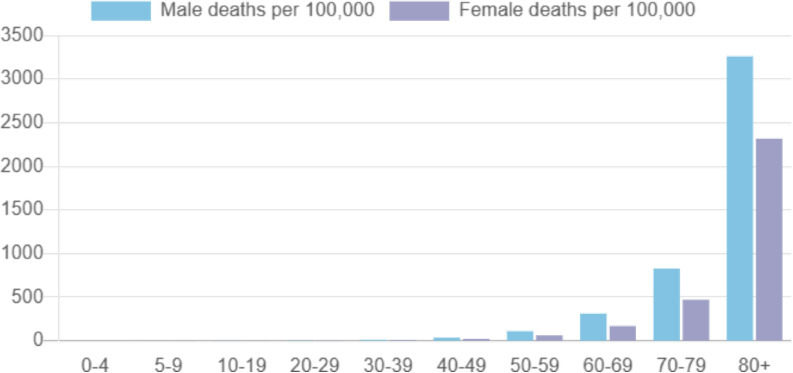
In comparison to females, males more frequently experience severe acute respiratory syndrome (SARS) caused by SARS-CoV-2 and also die more frequently from COVID-19, especially for the cohort older than 50 yr (Fig. 1 ) [1]. At this age, the most important endocrine difference by sex is the 10–20-fold higher testosterone levels among males, ranging from 12 to 35 ng/ml, while levels among females range from 1 to 3 ng/ml. Estrogen levels do not differ between men and women after the age of 50 yr.
Fig. 1.
Incidence of male and female deaths per 100 000 population by age group in years reported in England during the period May 6, 2020–May 16, 2021 (Global Health 50/50 [1]).
Testosterone has inhibitory effects on the immune system in terms of both cytokine production and lymphocyte proliferation. Apart from these general effects, there are two specific testosterone-dependent host cellular proteins related to viral cell entry and to serious COVID-19 disease. First, the SARS-CoV-2 virus uses ACE2 to enter the host cell. Second, the viral spike protein is primed by TMPRSS2, which is an androgen-regulated protease, and inhibition of TMPRSS2 using a protease inhibitor blocks viral cell entry. Serious COVID-19 is characterized by deterioration of lung function, and testosterone-stimulated TMPRSS2 expression in the lung may explain the greater susceptibility to serious COVID-19 lung infections among men [2]. Suppression of ACE2 and TMPRSS2 by inhibition of testosterone synthesis may be an effective treatment for COVID-19, by interfering with viral cell entry or activation. It has been shown that suppression of testosterone via androgen deprivation therapy (ADT) for the treatment of advanced prostate cancer also partly protects against serious COVID-19 infection [3].
Estrogens can blunt innate immune inflammatory responses and cytokine storms and can stimulate B-cell responses and antibody production, all expected to have a favorable effect on the course of COVID-19. In contrast to testosterone, estradiol reduces ACE2 expression and TMPRSS2 activity, thereby inhibiting viral cell entry and increasing viral clearance in females. These considerations have led to two ongoing clinical studies with the estrogens estradiol and estetrol in patients with COVID-19, focusing on clinical improvement in the acute stage of the disease (ClinicalTrials.gov NCT04359329 and NCT04801836). Recently, in a population of 1 863 478 women, significantly lower likelihood of all-cause mortality from COVID-19 has been reported for postmenopausal women using estrogen replacement therapy (adjusted odds ratio 0.22, 95% confidence interval 0.05–0.94) [4].
On the basis of these data, we propose to investigate the efficacy of ADT and high-dose estrogen (ADET) for the treatment of serious COVID-19 disease. We recently investigated this combination therapy for the treatment of advanced castration-sensitive prostate cancer, and observed strong antitestosterone and positive estrogenic effects [5]. The proposed ADET combined treatment will use gonadotropin-releasing hormone (GnRH) antagonists rather than luteinising hormone–releasing hormone agonists to avoid the initial testosterone increase that occurs with the latter [5].
To test the ADET hypothesis, we propose a clinical study in male and female patients admitted to an intensive care unit (ICU) because of SARS-CoV-2 infection. Patients would be randomized to no additional treatment or combined treatment with the GnRH antagonist degarelix and transdermal estradiol patches. All patients in the study would receive anticoagulant treatment. The primary endpoints would be death and duration of ICU stay.
Conflicts of interest: Herjan J.T. Coelingh Bennink is CEO of Pandora Endocrine Innovation, and president and shareholder of Pantarhei Oncology, an affiliate of Pantarhei Bioscience BV, Zeist, The Netherlands. He has submitted a patent application for the ADET treatment concept. Jean-Michel Foidart is cofounder of Mithra Pharmaceuticals and chairman of its scientific board. Mithra Pharmaceuticals holds a patent for estetrol treatment of severe viral infections. Frans M.J. Debruyne is Medical Director of Andros Men’s Health Institutes, The Netherlands, and is a paid consultant for Pantarhei Oncology BV, the company developing ADET for the treatment of advanced prostate cancer.
Acknowledgments:The authors wish to thank Jan Egberts (Terminal 4 Communications, Hilversum, The Netherlands) for providing manuscript preparation support.
References
- 1.Global Health 50/50. The COVID-19 sex-disaggregated data tracker: England. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker/?explore=country&country=england#search.
- 2.Younis J.S., Skorecki K., Abassi Z. The double edge sword of testosterone’s role in the COVID-19 pandemic. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.607179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montopoli M., Zumerle S., Vettor R. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dambha-Miller H, Hinton W, Joy M, Feher M, de Lusignan S. Mortality in COVID-19 amongst women on hormone replacement therapy or combined oral contraception: a cohort study. medRxiv preprint. 10.1101/2021.02.16.21251853. [DOI] [PMC free article] [PubMed]
- 5.Coelingh Bennink HJT, Van Moorselaar JA, Crawford ED. Estetrol cotreatment of androgen deprivation therapy in infiltrating or metastatic, castration-sensitive prostate cancer: a randomized, double-blind, phase II trial (PCombi) Eur Urol Open Sci. 2021;28:52–61. doi: 10.1016/j.euros.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]