Abstract
Objective
The goal of this study is to evaluate the long-term outcomes of patients with takotsubo syndrome and assess factors associated with death or recurrence.
Methods
This is a retrospective population-based cohort study of consecutive patients who presented to an integrated health system in Southern California with takotsubo syndrome between 2006 and 2016. Medical records were manually reviewed to confirm diagnosis and to identify predisposing factors, medication treatment and long-term outcomes. Factors associated with death or recurrent takotsubo syndrome were tested using Cox regression models.
Results
Between 2006 and 2016, there were 519 patients with a confirmed diagnosis of takotsubo syndrome. Patients were followed for 5.2 years (IQR 3.0–7.2). During the follow-up period, 39 (7.5%) had recurrent takotsubo syndrome and 84 (16.2%) died. In multivariate modelling, factors associated with higher risk of recurrence or death were age (HR 1.56 per 10-year increase, 95% CI 1.29 to 1.87), male sex (HR 2.52, 95% CI 1.38 to 4.60), diabetes (HR 1.6, 95% CI 1.06 to 2.43), pulmonary disease (HR 2.0, 95% CI 1.37 to 2.91) and chronic kidney disease (HR 1.58, 95% CI 1.01 to 2.47). Treatment with beta-blockers were associated with lower risk of recurrence or death (HR 0.46, 95% CI 0.29 to 0.72). No association was observed between treatment with ACE inhibitors or angiotensin-receptor blockers and recurrence or death (HR 0.92, 95% CI 0.59 to 1.42).
Conclusions
Recurrent takotsubo syndrome occurred in a minor subset of patients. Treatment with beta-blocker was associated with higher event-free survival.
Keywords: myocardial disease
Introduction
Takotsubo syndrome, also known as stress cardiomyopathy, is a clinical syndrome characterised by an acute and transient left ventricular dysfunction often related to an emotionally or physically stressful event. It is estimated to affect 1%–2% of patients with suspected acute coronary syndrome.1
Prognosis of patients with takotsubo syndrome was previously thought to be benign.2 However, recent studies have challenged this notion, reporting that long-term mortality is higher compared with mortality in the general population.2 Complications of takotsubo syndrome include acute heart failure, left ventricular outflow tract obstruction, mitral regurgitation, stroke and arrhythmias.3–5
A unique feature of takotsubo syndrome is the recovery of ventricular systolic function on cardiac imaging at follow-up, usually within 3 months.6 Recurrence of takotsubo syndrome has been reported.7–9 The triggering factors and the patterns of wall motion abnormalities may differ during recurrent events.8 It is not well-understood why some patients experience recurrence while others do not. The ideal management strategy has yet to be established. It is unclear if standard medical therapy used for heart failure with reduced ejection fraction confers similar benefits in patients with takotsubo syndrome. There are no randomised clinical trials to guide treatment decisions, and there is no established therapy to prevent recurrence.10
To evaluate the long-term outcomes of patients with takotsubo syndrome and assess factors associated with death or recurrence, we systematically identified patients with takotsubo syndrome within a large integrated healthcare delivery system in Southern California. The objectives of the study were to (1) report the long-term clinical outcomes including recurrence and death, (2) identify clinical factors associated with higher risk of recurrence or death and (3) determine if use of standard heart failure therapy including beta-blockers, ACE inhibitors (ACEi), angiotensin-receptor blockers (ARBs) and aldosterone antagonists were associated with higher event-free survival.
Methods
Study design and data source
This was a retrospective cohort study using data from the Kaiser Permanente Southern California (KPSC) Health System, a regional integrated healthcare system with >4 million members. Members enrol through the Kaiser Foundation Health Plan for comprehensive insurance including pharmaceutical benefits. The Kaiser system serves an ethnically and socioeconomically diverse population representative of the racial and ethnic groups within Southern California.11 Comprehensive medical information, which include demographics, administrative, pharmacy, laboratory and healthcare utilisation data from both ambulatory and inpatient encounters, is prospectively captured electronically through a centralised data warehouse. The data that support the findings of this study are available on reasonable request. It was not possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research due to the retrospective observational nature of the study.
Study population
Consecutive patients ≥18 years of age admitted to the hospital with a principal diagnosis of takotsubo syndrome between 1 January 2006 and 31 December 2016 were identified using International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) codes 429.83 and I51.81. For patients with multiple admissions for takotsubo syndrome, the first admission was included in the study. The date of admission was used as the index date. The 1-year period prior to the index date was defined as the baseline window. Patients who were not KPSC members or had been members for <1 year were excluded. Patients who did not undergo coronary angiogram were excluded. Detailed chart review and data abstraction was performed by two physicians (SC and RN) to confirm the diagnosis of takotsubo syndrome, identify medical comorbidities, determine the treatment provided and adjudicate outcomes.
Medical treatment was identified using outpatient pharmacy dispensing records. Medication dispense date, medication dosage and days supplied were abstracted. The proportion of days covered (PDC) was calculated by taking the total number of days covered by the dispensed medication during the follow-up period, and dividing by the total number of days during the follow-up period. A PDC >80% is considered adequate adherence. The primary analysis was performed using a modified intention-to-treat approach, where a patient was considered exposed to a medication during the follow-up period if the medication was dispensed during the first year of diagnosis. Additional sensitivity analyses performed were as follows: (1) excluding patients exposed to the medication prior to the index date; (2) only considering patients with >80% PDC as being exposed; (3) evaluating the effect of PDC as a continuous variable; (4) evaluating the effect of beta-blocker PDC as a time-varying variable and (5) evaluating the effect of prescribed dosage.
Patients were followed starting on the index date until they reached a study end point (recurrence of takotsubo syndrome or all-cause death) or the end of the study period.
Outcomes
Mortality data were extracted from a mortality datafile with integrated death information derived from multiple sources, including KPSC administrative records, California state death master files, Social Security Administrative death master files, hospital death records and insurance enrolment records. Recurrence of takotsubo syndrome was manually adjudicated by reviewing electronic medical records.
Statistical analysis
Descriptive statistics were used to examine covariate distribution. Interval level data were reported in means with SD. Nominal data were reported as counts and percentages. Differences in categorical data were compared by Fisher’s exact test. Differences in continuous data were compared by Student’s t-test.
Cox proportional hazard regression analyses were performed to identify factors associated with recurrent takotsubo syndrome or death. The proportional hazard assumption was assessed using Schoenfeld residuals. Variables tested included baseline demographics, baseline comorbidities and medications used. A multivariable adjustment analysis, including covariates with a univariate p<0.1 was performed in a Cox regression model. The multivariate Cox proportional hazard regression model included the following variables: age (per 10-year increase), sex, diabetes, pulmonary disease, chronic kidney disease and treatment with beta-blockers. Hazard ratio (HR) with corresponding 95% CIs were reported. A two-sided p value of <0.05 was considered statistically significant. Differences in outcomes between groups were depicted using Kaplan-Meier estimates and compared with the log-rank test. Statistical analyses were performed using STATA V.14 (StataCorp, College Station, Texas, USA).
Results
Baseline characteristics
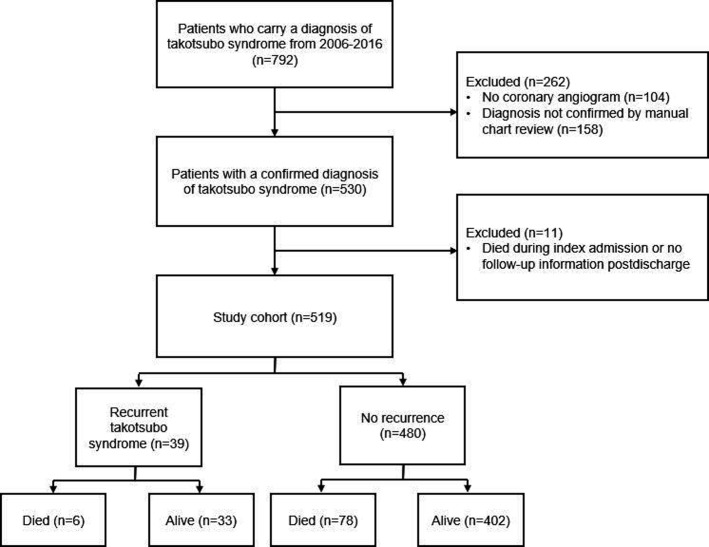
Between 2006 and 2016, 792 patients carried a diagnosis of takotsubo syndrome (figure 1). Of this, 530 had a confirmed diagnosis after reviewing coronary angiography, cardiac imaging and cardiac biomarkers. Patients who died during the index admission or had no follow-up information postdischarge were excluded from the analysis. The final cohort included 519 patients, among which 480 (92.5%) had no recurrence during follow-up and 39 (7.5%) had at least one episode of recurrence. During follow-up, 84 (16%) patients died, 6 in the group with recurrent takotsubo syndrome and 78 in the group without recurrence.
Figure 1.
Derivation of study cohort.
Table 1 shows the baseline characteristics of the cohort. Patients who survived without recurrence were younger. A higher proportion of patients who suffered death or recurrence were men. A higher proportion of patients who suffered death or recurrence had comorbidities and cardiac risk factors including hypertension, hyperlipidaemia and diabetes.
Table 1.
Baseline characteristics and clinical presentations
| Survival without recurrence (n=402) |
Recurrence (n=39) |
Death without recurrence (n=78) |
Death or recurrence (n=117) |
|
| Age (years) | 67.0±11.6 | 67.9±10.6 | 74±8.0 | 72.4±9.5 |
| <35 | 3 (0.8) | 0 (0) | 0 (0) | 0 (0) |
| 35–50 | 30 (7.5) | 4 (10.3) | 1 (1.3) | 5 (4.3) |
| 50–<65 | 139 (34.6) | 10 (25.6) | 3 (3.9) | 13 (11.1) |
| ≥65 | 230 (57.2) | 25 (64.1) | 74 (94.9) | 99 (84.6) |
| Female | 380 (94.5) | 33 (84.6) | 71 (91.0) | 104 (88.9) |
| Race/Ethnicity | ||||
| White | 252 (62.7) | 30 (76.9) | 50 (64.1) | 80 (68.4) |
| Black | 28 (7.0) | 1 (2.6) | 9 (11.5) | 10 (8.6) |
| Hispanic | 100 (24.9) | 8 (20.5) | 17 (21.8) | 25 (21.4) |
| Asian | 21 (5.2) | 0 (0) | 1 (1.3) | 1 (0.9) |
| Other | 1 (0.3) | 0 (0) | 1 (1.3) | 1 (0.9) |
| Income (US$) | ||||
| <45 000 | 81 (20.2) | 5 (12.8) | 20 (25.6) | 25 (21.4) |
| 45 000–80 000 | 186 (46.3) | 20 (51.3) | 36 (46.2) | 56 (47.9) |
| ≥80 000 | 115 (26.6) | 13 (33.3) | 19 (24.4) | 32 (27.4) |
| Unknown | 20 (5.0) | 1 (2.6) | 3 (3.9) | 4 (3.4) |
| Insurance | ||||
| Commercial | 142 (35.3) | 12 (30.8) | 5 (6.4) | 17 (14.5) |
| Medicare | 9 (2.2) | 19 (48.7) | 56 (71.8) | 75 (64.1) |
| Medicaid | 162 (40.3) | 0 (0) | 1 (1.3) | 1 (0.9) |
| Other | 89 (22.1) | 8 (20.5) | 16 (20.5) | 24 (20.5) |
| Hypertension | 259 (64.4) | 22 (56.4) | 67 (85.9) | 89 (76.1) |
| Hyperlipidaemia | 227 (56.5) | 21 (53.8) | 51 (65.4) | 72 (61.5) |
| Obesity | 87 (21.6) | 4 (10.3) | 20 (25.6) | 24 (20.5) |
| Diabetes | 77 (19.2) | 9 (23.1) | 25 (32.1) | 34 (29.1) |
| History of stroke/TIA | 35 (8.7) | 2 (5.1) | 16 (20.5) | 18 (15.4) |
| Hypothyroidism | 62 (15.4) | 7 (17.9) | 19 (24.4) | 26 (22.2) |
| Rheumatological diseases | 14 (3.5) | 1 (2.6) | 11 (14.1) | 12 (10.3) |
| Neurological diseases | 26 (6.5) | 2 (5.1) | 11 (14.1) | 13 (11.1) |
| Liver disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| COPD/Asthma | 87 (21.6) | 7 (17.9) | 41 (52.6) | 48 (41.0) |
| Chronic kidney disease | 55 (13.7) | 6 (15.4) | 20 (25.6) | 26 (22.2) |
| Depression | 112 (27.9) | 9 (23.1) | 32 (41.0) | 41 (35.0) |
| Dementia | 3 (0.8) | 0 (0) | 2 (2.6) | 2 (1.7) |
| Trigger | ||||
| Physical | 99 (24.6) | 11 (28.2) | 38 (48.7) | 49 (41.9) |
| Emotional | 158 (39.3) | 14 (35.9) | 13 (16.7) | 27 (23.1) |
| Both | 23 (5.7) | 3 (7.7) | 3 (3.9) | 6 (5.1) |
| No obvious trigger/unknown | 122 (30.4) | 11 (28.2) | 24 (30.8) | 35 (29.9) |
| STEMI on presentation | 103 (25.6) | 6 (15.4) | 27 (34.6) | 33 (28.2) |
| Baseline use of beta-blocker | 77 (19.2) | 9 (23.1) | 16 (20.5) | 25 (21.4) |
| Baseline use of ACEi/ARB | 145 (36.1) | 8 (20.5) | 28 (35.9) | 36 (30.8) |
Values are mean±SD or n (%).
ACEi, ACE inhibitor; ARB, angiotensin-receptor blocker; COPD, chronic obstructive pulmonary disease; STEMI, ST-elevation myocardial infarction; TIA, transient ischaemic attack.
Among the 39 patients with recurrent takotsubo syndrome, 35 had one episode of recurrence, 3 had two episodes and 1 individual had three recurrent episodes during the study period (table 2). The median time to recurrence was 2.87 years (IQR 1.02–5.79 years). There were 9 (1.7%) patients who experienced recurrence within 1 year of presentation. The number of days to first recurrence of takotsubo syndrome observed in our study cohort can be found in the supplemental material (online supplemental figure S1).
Table 2.
Number of recurrences and pharmacological treatment
| No of patients (n=519) |
||||
| Number of recurrences | ||||
| 0 | 480 (92.5) | |||
| 1 | 35 (6.7) | |||
| 2 | 3 (0.6) | |||
| 3 | 1 (0.2) | |||
| Recurrence within 1 year | 9 (1.7) | |||
| Pharmacological treatment within the first year of presentation | ||||
| Medications | Survival without recurrence (n=402) |
Recurrence (n=39) |
Death without recurrence (n=78) |
Death or recurrence (n=117) |
| P2Y12 | 66 (16.4) | 12 (30.8) | 16 (20.5) | 28 (23.9) |
| Statin | 335 (83.3) | 33 (84.6) | 56 (71.8) | 89 (76.1) |
| Beta-blocker | 356 (88.6) | 33 (84.6) | 58 (74.4) | 91 (77.8) |
| ACEi/ARB | 311 (77.4) | 35 (89.7) | 56 (71.8) | 91 (77.8) |
| Aldosterone antagonist | 16 (4.0) | 2 (5.1) | 5 (6.4) | 7 (6.0) |
| Diuretics | 100 (24.9) | 16 (41.0) | 35 (44.9) | 51 (43.6) |
Values are n(%)
ACEi, ACE inhibitor; ARB, angiotensin-receptor blocker.
heartjnl-2020-318028supp001.pdf (142.2KB, pdf)
Pharmacological treatment
Table 2 shows the pharmacological treatment used within the first year of presentation. The majority of patients were treated with standard heart failure therapy including beta-blockers (86.1%) and ACEi/ARB (77.5%). Use of aldosterone antagonist was rare (4.4%). Many patients were treated with statin therapy (81.7%).
Recurrence and all-cause mortality
During a median follow-up of 5.2 years (IQR 3.0–7.2 years), there were 117 patients with death or recurrence (figure 1). From univariable Cox regression analyses, factors associated with a higher risk of death or recurrence included age, male sex, diabetes, pulmonary disease and chronic kidney disease (table 3). Treatment with beta-blockers was associated with a lower risk of death or recurrence.
Table 3.
Univariable and multivariable predictors of recurrence or death
| Univariable | Multivariable Model 1 |
Multivariable Model 2 |
||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age* | 1.60 (1.33 to 1.91) | <0.001 | 1.56 (1.29 to 1.87) | <0.001 | 1.61 (1.34 to 1.95) | <0.001 |
| Male | 2.17 (1.22 to 3.86) | 0.009 | 2.52 (1.38 to 4.60) | 0.003 | 2.09 (1.14 to 3.82) | 0.016 |
| White | 1.24 (0.84 to 1.83) | 0.285 | ||||
| Diabetes | 1.94 (1.29 to 2.90) | 0.001 | 1.60 (1.06 to 2.43) | 0.026 | 1.59 (1.05 to 2.41) | 0.06 |
| COPD/Asthma | 2.29 (1.58 to 3.32) | <0.001 | 2.00 (1.37 to 2.91) | <0.001 | 1.95 (1.33 to 2.84) | 0.001 |
| Chronic kidney disease | 2.09 (1.35 to 3.24) | 0.001 | 1.58 (1.01 to 2.47) | 0.046 | 1.69 (1.07 to 2.66) | 0.023 |
| Treatment with beta-blocker | 0.53 (0.35 to 0.83) | 0.005 | 0.46 (0.29 to 0.72) | 0.001 | ||
| Beta-blocker proportion of days covered (per 10% increase) |
0.95 (0.91 to 0.99) | 0.018 | 0.92 (0.88 to 0.96) | <0.001 | ||
| Treatment with ACEi/ARB | 0.92 (0.59 to 1.42) | 0.705 | ||||
| Treatment with aldosterone antagonist | 1.42 (0.66 to 3.05) | 0.371 | ||||
*Per 10-year increase.
ACEi, ACE inhibitor; ARB, angiotensin-receptor blocker; COPD, chronic obstructive pulmonary disease.
In multivariable analysis, the following variables remained significant and were independently associated with a higher risk of recurrence or death: age (HR 1.56 per 10-year increase, 95% CI 1.29 to 1.87, p<0.001), male sex (HR 2.52, 95% CI 1.38 to 4.6, p=0.003), diabetes (HR 1.6, 95% CI 1.06 to 2.43, p=0.026), pulmonary disease (HR 2.0, 95% CI 1.37 to 2.91, p<0.001) and chronic kidney disease (HR 1.58, 95% CI 1.01 to 2.47, p=0.046) (table 3). Treatment with beta-blocker was associated with a significantly lower risk of recurrence or death (HR 0.46, 95% CI 0.29 to 0.72, p=0.001). No association with clinical outcomes was observed with use of ACEi/ARB, or with aldosterone antagonists.
Several sensitivity analyses yielded consistent results. Excluding patients with baseline exposure to beta-blocker did not affect the association between beta-blocker exposure and better clinical outcomes (online supplemental tables S1 and S2). PDC by beta-blocker during the entire follow-up period was evaluated (online supplemental figure S2). Each 10% increase in PDC was associated with an 8% lower risk of recurrence or death (table 3). Consistent results were observed when beta-blocker PDC was modelled as a time-varying exposure (online supplemental table S3). Similar observations were made when only patients with >80% PDC during the follow-up period were considered exposed. To further evaluate the association between exposure to beta-blocker for >80% PDC and outcome, we performed additional analyses using inverse probability of treatment weighting based on propensity score, which yielded consistent results (online supplemental table S4 and S5).
Figure 2 shows the Kaplan-Meier estimates for recurrence-free survival. Patients aged <65 years had higher recurrence-free survival compared with those aged ≥65 years. Women had higher recurrence-free survival compared with men. Patients treated with beta-blockers had higher recurrence-free survival compared with those not treated with beta-blockers. No significant difference in recurrence-free survival was observed between those treated versus not treated with ACEi/ARBs.
Figure 2.
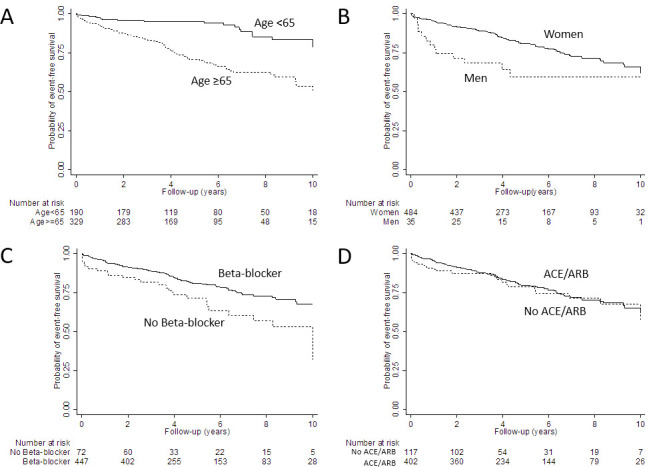
Survival free of recurrent takotsubo syndrome stratified by (A) age (logrank p<0.001); (B) sex (logrank p=0.005); (C) treatment with beta-blocker (logrank p=0.004) and (D) treatment with ACE inhibitors (ACEi)/angiotensin-receptor blockers (ARBs) (logrank p=0.647).
Discussion
In this large population-based cohort of patients with takotsubo syndrome, we assessed the long-term outcomes and explored clinical factors associated with recurrence or death. Recurrence of takotsubo syndrome occurred in 7.5% of patients over a median of 5.2 years of follow-up. The majority of patients with recurrence had a single episode of recurrence. A higher proportion of patients with recurrence were men. Factors associated with poor clinical outcomes included older age, male sex, diabetes, pulmonary disease and kidney disease. Treatment with beta-blocker was associated with a lower risk of recurrence or death, while no significant association was observed with ACEi/ARB.
Patients with takotsubo syndrome who survive the initial event generally have full recovery of their left ventricular systolic function.12 However, a subset of patients develops recurrent episodes. The rate of recurrence was reported to be 4.7% at a median follow-up of 2.5 years in one study, and 4% at a median follow-up of 2.2 years in another study.8 9 Recurrent cases have been reported to occur as early as 3 weeks and as late as 13 years after their initial event.13 Previous published series included patients referred to tertiary referral centres and represented a different population compared with our cohort. Our cohort was a population-based cohort with patients drawn from the community. We were able to systematically capture patients who comprised the entire spectrum of disease severity. This cohort was also unique in its racial and ethnic diversity, with more than one-third of the cohort from under-represented racial or ethnic minority groups. Despite differences in the characteristics of the population, the recurrence rate from this cohort was found to be 7.5% of patients over a median of 5.2 years of follow-up, which is largely consistent with what was reported in the literature. We also found the time to recurrence to span a wide range.
We found a higher proportion of patients with recurrence to be men. In our cohort, male sex was associated with a 2.5-fold higher risk of death or recurrence. Takotsubo syndrome was shown to be more prevalent in postmenopausal women.12 However, men who developed takotsubo syndrome had higher mortality rate and were more likely to suffer complications including cardiac and cerebrovascular events.12 14–16 This may be related to differences in baseline comorbidities between men and women. There is likely a hormonal influence on the clinical course of takotsubo syndrome, with oestrogen possibly being cardioprotective.10 17 Our findings are consistent with previous observations.
To date, there is no established therapy for takotsubo syndrome and there are no randomised clinical trials to guide treatment decisions.1 We found that many patients were treated with standard medical therapy used for heart failure with reduced ejection fraction including beta-blockers and ACEi/ARBs, which may be reasonable given most patients had left ventricular systolic dysfunction. However, whether standard heart failure medications confer similar benefits in patients with takotsubo syndrome is not known.
Data from the International Takotsubo Registry showed that the use of ACEi/ARBs was associated with improved survival at 1 year after propensity matching.12 In contrast, there was no evidence of survival benefits or reduced rate of recurrence with the use of beta-blockers.12 15 Another study found no benefit associated with either medication.18 Our findings were different. In our cohort, beta-blocker exposure was associated with lower mortality and recurrence, while no association was found with ACEi/ARBs. It is difficult to draw firm conclusions given these studies were observational in nature. However, differences in racial make-up of the study populations may have played a role. Our study included a number of patients from racial minority groups. Previous studies reported differential effectiveness of cardiac medications in patients from different racial backgrounds. For example, ACEi were found to not provide the same cardiovascular benefits in African-Americans compared with whites.19 20 In African-Americans, endothelial dysfunction is felt to result from abnormalities of nitric oxide production or metabolism.21 While angiotensin II is felt to play an important role in white patients, nitric oxide is hypothesised to be the dominant pathway mediating heart failure in African-Americans.22 Since coronary microcirculatory dysfunction likely plays an important role in the pathophysiology of takotsubo syndrome,23 the differential effect of ACEi/ARB in treating endothelial dysfunction in African-Americans could potentially translate into lower effectiveness for treating takotsubo syndrome in this population.
The association between beta-blockers and recurrence-free survival observed is consistent with the pathophysiological mechanism of takotsubo syndrome. Sympathetic stimulation and elevated levels of catecholamines are felt to be central to the pathogenesis of takotsubo syndrome.24 25 Beta-blocker therapy may be beneficial by attenuating sympathetic stimulation. Applying beta-blocker therapy at the appropriate time in the disease course may be important. A previous study showed that use of beta-blockers in the acute setting may not improve in-hospital mortality in patients with takotsubo syndrome.26 One possibility that we were able to observe an association between beta-blocker use and better clinical outcomes could be because we evaluated these patients in the chronic setting after they were discharged from the hospital. Patients were followed for several years, and beta-blocker use in the chronic setting may be helpful. A similar paradigm was seen in patients with other types of heart failure. In patients with heart failure with reduced ejection fraction, beta-blockers reduce mortality when used in long-term management, but can worsen heart failure and cause haemodynamic instability in the acute setting.27 It is possible that the potential benefits associated with beta-blocker exposure are only observed after prolonged use.
Limitations
Several limitations of this study should be acknowledged. This is a non-randomised observational analysis, so the diagnosis and management decisions were likely not uniform and were at the discretion of the treating clinicians. We attempted to account for many confounding variables, but similar to other observational studies, residual confounding may be present. Cases were initially identified using ICD‐9 and ICD‐10 codes, and it is possible that these codes did not capture all the cases of takotsubo syndrome. On the other hand, manual chart review was performed for all cases to allow for improved case ascertainment. A recurrence event was only captured if a patient was hospitalised. It is possible that some recurrence events were missed. The population studied was an insured population in the USA, with good access to healthcare. As such, the findings may not be generalisable to patients in the USA who lack health insurance or patients in low-income and middle-income countries with limited access to care.
Conclusions
In conclusion, we found that takotsubo syndrome recurrence occurred in 7.5% of patients over a median of 5.2 years. A higher proportion of patients with recurrence were men, who had a 2.5-fold higher risk of poor long-term outcomes. In this racially diverse population-based cohort, treatment with beta-blocker was associated with a lower risk of takotsubo syndrome recurrence or death, while no association was observed with ACEi/ARB.
Key messages.
What is already known on this subject?
Takotsubo syndrome is characterised by acute and transient left ventricular dysfunction related to a stressful event.
A subset of patients with takotsubo syndrome experiences recurrence.
Patients with takotsubo syndrome are often treated with standard medical therapy used for heart failure with reduced ejection fraction.
What might this study add?
In this large population-based cohort of patients with takotsubo syndrome, recurrence of takotsubo syndrome occurred in 7.5% of patients over a median of 5.2 years of follow-up.
Factors associated with poor clinical outcomes included older age, male sex, diabetes, pulmonary disease and kidney disease.
Treatment with beta-blocker was associated with a lower risk of recurrence or death, while no significant association was observed with ACE inhibitors/angiotensin-receptor blockers.
How might this impact on clinical practice?
Treatment with beta-blocker is associated with higher event-free survival.
Footnotes
Contributors: All of the authors contributed to study planning, data collection, data reporting and manuscript writing. CL and M-SL are responsible for the overall content of this manuscript as guarantors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. The data that support the findings of this study are available on reasonable request (https://www.kp-scalresearch.org/aboutus/contact-us/).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The present study was approved by the Kaiser Permanente Southern California Institutional Review Board. A waiver of informed consent was obtained because of the observational nature of the study.
References
- 1. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:1955–71. 10.1016/j.jacc.2018.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghadri JR, Kato K, Cammann VL, et al. Long-Term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol 2018;72:874–82. 10.1016/j.jacc.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 3. Pelliccia F, Pasceri V, Patti G, et al. Long-Term prognosis and outcome predictors in takotsubo syndrome: a systematic review and meta-regression study. JACC Heart Fail 2019;7:143–54. 10.1016/j.jchf.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 4. Santoro F, Stiermaier T, Tarantino N, et al. Left ventricular thrombi in takotsubo syndrome: incidence, predictors, and management: results from the GEIST (German Italian stress cardiomyopathy) registry. J Am Heart Assoc 2017;6:JAHA.117.006990. 10.1161/JAHA.117.006990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stiermaier T, Eitel C, Denef S, et al. Prevalence and clinical significance of life-threatening arrhythmias in takotsubo cardiomyopathy. J Am Coll Cardiol 2015;65:2148–50. 10.1016/j.jacc.2015.02.062 [DOI] [PubMed] [Google Scholar]
- 6. Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on takotsubo syndrome: a position statement from the Taskforce on takotsubo syndrome of the heart failure association of the European Society of cardiology. Eur J Heart Fail 2016;18:8–27. 10.1002/ejhf.424 [DOI] [PubMed] [Google Scholar]
- 7. Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–41. 10.1016/j.jacc.2009.08.057 [DOI] [PubMed] [Google Scholar]
- 8. Kato K, Di Vece D, Cammann VL, et al. Takotsubo recurrence: morphological types and triggers and identification of risk factors. J Am Coll Cardiol 2019;73:982–4. 10.1016/j.jacc.2018.12.033 [DOI] [PubMed] [Google Scholar]
- 9. El-Battrawy I, Santoro F, Stiermaier T, et al. Incidence and clinical impact of recurrent takotsubo syndrome: results from the GEIST registry. J Am Heart Assoc 2019;8:e010753. 10.1161/JAHA.118.010753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghadri J-R, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–62. 10.1093/eurheartj/ehy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Derose SF, Contreras R, Coleman KJ, et al. Race and ethnicity data quality and imputation using U.S. Census data in an integrated health system: the Kaiser Permanente southern California experience. Med Care Res Rev 2013;70:330–45. 10.1177/1077558712466293 [DOI] [PubMed] [Google Scholar]
- 12. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 13. Madias JE. Comparison of the first episode with the first recurrent episode of takotsubo syndrome in 128 patients from the world literature: pathophysiologic connotations. Int J Cardiol 2020;310:27–31. 10.1016/j.ijcard.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 14. Brinjikji W, El-Sayed AM, Salka S. In-Hospital mortality among patients with takotsubo cardiomyopathy: a study of the National inpatient sample 2008 to 2009. Am Heart J 2012;164:215–21. 10.1016/j.ahj.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 15. Singh K, Carson K, Usmani Z, et al. Systematic review and meta-analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy. Int J Cardiol 2014;174:696–701. 10.1016/j.ijcard.2014.04.221 [DOI] [PubMed] [Google Scholar]
- 16. Almendro-Delia M, Núñez-Gil IJ, Lobo M, et al. Short- and long-term prognostic relevance of cardiogenic shock in takotsubo syndrome: results from the RETAKO registry. JACC Heart Fail 2018;6:928–36. 10.1016/j.jchf.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 17. Schneider B, Athanasiadis A, Stöllberger C, et al. Gender differences in the manifestation of tako-tsubo cardiomyopathy. Int J Cardiol 2013;166:584–8. 10.1016/j.ijcard.2011.11.027 [DOI] [PubMed] [Google Scholar]
- 18. Kim H, Senecal C, Lewis B, et al. Natural history and predictors of mortality of patients with takotsubo syndrome. Int J Cardiol 2018;267:22–7. 10.1016/j.ijcard.2018.04.139 [DOI] [PubMed] [Google Scholar]
- 19. Norris K, Bourgoigne J, Gassman J, et al. Cardiovascular outcomes in the African American study of kidney disease and hypertension (AASK) trial. Am J Kidney Dis 2006;48:739–51. 10.1053/j.ajkd.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 20. Wright JT, Dunn JK, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005;293:1595–608. 10.1001/jama.293.13.1595 [DOI] [PubMed] [Google Scholar]
- 21. Franciosa JA, Ferdinand KC, Yancy CW, et al. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail 2010;16:27–38. 10.1111/j.1751-7133.2009.00118.x [DOI] [PubMed] [Google Scholar]
- 22. Cuyjet AB, Akinboboye O. Acute heart failure in the African American patient. J Card Fail 2014;20:533–40. 10.1016/j.cardfail.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 23. Galiuto L, De Caterina AR, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in apical ballooning or Tako-Tsubo syndrome. Eur Heart J 2010;31:1319–27. 10.1093/eurheartj/ehq039 [DOI] [PubMed] [Google Scholar]
- 24. Abraham J, Mudd JO, Kapur NK, et al. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol 2009;53:1320–5. 10.1016/j.jacc.2009.02.020 [DOI] [PubMed] [Google Scholar]
- 25. Kume T, Kawamoto T, Okura H, et al. Local release of catecholamines from the hearts of patients with tako-tsubo-like left ventricular dysfunction. Circ J 2008;72:106–8. 10.1253/circj.72.106 [DOI] [PubMed] [Google Scholar]
- 26. Isogai T, Matsui H, Tanaka H, et al. Early β-blocker use and in-hospital mortality in patients with takotsubo cardiomyopathy. Heart 2016;102:1029–35. 10.1136/heartjnl-2015-308712 [DOI] [PubMed] [Google Scholar]
- 27. Writing Committee M, Yancy CW, Jessup M, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. Circulation 2013;2013:e240–327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-318028supp001.pdf (142.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. The data that support the findings of this study are available on reasonable request (https://www.kp-scalresearch.org/aboutus/contact-us/).