Abstract
Animal behavior was classically considered to be determined exclusively by neuronal activity, whereas surrounding glial cells such as astrocytes played only supportive roles. However, astrocytes are as numerous as neurons in the mammalian brain, and current findings indicate a chemically based dialog between astrocytes and neurons. Activation of astrocytes by synaptically released neurotransmitters converges on regulating intracellular Ca2+ in astrocytes, which then can regulate the efficacy of near and distant tripartite synapses at diverse timescales through gliotransmitter release. Here, we discuss recent evidence on how diverse behaviors are impacted by this dialog. These recent findings support a paradigm shift in neuroscience, in which animal behavior does not result exclusively from neuronal activity but from the coordinated activity of both astrocytes and neurons. Decoding how astrocytes and neurons interact with each other in various brain circuits will be fundamental to fully understanding how behaviors originate and become dysregulated in disease.
Keywords: astrocytes, glia, Ca2+ signaling, gliotransmission, behavior, tripartite synapse
INTRODUCTION
Over the past decade, as reflected by the rapidly growing body of literature, the role of astrocytes in brain function and behavior has received a surge in interest. The increasing interest on the role of astrocytes can be attributed to three major reasons, which form the basis of this review. First, we provide a brief update on the basic cellular properties of astrocytes in the central nervous system, including a discussion of their physiological properties and responsiveness to neurotransmitters. Second, we describe the various functions in which astrocytes have been implicated, emphasizing their importance in driving or modulating behaviors. Third, we highlight pending questions that might further clarify or unify various competing models on how astrocytes interact with neuronal networks to organize behavior. While animal behavior encompasses a vast domain that can be studied with a plethora of experimental tasks, we focus the present discussion on paradigmatic examples in which the underlying cellular mechanisms have been at least partially identified. Moreover, although some models of pathology were instrumental to establish the relationship between brain function and behavior, we do not extensively discuss these models in this review.
Astrocytes sense synaptic activity by expressing a wide variety of G protein-coupled receptors (GPCRs), which bestow them with the aptitude to respond to neurotransmitters or neuromodulators (Araque et al. 2014, Kofuji & Araque 2020). GPCRs for many neurotransmitters have now been described in astrocytes, including small-molecule neurotransmitter receptors, neuropeptide receptors, and retrograde messenger receptors such as cannabinoid receptors type 1 (CB1Rs) (Kofuji & Araque 2020). Ca2+ elevation is a universal response by astrocytes when GPCRs are activated, independently of the type of G protein stimulated (Durkee et al. 2019), although activation of the Gi pathway may elicit a transient Ca2+ elevation followed by a later, more sustained Ca2+ decrease (Kol et al. 2020). Such Ca2+ elevations have been observed in vivo both in the astrocytic processes and in their somata (Durkee et al. 2019, Lines et al. 2020, Perez-Alvarez et al. 2014, Shigetomi et al. 2013, Stobart et al. 2018) (Figure 1). Mice deficient in inositol triphosphate type 2 receptors (IP3R2) show diminished astrocyte Ca2+ fluctuations (Srinivasan et al. 2015) and have been used extensively as an animal model of disrupted astrocytic excitability and impaired gliotransmitter release (Oliveira et al. 2015, Srinivasan et al. 2015). We note that there is controversy over whether gliotransmitters are released from astrocytes under physiological conditions (Fiacco & McCarthy 2018, Savtchouk & Volterra 2018). This is not a completely settled issue, but mounting recent data, including those mentioned in this review, indicate that gliotransmission is a universal feature in all neuronal-glial networks (Araque et al. 2014, Savtchouk & Volterra 2018).
Figure 1.
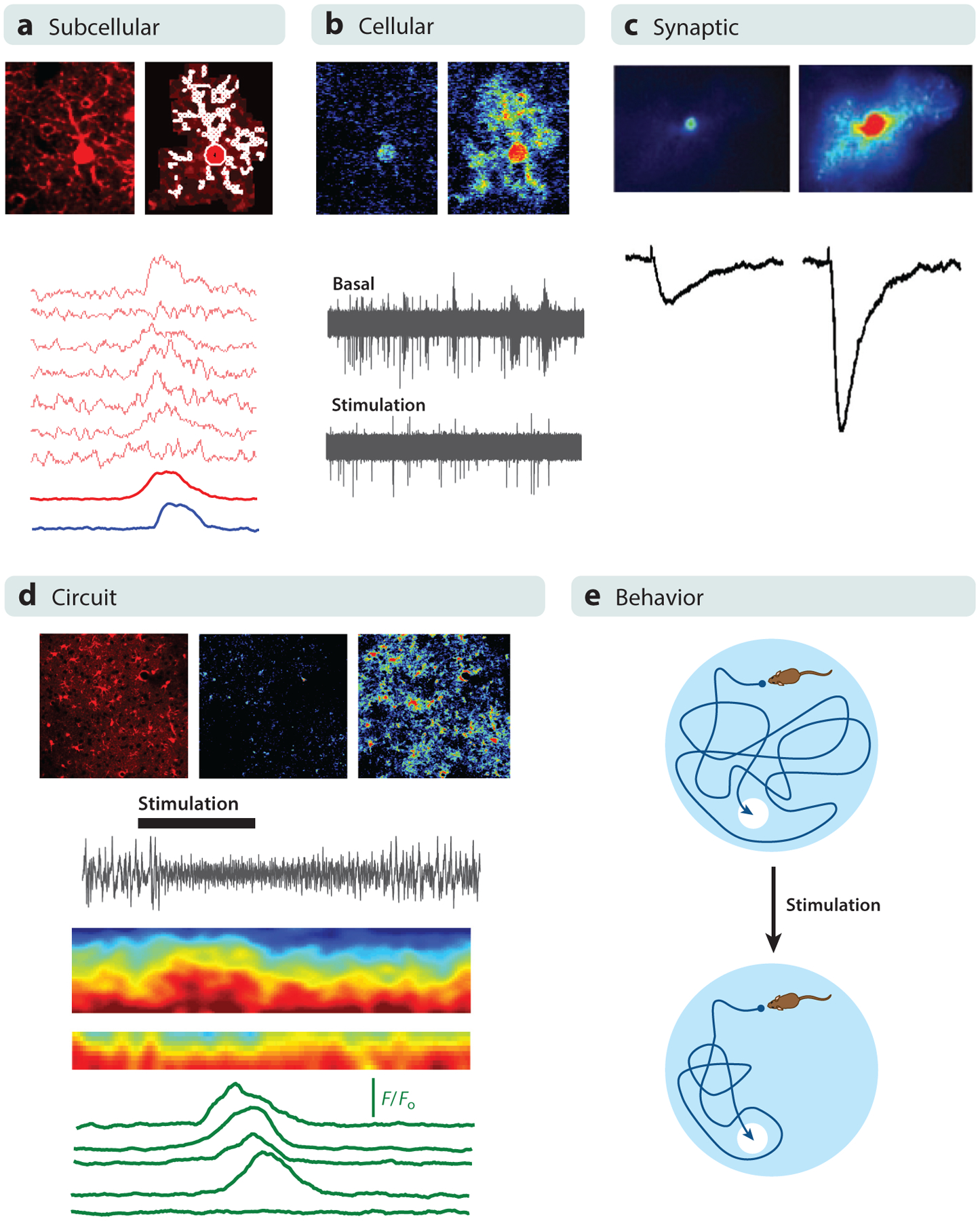
Astrocyte activity and astrocyte-neuron interactions at different levels of analysis. (a) At the subcellular level, astrocytes display calcium elevations in microdomains. (Top) Images represent an SR101-labeled astrocyte (left) with marked microdomains (right); (bottom) traces represent calcium levels at microdomains in response to sensory stimulation. Panel a adapted with permission from Lines et al. (2020). (b) At the cellular level, astrocytes display generalized calcium elevations that impact neuronal electrical activity. (Top) Images represent pseudocolor calcium levels in a single astrocyte; (bottom) traces represent extracellular field recordings before and after astrocyte stimulation. Panel b adapted with permission from Lines et al. (2020). (c) At the synaptic level, calcium elevations in astrocytes regulate synaptic transmission properties. (Top) Images represent pseudocolor calcium levels in a single astrocyte; (bottom) traces represent synaptic currents in an adjacent single synapse before and after astrocyte stimulation. Panel c adapted with permission from Martin et al. (2015). (d) At the circuit level, astrocyte populations display generalized calcium elevations that influence neuronal network activity. (Top) Images represent SR101-labeled cortical astrocytes and pseudocolor calcium levels in an astrocyte population before and after sensory stimulation; (bottom) electrocorticogram trace, color-coded spectrogram, and traces represent astrocyte calcium levels before and after sensory stimulation. Panel d adapted with permission from Lines et al. (2020). (e) At the behavioral level, astrocyte-neuron interactions may influence multiple animal behaviors such as spatial learning of a hidden platform in a Morris water maze.
Of particular relevance to our discussion of the role of astrocytes in behavior is that gliotransmitter release has been linked with all major forms of synaptic plasticity (De Pittà et al. 2016). Thus, astrocytic modulation of short-term synaptic excitation or depression, in a timescale of seconds to minutes, is described in diverse brain regions. Likewise, astrocytic modulation of long-lasting synaptic changes lasting tens of minutes, such as long-term potentiation (LTP) and long-term depression (LTD) of synaptic transmission, has also been extensively documented (Araque et al. 2014).
ASTROCYTES AND LEARNING AND MEMORY
Memory refers to the brain’s faculty to encode, store, and retrieve biologically relevant information to guide a desired behavioral output. Learning is viewed as an acquisition of information or encoding the information to memory. It is accepted that learning and memory are the results of interactions of millions of neurons in the brain and their coordinated activity. At the cellular level, learning and memory are thought to be associated with long-lasting changes in the strength of synaptic connections, such as those observed in LTP or LTD (Nicoll 2017).
What is the gliotransmitter released by astrocytes during learning and memory processes? A clear expectation would be a gliotransmitter with regulatory actions on glutamatergic NMDA receptors (NMDARs), as these receptors play a central role in synaptic plasticity, learning, and memory. NMDAR activation requires the binding of the coagonist glycine or d-serine (Hansen et al. 2018). Several studies suggest that d-serine is a gliotransmitter that modulates LTP in diverse brain regions (Han et al. 2015, Henneberger et al. 2010, Panatier et al. 2011, Yang et al. 2003). Accordingly, d-serine administration enhances performance in memory tasks (Andersen & Pouzet 2004, Bado et al. 2011, Zhang et al. 2008). Moreover, the genetic ablation of astrocytic CB1Rs reduces extracellular d-serine levels in the hippocampus and reduces the magnitude of hippocampal LTP (Robin et al. 2018). Behaviorally, this genetic maneuver results in mice with impaired object recognition memory (Robin et al. 2018). However, whether d-serine is primarily released by neurons or astrocytes is under debate (Benneyworth et al. 2012, Papouin et al. 2017, Wolosker et al. 2016).
Even if the mechanism of astrocytic modulation of LTP is not completely understood, the concept that astrocytic-neuronal communication regulates memory processes is now less controversial. One previous argument against the involvement of astrocytes in learning and memory was the seeming lack of deficits in memory tests for the IP3R2-knockout mouse in which the astrocytic Ca2+ signal is compromised (Petravicz et al. 2014). However, further examination of memory retention in longer timescales indeed showed memory impairments in this mouse model (Pinto-Duarte et al. 2019). While acquisition and short-term memory (24–48 h) were not affected, the IP3R2-knockout mouse performed much worse in all performed memory tests two or three weeks later (Pinto-Duarte et al. 2019).
Gain-of-function approaches have also been instrumental to bolstering the case of astrocytes as regulators of memory processes. The Goshen group (Adamsky & Goshen 2018) asked whether astrocytes in CA1 hippocampus regulate memory allocation and memory recall in mice. Astrocytic activation of the Gq pathway in CA1 hippocampus using chemogenetic approaches promoted the increase of spontaneous neuronal activity and triggered the induction of LTP. More importantly, in two memory tests, the T-maze and fear-conditioning tests, the astrocyte activation in the hippocampus also enhanced memory allocation, i.e., enhanced activation of specific neuronal assemblies in CA1 upon learning and memory recall on the following day. Astrocytic activation also resulted in improved performance in hippocampal-dependent memory tests such as contextual fear learning (Adamsky et al. 2018) (Figure 2). Recently, Gq pathway activation of astrocytes using melanopsin as an optogenetic actuator in CA1 hippocampal astrocytes also induced LTP of synapses and improved memory performance (Mederos et al. 2019).
Figure 2.
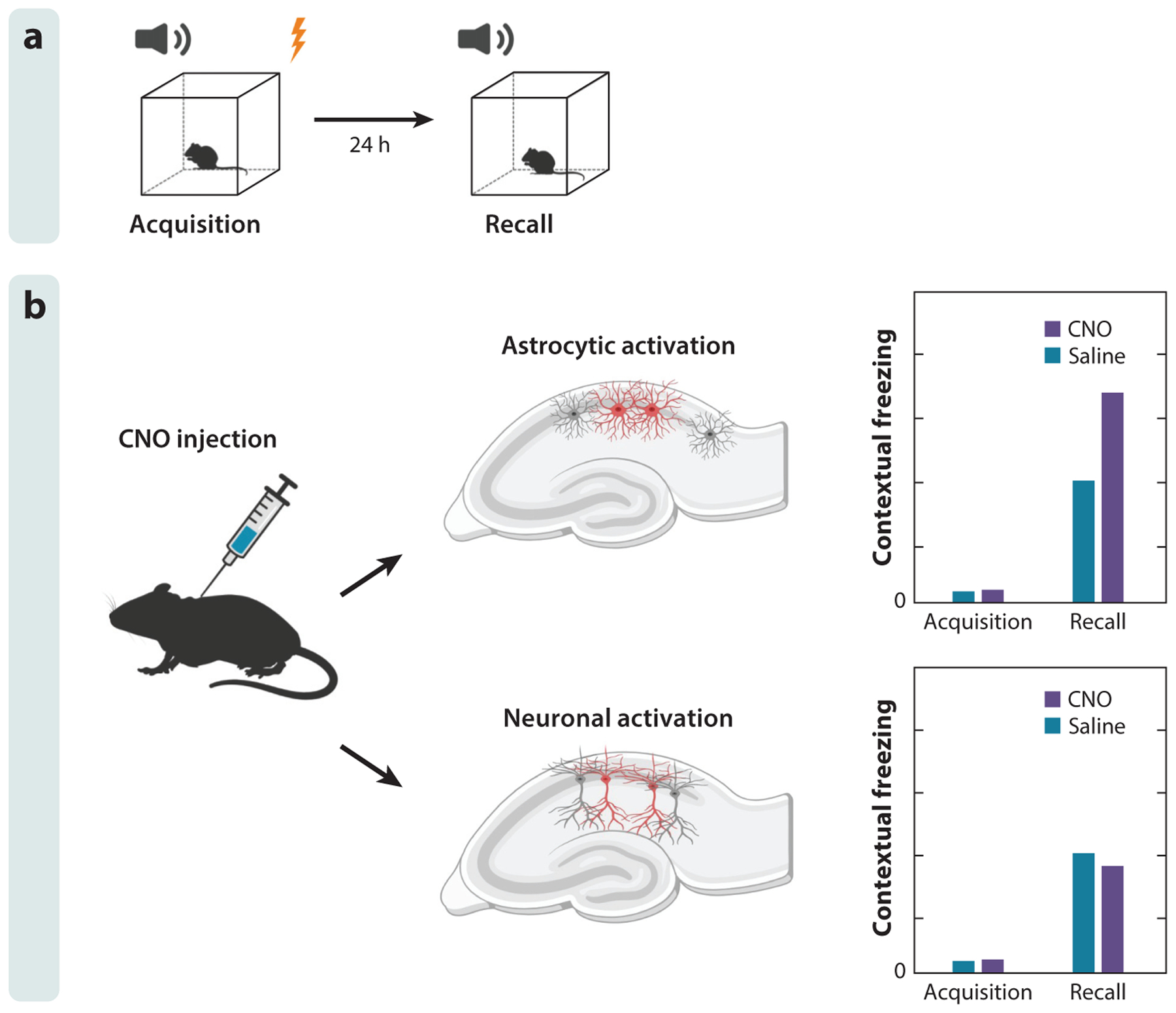
Astrocyte activation in the hippocampus is sufficient to induce memory enhancement. (a) A contextual fear conditioning paradigm is used to assess fear learning. Mice are exposed to electric shock and auditory cue during fear acquisition. Fear learning or recall is assessed by the freezing behavior displayed on exposure to auditory cue 24 h following fear acquisition. (b) Activation of CA1 astrocytes or neurons 30 min before fear acquisition has differential effects on fear learning. Chemogenetic activation of astrocytes in the mouse CA1 area of the hippocampus enhances fear learning, while chemogenetic activation of neuronal cells diminishes fear learning. Figure adapted with permission from Adamsky et al. (2018).
These results again highlight the relevant role of astrocyte-neuronal signaling in learning and memory processes. Nonetheless, it is important to note that optogenetic or chemogenetic stimulation might activate astrocytes outside of their normal physiological range, which may induce nonphysiological responses. Moving forward, complementary functional approaches to reversibly silence astrocyte signaling with temporal and spatial precision could provide further insights on the role of astrocytes in memory processes.
Intriguingly, activation of the Gi pathway in CA1 hippocampal astrocytes impacted another aspect of memory processes. Gi activation during training in contextual fear learning did not affect memory one day after training but impaired retrieval of memories one month later (Kol et al. 2020). Goshen and colleagues (Kol et al. 2020) present evidence that this impairment of remote memories was due to deficient astrocytic modulation of the pathway connecting CA1 neurons to anterior cingulate cortex (ACC) neurons during fear learning. This compromised CA1-ACC communication may impair the tagging of ACC circuits required for memory persistence (Kitamura et al. 2017).
Another form of astrocytic-neuronal signaling implicated in memory processes is what is known as the astrocyte-neuron lactate shuttle (ANLS) (Calì et al. 2019). Glucose derived from blood is the primary energy source for generating ATP in the brain, but an important energy reserve is the brain glycogen synthesized from glucose in astrocytes. In the brain, glycogen and the enzyme glycogen phosphorylase, which catalyzes glycogenolysis, are restricted to astrocytes and absent from neurons (Magistretti & Allaman 2018). Gibbs & Hertz (2008) were the first to show that inhibition of glycogenolysis interrupts memory consolidation in young chickens. These findings were further pursued by Suzuki et al. (2011) in a landmark study in rats. To test whether astrocytic glycogen metabolism in the hippocampus is required for short- and long-term memories, Suzuki et al. pharmacologically inhibited glycogenolysis by bilateral injection in the hippocampus of the glycogen phosphorylase inhibitor 1,4-dideoxy-1,4-imino-d-arabinitol (DAB). Blockade of astrocytic glycogenolysis in the hippocampus during memory acquisition prevented the retrieval of memories 24, 48, and 78 h later but not 1 h post-training. Thus, astrocytic glycogenolysis during learning acquisition is not required for short-term memory, but it is essential for long-term memory, suggesting a role in memory consolidation processes (Suzuki et al. 2011). Previously, it was shown that glycogenolysis results in lactate release from astrocytes (Brown et al. 2004, Dringen et al. 1993) and that the lactate transport between astrocytes and neurons proceeds via monocarboxylate transporters MCT1/4 in astrocytes and MCT2 in neurons. Accordingly, antisense knockdown of the astrocytic lactate transporters MCT1/MCT4 or the neuronal isoform MCT2 in the hippocampus impaired memory consolidation (Suzuki et al. 2011). Overall, these results make a compelling case for a tight metabolic coupling between astrocytes and neurons in which astrocytes provide the energy resources to neurons in the form of lactate during the energy-demanding phase of memory consolidation (Steinman et al. 2016).
How is lactate production in astrocytes triggered during memory formation? Production may be related to the actions of the neuromodulator norepinephrine (NE), which regulates multiple brain functions such as attention, perception, arousal, sleep, learning, and memory (O’Donnell et al. 2012). Activation of β2 adrenergic receptors in astrocytes by NE has been identified as a potent stimulus leading to l-lactate production during memory processing (Gao et al. 2016). Astrocyte-specific knockdown of the β2 adrenergic receptors prevents memory consolidation, and this effect is rescued by lactate infusion to the hippocampus. (Jensen et al. 2016).
Certain specific aspects of the ANLS model have been the subject of debate, which is beyond the scope of this review, but they have been argued in several review articles (Barros & Weber 2018, Chih et al. 2001, Magistretti & Allaman 2018, Mason 2017, Tang et al. 2014). One interesting question is whether lactate serves only as a fuel source to neurons or also as a signaling molecule that triggers synaptic changes in neurons (Magistretti & Allaman 2018). As pointed out by Magistretti & Allaman (2018), the fact that glucose does not rescue the effect of blocking glycogenolysis with DAB on memory consolidation suggests that lactate has functions independent of energy supply functions.
Finally, recent evidence has implicated astrocytes in LTD in the hippocampus and other brain areas (Chen et al. 2013, Han et al. 2012, Kakegawa et al. 2011). Establishing the functional role of LTD has proved more challenging than with LTP, due to the difficulty of inducing LTD in vivo and a lack of selective inhibitors for LTD (Collingridge et al. 2010). However, genetic mouse mutants with intact LTP but impaired LTD have deficits in hippocampal-dependent memory tasks (Brigman et al. 2010). Cannabinoid exposure in vivo in mice activates astrocytic CB1Rs to mediate AMPAR endocytosis-mediated expression of in vivo LTD at CA3-CA1 synapses and working memory impairment (Han et al. 2012). Moreover, in a recent study, Navarrete et al. (2019) identified astrocytes as the cells undergoing signaling through p38α MAPK, a key signaling molecule known to be necessary for hippocampal LTD, showing that selective deletion of p38α in astrocytes, but not in neurons, prevented LTD. Consistent with their results, which supported the idea that hippocampal LTD was mediated by elevations of astrocyte Ca2+ that stimulate glutamate release from astrocytes through a p38α MAPK-dependent mechanism that led to AMPAR internalization, they found that mice with p38α deletion in astrocytes, but not in neurons, increased the freezing response in the hippocampal-dependent contextual fear-conditioning task, indicating that astrocyte regulation of hippocampal LTD influenced long-term memory (Navarrete et al. 2019).
ASTROCYTES AND EMOTIONAL STATES
Astrocytic Dysregulation in Depression-Like Behaviors
Aberrant astrocyte function has been suggested to underlie some forms of depressive-like phenotype (DLP). For example, DLP manifested as anhedonia or behavioral despair was observed following the poisoning of astrocytes in the prefrontal cortex (PFC) of adult rats (Banasr & Duman 2008). A similar phenotype was observed following disruption of the astrocytic gap junctional network or astrocytic glutamate reuptake in the PFC (John et al. 2012, Sun et al. 2012). Using the chronic social defeat stress paradigm, Cao et al. (2013) found impaired astrocytic ATP release in DLP. In this study, the IP3R2-knockout and the dnSNARE mice with respectively impaired astrocytic Ca2+ signaling or vesicular gliotransmission showed a higher incidence of DLP behaviors than did control mice. Central or peripheral ATP administration reduced the DLP behaviors in the socially defeated mice by acting on P2X receptors in PFC neurons (Cao et al. 2013). In another study, while examining the role of insulin receptors in astrocytes, researchers found that lack of insulin receptors in astrocytes causes increased anxiety and DLP behaviors in mice. It appears that lack of insulin receptors in astrocytes leads to decreased exocytosis of ATP from astrocytes, resulting in decreased purinergic signaling on dopaminergic neurons (Cai et al. 2018). More recent work in mice has found that the antidepressant fluoxetine increased extracellular ATP from hippocampal astrocytes by a vesicular nucleotide transporter-dependent mechanism (Kinoshita et al. 2018). Taken together, these studies indicate that dysregulated astrocytic release of ATP is a potential contributing factor in some forms of DLP.
Interestingly, changes in the passive membrane properties of astrocytes may also underlie some forms of DLP. Proteomic analysis shows that an astroglial potassium channel (Kir4.1) is upregulated in the lateral habenula (LHb) in rat models of depression (Cui et al. 2018). The LHb is implicated in the coding of negative emotions (Matsumoto & Hikosaka 2007), and its dysregulation is implicated on the pathophysiology of major depression (Li et al. 2013). Electrophysiological recordings show that upregulation of Kir4.1 channels in LHb astrocytes causes the hyperpolarization of neighboring neurons and the shift of tonic-firing neurons into bursting modes. Such change of firing properties of LHb neurons is postulated to underlie the DLP (Cui et al. 2018).
Transcranial direct current stimulation (tDCS) is a noninvasive brain-stimulation procedure with potential therapeutic application in neuropsychiatric and neurological conditions, including mood disorders, in which the cellular mechanisms are unknown. Addressing this question, Hirase’s group (Monai et al. 2016) demonstrated that tDCS activated the calcium signaling in cortical astrocytes and that the tDCS-induced amelioration of chronic restraint stress-induced depression in mice required this astrocytic signal. These results revealed astrocytes as potential cellular targets in the treatment of depression.
Astrocytic Dysregulation in Drug Addiction
In rodent self-administration models of drug abuse, a decrease in expression and function of the glutamate transporter GLT-1 within the nucleus accumbens (NAc) has been described (Das et al. 2015; Gipson et al. 2013; Knackstedt et al. 2010; Reissner et al. 2014, 2015; Shen et al. 2014). The reduced glutamate reuptake by astrocytes may contribute to relapse susceptibility by excessive activation of the PFC-NAc pathway (Scofield & Kalivas 2014). Treatment with ceftriaxone or propentofylline, previously shown to increase GLT-1 expression, can attenuate cue-induced cocaine reinstatement (Fischer et al. 2013, Reissner et al. 2014). In the ventral tegmental area (VTA), the astrocytic GLT-1 transporter has been implicated in regulating avoidance behaviors (Gomez et al. 2019). Optogenetic manipulation of astrocyte GLT-1 activity in the VTA is sufficient to inhibit the behavioral expression of cocaine-induced conditioned place preference (CPP). Electrophysiological experiments show that glutamate transport activity by astrocytes in the VTA regulates the excitability of GABAergic neurons, which in turn regulate the activity of dopaminergic neurons to elicit avoidance behaviors (Gomez et al. 2019).
NAc neurons have long been known to undergo extensive alterations in their characteristics upon exposure to drugs of abuse (Nestler & Lüscher 2019). NAc neurons consist almost exclusively of medium spiny neurons (MSNs), commonly divided into two major subsets based on their expression of D1 dopamine receptors (D1R-MSNs) or D2 dopamine receptors (D2R-MSNs). Surprisingly, NAc astrocytes not only express D1Rs but also influence the NAc neuronal network via gliotransmitter release (Corkrum et al. 2020). Astrocytes in the NAc respond with Ca2+ elevations to synaptically released dopamine, which stimulates ATP/adenosine release; ATP/adenosine then depresses glutamatergic excitatory synaptic transmission in the NAc (Corkrum et al. 2020). Behaviorally, astrocytic D1Rs contribute to the locomotor hyperactivity induced by systemic injection of amphetamine. Thus, dopaminergic transmission in the NAc also engages the communication between astrocytes and neurons and may profoundly affect reward-related behaviors. A deeper understanding of the role of astrocytes in various reward circuits could provide new strategies for the treatment of addictive disorders.
Drug addiction has also been thought of as a disorder of aberrant learning and memory (Hyman et al. 2006). Thus, with progressive drug use, environmental cues and contexts can become associated with the rewarding effects of the drug to induce craving and relapse (Hyman et al. 2006). In rodents, the CPP paradigm is commonly used to study the associative learning of a drug with environmental cues. Using this paradigm, researchers have shown that disrupting the export of lactate from astrocytes to neurons in the basolateral amygdala (BLA) impairs the acquisition of cocaine-induced CPP in rats (Boury-Jamot et al. 2016, Zhang et al. 2016). The drug memory could be rescued by l-lactate coadministration (Boury-Jamot et al. 2016). Similar to the hippocampal memory discussed previously, the cocaine-induced memory could be reduced by antisense knockdown of the astrocytic MCT1 transporter or the neuronal MCT2 transporter in the BLA (Zhang et al. 2016).
Astrocytes as Integral Components of Fear Circuits
Numerous animal and human studies indicate that a highly conserved network of brain structures regulates the expression of fear and anxiety in mammals. The amygdala, in particular, plays a critical role in various aspects of emotional regulation, including the expression of innate fear responses or defensive behaviors, the acquisition of new fear responses as a result of experience, and the facilitation of memory by emotions (Janak & Tye 2015). The amygdala is often hyper-responsive in humans afflicted with anxiety disorders, and it is commonly believed that many anxiety disorders result, at least in part, from dysregulation of amygdala processes normally mediating fear/defensive behaviors (Ressler 2010).
The neuronal circuits underlying the conditioning and expression of acute fear responses have been extensively studied using classical auditory fear-conditioning as a model paradigm, in which animals learn to associate an initially neutral conditioned stimulus (CS; e.g., a tone) with an aversive unconditioned stimulus (US; e.g., a foot shock). These studies indicate that the acquisition of conditioned fear responses depends on activity-dependent functional changes in the BLA, which is composed of the lateral amygdala (LA) and basal amygdala (Duvarci & Pare 2014). Lesion and functional inactivation of LA are sufficient to block the formation of conditioned fear memory (Rodrigues et al. 2004). The fact that astrocytes are critical elements for memory formation in the hippocampus, as discussed above, prompted similar interrogations in the amygdala. Indeed, acquisition of fear memories could be prevented by in vivo infusion of a connexin 43 hemichannel blocker in the BLA (Stehberg et al. 2012). Restricted expression of connexin 43 to astrocytic membranes (Rash et al. 2001) and its purported role as a releasing pathway of gliotransmitters (Orellana & Stehberg 2014) argue for a direct role of astrocytes in fear learning. We have recently shown in brain slices that astrocytes fine-tune the synaptic circuits in the central medial amygdala (CeM), the output nucleus of the amygdala (Martin-Fernandez et al. 2017). We found that CeM astrocytes selectively regulate local neurons to dampen the excitability of output neurons (Martin-Fernandez et al. 2017). Moreover, chemogenetic activation of astrocytes in the CeM reduced fear expression in a fear-conditioning paradigm in mice (Martin-Fernandez et al. 2017) (Figure 3).
Figure 3.
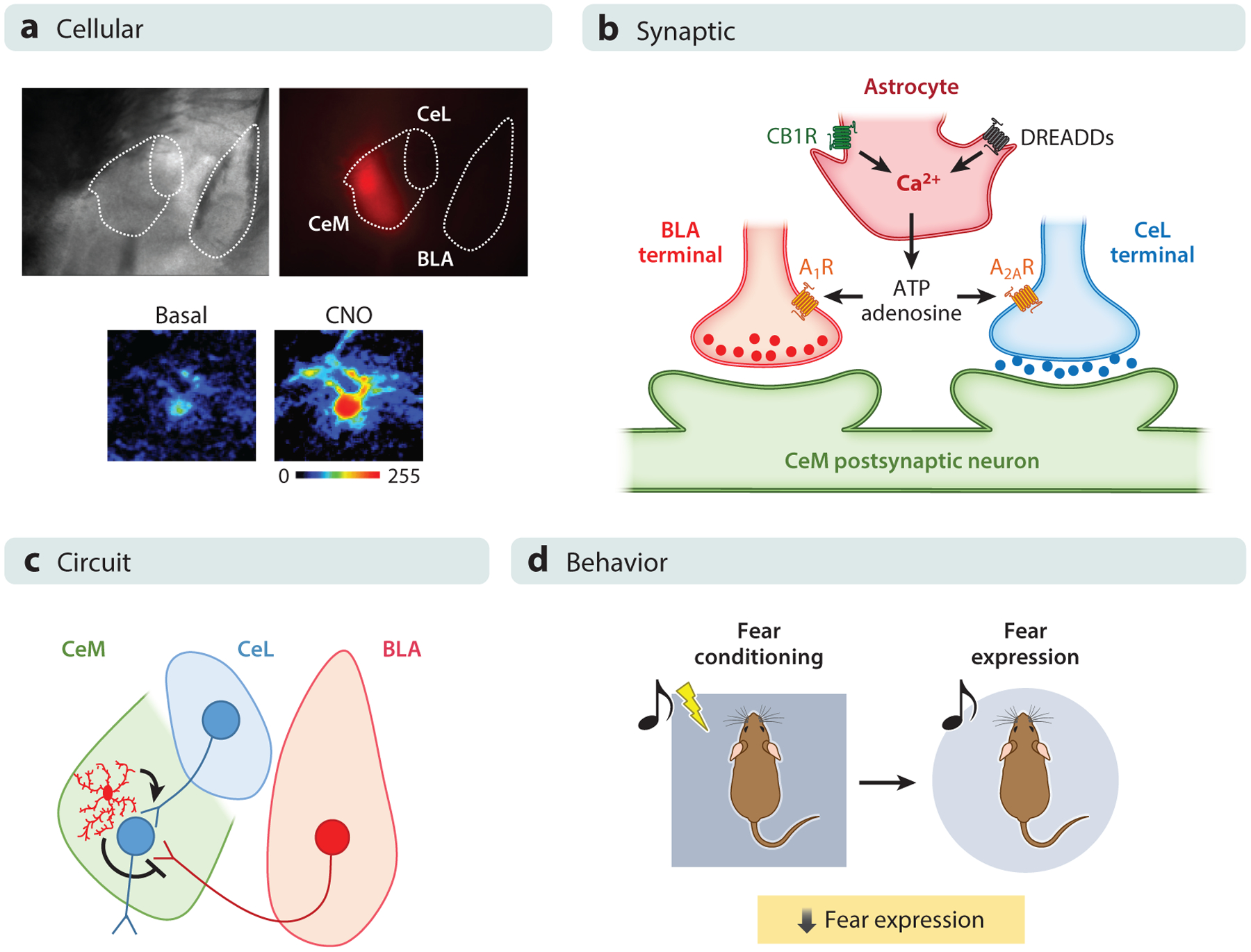
Astrocyte activity in the amygdala influences fear expression, which has been investigated at several levels in the central medial amygdala (CeM). (a) At the cellular level, expression of designer receptors exclusively activated by designer drugs (DREADDs) using clozapine-N-oxide (CNO) elicits calcium responses. (Top) Brightfield and fluorescence images show the localization of DREADDs in the CeM as reported by mCherry expression. (Bottom) Pseudocolor images of fluorescence intensities of a calcium indicator before and after local application of CNO are shown. (b) At the synaptic level, electrophysiological experiments show that the activation of astrocytes in the CeM, either by an endogenous stimulus or by a selective exogenous stimulus (DREADDs activation by CNO), is able to activate astrocytes and induce a regulation in central lateral amygdala (CeL)-CeM and basolateral amygdala (BLA)-CeM synapses. The astrocytic activity increases the synaptic probability of release in inhibitory CeL-CeM synapses through activation of A2A receptors and decreases the synaptic probability of release through activation of A1 receptors in excitatory BLA-CeM synapses. (c) At the circuit level, the combined and synergistic synaptic effects elicited upon astrocytic activation decrease the firing rate of CeM neurons. (d) At the behavioral level, the decrease of excitability of output CeM neurons results in decreases of behavioral fear expression as assessed using the cued fear-conditioning paradigm, in which an auditory stimulus is paired with an unconditioned stimulus. The fear expression is then tested by presenting only the conditioned stimulus in a novel surrounding context. Figure adapted with permission from Martin-Fernandez et al. (2017).
ASTROCYTES AND BODY HOMEOSTASIS
Astrocytes Regulate Circadian Rhythms
Circadian rhythms are controlled by a molecular clock in the suprachiasmatic nucleus (SCN) of the hypothalamus that is entrained daily to the environmental day-night cycles by photic input, synchronizing metabolism, cognition, and behavior (Hastings et al. 2018). Although the molecular machinery for circadian oscillations is found in virtually every cell of the body, the SCN is considered the master clock, as its lesioning abrogates circadian rhythmicity across all body tissues (Welsh et al. 2010). Astrocytes express the canonical clock genes Bmal1, Clock, Per, and Cry, and in culture conditions they can sustain circadian oscillations of clock genes for several days (Prolo et al. 2005). Simultaneous imaging of Ca2+ activity in neurons and astrocytes in the SCN has documented circadian oscillations of intracellular Ca2+ in neurons and astrocytes that are antiphasic to each other (Brancaccio et al. 2017). Moreover, knockdown or knockout of the essential canonical clock gene Bmal1 in SCN astrocytes lengthens the period of circadian locomotor activity in mice (Brancaccio et al. 2017, Tso et al. 2017). According to one working model, astrocytes influence the activity of SCN neurons by regulating the ambient levels of the neuromodulator glutamate around the synapses of the SCN neurons in a circadian fashion (Brancaccio et al. 2017). In another model, however, astrocytes influence the SCN clock by regulating the GABA levels via transporter uptake (Barca-Mayo et al. 2020). Thus, the precise mechanism(s) for astrocytic modulation of the SCN circadian clock remains unsettled.
Astrocytes and Sleep—Wake Cycles
The release of astrocytes of the somnogen ATP/adenosine was first suggested using the dnSNARE mouse model in which vesicular release of gliotransmitters was impaired (Halassa et al. 2009). The dnSNARE mice exhibited normal baseline sleep, but under sleep deprivation, they failed to exhibit an increase in sleep time (Halassa et al. 2009). This phenotype was interpreted as being produced by a reduced astrocytic supply of adenosine on neuronal A1Rs (Halassa et al. 2009). While the specificity of the dnSNARE mouse to targeting only astrocytes has been questioned (Fujita et al. 2014), additional independent lines of evidence provide support to the adenosine-astrocyte-sleep axis hypothesis. Bjorness et al. (2016) targeted the expression of the glial or neuronal adenosine kinase (AdK), the primary metabolizing enzyme for adenosine, and assessed the sleep drive of these mice. Mice deficient for astrocytic AdK or the neuronal adenosine A1 receptor exhibited a significantly decreased sleep drive. Neuronal knockout of AdK did not influence the homeostatic sleep drive.
Besides regulating adenosine levels in the brain, astrocytes may have a more localized impact on sleep-regulating brain regions. Thus, the global genetic ablation of connexin 43 in astrocytes causes enhanced sleepiness and fragmented wakefulness in mice (Clasadonte et al. 2017). This phenotype is also observed by the ablation of expression of connexin only in the astrocytes located in the lateral hypothalamic area (LHA), a sleep-regulating hypothalamic area (Arrigoni et al. 2019). This astrocyte-specific genetic manipulation silenced the wake-promoting orexin neurons in the LHA by impairing glucose and lactate trafficking through astrocytic networks (Clasadonte et al. 2017). Moreover, optogenetic stimulation of astrocytes in the posterior hypothalamus increases NREM and REM sleep at night (Pelluru et al. 2016).
Astrocytes and Feeding Behaviors
The hypothalamic arcuate nucleus (ARC) plays a key role by responding to metabolic signals, including leptin, insulin, and ghrelin, and signaling to other brain areas to drive feeding and energy expenditure (García-Cáceres et al. 2019). The primary ARC circuit includes neuropeptide Y/Agouti-related protein-producing (AgRP) and proopiomelanocortin-expressing (POMC) neurons, which provide orexigenic (appetite-inducing) and anorexigenic (appetite-suppressing) signals, respectively (Williams & Elmquist 2012). Astrocytes in the ARC express receptors for numerous hormones and metabolic factors, including the appetite-promoting hormone ghrelin, insulin, and leptin (García-Cáceres et al. 2019). Moreover, astrocytes in the arcuate show remarkable structural changes in response to dietary changes. A high-fat diet triggers hypothalamic inflammation with pronounced astrogliosis (García-Cáceres et al. 2019). Divergent results are described upon the chemogenetic activation of ARC astrocytes. In one study, the authors found that activation of ARC astrocytes reduced both basal- and ghrelin-evoked food intake by inactivation of AgRP neurons (Yang et al. 2015). On the other hand, another study found that activation of ARC astrocytes increased food intake with the enhancement of activity of AgRP neurons (Chen et al. 2016).
ASTROCYTES AND SENSORY-MOTOR RESPONSES
Astrocytes have been shown to respond to neuromodulators in the brain such as acetylcholine (Chen et al. 2012, Navarrete et al. 2012, Perea & Araque 2005, Takata et al. 2011) NE (Oe et al. 2020, Paukert et al. 2014) and serotonin (Carson et al. 1996), which are known to contribute to synaptic plasticity, neuronal network activity, and animals’ behavioral states. Although the involvement of astrocytes in the actions of neuromodulators has been only initially investigated, astrocytes’ responses to neuromodulators have been shown to impact sensory and motor processes. For example, the Mriganka Sur laboratory (Chen et al. 2012) has shown that stimulation of cholinergic inputs from the nucleus basils evoked robust calcium response in visual cortical astrocytes via muscarinic receptors and enhanced the visual responses of the neurons in the mouse primary visual cortex. This potentiation, however, was absent in the IP3R2-knockout mouse, which lacked relevant astrocytic calcium activation, indicating that astrocytes, via their responsiveness to the neuromodulator acetylcholine, are critical for the cholinergic-induced modulation of visual processing.
Astrocytes like neurons in the ferret primary visual cortex respond to visual stimuli with restricted spatial receptive fields and sharp tuning to visual stimulus features, including orientation and spatial frequency (Schummers et al. 2008). Optogenetic stimulation of astrocytes in the mouse primary visual cortex differentially affects the neuronal excitability (Perea et al. 2014). In response to visual stimuli, parvalbumin-positive neurons show increased baseline visual responses and reduced orientation selectivity to visual stimuli. Somatostatin-positive neurons, on the other hand, showed increases, decreases, or baseline visual responses and direction selectivity (Perea et al. 2014). Thus, astrocytes influence sensory processing in the primary visual cortex via differential modulation of neuronal subtypes.
Motor behavior is also influenced by astrocytes and other types of glial cells in various neuronal circuits. For example, conditional deletion of IP3R2 in astrocytes impaired motor-skill learning of a forelimb reaching task in mice (Padmashri et al. 2015). Ablation of AMPA receptor signaling in cerebellar Bergman glia impairs fine motor coordination in mice (Saab et al. 2012). Furthermore, astrocytes in the cerebellum may regulate cerebellar network function and thus motor coordination by tonically inhibiting neuronal excitability via the release of GABA as a gliotransmitter. Thus, reduction of tonic release of GABA from astrocytes by genetic ablation of the GABA-permeable bestrophin 1 (Best1) channel enhanced the excitability of cerebellar granule cells and performance in motor tests (Woo et al. 2018). Astrocytes are also implicated in the function of the dorsal striatum, another brain nucleus that is critical for motor function. Chemogenetic activation of astrocytes in the dorsal striatum results in hyperactivity and disrupted attention in mice (Nagai et al. 2019). At the cellular level, astrocyte activation in the dorsal striatum enhances the activity of MSNs by releasing thrombospondin-1 (Nagai et al. 2019).
Astrocytes have also been implicated in involuntary motor behaviors. A pioneer study demonstrated that astrocytes of the brain stem chemoreceptor areas are chemosensitive, responding to physiological pH changes with Ca2+ elevations that stimulate the release of ATP. The consequent regulation of the excitability of chemoreceptor neurons and breathing (Gourine et al. 2010) demonstrates the crucial role of astrocytes in mediating a physiological reflex of respiratory function. In the preBötzinger complex (preBötC), an essential nucleus for the generation of the respiratory rhythm (Smith et al. 1991), astrocytes are periodically activated preceding inspiratory neuronal activity, and stimulation of astrocytes induces inspiratory neuronal firings (Okada et al. 2012). Moreover, disruption of gliotransmitter vesicular release of astrocytes using viral expression of dnSNARE specifically in astrocytes in the preBötC induces a myriad of impairments in respiratory performance (Sheikhbahaei et al. 2018). The generation of rhythmic activity of neural networks, which is essential for generating stereotyped patterns for proper motor outputs, is also influenced by astrocytes. In the rat trigeminal sensorimotor circuit for mastication, astrocytes respond to sensory stimuli that induce neuronal rhythmic activity, and blocking astrocytic responses prevents neurons from generating a rhythmic bursting pattern (Morquette et al. 2015).
ASTROCYTES AND DECISION-MAKING
In simple terms, “decision making refers to the ability of humans and other animals to choose between competing courses of action based on the relative value of their consequences” (Balleine 2007, p. 8159). An example of an experimental paradigm designed to test decision-making is the rodent gambling task. In this test, the decision-making capacities in rats are evaluated via a conflict between immediate and long-term gratification of the food reward. Interestingly, rats with chronic visceral pain and hypersensitivity (VH) display poorer decision-making capacities in this test (Wang et al. 2017). This impairment of decision-making capacities in VH rats was correlated with LTP and spike-field coherence decreases in the BLA-ACC network (Cao et al. 2016). Wang et al. (2017) went on to demonstrate that the dysfunction of lactate transfer from astrocytes to neurons contributes to the impairment of decision-making in these animals. Indeed, there was an activity-dependent decrease of lactate release in the ACC of VH rats. Moreover, exogenous lactate infusion in the ACC rescued the deficits in decision-making. Finally, optogenetic astrocytic activation also improved decision-making performance. Thus, lactate shuttle from astrocytes to neurons optimizes the performance of the BLA-ACC network, leading to better behavioral performance in decision-making in this experimental paradigm. The medial prefrontal cortex (mPFC) is a brain area that is also critically involved in decision-making (Euston et al. 2012). Mederos et al. (2020) recently showed that in the mPFC, parvalbumin interneurons engage astrocytic activation via GABAB receptor signaling. This novel signaling pathway in the mPFC impacts local neuronal firing, gamma oscillations, working memory, and decision-making.
Gliotransmission also plays an important role in other experimental models of decision-making. Because the astrocytes signal to each other in a timescale slower than for neurons (Ca2+ signaling versus electrical signaling), they can integrate neuronal network activity over seconds to minutes, which is ideal for some forms of decision-making. In the zebrafish, repeated bouts of swimming episodes with no perceptual movement (perceived as futile efforts) trigger a passive behavioral state. Elegant experiments using large-scale optical imaging in zebrafish in vivo revealed that astrocytes trigger this passive behavioral state (giving up swimming) (Mu et al. 2019). The astrocytic network in the brain stem of zebrafish shows cumulative Ca2+ responses when swimming episodes do not generate perceptual movement. These astrocytes receive inputs from noradrenergic neurons that detect swim failures, and when the number of swim failures reaches a threshold, the astrocytic network engages the GABAergic neuronal network to suppress swimming (Mu et al. 2019). Thus, astrocytes function as signal integrators to perform computations critical to drive the behavioral state of giving up. This notion of astrocytes in tripartite synapses integrating and computing neural signals on slower temporal scales to influence neuronal networks may be more common than originally envisaged. In the hippocampus, some types of interneurons can generate long-lasting trains of action potentials (barrage firing) following repeated depolarizing stimuli (Deemyad et al. 2018). As in the zebrafish brain stem, the astrocytic network integrates the neural activity, and when the Ca2+ activity reaches a threshold, it triggers the barrage firing of populations of inhibitory interneurons (Deemyad et al. 2018).
CONCLUDING REMARKS
Optical, electrophysiological, genetic, and biochemical studies of model organisms have provided a compelling description of the modulatory control of astrocytes over many neuronal networks in the brain. Now that we better understand the molecular components of astrocyte-neuron interactions, the challenge arises of investigating how they integrate at physiological levels in complex animals. We have emphasized multiple mechanisms that astrocytes impact, including the inner workings of neuronal circuits that underlie behaviors. The dialog between astrocytes and neurons via gliotransmission, lactate signaling, and regulation of glutamate homeostasis is a common feature among many of these circuits. As we discussed above, each astrocyte is in a position to monitor and influence a large constellation of neurons. Thus, by integrating neuronal signals in time and space, astrocytes provide an extradimensional influence over neuronal networks and behavior. This intimate dialog between neurons and astrocytes might be an ancient paradigm that underlies brain functions that are essential for survival. This dialog has been and continues to be revealed by the development of novel and powerful analytical tools, and it is almost certainly the case that unsuspected and surprising mechanisms will be uncovered in the future. A better understanding of the astrocytic modulation of neuronal circuits and behavior will have a major impact on our ability to conceptualize novel pharmacological treatments for brain disorders.
FUTURE ISSUES.
A key question in the field is the nature of large-scale temporal and spatial Ca2+ dynamics in astrocytes and their impact on neuronal networks during behavior.
Much of our understanding of astrocyte-neuron interactions in vivo has been derived from the imaging of superficial cortical networks. Similar studies will be vital to link behavior and astrocyte-neuron networks in subcortically driven behaviors.
Astrocytes are likely to form functional networks. Connectomes of astrocytic networks at local and global scales need to be investigated.
The limited available methods to promote inactivation of astrocytes in a local and reversible manner restrict our ability to assess the role of astrocytes in behavior.
It is unclear whether and, if so, how astrocytes release multiple gliotransmitters in specific behaviors.
Neuromodulators like acetylcholine and norepinephrine are known to regulate multiple behavioral states through their action on a wide variety of circuits and nuclei. The involvement of astrocytes in the volume transmission regulatory effects of neuromodulators has only been initially investigated. Since astrocyte activity is highly sensitive to these transmitters, future studies are expected to reveal novel astrocyte-mediated mechanisms underlying the effects of neuromodulators in normal and disease behaviors.
ACKNOWLEDGMENTS
We wish to thank all of the scientists whose studies were reviewed in this paper and apologize to those authors whose work was not cited due to space limitations. We thank Marta Navarrete and Gertrudes Perea for their critical reading of the manuscript and our lab members for fruitful discussions. This work was supported by the National Institute of Neurological Disorders and Stroke (R01NS097312), National Institute on Drug Abuse (R01DA048822), and National Institute of Mental Health (R01MH119355).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adamsky A, Goshen I. 2018. Astrocytes in memory function: pioneering findings and future directions. Neuroscience 370:14–26 [DOI] [PubMed] [Google Scholar]
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, et al. 2018. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174:59–71.e14 [DOI] [PubMed] [Google Scholar]
- Andersen JD, Pouzet B. 2004. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of d-serine. Neuropsychopharmacology 29:1080–90 [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. 2014. Gliotransmitters travel in time and space. Neuron 81:728–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni E, Chee MJS, Fuller PM. 2019. To eat or to sleep: That is a lateral hypothalamic question. Neuropharmacology 154:34–49 [DOI] [PubMed] [Google Scholar]
- Bado P, Madeira C, Vargas-Lopes C, Moulin TC, Wasilewska-Sampaio AP, et al. 2011. Effects of low-dose d-serine on recognition and working memory in mice. Psychopharmacology 218:461–70 [DOI] [PubMed] [Google Scholar]
- Balleine BW. 2007. The neural basis of choice and decision making. J. Neurosci 27:8159–60 [Google Scholar]
- Banasr M, Duman RS. 2008. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol. Psychiatry 64:863–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barca-Mayo O, Boender AJ, Armirotti A, De Pietri Tonelli D. 2020. Deletion of astrocytic BMAL1 results in metabolic imbalance and shorter lifespan in mice. Glia 68:1131–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LF, Weber B. 2018. CrossTalk proposal: an important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J. Physiol 596:347–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT. 2012. Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol. Neurobiol 32:613–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness TE, Dale N, Mettlach G, Sonneborn A, Sahin B, et al. 2016. An adenosine-mediated glial-neuronal circuit for homeostatic sleep. J. Neurosci 36:3709–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boury-Jamot B, Carrard A, Martin JL, Halfon O, Magistretti PJ, Boutrel B. 2016. Disrupting astrocyte–neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol. Psychiatry 21:1070–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. 2017. Astrocytes control circadian time-keeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron 93:1420–35.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, et al. 2010. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J. Neurosci 30:4590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Baltan Tekkök S, Ransom BR. 2004. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem. Int 45:529–36 [DOI] [PubMed] [Google Scholar]
- Cai W, Xue C, Sakaguchi M, Konishi M, Shirazian A, et al. 2018. Insulin regulates astrocyte gliotransmission and modulates behavior. J. Clin. Investig 128:2914–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì C, Tauffenberger A, Magistretti P. 2019. The strategic location of glycogen and lactate: from body energy reserve to brain plasticity. Front. Cell. Neurosci 13:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Wang J, Mu L, Poon DC-H, Li Y. 2016. Impairment of decision making associated with disruption of phase-locking in the anterior cingulate cortex in viscerally hypersensitive rats. Exp. Neurol 286:21–31 [DOI] [PubMed] [Google Scholar]
- Cao X, Li L-P, Wang Q, Wu Q, Hu H-H, et al. 2013. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med 19:773–77 [DOI] [PubMed] [Google Scholar]
- Carson MJ, Thomas EA, Danielson PE, Sutcliffe JG. 1996. The 5HT5A serotonin receptor is expressed predominantly by astrocytes in which it inhibits cAMP accumulation: a mechanism for neuronal suppression of reactive astrocytes. Glia 17:317–26 [DOI] [PubMed] [Google Scholar]
- Chen J, Tan Z, Zeng L, Zhang X, He Y, et al. 2013. Heterosynaptic long-term depression mediated by ATP released from astrocytes. Glia 61:178–91 [DOI] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Kim J, Fu Z, Barak B, et al. 2016. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. eLife 5:e18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, et al. 2012. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. PNAS 109:E2832–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih C-P, Lipton P, Roberts EL. 2001. Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 24:573–78 [DOI] [PubMed] [Google Scholar]
- Clasadonte J, Scemes E, Wang Z, Boison D, Haydon PG. 2017. Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron 95:1365–80.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. 2010. Long-term depression in the CNS. Nat. Rev.Neurosci 11:459–73 [DOI] [PubMed] [Google Scholar]
- Corkrum M, Covelo A, Lines J, Bellocchio L, Pisansky M, et al. 2020. Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. Neuron 105:1036–47.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Yang Y, Ni Z, Dong Y, Cai G, et al. 2018. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554:323–27 [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. 2015. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 97:67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pittà M, Brunel N, Volterra A. 2016. Astrocytes: orchestrating synaptic plasticity? Neuroscience 323:43–61 [DOI] [PubMed] [Google Scholar]
- Deemyad T, Lüthi J, Spruston N. 2018. Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat. Commun 9:4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. 1993. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 623:208–14 [DOI] [PubMed] [Google Scholar]
- Durkee CA, Covelo A, Lines J, Kofuji P, Aguilar J, Araque A. 2019. Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 67:1076–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. 2014. Amygdala microcircuits controlling learned fear. Neuron 82:966–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. 2012. The role of medial prefrontal cortex in memory and decision making. Neuron 76:1057–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. 2018. Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J. Neurosci 38:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KD, Houston ACW, Rebec GV. 2013. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J. Neurosci 33:9319–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Chen MJ, Li B, Smith NA, Peng W, et al. 2014. Neuronal transgene expression in dominant-negative SNARE mice. J. Neurosci 34:16594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, et al. 2016. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. PNAS 113:8526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cáceres C, Balland E, Prevot V, Luquet S, Woods SC, et al. 2019. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci 22:7–14 [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Hertz L. 2008. Inhibition of astrocytic energy metabolism by d-lactate exposure impairs memory. Neurochem. Int 52:1012–18 [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith ACW, Stankeviciute N, et al. 2013. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. PNAS 110:9124–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JA, Perkins JM, Beaudoin GM, Cook NB, Quraishi SA, et al. 2019. Ventral tegmental area astrocytes orchestrate avoidance and approach behavior. Nat. Commun 10:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, et al. 2010. Astrocytes control breathing through pH-dependent release of ATP. Science 329:571–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, et al. 2009. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61:213–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Peng Y, Dong Z. 2015. d-Serine rescues the deficits of hippocampal long-term potentiation and learning and memory induced by sodium fluoroacetate. Pharmacol. Biochem. Behav 133:51–56 [DOI] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, et al. 2012. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148:1039–50 [DOI] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, et al. 2018. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol 150:1081–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Brancaccio M. 2018. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci 19:453–69 [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. 2010. Long-term potentiation depends on release of d-serine from astrocytes. Nature 463:232–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci 29:565–98 [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. 2015. From circuits to behaviour in the amygdala. Nature 517:284–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CJ, Demol F, Bauwens R, Kooijman R, Massie A, et al. 2016. Astrocytic β2 adrenergic receptor gene deletion affects memory in aged mice. PLOS ONE 11:e0164721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CS, Smith KL, Van’T Veer A, Gompf HS, Carlezon WA, et al. 2012. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology 37:2467–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, et al. 2011. d-Serine regulates cerebellar LTD and motor coordination through the δ2 glutamate receptor. Nat. Neurosci 14:603–11 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Hirayama Y, Fujishita K, Shibata K, Shinozaki Y, et al. 2018. Anti-depressant fluoxetine reveals its therapeutic effect via astrocytes. EBioMedicine 32:72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, et al. 2017. Engrams and circuits crucial for systems consolidation of a memory. Science 356:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. 2010. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry 67:81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Araque A. 2020. G-protein-coupled receptors in astrocyte–neuron communication. Neuroscience. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A, Adamsky A, Groysman M, Kreisel T, London M, Goshen I. 2020. Astrocytes contribute to remote memory formation by modulating hippocampal—cortical communication during learning. Nat. Neurosci 23:1229–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Zhou T, Liao L, Yang Z, Wong C, et al. 2013. βCaMKII in lateral habenula mediates core symptoms of depression. Science 341:1016–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines J, Martin ED, Kofuji P, Aguilar J, Araque A. 2020. Astrocytes modulate sensory-evoked neuronal network activity. Nat. Commun 11:3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I. 2018. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci 19:235–49 [DOI] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. 2015. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–34 [DOI] [PubMed] [Google Scholar]
- Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, et al. 2017. Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci 20:1540–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason S. 2017. Lactate shuttles in neuroenergetics—homeostasis, allostasis and beyond. Front. Neurosci 11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. 2007. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447:1111–15 [DOI] [PubMed] [Google Scholar]
- Mederos S, Hernández-Vivanco A, Ramírez-Franco J, Martín-Fernández M, Navarrete M, et al. 2019. Melanopsin for precise optogenetic activation of astrocyte-neuron networks. Glia 67:915–34 [DOI] [PubMed] [Google Scholar]
- Mederos S, Sanchez-Puelles C, Esparza J, Valero M, Ponomarenko A, Perea G. 2020. GABAergic signaling to astrocytes in prefrontal cortex sustains goal-directed behaviors. Nat. Neurosci In press [DOI] [PubMed] [Google Scholar]
- Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, et al. 2016. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat. Commun 7:11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquette P, Verdier D, Kadala A, Féthière J, Philippe AG, et al. 2015. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat. Neurosci 18:844–54 [DOI] [PubMed] [Google Scholar]
- Mu Y, Bennett DV, Rubinov M, Narayan S, Yang C-T, et al. 2019. Glia accumulate evidence that actions are futile and suppress unsuccessful behavior. Cell 178:27–43.e19 [DOI] [PubMed] [Google Scholar]
- Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, et al. 2019. Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell 177:1280–92.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Cuartero MI, Palenzuela R, Draffin JE, Konomi A, et al. 2019. Astrocytic p38α MAPK drives NMDA receptor-dependent long-term depression and modulates long-term memory. Nat. Commun 10:2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, de Sevilla DF, Gómez-Gonzalo M, Núñez A, et al. 2012. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLOS Biol. 10:e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Lüscher C. 2019. The molecular basis of drug addiction: linking epigenetic to synaptic and circuit mechanisms. Neuron 102:48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA. 2017. A brief history of long-term potentiation. Neuron 93:281–90 [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. 2012. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res 37:2496–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oe Y, Wang X, Patriarchi T, Konno A, Ozawa K, et al. 2020. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat. Commun 11:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Sasaki T, Oku Y, Takahashi N, Seki M, et al. 2012. Preinspiratory calcium rise in putative pre-Bötzinger complex astrocytes. J. Physiol 590:4933–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JF, Sardinha VM, Guerra-Gomes S, Araque A, Sousa N. 2015. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 38:535–49 [DOI] [PubMed] [Google Scholar]
- Orellana JA, Stehberg J. 2014. Hemichannels: new roles in astroglial function. Front. Physiol 5:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmashri R, Suresh A, Boska MD, Dunaevsky A. 2015. Motor-skill learning is dependent on astrocytic activity. Neural Plast. 2015:938023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. 2011. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146:785–98 [DOI] [PubMed] [Google Scholar]
- Papouin T, Henneberger C, Rusakov DA, Oliet SHR. 2017. Astroglial versus neuronal d-serine: fact checking. Trends Neurosci. 40:517–20 [DOI] [PubMed] [Google Scholar]
- Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. 2014. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82:1263–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelluru D, Konadhode RR, Bhat NR, Shiromani PJ. 2016. Optogenetic stimulation of astrocytes in the posterior hypothalamus increases sleep at night in C57BL/6J mice. Eur. J. Neurosci 43:1298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. 2005. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci 25:2192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Yang A, Boyden ES, Sur M. 2014. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat. Commun 5:3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. 2014. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J. Neurosci 34:12738–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J, Boyt KM, McCarthy KD. 2014. Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front. Behav. Neurosci 8:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Duarte A, Roberts AJ, Ouyang K, Sejnowski TJ. 2019. Impairments in remote memory caused by the lack of Type 2 IP3 receptors. Glia 67:1976–89 [DOI] [PubMed] [Google Scholar]
- Prolo LM, Takahashi JS, Herzog ED. 2005. Circadian rhythm generation and entrainment in astrocytes. J. Neurosci 25:404–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE, Nagy JI. 2001. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J. Neurosci 21:1983–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Brown RM, Spencer S, Tran PK, Thomas CA, Kalivas PW. 2014. Chronic administration of the methylxanthine propentofylline impairs reinstatement to cocaine by a GLT-1-dependent mechanism. Neuropsychopharmacology 39:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. 2015. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict. Biol 20:316–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ. 2010. Amygdala activity, fear, and anxiety: modulation by stress. Biol. Psychiatry 67:1117–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, et al. 2018. Astroglial CB1 receptors determine synaptic d-serine availability to enable recognition memory. Neuron 98:935–44.e5 [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. 2004. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron 44:75–91 [DOI] [PubMed] [Google Scholar]
- Saab AS, Neumeyer A, Jahn HM, Cupido A, Šimek AAM, et al. 2012. Bergmann glial AMPA receptors are required for fine motor coordination. Science 337:749–53 [DOI] [PubMed] [Google Scholar]
- Savtchouk I, Volterra A. 2018. Gliotransmission: beyond black-and-white. J. Neurosci 38:14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. 2008. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320:1638–43 [DOI] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW. 2014. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist 20:610–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, et al. 2018. Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat. Commun 9:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-W, Scofield MD, Boger H, Hensley M, Kalivas PW. 2014. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J. Neurosci 34:5649–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, et al. 2013. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol 141:633–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. 1991. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254:726–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, et al. 2015. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat. Neurosci 18:708–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehberg J, Moraga-Amaro R, Salazar C, Becerra A, Echeverría C, et al. 2012. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 26:3649–57 [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Gao V, Alberini CM. 2016. The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front. Integr. Neurosci 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart JL, Ferrari KD, Barrett MJP, Glück C, Stobart MJ, et al. 2018. Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 98:726–35.e4 [DOI] [PubMed] [Google Scholar]
- Sun J-D, Liu Y, Yuan Y-H, Li J, Chen N-H. 2012. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 37:1305–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, et al. 2011. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144:810–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Lane S, Korsak A, Paton JFR, Gourine AV, et al. 2014. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun 5:3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, et al. 2011. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci 31:18155–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, Herzog ED. 2017. Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Curr. Biol 27:1055–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tu J, Cao B, Mu L, Yang X, et al. 2017. Astrocytic l-lactate signaling facilitates amygdala-anterior cingulate cortex synchrony and decision making in rats. Cell Rep. 21:2407–18 [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. 2010. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol 72:551–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. 2012. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat. Neurosci 15:1350–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Balu DT, Coyle JT. 2016. The rise and fall of the d-serine-mediated gliotransmission hypothesis. Trends Neurosci. 39:712–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Min JO, Kang D-S, Kim YS, Jung GH, et al. 2018. Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. PNAS 115:5004–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qi Y, Yang Y. 2015. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep. 11:798–807 [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, et al. 2003. Contribution of astrocytes to hippocampal long-term potentiation through release of d-serine. PNAS 100:15194–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xue Y, Meng S, Luo Y, Liang J, et al. 2016. Inhibition of lactate transport erases drug memory and prevents drug relapse. Biol. Psychiatry 79:928–39 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gong N, Wang W, Xu L, Xu T-L. 2008. Bell-shaped d-serine actions on hippocampal long-term depression and spatial memory retrieval. Cereb. Cortex 18:2391–401 [DOI] [PubMed] [Google Scholar]


