Abstract
Background
Although tricuspid valve surgery improves functional capacity in patients with Ebstein anomaly, it is not always associated with improvement in aerobic capacity. The purpose of this study was to identify the determinants of improved aerobic capacity after tricuspid valve surgery in adults with Ebstein anomaly with severe tricuspid regurgitation.
Methods
Retrospective study of patients with severe tricuspid regurgitation due to Ebstein anomaly that had tricuspid valve surgery at Mayo Clinic Rochester (2000–2019) and had preoperative and postoperative cardiopulmonary exercise tests and echocardiograms. The patients were divided into aerobic capacity(+) and aerobic capacity(-) groups depending on whether they had postoperative improvement in %-predicted peak oxygen consumption (VO2).
Results
Of 76 patients with severe tricuspid regurgitation due to Ebstein anomaly, 28 (37%) and 48 (63%) were in aerobic capacity(+) and aerobic capacity(-) groups, respectively. The average improvement in peak VO2 was 2.1±1.4 mL/kg/min and −0.9±0.4 mL/kg/min in the in aerobic capacity(+) and aerobic capacity(-) groups, respectively. Although both groups had similar severity of residual tricuspid regurgitation, the aerobic capacity(+) group had more postoperative improvement in right atrial (RA) function, left atrial (LA) function and left ventricular preload and stroke volume. Of the preoperative variables analysed, RA reservoir strain (relative risk 1.12; 95% CI 1.06 to 1.18); LA reservoir strain (relative risk 1.09; 95% CI 1.04 to 1.14) and LV stroke volume index (OR 1.04; 95% CI 1.01 to 1.07) were predictors of postoperative improvement in peak VO2.
Conclusions
One-third of patients with severe tricuspid regurgitation due to Ebstein anomaly had postoperative improvement in aerobic capacity, and atrial function indices were the best predictors of postoperative improvement in aerobic capacity. These data provide new insight into the haemodynamic determinants of exercise capacity and lay the foundation for further studies to determine whether postoperative improvement in aerobic capacity translates to improved long-term survival, and whether timing of tricuspid valve surgery based on these echocardiographic indices will improve long-term outcomes.
Keywords: congenital heart disease surgery
INTRODUCTION
Congenital heart disease is the leading cause of heart failure and cardiovascular death in young adults less than 50 years of age, and among patients with congenital heart disease, Ebstein anomaly is an important cause of right heart failure and cardiovascular death.1 Tricuspid regurgitation and right ventricular (RV) cardiomyopathy are the primary haemodynamic lesions in Ebstein anomaly, and over time, they lead to right heart failure symptoms such as atrial and ventricular arrhythmias, systemic congestion and exercise intolerance.2 Tricuspid valve surgery restores tricuspid valve competence, and in turn, improves right heart failure symptoms and functional capacity (a subjective metric of exercise tolerance).2 3 However, the effect of tricuspid valve surgery on aerobic capacity remains unclear, with some studies showing improvement in aerobic capacity while others report lack of improvement in aerobic capacity after surgery.4 5 More importantly, the preoperative indices associated with postoperative improvement in aerobic capacity have not been described. Such data will be clinically relevant, as it will help guide the timing of intervention in these patients. The purpose of this study was to identify the determinants of improved aerobic capacity after tricuspid valve surgery in adults with Ebstein anomaly through a detailed analysis of clinical, surgical and haemodynamic indices.
Methods
Study population
We reviewed the MACHD (Mayo Adult Congenital Heart Disease) Registry and identified adults (age >18 years) with Ebstein anomaly that underwent tricuspid valve surgery from 1 January 2000 to 31 December 2018. From this cohort, we identified consecutive patients that had transthoracic echocardiogram and cardiopulmonary exercise test pretricuspid and post-tricuspid valve surgery. We excluded patients with pacemakers. The Mayo Clinic Institutional Review Board approved the study and waived informed consent for patients that provided research authorisation. This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Cardiopulmonary exercise test
We analysed the last exercise test performed within 1-year prior to tricuspid valve surgery, and the first exercise test performed at least 1 year after tricuspid valve surgery. Only symptom-limited exercise tests performed using treadmill ergometer (modified Bruce protocol) with a respiratory exchange ratio >1.1 were included in our analysis.6 Improvement in aerobic capacity was defined as any postoperative improvement in %-predicted peak oxygen consumption (VO2) and was calculated as peak VO2 post-tricuspid valve surgery minus peak VO2 pretricuspid valve surgery. We used %-predicted peak VO2 as the outcomes metric because of differences in the age of the patients. We divided the cohort into two groups based on whether they had postoperative improvement in aerobic capacity defined as delta (∆) peak VO2 >0 (aerobic capacity (+) group) versus no improvement in aerobic capacity defined as ∆ peak VO2 ≤0 (aerobic capacity (-) group).
Echocardiography
We analysed the last transthoracic echocardiogram performed within 1-year prior to tricuspid valve surgery and the first echocardiogram performed at least 1 year after tricuspid valve surgery. Two experienced sonographers (JW and KT) performed offline measurements of all echocardiographic indices.
Right atrial (RA) and RV function were assessed using speckle tracking strain imaging obtained using Vivid E9 and E95 (General Electric, Fairfield, Connecticut, USA) with M5S and M5Sc-D transducers (1.5–4.6 MHz) at frame rate of 40–80 Hz.7 Three-beat cine-loop clips were obtained from RV-focused apical four-chamber views. These images were exported (DICOM) and then analysed offline using TomTec (TomTec Imaging Systems, Unterschleissheim, Germany). Offline analysis involved manual endocardial tracing of a single frame at end-systole by a point-click approach, starting from the lateral tricuspid annulus to the ‘normal/undisplaced’ septal tricuspid (<8 mm/m2 apical to the septal mitral annulus) as previously described.8 RA volume was also assessed using the same medial and lateral tricuspid annulus reference points. The periodic displacement of the tracing was automatically tracked in subsequent frames. Adequate tracking by the software was visually verified and retraced if necessary until adequate tracking was achieved. RA compliance was assessed using a ratio RA reservoir strain/RA maximum (end-systolic) volume and tricuspid regurgitation was assessed using qualitative Doppler method.9–11
Left ventricular (LV) volumes and ejection fraction were assessed using biplane Simpson’s method, and left atrial (LA) and LV strain imaging were performed as per guidelines. Diastolic LV eccentricity index, a measure of ventricular interdependence, was assessed from the parasternal short axis window using 2D echocardiogram. The major axis of the LV perpendicular to the ventricular septum (D1) and the minor axis of the LV parallel to the ventricular septum (D2) were measured at the mid papillary level in end-diastole. Diastolic LV eccentricity index was calculated as D2/D1, and higher values indicate greater septal flattening and therefore greater ventricular interdependence.12 13 Figures 1–3 show representative images for the assessment of atrial strain, ventricular strain and eccentricity index.
Figure 1.
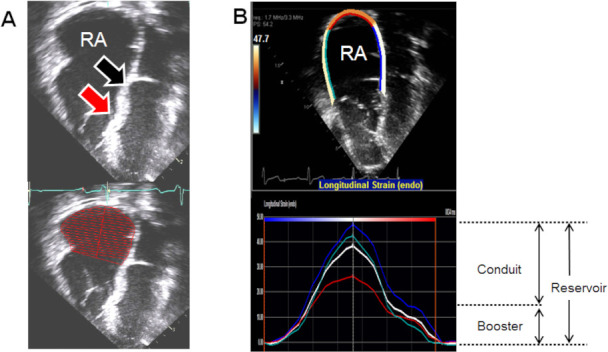
(A) 2D images showing location of apically displaced septal attachment of the tricuspid valve (red arrow) and the location of the ‘normal/undisplaced’ tricuspid annulus (black arrow). The RA volume was traced using the ‘normal/undisplaced’ location of the tricuspid annulus as the reference. (B) 2D and graphical tracing of RA strain which is the average of strain indices from atrial septum, free wall and roof of the RA. Reservoir strain is measured at end of ventricular systole; booster strain was measured at the end of atrial systole, and conduit strain was calculated as the difference between reservoir strain and booster strain. RA, right atrium.
Figure 2.
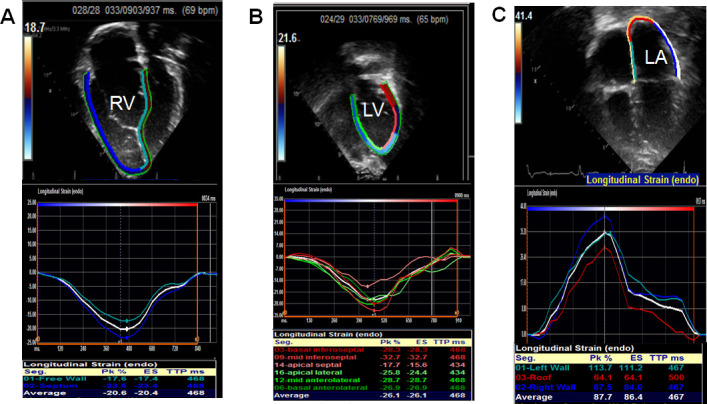
2D and graphical tracing from apical four chamber view showing right ventricular longitudinal strain (A), left ventricular longitudinal strain (B) and left atrial strain (C). RV: right ventricle; LV: left ventricle, LA: left atrium.
Figure 3.
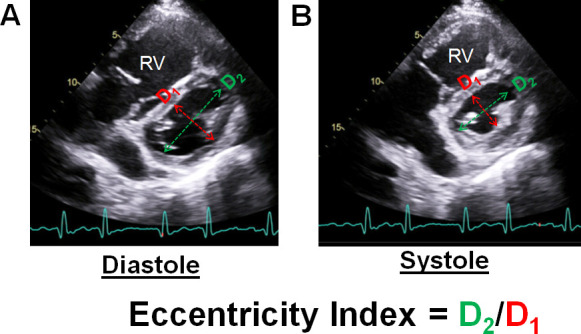
2D images of the left ventricle at the mid papillary level in end-diastole (A) and end-systole (B) from the parasternal short axis window. The major axis (D1) is measured perpendicular to the ventricular septum, and the minor axis (D2) is measured parallel to the ventricular septum. Left ventricular eccentricity index, a measure of ventricular interdependence, is calculated as D2/D1.
We assessed disease severity using the Carpentier classification as follows: Type A: the volume of the true RV is adequate; Type B: a large atrialised component of the RV exists, but the anterior leaflet of the tricuspid valve moves freely; Type C: the anterior leaflet is severely restricted in its movement and may cause significant obstruction of the RV outflow tract and Type D: almost complete atrialisation of the ventricle except for a small infundibular component.14
Reproducibility analysis
The reproducibility of atrial strain, atrial volumes and global longitudinal strain was assessed in 20 randomly selected patients. Intraobserver and interobserver agreement was evaluated after the same observer and another experienced reader repeated the analysis using intraclass correlation coefficient and mean absolute difference. Test-retest reproducibility was assessed in 20 patients by measuring two different image sets.
Statistical analysis
Data were presented as mean±SD, median (IQR) and count (%). Between-group comparisons were performed using Fisher’s exact test, unpaired t-test and Wilcoxon rank sum test as appropriate. Linear regression analysis was used to assess the correlations between continuous variables. Univariate logistic regression analysis was used to assess the relationship between baseline data (surgical, clinical and echocardiographic indices) and postoperative improvement in peak VO2. Next, a multivariate logistic regression model was built using forward stepwise selection based on data from univariate analysis with p<0.25 required for entry and <0.1 required to remain in the models. P<0.05 was considered statistically significant. All statistical analyses were performed with JMP software (V.14.0; SAS Institute, Cary, North Carolina, USA).
Results
Clinical and surgical data
A total of 76 patients met the inclusion criteria, and the median age at the time of surgery was 36 (24–51) years and 34 (45%) were men. Based on predefined criteria, 28 (37%) patients had improvement in aerobic capacity (aerobic capacity (+) group), while 48 (63%) had no improvement in aerobic capacity after tricuspid valve surgery (aerobic capacity (-) group) (table 1). There were no significant between-group differences in baseline clinical characteristics (table 1). Of the 76 patients, we were able to ascertain the Carpenter’s classification in 72 of the patients. Of these 72 patients, 39 patients were Type A, 31 patients were Type B and 2 patients were Type C.
Table 1.
Baseline characteristics
| Aerobic capacity+ (n=48) | Aerobic capacity- (n=28) | P value | |
| Age, years | 34 (22–48) | 38 (25–51) | 0.09 |
| Male | 12 (43%) | 22 (46%) | 0.4 |
| Body mass index, kg/m2 | 27±5 | 29±6 | 0.1 |
| Prior tricuspid valve repair | 6 (21%) | 13 (27%) | 0.6 |
| NYHA II–IV | 21 (75%) | 43 (90%) | 0.09 |
| Comorbidities | |||
| Hypertension | 5 (18%) | 9 (19%) | 0.7 |
| Diabetes | 1 (4%) | 2 (4%) | 0.8 |
| Coronary artery disease | – | 2 (4%) | – |
| Atrial flutter/tachycardia | 6 (21%) | 12 (25%) | 0.4 |
| Atrial fibrillation | 4 (14%) | 9 (18%) | 0.6 |
| Supraventricular tachycardia | 3 (11%) | 6 (13%) | 0.8 |
| Non-sustained ventricular tachycardia | 1 (4%) | 1 (2%) | 0.7 |
| Laboratory data | |||
| NT proBNP, pg/mL | 137 (91–322) | 152 (73–412) | 0.4 |
| Haemoglobin, g/dL | 13.8±1.4 | 14.1±2.1 | 0.3 |
| Estimated GFR, mL/min/1.73 m2 | 85±20 | 87±16 | 0.4 |
| Medications | |||
| Loop diuretics | 11 (39%) | 21 (44%) | 0.3 |
| Beta blockers | 8 (29%) | 11 (23%) | 0.5 |
| ACEI/ARB | 5 (18%) | 10 (21%) | 0.7 |
| Aldosterone antagonist | – | 1 (2%) | – |
ACEI, ACE inhibitor; ARB, angiotensin II receptor blockers; GFR, glomerular filtration rate; NT proBNP, N-terminal pro-brain natriuretic peptide; VO2, oxygen consumption.
Of the 76 patients, 51 (67%) had tricuspid valve repair while 25 (33%) had tricuspid valve replacement with a bioprosthesis. The indication for surgery was severe tricuspid regurgitation in 68 (90%) patients and a combination of tricuspid regurgitation and stenosis in 8 (10%) patients. All the 51 patients that underwent tricuspid valve repair, the cone technique was used in 48 patients. Of the 25 patients that had tricuspid valve replacement, 17 were reoperations after a prior tricuspid valve repair. Concomitant RA Maze, biatrial Maze and closure of atrial level shunts were performed in 21 (28%), 1 (1%) and 44 (58%), respectively. There were no significant between-group differences in the type of surgical procedures performed for the aerobic capacity (+) group versus aerobic capacity (-) group: tricuspid valve repair 21 (75%) vs 30 (63%), p=0.3; RA Maze 8 (29%) vs 13 (27%), p=0.9; closure of atrial shunt 18 (64%) vs 26 (54%), p=0.7 and redo tricuspid valve surgery 6 (21%) vs 13 (27%), p=0.6. At the time of hospital discharge, 49 (65%) had none/trivial tricuspid regurgitation, 27 (35%) had mild or mild/moderate tricuspid regurgitation, and no patient had moderate or more tricuspid regurgitation. There were no between-group differences in the severity of residual tricuspid regurgitation or postoperative tricuspid valve mean gradient (2±1 vs 3±1 mm Hg, p=0.6).
None of the patients required reoperation between the time of surgery and follow-up exercise test. At the time of 1-year postoperative follow-up, there were no between-group differences in the severity of residual tricuspid regurgitation or postoperative tricuspid valve mean gradient. Altogether, the entire cohort had a median follow-up of 64 (33-102) months yielding a total follow-up of 383 patient-years. A total of three patients died during follow-up, and the cause of death was end-stage right heart failure (n=1), sepsis/multi organ dysfunction (n=1) and unknown cause (n=1). Two of the deaths occurred in the aerobic capacity (+) group while one case occurred in the aerobic capacity (-) group. We did not perform between group comparison of mortality risk because of small sample size and low event rates.
Haemodynamic and exercise data
The average interval from the baseline echocardiogram to the time of surgery was 8±4 days and from the baseline exercise test to the time of surgery was 52±37 days. Compared with the aerobic capacity (-) group, the aerobic capacity (+) group had better RA, RV, LA and LV function and stroke volume prior to surgery (table 2). There was good intraobserver, interobserver and test-retest reproducibility for the assessment of echocardiographic indices of right and left heart function (online supplemental table 1). The aerobic capacity (+) group had higher peak VO2 and oxygen pulse and lower VE/VCO2 preoperatively. Cardiac magnetic resonance imaging (CMRI) data were available in 51 patients (67%). A subgroup analysis performed in the patients with CMRI data showed that although both groups had similar anatomic RV stroke volume, the aerobic capacity (+) group had higher RV physiologic (effective) stroke volume and lower tricuspid regurgitation volume (table 2).
Table 2.
Preoperative imaging and exercise data
| Aerobic capacity+ (n=48) | Aerobic capacity- (n=28) | P value | |
| Echocardiography | |||
| RA reservoir strain, % | 29±6 | 24±6 | 0.001 |
| RA booster strain, % | 12±4 | 11±4 | 0.3 |
| RA conduit strain, % | 17±5 | 13±4 | <0.001 |
| RA volume index, mL/m2 | 56±17 | 64±19 | 0.08 |
| RA reservoir strain/RA max volume | 0.52±0.23 | 0.38±0.17 | 0.02 |
| RV global longitudinal strain,% | 17±3 | 15±4 | 0.04 |
| TAPSE, mm | 27±7 | 25±5 | 0.08 |
| RV s’, cm/s | 14±6 | 12±4 | 0.1 |
| RV fractional area change, % | 34±9 | 33±8 | 0.2 |
| IVC diameter-expiration, mm | 19±4 | 19±4 | 0.8 |
| IVC diameter-inspiration, mm | 9±2 | 10±2 | 0.8 |
| IVC collapsibility,% | 54±13 | 47±10 | 0.02 |
| Estimated RA pressure, mm Hg | 8±2 | 10±3 | 0.002 |
| ≥Moderate TR severity | 28 (100%) | 48 (100%) | 0.9 |
| TR velocity, m/s | 2.7±0.4 | 2.7±0.5 | 0.8 |
| Estimated RVSP, mm Hg | 37±6 | 39±7 | 0.2 |
| LA reservoir strain, % | 31.2±5.3 | 27.6±5.8 | 0.01 |
| LA volume index, mL/m2 | 25±9 | 27±10 | 0.4 |
| LA reservoir strain/LA max volume | 1.2±0.3 | 1.0±0.4 | 0.02 |
| LV end-diastolic volume index, mL/m2 | 53±8 | 48±7 | 0.01 |
| LV end-systolic volume index, mL/m2 | 21±6 | 20±5 | 0.05 |
| LV stroke volume index, mL m2 | 31±4 | 28±4 | <0.001 |
| LV ejection fraction, % | 58±7 | 57±9 | 0.5 |
| LV global longitudinal strain, % | 21±4 | 19±4 | 0.04 |
| Diastolic LV eccentricity index | 1.37±0.21 | 1.49±0.21 | 0.02 |
| Lateral mitral E/e’ | 9±3 | 10±4 | 0.3 |
| Exercise test | |||
| Peak VO2, mL/kg/min | 23.4±4.2 | 20.8±5.2 | 0.03 |
| Predicted peak VO2, % | 65±16 | 56±17 | 0.02 |
| Predicted peak heart rate, % | 87±11 | 85±15 | 0.3 |
| Oxygen pulse, mL | 10.6±3.1 | 8.3±2.7 | <0.001 |
| VE/VCO2 slope | 26±5 | 31±7 | <0.001 |
| Oxygen saturation at rest, % | 98±1 | 98±1 | 0.9 |
| Oxygen saturation at peak, % | 96±2 | 94±2 | 0.3 |
| Cardiac MRI (n=51) | |||
| RV end-diastolic volume index, mL/m2 | 154±37 | 159±41 | 0.5 |
| RV end-systolic volume index, mL/m2 | 86±36 | 89±42 | 0.6 |
| TR volume index*, mL/m2 | 34±14 | 43±16 | 0.02 |
| RV ejection fraction, % | 46±13 | 44±12 | 0.2 |
| LV end-diastolic volume index, mL/m2 | 56±11 | 50±13 | 0.04 |
| LV end-systolic volume index, mL/m2 | 23±9 | 22±7 | 0.3 |
| LV stroke volume index, mL/m2 | 32±7 | 28±6 | 0.007 |
| LV ejection fraction, % | 57±6 | 55±9 | 0.4 |
*TR volume index was calculated as RV anatomic stroke volume (end-diastolic minus end-systolic volume) minus RV effective stroke volume (LV stroke volume).
E/e’, ratio of mitral inflow pulsed wave Doppler early velocity to mitral annular tissue Doppler early velocity; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; s’, annular tissue Doppler systolic velocity; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; VE/VCO2, ventilatory equivalent for carbon dioxide; VO2, oxygen consumption.
heartjnl-2020-317756supp001.pdf (107KB, pdf)
The average interval from the time of surgery to the follow-up echocardiogram and exercise test was 15 (12–19) months. By design, the aerobic capacity (+) group had more improvement in peak VO2 and oxygen pulse (table 3, figure 4). There was no between-group difference in the number of patients on beta-blocker therapy at the time of follow-up exercise test (7 (25%) vs 13 (27%), p=0.7). Although both groups had similar severity of residual tricuspid regurgitation, the aerobic capacity (+) group had more improvement in RA function (RA strain and RA compliance), right heart filling pressure (estimated RA pressure), LA function (LA strain and LA compliance) and LV preload and stroke volume (table 3). The average RA reservoir strain for the entire cohort was −27±5% preoperatively, and this increased to 30%±6% postoperatively. These values were lower than published reference values of RA reservoir strain in healthy subjects (mean+SD −49±13%; 5% to 95% percentile: −31 to −71%).15
Table 3.
Postoperative change in haemodynamic and exercise indices
| Aerobic capacity+ (n=48) | Aerobic capacity- (n=28) | P value | |
| Echocardiography | |||
| RA reservoir strain, % | 3.2±1.1 | 0.4±0.7 | <0.001 |
| RA volume index, mL/m2 | −16±5 | −18±7 | 0.4 |
| RA reservoir strain/RA max volume | 0.31±0.1 | 0.12±0.1 | <0.001 |
| RV global longitudinal strain,% | 2.6±1.9 | 1.3±1.7 | 0.2 |
| TAPSE, mm | −3±2 | −2±2 | 0.5 |
| RV s’, cm/s | 2±1 | 2±2 | 0.4 |
| RV fractional area change, % | −5±3 | −7±4 | 0.2 |
| IVC collapsibility,% | 18±11 | 11±9 | 0.004 |
| Estimated RA pressure, mm Hg | −2.4±1.1 | −1.3±1.9 | 0.02 |
| LA reservoir strain, % | 1.9±0.5 | 1.1±0.8 | 0.008 |
| LA reservoir strain/LA max volume | 0.1±0.1 | 0.1±0.1 | 0.9 |
| LV end-diastolic volume index, mL/m2 | 8±3 | 5±3 | <0.001 |
| LV end-systolic volume index, mL/m2 | 2±1 | 2±2 | 0.9 |
| LV stroke volume index, mL/m2 | 6±3 | 3±2 | <0.001 |
| LV ejection fraction, % | 3±2 | 3±3 | 0.7 |
| LV global longitudinal strain, % | −0.6±0.8 | −0.4±0.6 | 0.4 |
| Diastolic LV eccentricity index | −0.31±0.19 | −0.22.±0.13 | 0.03 |
| Lateral mitral E/e’ | −1±1 | −1±1 | 0.9 |
| Exercise test | |||
| Peak VO2, mL/kg/min | 2.1±1.4 | −0.9±0.4 | <0.001 |
| Predicted peak VO2, % | 6±2 | −3±2 | <0.001 |
| Predicted peak heart rate, % | 3±2 | 3±2 | 0.9 |
| Oxygen pulse, mL | 2.3±1.1 | 1.1±2.3 | 0.006 |
| VE/VCO2 slope | −3±2 | −2±3 | 0.2 |
| Oxygen saturation at rest, % | 0±1 | 0±1 | 0.9 |
| Oxygen saturation at peak, % | 1±1 | 2±1 | 0.7 |
E/e’, ratio of mitral inflow pulsed wave Doppler early velocity to mitral annular tissue Doppler early velocity; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; TR, tricuspid regurgitation; VE/VCO2, ventilatory equivalent for carbon dioxide; VO2, oxygen consumption.
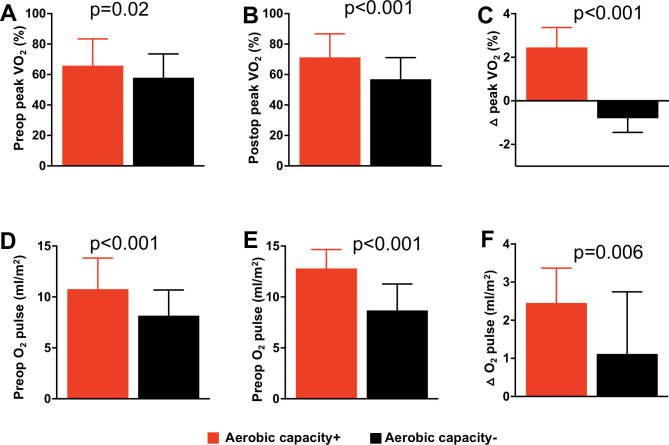
Figure 4.
Box and whisker plot showing between-group comparison of preoperative per cent-predicted peak oxygen consumption (VO2) (A); postoperative peak VO2 (B); postoperative change in peak VO2 (C); preoperative oxygen (O2) pulse (D); preoperative O2 (E); postoperative change in O2 (F).
The aerobic capacity (+) group had a lower diastolic LV eccentricity index at baseline (lower value signifies less ventricular interdependence) and also had a greater reduction in diastolic LV eccentricity index (table 3). Postoperative change in diastolic LV eccentricity index correlated with postoperative change in peak VO2 (r=0.72, p<0.001), RA reservoir strain (r=0.66, p<0.001), LV end-diastolic volume (r=0.58, p=0.008) and LV stroke volume (r=0.75, p<0.001). This relationship was independent of patients’ demographic characteristics, type of tricuspid valve surgery and the severity of residual tricuspid regurgitation.
Determinants of postoperative improvement in aerobic capacity
Online supplemental table 2 shows a univariate analysis of the relationship between baseline data (surgical, clinical and echocardiographic indices) and improvement in aerobic capacity. On multivariate analysis, the echocardiographic indices associated with postoperative improvement in aerobic capacity were RA reservoir strain (relative risk 1.12; 95% CI 1.06 to 1.18 per 1%-point increase); LA reservoir strain (relative risk 1.09; 95% CI 1.04 to 1.14 per 1%-point increase) and LV stroke volume index (relative risk 1.04; 95% CI 1.01 to 1.07 per 1 mL/m2 increase).
Discussion
Patients with Ebstein anomaly have reduced aerobic capacity, and without tricuspid valve surgery, most patients experience further deterioration in aerobic capacity during follow-up.5 8 16 17 Tricuspid valve surgery restores tricuspid valve competence and improves cardiac output and right heart failure symptoms.18–22 However, a successful tricuspid valve surgery does not result in improved aerobic capacity in all patients, and the preoperative factors responsible for these differences in outcomes are not well defined. In this study, we showed that 37% of patients with Ebstein anomaly had improvement in aerobic capacity after tricuspid valve surgery, and that atrial function at the time of surgery was the strongest determinant of postoperative improvement in aerobic capacity.
Similar to the findings in the current study, Ibrahim et al,22 Müller et al 4 and Kühn et al 21 reported improvement in peak VO2 after tricuspid valve surgery based on retrospective case series of 27 patients, 21 patients and 16 patients with Ebstein anomaly, respectively. However, these previous studies did not describe the preoperative factors associated with postoperative improvement in aerobic capacity likely because of small sample size. The current study identified the echocardiographic predictors of postoperative improvement in aerobic capacity, hence bridging this knowledge gap. This novel finding is clinically relevant because it opens new horizons for further prospective studies to determine the prognostic implication of timing of tricuspid valve surgery based on these echocardiographic indices and also to determine if postoperative improvement in aerobic capacity translates to improved long-term survival.
On the other hand, Müller et al 5 reported no improvement in exercise capacity after tricuspid valve surgery based on a retrospective cohort study of 37 patients with Ebstein anomaly. In that study, exercise capacity was assessed using peak workload (W/kg) instead of predicted peak VO2 (%) and hence did not consider the effect of difference in age and interval between exercise tests. Another study that reported lack of postoperative improvement in aerobic capacity was a previous study from our institution by Morrical et al based on a review of 32 patients with Ebstein anomaly that underwent tricuspid valve surgery.23 The apparent difference in the results of the current study as compared with the previous study by Morrical et al 23 may be related to differences in study design and inclusion criteria used for both studies. The Morrical et al 23 study was based on exercise data generated from different types of exercise test (some with measured VO2 and others with VO2 estimated from metabolic equivalent), different ergometers (cycle and treadmill) and different levels of effort (both maximum effort and submaximal effort). Since aerobic capacity can differ significantly in the same patient based on the type of exercise protocols used, combining exercise indices from different types of exercise tests could have introduced significant confounders. In contrast, the current study analysed only cardiopulmonary exercise test (with measured VO2) performed using treadmill ergometer in subjects that exerted maximum effort defined as respiratory exchange ratio >1.1.
Potential mechanism for postoperative improvement in aerobic capacity
There are two factors that determine VO2, and these factors are oxygen delivery (dependent on cardiac output and blood oxygen concentration) and oxygen extraction (dependent on the efficiency of the skeletal muscle metabolism to extract oxygen from the blood).24 An increase in aerobic capacity as measured by peak VO2 requires an increase in oxygen delivery and/or oxygen extraction.24 Since there was no postoperative change in peak heart rate and haemoglobin concentration in our cohort, and there is reason to expect a change in skeletal muscle metabolism (oxygen extraction) postoperatively, then postoperative improvement in peak VO2 can only occur due to an increase in stroke volume which is consistent with our results. Since none of the patients had concomitant left heart intervention at the time of tricuspid valve surgery, we postulated that a change in ventricular interdependence was the likely mechanism transmitting improvement in right heart haemodynamics to measurable improvement in left heart output (stroke volume) post tricuspid valve surgery. This is consistent with observed correlations between change in LV eccentricity index (measure of ventricular interdependence) and improvement in LV preload and stroke volume.
The aerobic capacity (+) group had better atrial function (RA reservoir strain and LA reservoir strain) at baseline, and atrial function indices were the strongest predictors of postoperative improvement in aerobic capacity. RA strain is a composite metric of RA function, remodelling and dispensability and becomes progressively more impaired in the setting right heart haemodynamic stress form tricuspid regurgitation and RV diastolic dysfunction.8 25 26 Hence the aerobic capacity (-) group had worse RA function as evidenced by lower RA reservoir strain and higher RA pressure. Since RA pressure reflects intra-pericardial pressure and pericardial restraint,27 28 the elevated right heart filling pressures in the aerobic capacity (-) group is transmitted to the left heart via increased ventricular interdependence as evidenced by higher LV eccentric index and over time results in LA remodelling and dysfunction as evidenced by a lower LA reservoir strain in this group.29 This is consistent with previous cross-sectional studies that have also shown an association between RA strain, ventricular interdependence, LV volumes and peak VO2 in Ebstein anomaly.8 30
We postulated that improvement in RA function in the aerobic capacity (+) group suggests a more favourable haemodynamic response to right heart unloading resulting in more profound reduction in right heart filling pressure and pericardial restraint, and in turn, less ventricular interdependence, and improved LV preload. Perhaps these patients had more favourable response because of a less advanced atrial dysfunction at baseline as evidence by higher RA and LA reservoir strain preoperatively. A potential clinical application of this finding will be to use atrial function indices in determining the timing of tricuspid valve surgery.
Limitations
This was a retrospective single-centre study and therefore prone to selection bias and other limitations inherent in retrospective study design. Additionally, we did not have CMRI data (the gold standard of RV volumetric analysis) in all patients and hence were unable to evaluate the relationship between RV volumetric indices and postoperative change in aerobic capacity. Furthermore, the cohort had limited follow-up and as a result we were unable to determine whether patients with postoperative improvement in aerobic capacity had better long-term survival. The current study was limited by small sample size which increases the probability of type II error.
Conclusion
The current study showed that one-third of patients with Ebstein anomaly had improvement in aerobic capacity after tricuspid valve surgery, and that improvement in aerobic capacity was related to postoperative changes in LV volumes and output. RA and LA function were the most robust echocardiographic predictors of postoperative improvement in aerobic capacity, and that change in ventricular interdependence in response to right heart unloading was mechanistically linked to improvement in left heart preload and output, and in turn, improvement in peak VO2. This study provides a foundation for further studies to determine whether postoperative improvement in aerobic capacity translates to improve long-term survival, and whether the preoperative echocardiographic indices identified in the study will assist in determining optimal timing for operative intervention in asymptomatic or minimally symptomatic patients with Ebstein anomaly.
Key messages.
What is already known on this subject?
Ebstein anomaly is an important cause of right heart failure symptoms suggesting impaired aerobic capacity among patients with congenital heart disease. Although tricuspid valve surgery restores tricuspid valve competence, it is not always associated with improved aerobic capacity in this population. The haemodynamic determinants of postoperative improvement in aerobic capacity in patients with Ebstein anomaly undergoing tricuspid valve surgery have not been defined.
What might this study add?
Postoperative improvement in aerobic capacity occurred in one-third of patients with Ebstein anomaly after a successful tricuspid valve surgery, and the average improvement in peak oxygen consumption was 2.1±1.4 mL/kg/min (6±2%-predicted peak oxygen consumption). Postoperative improvement in aerobic capacity is not related to patient’s demographic characteristics or type/technique of tricuspid valve surgery, but rather it is related to right and left atrial function indices.
How might this impact on clinical practice?
Echocardiographic indices of atrial function can potentially assist in determining optimal timing for operative intervention in asymptomatic or minimally symptomatic patients with Ebstein anomaly.
Footnotes
Contributors: AE was responsible for the planning, conduct, reporting, drafting and critical review of the manuscript and takes responsibility for the overall content. JD, WM and HC were responsible for the drafting, critical review and final approval of the manuscript.
Funding: Dr Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grant K23 HL141448. The MACHD Registry is supported by the Al-Bahar Research grant.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. egbe.alexander@mayo.edu.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation 2016;134:101-9. 10.1161/CIRCULATIONAHA.115.019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Attenhofer Jost CH, Connolly HM, Scott CG, et al. Outcome of cardiac surgery in patients 50 years of age or older with Ebstein anomaly: survival and functional improvement. J Am Coll Cardiol 2012;59:2101–6. 10.1016/j.jacc.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 3. Brown ML, Dearani JA, Danielson GK, et al. Functional status after operation for Ebstein anomaly: the Mayo clinic experience. J Am Coll Cardiol 2008;52:460–6. 10.1016/j.jacc.2008.03.064 [DOI] [PubMed] [Google Scholar]
- 4. Müller J, Kühn A, Vogt M, et al. Improvements in exercise performance after surgery for Ebstein anomaly. J Thorac Cardiovasc Surg 2011;141:1192–5. 10.1016/j.jtcvs.2010.08.083 [DOI] [PubMed] [Google Scholar]
- 5. Müller J, Kühn A, Tropschuh A, et al. Exercise performance in Ebstein's anomaly in the course of time - Deterioration in native patients and preserved function after tricuspid valve surgery. Int J Cardiol 2016;218:79–82. 10.1016/j.ijcard.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 6. Egbe A, Khan AR, Miranda WR, et al. Mechanism for temporal changes in exercise capacity after Fontan palliation: role of Doppler echocardiography. Am Heart J 2018;196:144–52. 10.1016/j.ahj.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 7. Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018;19:591–600. 10.1093/ehjci/jey042 [DOI] [PubMed] [Google Scholar]
- 8. Akazawa Y, Fujioka T, Kühn A, et al. Right ventricular diastolic function and right atrial function and their relation with exercise capacity in Ebstein anomaly. Can J Cardiol 2019;35:1824–33. 10.1016/j.cjca.2019.05.036 [DOI] [PubMed] [Google Scholar]
- 9. Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of echocardiography developed in collaboration with the Society for cardiovascular magnetic resonance. J Am Soc Echocardiogr 2017;30:303–71. 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 10. Lancellotti P, Moura L, Pierard LA, et al. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307–32. 10.1093/ejechocard/jeq031 [DOI] [PubMed] [Google Scholar]
- 11. Reddy YNV, Obokata M, Egbe A, et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:891–900. 10.1002/ejhf.1464 [DOI] [PubMed] [Google Scholar]
- 12. Ryan T, Petrovic O, Dillon JC, et al. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol 1985;5:918–24. 10.1016/S0735-1097(85)80433-2 [DOI] [PubMed] [Google Scholar]
- 13. Friedberg MK. Imaging Right-Left ventricular interactions. JACC Cardiovasc Imaging 2018;11:755–71. 10.1016/j.jcmg.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 14. Carpentier A, Chauvaud S, Macé L, et al. A new reconstructive operation for Ebstein's anomaly of the tricuspid valve. J Thorac Cardiovasc Surg 1988;96:92–101. 10.1016/S0022-5223(19)35302-4 [DOI] [PubMed] [Google Scholar]
- 15. Padeletti M, Cameli M, Lisi M, et al. Reference values of right atrial longitudinal strain imaging by two-dimensional speckle tracking. Echocardiography 2012;29:147–52. 10.1111/j.1540-8175.2011.01564.x [DOI] [PubMed] [Google Scholar]
- 16. Kipps AK, Graham DA, Lewis E, et al. Natural history of exercise function in patients with Ebstein anomaly: a serial study. Am Heart J 2012;163:486–91. 10.1016/j.ahj.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 17. Chen SSM, Dimopoulos K, Sheehan FH, et al. Physiologic determinants of exercise capacity in patients with different types of right-sided regurgitant lesions: Ebstein's malformation with tricuspid regurgitation and repaired tetralogy of Fallot with pulmonary regurgitation. Int J Cardiol 2016;205:1–5. 10.1016/j.ijcard.2015.10.175 [DOI] [PubMed] [Google Scholar]
- 18. Brown ML, Dearani JA, Danielson GK, et al. The outcomes of operations for 539 patients with Ebstein anomaly. J Thorac Cardiovasc Surg 2008;135:1120–36. 10.1016/j.jtcvs.2008.02.034 [DOI] [PubMed] [Google Scholar]
- 19. Holst KA, Dearani JA, Said S, et al. Improving results of surgery for Ebstein anomaly: where are we after 235 cone repairs? Ann Thorac Surg 2018;105:160–8. 10.1016/j.athoracsur.2017.09.058 [DOI] [PubMed] [Google Scholar]
- 20. Stulak JM, Sharma V, Cannon BC, et al. Optimal surgical ablation of atrial tachyarrhythmias during correction of Ebstein anomaly. Ann Thorac Surg 2015;99:1700–5. 10.1016/j.athoracsur.2015.01.037 [DOI] [PubMed] [Google Scholar]
- 21. Kühn A, De Pasquale Meyer G, Müller J, et al. Tricuspid valve surgery improves cardiac output and exercise performance in patients with Ebstein's anomaly. Int J Cardiol 2013;166:494–8. 10.1016/j.ijcard.2011.11.033 [DOI] [PubMed] [Google Scholar]
- 22. Ibrahim M, Tsang VT, Caruana M, et al. Cone reconstruction for Ebstein's anomaly: patient outcomes, biventricular function, and cardiopulmonary exercise capacity. J Thorac Cardiovasc Surg 2015;149:1144–50. 10.1016/j.jtcvs.2014.12.074 [DOI] [PubMed] [Google Scholar]
- 23. Morrical BD, Dearani JA, Bonnichsen CR, et al. Exercise capacity after repair of Ebstein anomaly in adults. Pediatr Cardiol 2019;40:726–32. 10.1007/s00246-019-02056-9 [DOI] [PubMed] [Google Scholar]
- 24. Poole DC, Richardson RS. Determinants of oxygen uptake. Implications for exercise testing. Sports Med 1997;24:308–20. 10.2165/00007256-199724050-00003 [DOI] [PubMed] [Google Scholar]
- 25. Steinmetz M, Broder M, Hösch O, et al. Atrio-ventricular deformation and heart failure in Ebstein's Anomaly - A cardiovascular magnetic resonance study. Int J Cardiol 2018;257:54–61. 10.1016/j.ijcard.2017.11.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciepłucha A, Trojnarska O, Kociemba A, et al. Clinical aspects of myocardial fibrosis in adults with Ebstein's anomaly. Heart Vessels 2018;33:1076–85. 10.1007/s00380-018-1141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tyberg JV, Taichman GC, Smith ER, et al. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 1986;73:428–32. 10.1161/01.CIR.73.3.428 [DOI] [PubMed] [Google Scholar]
- 28. Smiseth OA, Frais MA, Kingma I, et al. Assessment of pericardial constraint: the relation between right ventricular filling pressure and pericardial pressure measured after pericardiocentesis. J Am Coll Cardiol 1986;7:307–14. 10.1016/S0735-1097(86)80496-X [DOI] [PubMed] [Google Scholar]
- 29. Egbe AC, Bonnichsen C, Reddy YNV, et al. Pathophysiologic and prognostic implications of right atrial hypertension in adults with tetralogy of Fallot. J Am Heart Assoc 2019;8:e014148. 10.1161/JAHA.119.014148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujioka T, Kühn A, Sanchez-Martinez S, et al. Impact of interventricular interactions on left ventricular function, stroke volume, and exercise capacity in children and adults with Ebstein's anomaly. JACC Cardiovasc Imaging 2019;12:925–7. 10.1016/j.jcmg.2018.09.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-317756supp001.pdf (107KB, pdf)
Data Availability Statement
Data are available on reasonable request. egbe.alexander@mayo.edu.