rodrigo E. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation. 2020;104:2225–2233. doi: 10.1097/TP.0000000000003413. [DOI] [PubMed] [Google Scholar]
- 9.Registro Español de Enfermos Renales Informe de Diálisis y Trasplante. 2019. https://www.senefro.org/contents/webstructure/INFORME_REER_SEN_2020_WEB_SEN.pdf Accessed February 1, 2021.
- 10.Registro S.E.N COVID-19. Informe 20. Spanish Society of Nephrology. https://mailchi.mp/senefro/registro-epidemiolgico-vhc-vhb-vih-1315046 Accessed February 27, 2021.
- 11.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The RECOVERY Collaborative Group Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/nejmoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosas I.O., Bräu N., Waters M. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal V., Mahapure K.S., Bhurwal A. Mortality Benefit of Remdesivir in COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2021;7:606429. doi: 10.3389/fmed.2020.606429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman J.D., Lye D.C.B., Hui D.S. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/nejmoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adamsick M.L., Gandhi R.G., Bidell M.R. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol. 2020;31:1384–1386. doi: 10.1681/ASN.2020050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/nejmoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antinori S., Cossu M.V., Ridolfo A.L. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol Res. 2020;158:104899. doi: 10.1016/j.phrs.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan V.C., Muller F.L. Captisol and GS-704277, but not GS-441524, are credible mediators of remdesivir’s nephrotoxicity. Antimicrob Agents Chemother. 2020;64:e01920-20 doi: 10.1128/AAC.01920-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinner C.D., Gottlieb R.L., Criner G.J. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakare S., Gandhi C., Modi T. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Reports. 2021;6:206–210. doi: 10.1016/j.ekir.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aiswarya D., Arumugam V., Dineshkumar T. Use of remdesivir in patients with COVID-19 on hemodialysis: a study of safety and tolerance. Kidney Int Reports. 2021;6:586–593. doi: 10.1016/j.ekir.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gérard A., Laurain A., Fresse A. Remdesivir and acute renal failure: a potential safety signal from disproportionality analysis of the WHO safety database. Clin Pharmacol Ther. 2021;109:1021–1024. doi: 10.1002/cpt.2145. [DOI] [PubMed] [Google Scholar]
- 27.Fisher M., Neugarten J., Bellin E. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31:2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elec A.D., Oltean M., Goldis P. COVID-19 after kidney transplantation: early outcomes and renal function following antiviral treatment. Int J Infect Dis. 2021;104:426–432. doi: 10.1016/j.ijid.2021.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez-Sáez M.J., Blasco M., Redondo-Pachón D. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3182–3190. doi: 10.1111/ajt.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 32.Fontana R.J., Watkins P.B., Bonkovsky H.L. Drug-Induced Liver Injury Network (DILIN) prospective study. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO Solidarity Trial Consortium. Pan H., Peto R., Henao-Restrepo A.M. Repurposed antiviral drugs for Covid-19— interim WHO Solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/nejmoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Food and Drug Administration Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. May 1, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment Accessed February 27, 2021.
- 35.European Medicines Agency First COVID-19 treatment recommended for EU authorisation. June 25, 2020. https://www.ema.europa.eu/en/news/first-covid-19-treatment-recommended-eu-authorisation Accessed February 27, 2021.
- 36.Estiverne C., Strohbehn I.A., Mithani Z. Remdesivir in patients with estimated GFR <30 ml/min per 1.73 m2 or on renal replacement therapy. Kidney Int Rep. 2021;6:835–838. doi: 10.1016/j.ekir.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peyko V., Ladd H., Cutrona A. The safe administration of remdesivir in a patient with acute kidney injury requiring hemodialysis. Case Rep Infect Dis. 2020;2020:8811798. doi: 10.1155/2020/8811798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Medicines Agency Summary on compassionate use: Remdesivir Gilead. April 3, 2020. https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf Accessed February 28, 2021.
- 39.Metha R.M., Bansal S., Bysani S., Kalpakam H. A shorter symptom-onset to remdesivir treatment (SORT) interval is associated with a lower mortality in moderate-to-severe COVID-19: a real-world analysis. Int J Infect Dis. 2021;106:71–77. doi: 10.1016/j.ijid.2021.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pascual J., Melilli E., Jiménez-Martín C. COVID-19–related mortality during the first 60 days after kidney transplantation. Eur Urol. 2020;78:641–643. doi: 10.1016/j.eururo.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crespo M., Pérez-Sáez M.J., Redondo-Pachón D. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20:2883–2889. doi: 10.1111/ajt.16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.