Abstract
The pubertal process is initiated as a result of complex neuroendocrine interactions within the preoptic and hypothalamic regions of the brain. These interactions ultimately result in a timely increase in the secretion of gonadotropin-releasing hormone (GnRH). Researchers for years have believed that this increase is due to a diminished inhibitory tone which has applied a prepubertal brake on GnRH secretion, as well as to the gradual development of excitatory inputs driving the increased release of the peptide. Over the years, insulin-like growth factor −1 (IGF-1) has emerged as a prime candidate for playing an important role in the onset of puberty. This review will first present initial research demonstrating that IGF-1 increases in circulation as puberty approaches, is able to induce the release of prepubertal GnRH and can advance the timing of puberty. More recent findings depict an early action of IGF-1 to activate a pathway that releases the inhibitory brake on prepubertal GnRH secretion provided by dynorphin (DYN), as well as demonstrating that IGF-1 can also act later in the process to regulate the synthesis and release of kisspeptin (Kp), a potent stimulator of GnRH at puberty.
Keywords: Insulin-like growth factor-1, puberty, Gonadotropin-releasing hormone, Neurokinin B
Introduction
The onset of puberty results from a series of neuronal and glial mediated events within the preoptic area (POA) and hypothalamus that promote the increased secretion of gonadotropin-releasing hormone (GnRH), the peptide responsible for stimulation of pituitary gonadotropin output driving the pubertal process to sexual maturity. This increased secretion of GnRH has been attributed to a gradual removal of prepubertal inhibitory influences, as well as to enhanced responsiveness to excitatory influences. With regard to inhibitory influences, gamma aminobutyric acid, as well as the opioid peptides, β-endorphin (β-END) and dynorphin (DYN) [1–4], are synthesized within the medial basal hypothalamus (MBH), which includes the arcuate nucleus (ARC). Each of these neuromodulators are capable of suppressing GnRH secretion and thus, contribute to the brake on pubertal development during childhood. Regarding the excitatory influences, norepinephrine [5], excitatory amino acids [6–8]; leptin [9,10], transforming growth factor-α [11], insulin like growth factor-1 (IGF-1) [12–14], the kisspeptins (Kp) [15–18] and neurokinin B (NKB); [3, 19, 20] have emerged as important stimulators of GnRH during pubertal development. Among these stimulatory substances, IGF-1 has emerged as playing a pivotal role in puberty-related events.
IGF-1 is a 70 amino acid polypeptide mitogen that is essential for mammalian growth. Although this peptide is synthesized in many tissues including the brain, it is predominantly produced in the liver [21]. As puberty approaches the amplitude and frequency of growth hormone secretion increases [22], which induces the liver to synthesize and then secrete IGF-1 into the systemic circulation [23, 24] Once in circulation, IGF-1 binds to a GH-dependent, IGF-binding protein-3 (IGFBP-3) and an acid labile subunit [For review see 25]. To cross the blood brain barrier, the peptide is released from the ternary unit via proteases, and can now bind the type 1 IGF receptor (IGF-1R). This tyrosine kinase receptor is located in many organs including the brain. Although located throughout the brain, the highest concentration of these receptors is within the median eminence (ME) of the hypothalamus [26–30].
Serum IGF-1 levels increase markedly during pubertal development in rodents [31,32], and ruminants [33,34], as well as in primates [35, 36], including humans [37, 38]. Thus, this trophic factor has been suspected of linking somatic growth to the activation of the reproductive hypothalamus at puberty [39]; however, determining whether the peptide acts centrally to induce prepubertal GnRH secretion was needed to support this hypothesis. In 1991, research showed that IGF-1 stimulated GnRH release directly from the prepubertal ME in vitro [12] and therefore, demonstrated the first indication that IGF-1 may play a pivotal role in the onset of puberty. Subsequent research efforts over the last two decades have utilized numerous physiological, toxicological and molecular approaches to discern the extent to which this peptide influences the timing of puberty. During this time, studies have shown mechanisms and pathways by which IGF-1 regulates GnRH and helps to drive the pubertal process. This review will first discuss important research using prepubertal rodents and primates demonstrating the hypothalamic site of action of IGF-1 resulting in the stimulation of prepubertal GnRH and thereby, luteinizing hormone (LH) release. We will next present recently obtained evidence revealing an action of IGF-1 to stimulate a peptidergic pathway within the medial basal hypothalamus (MBH) that contributes to the removal of the prepubertal brake on GnRH secretion and thus, further allowing for the initial puberty-related increase in the release of the GnRH peptide. We will also describe the actions and interactions of estradiol (E2) and IGF-1, as well as recently described interactions between IGF-1, Kp and GnRH within the preoptic and rostral hypothalamic areas (POA/RHA) of the brain as puberty progresses through the peripubertal period of development to maturity.
Early Evidence for an IGF-1 Involvement in the Pubertal Process
With the knowledge that the ME is outside the blood brain barrier, synthesizes significant concentrations of IGF-1R [26–30], and contains the GnRH nerve terminals, it was hypothesized that IGF-1 may be a peripheral metabolic signal capable of acting centrally to stimulate GnRH at the time of puberty. This hypothesis was first addressed in vitro with the results revealing a dose-dependent action of IGF-1 to stimulate GnRH secretion from isolated MEs removed from female rats during the late juvenile phase development [12]. Since IGF-1Rs were not observed directly on GnRH nerve terminals in the ME [40], and because glial cells were shown to have close associations with GnRH nerve terminals [41, 42], it appeared that the IGF-1-induced GnRH release was mediated by glial-glial and glial-neuronal communications. Several lines of evidence support that IGF-1Rs are expressed on glial cells in the ME [26,27], and through glial-glial interactions, IGF-1 stimulates the release of glial derived prostaglandin-E2 in vitro [43]. Importantly, once the prostaglandin-E2 (PGE2) is secreted it binds to its receptors on nearby GnRH nerve terminals and causes release of the GnRH peptide [44, 45].
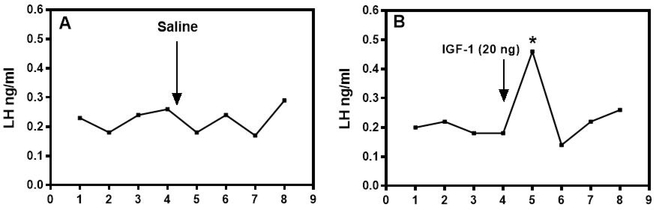
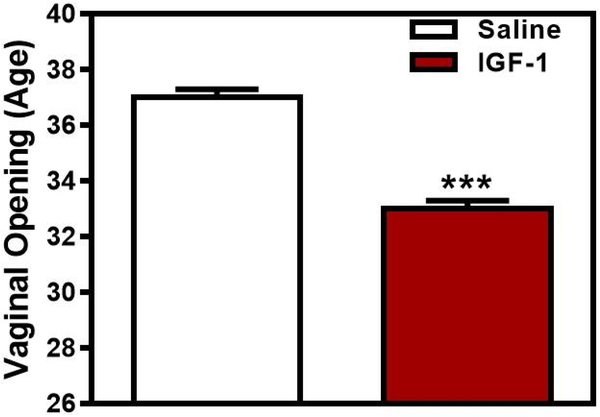
Subsequent in vivo studies further suggested central effects of IGF-1 on GnRH secretion. In this regard, central administration of IGF-1 induced the secretion of LH in immature female rats [13], while an similar effect of IGF-1 was shown in male sheep following systemic administration of the peptide [46]. Examples of representative secretion profiles using late juvenile female rats demonstrate the lack of an LH response to saline, compared to the increase in LH released after administration of IGF-1 (Fig. 1). Importantly, this LH response to IGF-1 was blocked by prior immunoneutralization of GnRH. Furthermore, when IGF-1 was administered centrally to immature female rats twice daily, each late in the afternoon, in order to mimic the peripubertal afternoon increase in GnRH secretion, it advanced the onset of puberty (Fig. 2). Several other studies later confirmed this action of IGF-1 on puberty. In primates, an increase in serum IGF-1 advanced first ovulation in rhesus monkeys [14]. In mice, replacement of IGF-1 advanced puberty in GH-receptor knockout mice expressing very low levels of the peptide [47]. IGF-1 administration to wild type mice showed precocious puberty in females but this did not occur in males, while both male and female transgenic mice lacking the IGF-1R on their GnRH neurons showed delayed puberty [48]. Furthermore, central delivery of IGF-1 antiserum to prepubertal male rats caused delayed puberty [49].
Fig. 1.
The effect of third ventricular administration of IGF-1 on LH release from late juvenile female rats. Representative LH secretory profiles from rats before and after receiving either saline (A) or 20 ng IGF-1 (B). Note that the IGF-1 induced a marked post-injection increase in LH secretion. Samples were taken at ten minute intervals and the arrows denote injections of either saline or IGF-1 after the fourth basal sample. *, p < 0.05. (With permission, Copyright © 1996 by The Endocrine Society, [13] Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL; Endocrinology published by Oxford University Press.)
Fig. 2:
The central administration of IGF-1 advanced the timing of puberty. Open and solid bars represent the mean (±SEM) age in days at vaginal opening for saline and IGF-1-treated animals, respectively. Note that IGF-1 advanced vaginal opening by 4.9 days. N = 6–7/bar. *** p<0.001. (With permission, © 1996 by The Endocrine Society, [13] Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL; Endocrinology published by Oxford University Press.)
In addition to stimulating prepubertal GnRH secretion, other evidence also supports the concept that peripherally derived IGF-1 plays an early role in the pubertal process. Importantly, there are developmental differences in IGF-1 synthesis between central and peripheral sources in the days leading up to puberty in the female rat [13]. In this regard, IGF-1 gene expression did not change in the POA or MBH during late juvenile/peripubertal development (days 30–37). However, an increase in liver IGF-1 gene expression was observed on the day of first proestrus [13], a change that was accompanied by elevated serum levels of IGF-1, along with serum LH and E2 during development. Importantly, the increase in serum IGF-1 was accompanied by an increase in IGF-1 receptor gene expression in the ME. Similar results were shown in both male and female mice indicating that hypothalamic IGF-1 gene and protein expression levels were elevated during the neonatal period, decreased through 20 days of life, and then increased by day 30 and again by day 60 [50]. Those investigators also showed that IGF-1R gene and protein expression increase developmentally in the POA and MBH/ME of male and female mice [40]. Overall, the prepubertal increase in serum IGF-1 followed by elevated hypothalamic-pituitary-ovarian activity demonstrates the general influence of IGF-1 to facilitate GnRH secretion and the pubertal process. It is important to note that more recent research, which is detailed below, has revealed specific mechanisms of IGF-1 actions within the MBH, as well as within the more rostral/anterior brain regions that occur both early and late, respectively, in the pubertal process.
Recent Evidence Revealing Puberty-Related Mechanisms of IGF-1 Actions
Novel information has emerged revealing specific IGF-1 actions and interactions that occur within the MBH, as well as the POA and rostral hypothalamic area (RHA), the principal brain regions responsible for pubertal development. While this review deals mainly with the rodent model for puberty, there are some species differences regarding GnRH localization within these brain regions that warrant mentioning. In all species, there is an abundance of GnRH nerve fibers coursing through the MBH region in route to the ME, where the peptide is released from their nerve terminals directly into the hypophyseal portal blood. In addition, neurons that synthesize GnRH are present in large numbers within the ARC nucleus of several species such as sheep [51], guinea pigs [52, 53], hamsters [54], horses [55], cattle [56], and primates [57–59], including humans [60,61]. Conversely, GnRH neurons are not present in the ARC nucleus of rats [62], and in mice, they are either not present [63] or have only been observed in very small numbers [64]. However, it is important to note that in all species, including rats and mice, most of the GnRH neurons are located rostrally within the POA/RHA region. The perikarya of these neurons express the IGF-1R [40, 47, 50, 65], and some of their nerve processes course caudally through the MBH into the ME. Even though differences are observed in GnRH neuronal location, it is well-accepted that the mechanisms governing release of GnRH from its nerve terminals are similar across species. Importantly, IGF-1 interacts with several other neuropeptides in the MBH/ARC, as well as within the POA/RHA regions. Because each of these areas exhibit different physiological roles during pubertal development they will be discussed separately.
Actions within the MBH
The importance of increased communication between neuronal circuitries and glial elements within the MBH that facilitates the early actions of IGF-1 to induce prepubertal GnRH release has already been discussed. In addition to the dense population of GnRH nerve fibers in this region, there are also numerous other neuropeptides present within nerves and glial elements that are capable of interacting with the GnRH-containing nerve processes to alter release of the peptide. Among these peptides are DYN, NKB, Kp and IGF-1. The sections below will describe recent research depicting some of the specific actions and interactions of these peptides within the MBH on GnRH secretion.
Action of DYN on Prepubertal GnRH Secretion
We have already discussed the early effect of IGF-1 to cross the blood brain barrier and stimulate GnRH release from the ME; however, for years it has been proposed that another important factor in the onset of puberty is the removal of the prepubertal brake on GnRH secretion. DYN is an endogenous opioid peptide that has emerged as a candidate for such a role since it is recognized for its ability to inhibit prepubertal GnRH/LH secretion [3, 4, 66]. Other evidence of this comes from studies showing that the administration of anti-DYN resulted in increased serum LH [67], and that a kappa opioid receptor (KOR-1) antagonist increased the pulsatile secretion of LH and advanced puberty [68,69]. Another study demonstrated that prepubertal alcohol administration stimulated the hypothalamic synthesis and secretion of DYN [70,71]; hence, suggesting that DYN is an important component of the prepubertal inhibition in GnRH/LH release which accompanies alcohol induced delayed pubertal development observed following chronic exposure to the drug in rats [72–74] and monkeys [75,76], as well as humans [77, 78]. Although these studies clearly depicted the inhibitory nature of DYN on prepubertal GnRH secretion, there was a lack of information regarding upstream substance(s) involved in controlling its synthesis and release within the MBH. Even though DYN is produced in several regions of the hypothalamus, neurons in the ARC nucleus are considered the major source of its synthesis. Importantly, a subpopulation of these DYN producing neurons in the ARC also co-express NKB and Kp [79]. While these peptides are found in separate neurons within other brain nuclei, it is unique that they co-localize to these specific neurons within the ARC nucleus, although the extent of their overlap varies between species [3,4,19,80,81]. Interestingly, as opposed to DYN, stimulatory actions on prepubertal GnRH/LH release have been shown in several species for both NKB [20, 82–85] and Kp [16–18]. An important question for several years has been what may first suppress the prepubertal DYN inhibitory tone on GnRH release and thus, allowing for increased secretion of the GnRH peptide at the onset of puberty. Therefore, NKB and Kp were considered prime candidates for such a role based on their presence in the cells of the ARC nucleus and ability to stimulate GnRH release.
Action of NKB on Prepubertal DYN Secretion
The fact that most DYN neurons in the MBH express the neurokinin B receptor (NK3R) [81] suggests that NKB likely contributes to the regulation of prepubertal DYN secretion. Therefore, it was important to determine whether the tachykinin, NKB, which increases in the MBH as puberty approaches [86], would cause the suppression of prepubertal DYN release and thus, facilitate the puberty-related rise in GnRH/LH secretion [20,82]. An initial study [87] assessed MBH explants from late juvenile female rats exposed to senktide, an NK3R agonist, and revealed a dose dependent inhibition of DYN release, which coincided with an increase in GnRH release from the same tissue block. That same study also showed that both actions of senktide were blocked by the prior central administration of an antagonist to NK3R. This antagonist has been shown to delay the onset of puberty in female rats [88] and to block the NKB stimulation of LH release [20,82, 83]. Importantly, the release of β-END, another opioid inhibitor of GnRH secretion [63] produced in the ARC, was unaffected by senktide [87].
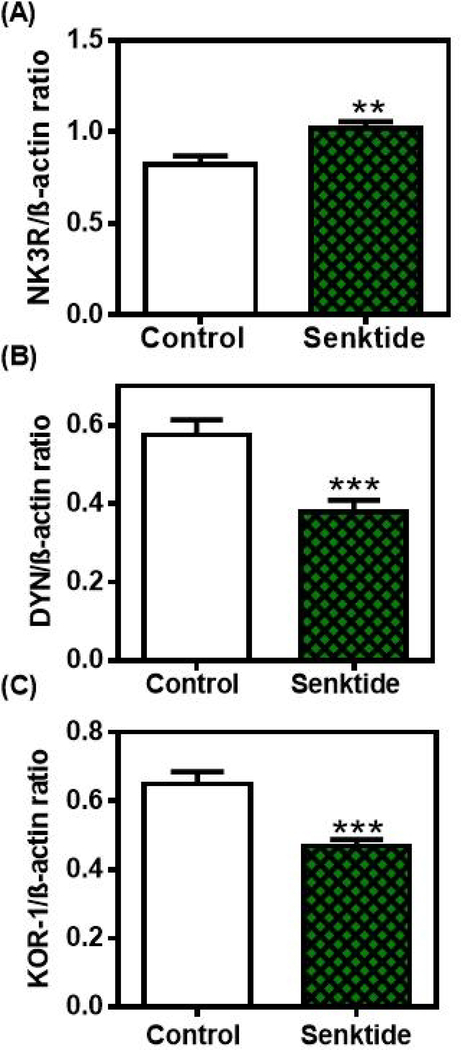
While the above in vitro study suggested that NKB inhibits DYN secretion, animal studies were needed to discern a better understanding of this action within the MBH. In this regard, that same study [87] showed an increase in protein expression of the NK3R (Fig. 3A), along with suppressed protein expressions of DYN (Fig. 3B) and its receptor, KOR-1 (Fig. 3C) following 4 days of senktide administration into the third ventricle of late juvenile female rats. Furthermore, in vitro incubation of MBH tissues following 4 days of senktide administration in vivo resulted in suppressed DYN and increased GnRH secretion. Previous studies have demonstrated that NKB elicits prepubertal GnRH release in several species [20,82,83]. Collectively, these results not only further support this action of NKB, but also suggests that the activation of the NK3R causes a suppression of the DYN brake on prepubertal GnRH secretion and, at least in part, allows for the rise in the GnRH peptide to occur. Since NK3R are localized on GnRH nerve fibers in the MBH [85, 89, 90], it is possible that GnRH was also released by a direct action of senktide on these nerve fibers.
Fig. 3.
The effects of senktide administered centrally for four days on NK3R, DYN and KOR-1 protein expressions in the MBH. Western blots of all three proteins were assessed from animals that received either a daily third ventricular (3V) injection of saline or senktide (gels not shown). Each of the above proteins were normalized to β-actin protein. The bars presented represent the mean ± SEM of the densitometric quantification of all bands assessing A) NK3R, B) DYN and C) KOR-1 proteins. Note that senktide-induced an increase in NK3R protein expression, but it suppressed DYN and KOR-1 protein expressions over the levels of their respective saline controls. Open bars, saline controls; Hatched bars, senktide-treated. N=14/bar. Unpaired t-test was used to compare control vs senktide-treated animal groups; **p<0.01, ***p<0.001. (With permission Copyright © 2019 [87] Dees WL, Hiney JK, Srivastava VK. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.)
The kisspeptins are peptide products of the KiSS-1 gene [For review see 91] and are considered potent stimulators of prepubertal GnRH and critical for driving the pubertal process [15, 17, 18, 92]. Importantly, since Kp is also synthesized by neurons in the ARC nucleus [93,94] its secretion was also assessed from the same MBH explants described above to determine if Kp was involved in the senktide/NKB induced suppression of DYN, along with the increased release of GnRH. In this regard, senktide did not affect Kp release; thus, indicating Kp was not involved in the senktide stimulation of GnRH release in these juvenile female rats [87]. A study using prepubertal female monkeys showed that senktide was able to stimulate Kp release following delivery into the stalk ME [20] however, the release observed was markedly less than that produced in pubertal monkeys; an action attributed to differences in circulating E2 levels. The differences noted between the rodent and primate studies regarding senktide stimulation of Kp could be due to a species difference or to the low levels of E2 in the juvenile female rats. Interestingly, the study using prepubertal female monkeys revealed that NKB and Kp take independent signaling paths to affect the release of GnRH [20]. Also, a study using tissue slices from adult male transgenic mice indicated that NK3R activated GnRH release was Kp-independent [85]. Furthermore, the study detailed above showing that senktide administration caused increased GnRH release without affecting Kp release in juvenile female rats [87], also supports the concept that NK3R activated GnRH release is independent of Kp, at lease at this early stage of development. This is important because it suggests Kp is not associated with the removal of the DYN inhibitory tone on prepubertal GnRH secretion; hence, indicating an earlier role for NKB in the pubertal process than that of Kp.
Action of IGF-1 on Prepubertal NKB Secretion
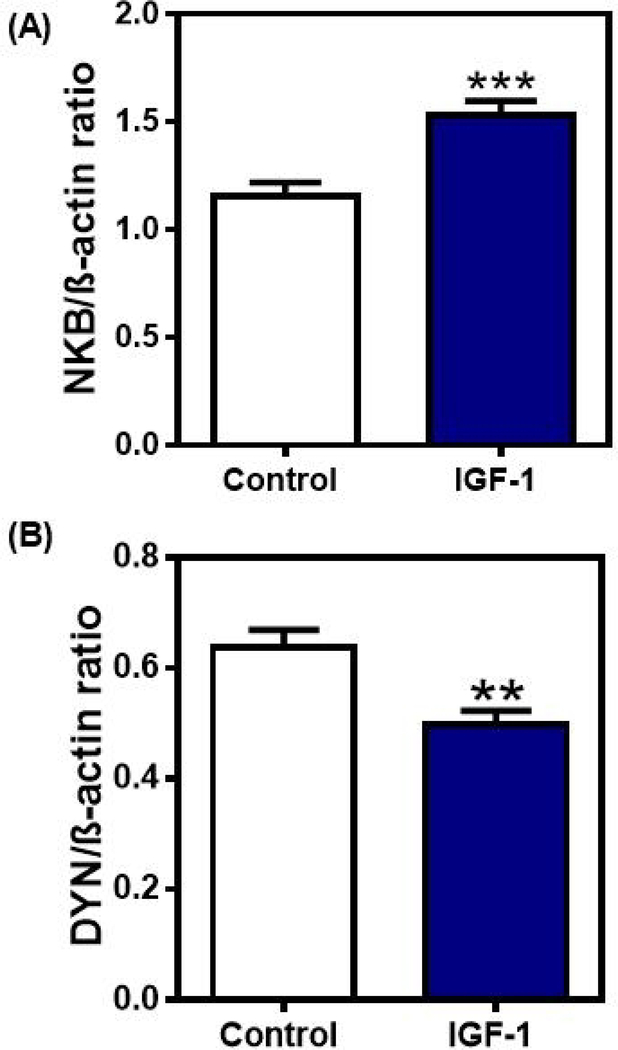
The observation that senktide/NKB can suppress prepubertal DYN secretion raised the question as to what neuronal substance may regulate the puberty-related synthesis and release of NKB within the MBH. We have already discussed a direct early action of IGF-1 within the MBH to stimulate the prepubertal release of GnRH, however, it is also possible that there is another means by which this metabolic signal can facilitate GnRH secretion at the time of puberty. A recent study supports this hypothesis by showing an early action of IGF-1 to regulate NKB [87]. Specifically, central delivery of IGF-1 into the third ventricle of late juvenile female rats for 4 days caused increased NKB and decreased DYN protein expressions within the MBH (Fig. 4). IGF-1 administration, however, did not alter Kp expression in this brain region [87]. This lack of an effect of IGF-1 on Kp protein expression agrees with a previous study showing that KiSS-1 gene expression was also unaltered in the ARC nucleus [95]. Furthermore, after 4 days of IGF-1 administration there was no change observed in the in vitro release of Kp; however, there was an increase noted in the release of NKB, an action that was blocked by the IGF-1R antagonist, JB-1. The exact site of this interaction between IGF-1 and NKB has yet to be determined because it is presently unknown whether NKB neurons express IGF-1Rs or whether IGF-1 is binding to its receptors expressed on an interneuron. While additional anatomical work in this regard is needed, the physiological information presented to date clearly demonstrates that IGF-1 can act as an upstream regulator of prepubertal NKB synthesis and release within the MBH, but not that of Kp. Although not related to IGF-1, another study showed that the KiSS-1 gene is expressed consistently in the ARC of prepubertal pigs and that there was no association between expression levels in this nucleus and the timing of puberty [96]. Those authors suggested that secretion levels of Kp is likely a more reliable indicator of puberty than gene expression.
Fig. 4.
The effects of IGF-1 administered centrally for four days on NKB and DYN protein expressions in the MBH. Western blots of NKB and DYN were assessed from animals that received either a daily third ventricular (3V) injection of saline or IGF-1 (gels not shown). Each of the above proteins were normalized to β-actin protein. The bars presented represent the mean ± SEM of the densitometric quantification of all bands assessing A) NKB and B) DYN proteins. Note that IGF-1 induced an increase in NKB protein expression, but it suppressed DYN protein expression over the levels of their respective saline controls. Closed bars, saline controls, N=28; Open bars, IGF-1-treated, N=29. Unpaired t-test was used to compare control vs IGF-1-treated animal groups. **p<0.01, ***p<0.001. (With permission Copyright © 2019 [87] Dees WL, Hiney JK, Srivastava VK. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.)
Importance of the IGF-1/NKB/DYN Pathway on Prepubertal GnRH Secretion
Increasing amounts of peripherally derived IGF-1 entering the brain as puberty approaches is well known [for review, see 13]. Recently, we suggested that a novel pathway induced by IGF-1 is involved in early puberty-related events within the MBH [87]. Specifically, IGF-1 is capable of inducing NKB secretion and the subsequent NK3R activated suppression of DYN secretion. Thus, activation of this pathway contributes to the removal of the DYN inhibitory tone on prepubertal GnRH secretion; an action that allows for the puberty-related increase in GnRH to begin. Importantly, Kp is not involved in the action of this pathway to suppress DYN, although it may participate with both NKB and DYN within the MBH in regulating pulsatile GnRH secretion later during pubertal development and during adulthood [For review see 97]. Overall, we suggest that the newly described action of the IGF-1/NKB/DYN pathway within the MBH, along with the actions of both NKB and IGF-1 to independently stimulate GnRH release from the nerve terminals in the ME, facilitate the gradual rise in GnRH secretion at the onset of puberty.
Actions within the POA/RHA
As E2 levels begin to increase and the pubertal process transitions from juvenile to peripubertal development, the more rostral/anterior brain regions play an increasingly important role. We have already established that this region is the major site of GnRH producing neurons. Importantly, in addition to Kp synthesis within the MBH, the peptide is also produced by neurons in the RHA, specifically, within the anteroventral periventricular (AVPV) nucleus. KiSS-1 gene expression and that of the Kp receptor, GPR54, increases during pubertal development [16], and changes depending upon the steroid milieu [98, 99]. Furthermore, as E2 levels rise during proestrus the IGF-1 peptide demonstrates an increased efficiency to stimulate GnRH secretion [13]. Because of the contributions of both IGF-1 [13,14] and Kp [for review see 100] to the regulation of prepubertal GnRH secretion and the timing of puberty in several mammalian species, including humans, it became important to obtain a better understanding of the potential interactions between these two puberty-related peptides.
Actions of IGF-1 in the regulation of Kp synthesis
As stated earlier, IGF-1 administration did not alter expression of the KiSS-1 gene in the MBH of prepubertal female rats; however, the peptide did effectively induce expressions of the KiSS-1 gene and Kp protein within the RHA/AVPV brain region, actions that were blocked by the prior administration of the IGF-1 receptor blocker, JB-1 [95]. That increase in KiSS-1 gene occurred in juvenile female rats with low levels of E2, but later revealed that this response was E2 dependent, since IGF-1 was ineffective in stimulating KiSS-1 gene expression in the AVPV region 20 days after ovariectomy [95]. Subsequently, it was shown that the increase in KiSS-1 gene expression induced by IGF-1 was the result of an Akt-mediated action [101]. This agrees with a previous report showing that Akt is a transduction mediator of some other effects of IGF-1 [102]. More recently, it was revealed that both the stimulation of endogenous IGF-1, as well as the direct central delivery of the peptide, caused an increase in Kp protein synthesis in this brain region, and that this stimulatory action of IGF-1 on Kp is mediated by an IGF-1R activated Akt/mammalian target of rapamycin (mTOR) pathway [103]. Specifically, IGF-1 administered into the third ventricle induced the phosphorylation of insulin receptor substrate 1 (IRS1) by 6 hours post-injection, thus confirming activation of the IGF-1R receptor/IRS1complex. This action caused the phosphorylation of Akt, which in turn initiates the critical downstream phosphorylation of tuberous sclerosis complex 2 (TSC2). Phosphorylation of the TSC2 complex deactivates the inhibitory tone that TSC2 has on ras homologue enriched in brain (Rheb) and as a result Rheb levels rise. The increased levels of Rheb is associated with the phosphorylation of mTOR, and finally increased Kp synthesis. Interestingly, this pathway to Kp was shown to be inhibited by alcohol blocking the IGF-1R/IRS1 complex [103].
Actions of IGF-1 in the regulation of Kp release
As puberty progresses through the peripubertal period, Kp synthesizing neurons in the RHA/AVPV respond to the positive feedback effect of rising serum levels of E2 [4, 104, 105]. This response ultimately promotes an increase in the synthesis and release of GnRH; an action that drives the pubertal process to the preovulatory surge [104–106]. Because IGF-1 is also sensitive to the positive effect of E2 regarding stimulation of GnRH/LH secretion as puberty proceeds [13,107,108], it was important to discern whether IGF-1 played an upstream role in stimulating release of Kp from the RHA/AVPV region. In this regard, IGF-1 did not induce the in vitro release of Kp from tissue incubates collected during juvenile development when E2 levels were low; but, was effective in stimulating the release of Kp from tissues collected during first proestrus when E2 levels were higher. This action of IGF-1 to stimulate Kp secretion was shown to be inhibited by alcohol, a known blocker of the IGF-1R [103].
The observation that IGF-1 can regulate the secretion of Kp by an action within the RHA and thereby affect GnRH synthesis and secretion is further supported by anatomical and physiological information. While Kp neurons in this brain region project some of their nerve processes caudally to the MBH, there are also processes that project rostrally to adjacent GnRH synthesizing neurons in other areas of the RHA, as well as the POA [109, 110]. Importantly, most of these GnRH neurons express Kp receptors [111–113]. Thus, evidence indicates that once Kp is released from the RHA [103], it acts directly on GnRH neurons to stimulate the synthesis and release of the GnRH peptide. These interactions are important since, as stated above, Kp is a potent stimulator of GnRH/LH, especially at the time of proestrus. Interestingly, resumption of ad libidum feeding to previously food restricted rats caused vaginal proestrus and a surge-like increase in LH associated with an increase in KiSS-1 gene expression in the AVPV nucleus [114]. Furthermore, the firing rates of Kp neurons increase at proestrus [115], a time when the peptide has been shown to directly enhance GnRH neuronal firing in vitro [116–118]. Collectively, these facts further support the evidence that Kp neurons in the RHA region play an important role in relaying the positive feedback effects of rising E2 in the control of GnRH neuronal function during pubertal development [119, 120]. Thus, the fact that IGF-1 stimulates Kp release within the RHA is important, since it ultimately contributes to the increased synthesis and secretion of GnRH as puberty approaches [103].
Conclusions
There are important actions and interactions of IGF-1 that occur within the MBH, as well as the POA/RHA, the principal brain regions responsible for pubertal development. This review describes research over the years supporting the hypothesis that IGF-1 is a metabolic signal capable of activating and enhancing GnRH secretion at the time of puberty. Specifically, early work showed that the hepatic synthesis of IGF-1, followed by its rising levels in peripheral circulation, markedly increases as puberty approaches. The peptide crosses into the brain at the level of the ME and acts through the IGF-1R to facilitate GnRH secretion. Furthermore, it is now well known that the peptide increasingly stimulates LH secretion throughout pubertal development via a centrally mediated effect, and that repeated exposure of the hypothalamus to increased levels of the peptide effectively accelerates the timing of puberty. We discussed that the action of IGF-1 to stimulate prepubertal GnRH secretion is coordinated by a complex series of glial and neuronal mediated events. Regarding glial interactions, one example that occurs within the MBH is that IGF-1 binds to its receptors located on glial cells that secrete PGE2. Once the PGE2 is secreted, it then binds to its receptors on nearby GnRH nerve terminals within the ME and causes release of the GnRH peptide. Regarding neuronal interactions, there are several peptides produced by neurons that are known to have either positive or negative effects on prepubertal GnRH release. It is becoming increasingly clear that IGF-1 has the capability of regulating some of these neurons in specific brain regions. In this regard, IGF-1 has been shown to act within the MBH to stimulate the synthesis and secretion of prepubertal NKB, which subsequently binds to NK3R on DYN producing neurons and causing the suppressed release of DYN. This suggests that the NKB-induced suppression of the DYN brake on prepubertal GnRH secretion, at least in part, facilitates the puberty-related rise in GnRH/LH secretion. Furthermore, a direct action of NKB to stimulate GnRH release is also likely since NK3R are localized on GnRH nerve fibers in the MBH. These actions and interactions of IGF-1, NKB and DYN within the MBH begin early in the developmental process and are shown in the schematic revealed in Fig. 5. Moreover, during the transition from juvenile into peripubertal development, neurons in more rostral regions of the brain begin to show increased sensitivity to the rising levels of E2. As the IGF-1 levels increase in the region, induction of the IGF-1R activates an Akt-mediated pathway to mTOR, ultimately resulting in increased synthesis and release of Kp from its E2 sensitive neurons in the AVPV region of the RHA. Finally, once released Kp then binds to its receptors on GnRH producing neurons in the RHA, as well as in the POA and other rostral nuclei, further driving the preovulatory surge in GnRH/LH release and hence, resulting in first ovulation. These peripubertal actions and interactions between IGF-1, Kp and GnRH within the more rostral brain regions are shown in the schematic revealed in Fig. 6.
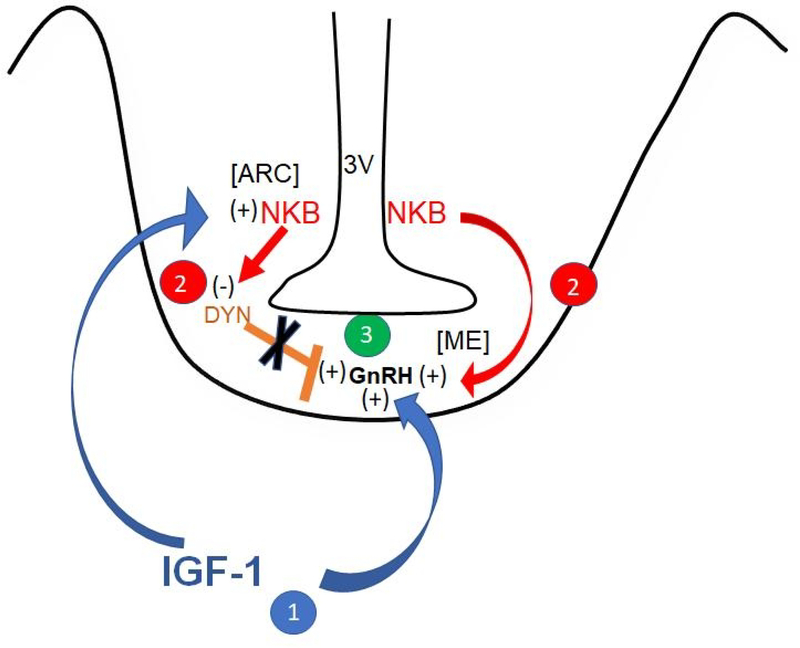
Fig. 5.
Schematic showing early actions of IGF-1, NKB and DYN within the medial basal hypothalamus contributing to the prepubertal regulation of GnRH secretion. 1. Blue arrows indicate that IGF-1 directly stimulates GnRH release from the median eminence (ME), while also stimulating NKB synthesis and release from neurons in the arcuate (ARC) nucleus. 2. Red arrows indicate that NKB likewise stimulates GnRH release from the ME, while also inhibiting DYN (orange bar) synthesis and release from neurons in the ARC nucleus. 3. The combined actions of increased IGF-1 and NKB and the removal of the inhibitory tone of DYN results in increased GnRH secretion during the initiation of puberty. 3V, third ventricle; (+) = stimulation; (−) = inhibition. (with permission, Copyright © 2019 [87] Dees WL, Hiney JK, Srivastava VK. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.)
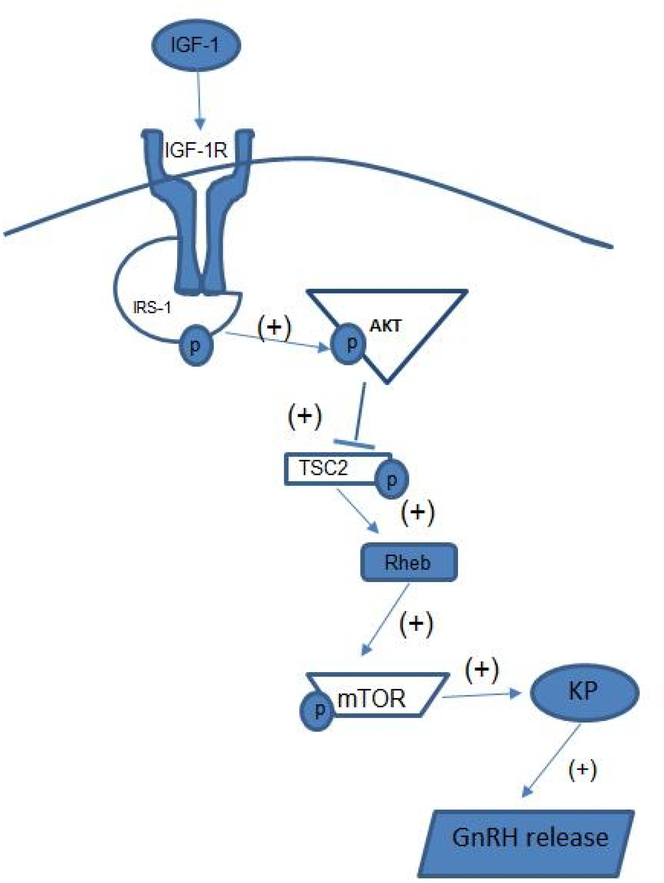
Fig. 6.
Schematic drawing showing the effects of IGF-1 in the preoptic area/ rostral hypothalamic region on prepubertal Kp and GnRH synthesis and release. IGF-1 activates the IGF-1R/IRS-1 complex, causing phosphorylation of Akt. This activation of Akt initiates the downstream phosphorylation of TSC2 and thus, removing the inhibitory tone on Rheb. The increase in Rheb induces the phosphorylation of mTOR leading to a stimulation in the synthesis and release of Kp, as well as GnRH. P, phosphorylated site; IGF-1, Insulin-like growth factor 1; IGF-1R; insulin-like growth factor receptor 1; IRS-1, insulin receptor substrate 1; TSC2, tuberous sclerosis complex 2; Rheb, ras homologue enriched in brain, mTOR, mammalian target of rapamycin; Kp, kisspeptin; GnRH, gonadotropin hormone- releasing hormone.
Acknowledgments
Funding Source: Supported by the National Institutes of Health Grant AA-07216 (to W.L.D.).
Footnotes
Conflict of Interest: Authors have no conflict of interest to declare.
References
- 1.Faletti AG, Mastonardi CA, Lomniczi A, Seilicovich A, Gimeno M, McCann SM, Rettori V. ß-endorphin blocks luteinizing hormone-releasing hormone release by inhibiting the nitricoxidergic pathway controlling its release. Proc Natl Acad Sci. USA. 1999. February16; 96(4):1722–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terasawa E, Fernandez DL. Neurobiological-mechanisms of the onset of puberty in primates. Endocr Rev. 2001. February; 22(1):111–51. [DOI] [PubMed] [Google Scholar]
- 3.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009. September 23; 29(38):11859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010. August; 151(8):3479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarkar DK, Smith GC, Fink G. Effect of manipulating central catecholamines on puberty and the surge of luteinizing hormone and gonadotropin releasing hormone induced by pregnant mare serum gonadotropin in female rats. Brain Res.1981. June 1; 213(2):335–49. [DOI] [PubMed] [Google Scholar]
- 6.Claypool LE, Kasuya E, Saitoh Y, Marzban F, Terasawa E. N-methyl-D,L-aspartate induces the release of LHRH in the prepubertal and pubertal female rhesus monkey as measured by in vivo push-pull perfusion in the stalk-median eminence. Endocrinology. 2000. January; 141(1): 219–28. [DOI] [PubMed] [Google Scholar]
- 7.Gay VL, Plant TM. N-methyl-DL-aspartate elicits hypothalamic gonadotropin-releasing hormone release in prepubertal male rhesus monkeys (Macaca mulatta). Endocrinology. 1987. June; 120(6):2289–96. [DOI] [PubMed] [Google Scholar]
- 8.Urbanski HF, Ojeda SR. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990. March; 126(3):1774–1776. [DOI] [PubMed] [Google Scholar]
- 9.Dearth RK, Hiney JK, Dees WL. Leptin acts centrally to induce the prepubertal secretion of luteinizing hormone in the female rat. Peptides. 2000. March; 21(3): 387–92. [DOI] [PubMed] [Google Scholar]
- 10.Lebrethon MC, Vandersmissen E, Gérard A, Parent AS, Junien JL, Bourguignon JP. In vitro stimulation of the prepubertal rat gonadotropin-releasing hormone pulse generator by leptin and neuropeptide Y through distinct mechanisms. Endocrinology. 2000. April;141(4):1464–9. [DOI] [PubMed] [Google Scholar]
- 11.Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci USA. 1990. December; 87(24):9698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiney JK, Ojeda SR, Dees WL. Insulin-like growth factor (IGF-I) stimulates LHRH release from the prepubertal female median eminence in vitro. Neuroendocrinology. 1991. October; 54(4): 420–23. [DOI] [PubMed] [Google Scholar]
- 13.Hiney JK, Srivastava VK, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor-1 (IGF1) of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996. September; 137(9): 3717–27. [DOI] [PubMed] [Google Scholar]
- 14.Wilson ME. Premature elevation in serum insulin-like growth factor-1 advances first ovulation in monkeys. J Endocrinol. 1998. August; 158(2): 247–57. [DOI] [PubMed] [Google Scholar]
- 15.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004; 561: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barriero ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated mRNA expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent LH-releasing activity of KiSS-1 peptide. Endocrinology. 2004. December 1; 145(10):4565–74. [DOI] [PubMed] [Google Scholar]
- 17.Shahab M, Mastronardi C, Seminara S, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005. February 8; 102(6): 2129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004. October; 16(10):850–58. [DOI] [PubMed] [Google Scholar]
- 19.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010. September; 151(9):4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Kisspeptin and neurokinin B signaling network underlies the pubertal increase in GnRH release in female rhesus monkeys. Endocrinology. 2017. October 1; 158(10):3269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiological Reviews. 1990. July; 70(3):591–614. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992. November; 56(5):619–28. [DOI] [PubMed] [Google Scholar]
- 23.Froesch ER, Schmid C, Schwander J, Zapf J. Actions of insulin-like growth factors. Ann Rev Physiol. 1985; 47:443–67. [DOI] [PubMed] [Google Scholar]
- 24.D’Ercole AJ, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: Further evidence for multiple sites of synthesis and paracrine or autocrine mechanism of action. Proc Natl Acad Sci USA. 1984. February:81(3):935–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr. Rev. 1997. 18, 801–831. [DOI] [PubMed] [Google Scholar]
- 26.Aguado F, Rodrigo J, Cacicedo L, Mellstrom B. Distribution of insulin-like growth factor-1 receptor mRNA in rat brain. Regulation in the hypothalamo-neurohypophysial system. J Mol Endocrinol. 1993. October; 11(2):231–9. [DOI] [PubMed] [Google Scholar]
- 27.Bohannon NJ, Figlewicz DP, Corp ES, Wilcox J, Porte D Jr, Baskin DG. Identification of binding sites for an insulin-like growth factor (IGF-1) in the median eminence of the rat brain by quantitative autoradiography. Endocrinology.1986. August: 119(2):943–45. [DOI] [PubMed] [Google Scholar]
- 28.Lesniak MA, Hill JM, Kiess W, Rojeski M, Candace BP, Roth J. Receptors for insulin-like growth factors I and II: Autoradiographic localization in rat brain and comparison to receptor for insulin. Endocrinology.1988. October; 123(4):2089–99. [DOI] [PubMed] [Google Scholar]
- 29.Marks JL, Porte D Jr, Baskin DG. Localization of type 1 insulin-like growth factor receptor messenger RNA in the adult rat brain by in situ hybridization. Mol Endocrinol. 1991. August; 5(8):1158–68. [DOI] [PubMed] [Google Scholar]
- 30.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Mendelsohn FA. Localization and characterization of IGF-1 receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. A distinct distribution from insulin receptors. J Neuroendocrinol. 1989. October 1: 1(5):369–77. [DOI] [PubMed] [Google Scholar]
- 31.Crawford BA, Singh JM, Simpson JM, Handelsman DJ. Androgen regulation of circulating insulin-like growth factor-1 during puberty in male hypogonadal mice. J Endocrinol.1993. October; 139(1):57–65. [DOI] [PubMed] [Google Scholar]
- 32.Handelsman DJ, Spaliviero JA, Scott CD, Baxter RC. Hormonal regulation of the peripubertal surge of insulin-like growth factor-1 in the rat. Endocrinology. 1987. February; 120(2):491–96. [DOI] [PubMed] [Google Scholar]
- 33.Jones EJ, Armstrong JD, Harvey RW. Changes in metabolites, metabolic hormones, and luteinizing hormone before puberty in Angus, Braford, Charolais, and Simmental heifers. J Animal Sci. 1991. April; 69(4):1607–12. [DOI] [PubMed] [Google Scholar]
- 34.Roberts CA, McCutcheon SN, Blair HT. Developmental patterns of plasma insulin-like growth factor-1 concentrations in sheep. Domest Anim Endo. 1990. October; 7(4):457–63. [DOI] [PubMed] [Google Scholar]
- 35.Copeland KC, Kuehl TJ, Castracane VD. Pubertal endocrinology of the baboon: Elevated somatomedin-C/insulin-like growth factor 1 at puberty. J Clin Endocrinol Metab. 1982. December; 55(6):1198–201. [DOI] [PubMed] [Google Scholar]
- 36.Copeland KC, Eichberg JW, Parker CR, Bartke A. Puberty in the chimpanzee: Somatomedin-C and its relationship to somatic growth and steroid hormone concentrations. J Clin Endocrinol Metab. 1985. June; 60(6):1154–60. [DOI] [PubMed] [Google Scholar]
- 37.Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: Relation to age, sex, stage of puberty, testicular size and body mass index. J Clin Endocrinol Metab. 1994. March; 78(3):744–52 [DOI] [PubMed] [Google Scholar]
- 38.Tam CS, deZegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006. November; 91(11):4369–73. [DOI] [PubMed] [Google Scholar]
- 39.Plant TM 1988. Puberty in primates. In: Knobil E, Neill JD (eds) The Physiology of Reproduction. Raven Press, New York, vol. 2:1763–1788 [Google Scholar]
- 40.Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormone neurons during postnatal development. J Neuroendocrinol. 2004. February; 16(2):160–9. [DOI] [PubMed] [Google Scholar]
- 41.Ma YJ., Berg-von der Emde K, Moholt-Siebert M, Hill DF, Ojeda SR. Region specific regulation of transforming growth factor-α (TGFα) gene expression in astrocytes of the neuroendocrine brain. J Neurosci. 1994. September; 14(9):5644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojeda SR, Lomniczi A, Sandau US. Glial-gonadotropin hormone (GnRH) neuron interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol. 2008; 20():732–42. [DOI] [PubMed] [Google Scholar]
- 43.Hiney JK, Srivastava VK, Lara F, Dees WL. Ethanol blocks the central action of IGF1 to induce luteinizing hormone secretion in the prepubertal female rat. Life Sci. 1998; 62(4): 301–8. [DOI] [PubMed] [Google Scholar]
- 44.Hiney JK, Dees WL. Ethanol inhibits LHRH release from the median eminence of prepubertal female rats in vitro: investigation of its action on norepinephrine and prostaglandin E2. Endocrinology. 1991. March; 128(3):1404–08. [DOI] [PubMed] [Google Scholar]
- 45.Ojeda SR, Negro-Vilar A, McCann SM. Release of prostaglandins Es (PGEs) by hypothalamic tissue: evidence of their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology. 1979. March; 104(3):617–24. [DOI] [PubMed] [Google Scholar]
- 46.Adam CL, Findlay PA, Hotston Moore A. Effects of insulin-like growth factor-1 on luteinizing hormone secretion in sheep. Animal Reprod Sci 1997. 50: 45–56. [DOI] [PubMed] [Google Scholar]
- 47.Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role of IGF-1. Endocrinology. 1999. June; 140(6):2637–40. [DOI] [PubMed] [Google Scholar]
- 48.DiVall S, Williams TR, Carver SE, Koch L, Bruning JC, Kahn CR, et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010. August; 120(8): 2900–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pazos F, Sanchez-Franco F, Balsa J, Lopez-Fernandez J, Escalada J, Cacicedo L. Regulation of gonadal and somatotropic axis by chronic intraventricular infusion of Insulin-like growth factor 1 antibody at the initiation of puberty in male rats. Neuroendocrinology 1999. June; 69(6):408–16. [DOI] [PubMed] [Google Scholar]
- 50.Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: Relationship to gonadotropin-releasing hormone neurons. Endocrinology 2003. May; 144(5): 2034–45. [DOI] [PubMed] [Google Scholar]
- 51.Dees WL, Sorensen AM, Kemp WM, McArthur NH. Immunohistochemical localization of gonadotropin-releasing hormone (GnRH) in the brain and infundibulum of the sheep. Cell Tissue Res. 1981;215(1):181–91. [DOI] [PubMed] [Google Scholar]
- 52.Barry J, Dubois MP, Carrette B. Immunofluorescence study of the preoptico-infundibular LRF neurosecretory pathway in the normal, castrated or testosterone -treated male guinea. Endocrinology. 1974; 95(5):1416–23. [DOI] [PubMed] [Google Scholar]
- 53.Silverman AJ. Distribution of luteinizing hormone releasing hormone (LHRH) in the guinea pig brain. Endocrinology 1976. July; 99(1):30–41. [DOI] [PubMed] [Google Scholar]
- 54.Lamperti AA, Pickard GE. Immunohistochemical localization of luteinizing hormone-releasing hormone (LHRH) in the hypothalamus of adult female hamsters treated neonatally with monosodium glutamate or hypertonic saline. Anat Rec. May; 1984; 209(1):131–41. [DOI] [PubMed] [Google Scholar]
- 55.Dees WL, Sorensen AM Jr, Kemp WM, McArthur NH. GnRH localization in the equine brain and infundibulum: an immunohistochemical study. Brain Res. 1981; 208(1):123–3 [DOI] [PubMed] [Google Scholar]
- 56.Dees WL, McArthur NH Immunohistochemical localization of gonadotropin releasing hormone (GnRH) in the bovine hypothalamus and infundibulum. Anat Rec. 1981; July;200(3):281–5. [DOI] [PubMed] [Google Scholar]
- 57.Barry J, Carette B. Immunofluorescence study of LRF neurons in primates. Cell Tiss Res. 1975. December 2; 164 (2): 163–78. [DOI] [PubMed] [Google Scholar]
- 58.Marshall PE and Goldsmith PC. Neuroregulatory and neuroendocrine pathways in the hypothalamus and forebrain of the baboon. 1980. July 14; Brain Res. 193(2):353–72. [DOI] [PubMed] [Google Scholar]
- 59.Silverman AJ, Antunes JL, Abrams GM, Nilaver G, Thau R, Robinson JA, et al. The luteinizing hormone-releasing hormone pathways in rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) monkeys: new observations on thick, unembedded sections. J Comp Neurol. 1982; 211(3):309–317 [DOI] [PubMed] [Google Scholar]
- 60.Bugnon C, Bloch B, Lenys D, Fellmann D. Ultrastructural study of the LH-RH containing neurons in the human fetus. Brain Res. 1977. November 25; 137(1):175–80. [DOI] [PubMed] [Google Scholar]
- 61.Paulin C, Dubois MP, Barry J, Dubois PM. Immunofluorescence study of LH-RH producing cells in the human fetal hypothalamus. Cell Tissue Res.1977. August 26; 182(3): 341–5. [DOI] [PubMed] [Google Scholar]
- 62.Kozlowski GP, Dees WL. Immunocytochemistry for LHRH neurons in the arcuate nucleus area of the rat: fact or artifact? J Histochem Cytochem. 1984. January; 32(1):83–91. [DOI] [PubMed] [Google Scholar]
- 63.Gross DS. Distribution of gonadotropin-releasing hormone in the mouse brain as revealed by immunohistochemistry. Endocrinology. 1976. 98(6): 1408–1417. [DOI] [PubMed] [Google Scholar]
- 64.Zimmerman EA, Hsu KC, Ferin M, Kozlowski GP. Localization of gonadotropin - releasing hormone (Gn-RH) in the hypothalamus of the mouse by immunoperoxidase technique. Endocrinology. 1974. 95(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 65.Miller BH, Gore AC. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and aging. J Neuroendocrinol. 2001. August;13(8):728–36 [DOI] [PubMed] [Google Scholar]
- 66.Lopez JA, Bedenbaugh MN, McCosh RB, Weems PW, Meadows LJ, Wisman B, et al. Does dynorphin play a role in the onset of puberty in female sheep? J Neuroendo. 2016. December; 28(12): doi: 10.1111/jne.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schulz R, Wilhelm A, Pirke KM, Gramsch C, Herz A. Beta-endorphin and dynorphin control serum luteinizing hormone level in immature female rats. Nature. 1981. December 24; 294(5843):757–59. [DOI] [PubMed] [Google Scholar]
- 68.Sirinathsinghji DJS, Motta M, Martini L. Induction of precocious puberty in the female rat after chronic naloxone administration during neonatal period: the opiate “brake” on pubertal gonadotrophin secretion. J Endocrinol. 1985. February; 104(2):299–307. [DOI] [PubMed] [Google Scholar]
- 69.Nakahara T, Uenoyama Y, Iwase A, Oishi S, Nakamura S, Minabe S, et al. Chronic peripheral administration of kappa-opioid receptor antagonist advances puberty onset association with acceleration of pulsatile luteinizing hormone secretion in female rats. J Reprod Dev. 2013; 59:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srivastava VK, Hiney JK, Stevener K, Dees WL. Differential effects of alcohol on excitatory and inhibitory puberty-related peptides in the basal hypothalamus of the female rats. Alcohol Clin Exp Res. 2015. December; 39(12):2386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srivastava VK, Hiney JK, Dees WL. Alcohol delays the onset of puberty in the female rat by altering key hypothalamic events. Alcohol Clin Exp Res. 2018. July; 42(7):1166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramaley JA. The regulation of gonadotropin secretion in immature ethanol-treated male rats. J Androl. 1982; 3:248–52. [Google Scholar]
- 73.Bo WJ, Krueger WA, Rudeen PK, Symmes SK. Ethanol-induced alterations in the morphology and function of the rat ovary. Anat Rec. 1982. February; 202(2):255–60. [DOI] [PubMed] [Google Scholar]
- 74.Dees WL, Skelley CW. Effects of ethanol during the onset of female puberty. Neuroendocrinology; 1990. January; 51(1):64–9. [DOI] [PubMed] [Google Scholar]
- 75.Dees WL, Dissen GA, Hiney JK, Lara F, Ojeda SR. Alcohol ingestion inhibits the increased secretion of puberty-related hormones in the developing female rhesus monkey. Endocrinology. 2000. April; 141(4):1325–31. [DOI] [PubMed] [Google Scholar]
- 76.Dissen GA, Dearth RK, Scott H, Ojeda SR, Dees WL. Alcohol alters luteinizing hormone secretion in immature female rhesus monkeys by a hypothalamic action. Endocrinology. 2004. October; 145(4):4558–64. [DOI] [PubMed] [Google Scholar]
- 77.Richards MA, Oinonen KA. Age at menarche is associated with divergent alcohol use patterns in early adolescence and early adulthood. J Adolesc. 2011. October; 34(5):1065–76. [DOI] [PubMed] [Google Scholar]
- 78.Peck JD, Peck BM, Skaggs VJ, Fukushima M, Kaplan HB. Socio-environmental factors associated with pubertal development in female adolescents: The role of tobacco and alcohol use. J.Adolesc Health. 2011. March; 48(3):241–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007. December; 148(12):5752–60. [DOI] [PubMed] [Google Scholar]
- 80.Hrabovszky E, Sipos MT, Molnar CS, Vida B, Ciofi P, Borsay BA, et al. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012. October; 153(10):4978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burke MC, Letts PA, Krajewski SJ, Rance NE. Co-expression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006. October; 498(5):712–26. [DOI] [PubMed] [Google Scholar]
- 82.Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS ONE. 2012; 7(9): e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nestor CC, Briscoe AMS, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012. June; 153(6):2756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, et al. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010. August; 151(8):3836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013. November; 154(11):3984–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gill JC, Navarro VM, Kwong C, Noel SD, Martin C, Xu S, et al. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology. 2012. October; 153(10):4883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dees WL, Hiney JK, Srivastava VK. Regulation of prepubertal dynorphin secretion in the medial basal hypothalamus of the female rat. J Neuroendocrinol. 2019. December; 31(12):e12810. doi: 10.1111/jne.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li SY, Li XF, Hu MH, Shao B, Poston L, Lightman SL, O’Byrne KT. Neurokinin B receptor antagonism decreases luteinizing hormone pulse frequency and amplitude and delays puberty onset in the female rat. J Neuroendocrinol. 2014. August; 26(8):521–27. [DOI] [PubMed] [Google Scholar]
- 89.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005. August 29; 489(3):372–86. [DOI] [PubMed] [Google Scholar]
- 90.Hrabovszky E, Molnar CS, Sipos M, Vida B, Ciofi P, Borsay BA, et al. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front Endocrinol (Lausanne) 2011. December 1;2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roa J, Tena-Sempere M. KiSS-1 system and reproduction: Comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen Comp Endocrinol. 2007. August-September; 153(1–3): 132–140. [DOI] [PubMed] [Google Scholar]
- 92.Keen KL, Wegner FH, Bloom SR, Ghatei Ma, Terasawa E An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008. August; 149(8): 4151–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006. December; 147(12): 5817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology. 2013. August; 154(8):2739–49. [DOI] [PubMed] [Google Scholar]
- 95.Hiney JK, Srivastava VK, Pine MD, Dees WL. Insulin-like growth factor-1 activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009. January; 150(1): 376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ieda N, Uenoyama Y, Tajima Y, Nakata T, Kano M, Naniwa Y, et al. KiSS-1 gene expression in the developing brain of female pigs in pre- and peripubertal periods. J Reprod Dev 2014. 60 (4): 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018. September; 159(9): 3219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005. September; 146(9):3686–92. [DOI] [PubMed] [Google Scholar]
- 99.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007. March; 148(3):1150–57. [DOI] [PubMed] [Google Scholar]
- 100.Tena-Sempere M KiSS-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006; 83(5–6): 275–81. [DOI] [PubMed] [Google Scholar]
- 101.Hiney JK, Srivastava VK, Dees WL. Insulin-like growth factor-1 stimulates hypothalamic Kiss-1 gene expression by activating Akt: Effect of alcohol. Neuroscience. 2010. March 17; 166(2):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cardona-Gomez GP, Mendez P and Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-1 in the activation of P13K/Akt signaling in the adult rat hypothalamus. Brain Res Mol Brain Res. 2002. October 30; 107(1):80–8. [DOI] [PubMed] [Google Scholar]
- 103.Hiney JK, Srivastava VK, Vaden Anderson DN, Hartzoge NL, Dees WL. Regulation of kisspeptin synthesis and release in the preoptic/anterior hypothalamic region of prepubertal female rats: Actions of IGF-1 and alcohol. Alcoholism: Clin Exp Res. 2018. June; 42(7):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanism underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010. August 5; 324(1–2):45–50. [DOI] [PubMed] [Google Scholar]
- 105.Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinizing hormone surge. J Neuroendocrinol. 2009. March; 21(4):305–11. [DOI] [PubMed] [Google Scholar]
- 106.Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008; 70:213–38. [DOI] [PubMed] [Google Scholar]
- 107.Quesda A, Etgen A. Functional interactions between estrogen and insulin-like growth factor-1 in the regulation of α1ß-adenoreceptors and female reproductive function. J Neurosci. 2002. March 15; 22(6):2401–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hiney JK, Srivastava VK, Dees WL. Influence of estradiol on insulin-like growth factor-1-induced luteinizing hormone secretion. Brain Res. 2004. July 2; 1013(1)91–7. [DOI] [PubMed] [Google Scholar]
- 109.Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus interactions with GnRH neuronal system. J Chem Neuroanat. 2008. December; 36(3–4) 131–37. [DOI] [PubMed] [Google Scholar]
- 110.Smith JT, Collen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008. November; 149(11): 5770–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotrop-inreleasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neuroscience. 2005. December 7; 25(49):11349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Nat Acad Sci USA. 2005. February 1; 102(5): 1761–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology. 2004. August; 145(8): 3613–18. [DOI] [PubMed] [Google Scholar]
- 114.Majarune S, Nima P, Sugimoto A, Nagae M, Inoue N, Tsukamura H, et al. Ad libitum feeding triggers puberty onset associated with increases in arcuate KiSS1 and Pdyn expression in growth-retarded rats. J Reprod Dev 2019. 65 (5) 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ducret E, Gaidamaka G, Herbison AE. Electrical and morphological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinology. 2010. May; 151(5): 2223–32. [DOI] [PubMed] [Google Scholar]
- 116.Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008. September; 149(9):4605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008. April; 149(4):1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008; 28:4423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terasawa E, Wiegand SJ, Bridson WE. A role for medial preoptic nucleus on afternoon of proestrus in female rats. Am J Physiol. 1980. June; 238(6): E533–E539. [DOI] [PubMed] [Google Scholar]
- 120.Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006. October 19; 52(2): 271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]