To the Editor:
There have been multiple reports of nephrotic syndrome following the Pfizer-BioNTech coronavirus disease 2019 vaccine.1, 2, 3 We report a similar case of nephrotic syndrome which developed shortly after receiving ChAdOx1 nCoV-19 (Chimpanzee Adenovirus vectored Oxford university - novel CoronaVirus 2019 vaccine) vaccine against severe acute respiratory syndrome coronavirus 2 developed by Oxford University and AstraZeneca (Covishield, Serum Institute of India, Pune, India). It is a viral vector vaccine developed using the modified chimpanzee adenovirus ChAdOx1 as a vector.
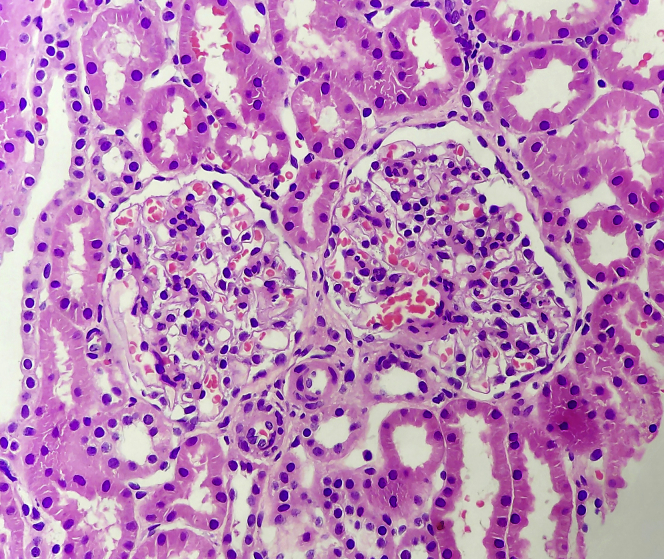
A 19-year-old girl presented with generalized body swelling, which started 8 days after the first dose of the Covishield coronavirus disease 2019 vaccine. Clinical examination was unremarkable except for generalized edema. Blood tests revealed serum creatinine, 1.09 mg/dl; albumin, 2.15 g/dl; and total cholesterol, 274 mg/dl; and a urine protein creatinine ratio of 3.18 g/g. Additional evaluation for secondary causes was negative. Kidney biopsy results revealed 11 glomeruli with normal capillary walls with diffuse and global mesangial cell proliferation on light microscopy (Figure 1). The glomerular walls did not stain for immunoglobulins or complement, but there was mesangial trapping of immunoglobulin M (IgM[1+]) and C3(1+). A diagnosis of a mesangial proliferative variant of minimal change disease was made. She responded to oral prednisone 1 mg/kg body weight with clinical and biochemical remission.
Figure 1.
Glomerulus revealing mild to moderate increase in the mesangial cellularity globally (hematoxylin and eosin stain, original magnification ×40).
To the best of our knowledge, this is a first ever report of nephrotic syndrome after the ChAdOx1 nCoV-19 vaccine. The temporal profile of nephrotic syndrome after the coronavirus disease 2019 vaccination and absence of any other precipitating factors points toward the vaccine as a possible trigger. It is uncertain if she should be advised to take the second dose and when it can be safely taken.
References
- 1.Lebedev L., Sapojnikov M., Wechsler A. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.03.010. S0272-6386(21)00509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Agati V.D., Kudose S., Bomback A.S., Adamidis A., Tartini A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. Published ahead of print 14, May 2021. [DOI] [PMC free article] [PubMed]
- 3.Maas R.J., Gianotten S., van der Meijden W.A.G. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. Published online May 12, 2021. [DOI] [PMC free article] [PubMed]