Abstract
The impact of infection on the prognosis of trauma patients according to severity remains unclear. We assessed the impact of infection complications on in-hospital mortality among patients with trauma according to severity. This retrospective cohort study used a nationwide registry of trauma patients. Patients aged ≥ 18 years with blunt or penetrating trauma who were admitted to intensive care units or general wards between 2004 and 2017 were included. We compared the baseline characteristics and outcomes between patients with and without infection and conducted a multivariable logistic regression analysis to investigate the impact of infection on in-hospital mortality according to trauma severity, which was classified as mild [Injury Severity Score (ISS) < 15], moderate (ISS 15–29), or severe (ISS ≥ 30). Among the 150,948 patients in this study, 10,338 (6.8%) developed infections. Patients with infection had greater in-hospital mortality than patients without infection [1085 (10.5%) vs. 2898 (2.1%), p < 0.01]. After adjusting for clinical characteristics, in-hospital mortality differed between trauma patients with and without infection according to trauma severity [17.1% (95% CI 15.2–18.9%) vs. 2.9% (95% CI 2.7–3.1%), p < 0.01, in patients with mild trauma; 14.8% (95% CI 13.3–16.3%) vs. 8.4% (95% CI 7.9–8.8%), p < 0.01, in patients with moderate trauma; and 13.5% (95% CI 11.2–15.7%) vs. 13.7% (95% CI 12.4–14.9%), p = 0.86, in patients with severe trauma]. In conclusion, the effect of infection complications in patients with trauma on in-hospital mortality differs by trauma severity.
Subject terms: Trauma, Infectious diseases
Introduction
Infection after trauma, including sepsis, is the most common complication affecting trauma patients; unfortunately, it has a poor prognosis. However, some infections may be preventable or recognized early because infections are typically acquired during hospitalization after the onset of trauma1. Therefore, complication rates and the failure to rescue including infection are considered indicators of the quality of trauma care2.
Previous studies have reported that the overall mortality from trauma has gradually decreased in western countries3 and in Japan4. Additionally, many studies have shown the improvement in outcomes for patients with sepsis following the development subsequent revision of definitions, guidelines, and bundles5–8. Conversely, only limited information is available on the development of infection and sepsis in patients with trauma1, likely because the definition of sepsis continues to change9. Patients with trauma often arrive with organ dysfunction, which adds complexity and inaccuracy to applying the definition of sepsis using organ failure scores such as sequential organ failure assessment scores10. It is difficult to determine whether severity scores and organ damage are caused by trauma or the subsequent infection. Furthermore, few studies have assessed the impact of infection and its prognosis among trauma patients. Prognosis among trauma patients complicated with infection may be influenced by trauma severity.
Therefore, we aimed to assess the impact of infection complications on in-hospital mortality among patients with trauma according to trauma severity using a national database in Japan.
Methods
Study design and data source
This retrospective cohort study used the Japan Trauma Data Bank (JTDB) database from 2004 and 2017. The JTDB was established in 2003 and is authorized and maintained by the Japanese Association for the Surgery of Trauma and the Japanese Association for Acute Medicine to improve and assure the quality of trauma care in Japan. A total of 272 hospitals, including more than 75% of the certified tertiary emergency medical centers in Japan, contributed to the JTDB in March 201811.
Data collection
The JTDB includes data related to patient and hospital information such as patient demographics, Abbreviated Injury Scale scores, Injury Severity Score (ISS), prehospital and in-hospital procedures, complications, and treatment and emergency procedures including transfusion within 24 h. The JTDB also records outcome data such as emergency department (ED) mortality, in-hospital mortality, and length of hospital stay. Data collection was performed as a part of routine clinical patient management.
Study participants
Patients aged ≥ 18 years with blunt or penetrating trauma who were admitted to the intensive care unit or a general ward were enrolled in this study. We excluded patients who died < 7 days after admission, similar to previous reports12,13, to exclude the effects of first trauma impact on in-hospital mortality and because infection usually occurred approximately seven days after trauma14. We also excluded patients who met the following criteria: missing data on sex and ISS, an Abbreviated Injury Scale score of 6 (i.e., non-survivable injury), inconceivable vital signs in the ED (e.g., systolic blood pressure ≤ 40 mmHg), hospital stay for ≥ 1 year or missing, or missing data on in-hospital death.
Definitions
Infection and sepsis were clinically diagnosed by a physician in charge. Sepsis was identified a composite variable, “sepsis/multiple organ failure”, in the JTDB database. This definition is similar to the definition of severe sepsis in the Sepsis-2 criteria15. We divided trauma severity into three groups based on the ISS to reflect the clinically relevant categories, similar to previous reports1,16: ISS < 15 (mild), ISS 15–29 (moderate), and ISS ≥ 30 (severe). Types of infections included pneumonia, urinary tract infection, surgical site infection, myelitis, meningitis, abdominal abscess, cholecystitis, enterocolitis, empyema, and bacteremia. The definition of a complication was in accordance with the JTDB. All emergency procedures were operated as part of the resuscitation or initial management at the ED.
Statistical analysis
Continuous variables were presented as the median and interquartile range and were compared using the Mann–Whitney U test because none of the variables were normally distributed. Categorical variables were presented as numbers and percentages and compared using the Chi-square test. We compared the baseline characteristics such as age, sex, site of injury, comorbidities, emergency procedures, concomitant complications, and outcomes between the patients with and without infection.
We performed a multivariable logistic regression analysis to investigate the influence of infection on in-hospital mortality for trauma patients. The adjusted variables included age, sex, number of comorbidities, transfusion, emergency procedures, admission disposition, any operations, and concomitant complications; these variables were chosen based on previous reports and clinical relevance1,16–18. We assessed the multicollinearity of variables using the variance inflation factor, and the tolerance value was set at less than 2. We then used marginal standardization based on probability determined from the previous analysis to estimate the adjusted in-hospital mortality rate according to trauma severity. The results were reported as adjusted in-hospital mortality rates with 95% confidence intervals (CIs). In a subgroup analysis, we evaluated the impact of infection on in-hospital mortality after excluding patients with hospital stays < 14 days, to reduce the potential for immortal time bias.
All p values were two-sided, with p < 0.05 considered statistically significant. The data were statistically analyzed using Stata software, version 15.1 (StataCorp, College Station, TX, USA).
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Research Ethics Committee of the Faculty of Medicine of the Juntendo University (IRB No.19-010). The Research Ethics Committee of the Faculty of Medicine of the Juntendo University waived the need to obtain informed consent from the study participants given the retrospective and anonymized nature of this study in routine care. The JTDB administrators also provided permission to use the data from their database. Our study was performed in accordance with the amended Declaration of Helsinki.
Results
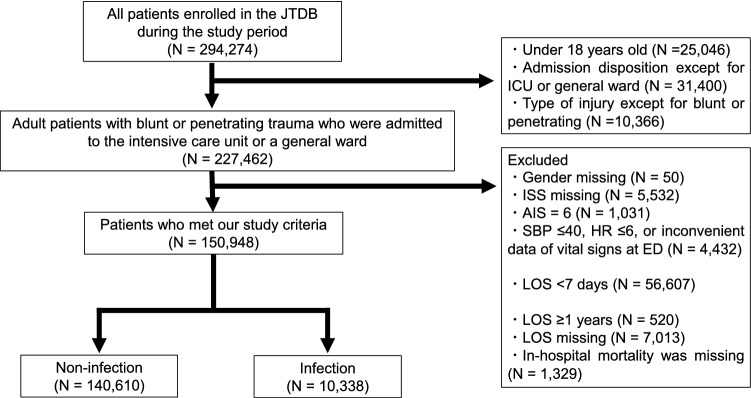
Of the 294,274 patients in the JTDB from 2004 to 2017, 227,462 adult patients with blunt or penetrating trauma who were admitted to the intensive care unit or a general ward were identified. After eliminating those who met the exclusion criteria, the remaining 150,948 patients were included in this study (Fig. 1).
Figure 1.
Patient selection. JTDB Japan Trauma Data Bank, ICU intensive care unit, ISS Injury Severity Score, AIS Abbreviated Injury Scale, SBP systolic blood pressure, HR heart rate, ED emergency department, LOS length of hospital stay.
Of those patients, 10,338 (6.8%) with infection were identified. A total of 1130 (10.9%) patients had sepsis. The demographic characteristics among the patients with and without infection are shown in Table 1. Patients with infection were older than those without [71 (53–82) vs. 67 (47–80) years, p < 0.01]. Patients with infection had more comorbidities [6921 (66.9%) vs. 80,450 (57.2%), p < 0.01, Supplementary Table 1]. Patients with infection received more emergency procedures [5313 (51.4%) vs. 38,874 (27.6%), p < 0.01] and transfusions [3162 (31.0%) vs. 19,077 (13.9%), p < 0.01] than those without infection. The use of steroids or immunosuppressants did not differ between the patients with or without infection [48 (0.5%) vs. 532 (0.4%), p = 0.17, and 18 (0.2%) vs. 204 (0.1%), p = 0.46, respectively]. The severity of trauma was greater in patients with infection than those without [mild, 3901 (37.7%) vs. 84,042 (59.8%); moderate, 4584 (44.3%) vs. 47,743 (34.0%); severe, 1853 (17.9%) vs. 8825 (6.3%), p < 0.01].
Table 1.
Characteristics of trauma patients with and without infection.
| Non-infection | Infection | p value | |
|---|---|---|---|
| Number | 140,610 (93.2) | 10,338 (6.8) | |
| Age | 67 (47–80) | 71 (53–82) | < 0.01 |
| Gender (male) | 81,187 (57.7) | 6871 (66.5) | < 0.01 |
| Mechanism of injury | |||
| Blunt | 136,427 (97.0) | 10,070 (97.4) | 0.04 |
| Penetrating | 4183 (3.0) | 268 (2.6) | |
| Injury site (AIS ≥ 3) | |||
| Head | 38,793 (27.6) | 4082 (39.5) | < 0.01 |
| Face | 1008 (0.7) | 99 (1.0) | < 0.01 |
| Neck | 468 (0.3) | 58 (0.6) | < 0.01 |
| Thorax | 29,090 (20.7) | 2860 (27.7) | < 0.01 |
| Abdomen and pelvis | 8184 (5.8) | 984 (9.5) | < 0.01 |
| Spine | 14,732 (10.5) | 1616 (15.6) | < 0.01 |
| Upper extremity | 7383 (5.3) | 471 (4.6) | < 0.01 |
| Lower extremity | 53,510 (38.1) | 3940 (38.1) | 0.91 |
| Others | 35 (0.0) | 9 (0.1) | < 0.01 |
| Trauma severity (ISS category) | < 0.01 | ||
| Mild (ISS < 15) | 84,042 (59.8) | 3901 (37.7) | |
| Moderate (ISS 15–29) | 47,743 (34.0) | 4584 (44.3) | |
| Severe (ISS ≥ 30) | 8825 (6.3) | 1853 (17.9) | |
| Number of comorbidities | |||
| 0 | 60,160 (42.8) | 3417 (33.1) | < 0.01 |
| 1 | 42,810 (30.5) | 3197 (30.9) | |
| 2 | 22,303 (15.9) | 1983 (19.2) | |
| 3 | 9975 (7.1) | 1058 (10.2) | |
| ≥ 4 | 5362 (3.8) | 683 (6.6) | |
| Medication | |||
| Steroid | 532 (0.4) | 48 (0.5) | 0.17 |
| Immunosuppressant | 204 (0.1) | 18 (0.2) | 0.46 |
| Anticoagulant | 2631 (1.9) | 302 (2.9) | < 0.01 |
| Vital signs at emergency department | |||
| GCS | 15 (14–15) | 14 (11–15) | < 0.01 |
| SBP | 138 (119–159) | 134 (110–158) | < 0.01 |
| HR | 82 (71–95) | 86 (73–102) | < 0.01 |
| Temperature | 36.5 (36.0–37.0) | 36.4 (35.8–36.9) | < 0.01 |
| RR | < 0.01 | ||
| ≤ 17 (quartile 1) | 31,165 (25.9) | 1960 (21.2) | |
| 18–23 (quartile 2–3) | 54,057 (45.0) | 3746 (40.5) | |
| ≥ 24 (quartile 4) | 35,042 (29.1) | 3537 (38.3) | |
| Number of emergency procedures | |||
| 0 | 101,736 (72.4) | 5025 (48.6) | < 0.01 |
| 1 | 26,264 (18.7) | 2025 (19.6) | |
| 2 | 7208 (5.1) | 1397 (13.5) | |
| ≥ 3 | 5402 (3.8) | 1891 (18.3) | |
| Emergency procedures | |||
| Intubation | 11,837 (8.4) | 3257 (31.5) | < 0.01 |
| Ventilator use or assisted ventilation | 9461 (6.7) | 2476 (24.0) | < 0.01 |
| REBOA | 291 (0.2) | 105 (1.0) | < 0.01 |
| Chest drainage | 8154 (5.8) | 1133 (11.0) | < 0.01 |
| Craterization | 653 (0.5) | 297 (2.9) | < 0.01 |
| Emergency TAE | 4050 (2.9) | 830 (8.0) | < 0.01 |
| Central venous line use | 4262 (3.0) | 1454 (14.1) | < 0.01 |
| Vasopressor use | 1481 (1.1) | 518 (5.0) | < 0.01 |
| Open bone traction | 10,228 (7.3) | 846 (8.2) | < 0.01 |
| External skeletal fixation | 4115 (2.9) | 607 (5.8) | < 0.01 |
| Other emergency bone fixation | 5430 (3.9) | 442 (4.3) | 0.04 |
| Blood transfusion | 19,077 (13.9) | 3162 (31.0) | < 0.01 |
| Any operation | 80,186 (57.0) | 6883 (66.6) | < 0.01 |
Continuous variables were compared using the Mann–Whitney U test. Categorical variables were compared using the Chi-square test.
Missing: GCS = 13,085, SBP = 2281, HR = 5577, Temperature = 15,926, RR = 21,441, Blood transfusion = 3421.
AIS Abbreviated Injury Scale, ISS Injury Severity Score, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, HD hemodialysis, GCS Glasgow coma scale, SBP systolic blood pressure, HR Heart rate, RR respiratory rate, REBOA resuscitative endovascular balloon occlusion of the aorta, TAE transcatheter arterial embolization.
Patients with infection had more concomitant complications than patients without infection (Table 2). Specifically, atelectasis [1048 (10.1%) vs. 906 (0.6%), p < 0.01], higher brain dysfunction [954 (9.2%) vs. 2529 (1.8%), p < 0.01], and disseminated intravascular coagulation and coagulation disorder [758 (7.3%) vs. 706 (0.5%), p < 0.01] were more common in patients with infection than in those without infection.
Table 2.
Concomitant complications in patients with and without infection.
| Non-infection | Infection | p value | |
|---|---|---|---|
| Number | 140,610 (93.2) | 10,338 (6.8) | |
| Number of concomitant complications | |||
| 0 | 129,290 (91.8) | 5022 (49.4) | < 0.01 |
| 1 | 9165 (6.5) | 2748 (26.6) | |
| 2 | 1632 (1.2) | 1178 (11.4) | |
| 3 | 360 (0.3) | 568 (5.5) | |
| ≥ 4 | 163 (0.1) | 822 (8.0) | |
| Central nervous system | |||
| Diabetes insipidus | 194 (0.1) | 116 (1.1) | < 0.01 |
| Hydrocephalus | 209 (0.2) | 166 (1.6) | < 0.01 |
| Fat embolism | 84 (0.1) | 152 (1.5) | < 0.01 |
| Cerebrospinal fluid leakage | 235 (0.2) | 115 (1.1) | < 0.01 |
| Higher brain dysfunction | 2529 (1.8) | 954 (9.2) | < 0.01 |
| Mental disorders (PTSD, etc.) | 597 (0.4) | 201 (2.0) | < 0.01 |
| Others | 1535 (1.1) | 512 (5.0) | < 0.01 |
| Circulation | |||
| Acute coronary syndrome | 63 (0.0) | 38 (0.4) | < 0.01 |
| Refractory shock | 221 (0.2) | 176 (1.7) | < 0.01 |
| Acute kidney injury | 186 (0.1) | 267 (2.6) | < 0.01 |
| Abdominal compartment syndrome | 32 (0.0) | 38 (0.4) | < 0.01 |
| Others | 759 (0.5) | 328 (3.2) | < 0.01 |
| Respiratory | |||
| Lung edema | 120 (0.1) | 167 (1.6) | < 0.01 |
| Atelectasis | 906 (0.6) | 1048 (10.1) | < 0.01 |
| Pulmonary embolism | 289 (0.2) | 495 (4.8) | < 0.01 |
| ARDS and respiratory failure | 343 (0.2) | 618 (6.0) | < 0.01 |
| Others | 459 (0.3) | 199 (1.9) | < 0.01 |
| Gastroenterology and hepato-biliary | |||
| Ulcer and upper GI bleeding | 425 (0.3) | 325 (3.1) | < 0.01 |
| Ileus | 219 (0.2) | 159 (1.5) | < 0.01 |
| Pancreatitis | 65 (0.1) | 52 (0.5) | < 0.01 |
| Hyperbilirubinemia and liver failure | 111 (0.1) | 165 (1.6) | < 0.01 |
| Others | 560 (0.4) | 345 (3.3) | < 0.01 |
| Bone and joint | |||
| Compartment syndrome | 219 (0.2) | 346 (3.4) | < 0.01 |
| Refracture | 62 (0.0) | 342 (3.3) | < 0.01 |
| Pseudoarthrosis | 57 (0.0) | 377 (3.7) | < 0.01 |
| Others | 381 (0.3) | 160 (1.6) | < 0.01 |
| Coagulation | |||
| DIC and coagulation disorder | 706 (0.5) | 758 (7.3) | < 0.01 |
| Thrombopenia (< 50,000) | 285 (0.2) | 347 (3.4) | < 0.01 |
| Others | 355 (0.3) | 109 (1.1) | < 0.01 |
| Others | |||
| Wound disruption | 177 (0.1) | 321 (3.1) | < 0.01 |
| Decubitus | 402 (0.3) | 410 (4.0) | < 0.01 |
| Hypothermia (< 35 °C) | 203 (0.1) | 141 (1.4) | < 0.01 |
| Drug allergy | 116 (0.1) | 76 (0.7) | < 0.01 |
| Others | 1123 (0.8) | 428 (4.1) | < 0.01 |
PTSD post trauma stress disorder, ARDS acute respiratory destress syndrome, GI gastrointestinal, DIC disseminated intravascular coagulopathy.
Patients with infection had higher in-hospital mortality [1085 (10.5%) vs. 2898 (2.1%), p < 0.01], a longer hospital stay [42 (25–70) vs. 22 (14–38) days, p < 0.01], and less discharge at home [2469 (26.7%) vs. 61,702 (44.9%), p < 0.01] than patients without infection (Table 3).
Table 3.
Outcome of trauma patients with and without infection.
| Non-infection | Infection | p value | |
|---|---|---|---|
| Number | 140,610 (93.2) | 10,338 (6.8) | |
| Admission | |||
| ICU | 79,966 (56.9) | 7315 (70.8) | < 0.01 |
| General ward | 60,644 (43.1) | 3023 (29.2) | |
| In-hospital mortality | 2898 (2.1) | 1085 (10.5) | < 0.01 |
| Place after discharge | |||
| Home | 61,702 (44.9) | 2469 (26.7) | < 0.01 |
| Transfer | 72,833 (53.0) | 6532 (70.7) | |
| Other | 2925 (2.1) | 241 (2.6) | |
| LOS | 22 (14–38) | 42 (25–70) | < 0.01 |
Continuous variables were compared using the Mann–Whitney U test. Categorical variables were compared using the Chi-square test.
Missing: place after discharge = 263.
ICU intensive care unit, LOS length of hospital stay.
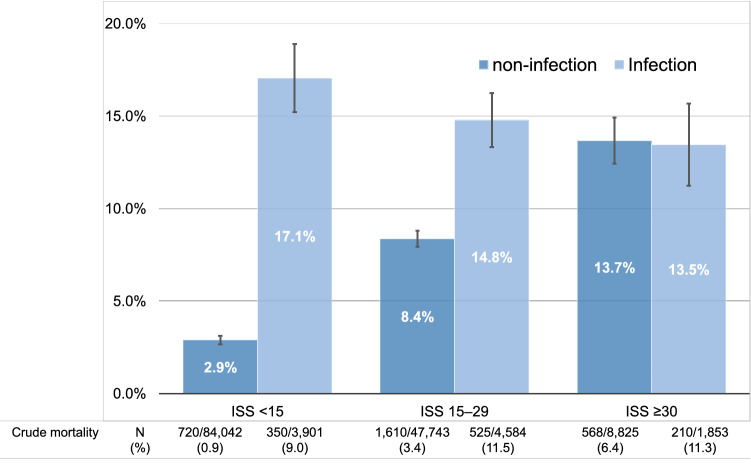
Figure 2 shows the effect of infection on trauma patients according to trauma severity. The in-hospital mortality rate between trauma patients with infection and those without infection differed according to trauma severity [17.1% (95% CI 15.2%–18.9%) vs. 2.9% (95% CI 2.7%–3.1%), p < 0.01, in patients with mild trauma; 14.8% (95% CI 13.3%–16.3%) vs. 8.4% (95% CI 7.9%–8.8%), p < 0.01, in patients with moderate trauma; and 13.5% (95% CI 11.2%–15.7%) vs. 13.7% (95% CI 12.4%–14.9%), p = 0.86, in patients with severe trauma]. Details on the multivariable logistic regression analysis are shown in Supplementary Table 2.
Figure 2.
Crude and adjusted in-hospital mortality in patients with and without infection by trauma severity. The crude mortalities are summarized in the table and adjusted mortalities and its 95% confidence interval calculated by the multivariable logistic regression analysis are demonstrated with a bar graph with error bars. ISS Injury Severity Score.
We analyzed a subgroup of patients with hospital stays of longer than 14 days from admission to reduce the impact of immortal time bias. Infection occurred in 8.2% of patients. The outcomes in this subgroup analysis were similar to the primary results (Supplementary Table 3). Multivariable logistic regression analysis also showed differences in the impact of infection according to trauma severity: the impact of infections was higher in patients with mild trauma (Supplementary Table 4).
Discussion
We assessed the impact of infection complications on in-hospital mortality among trauma patients. The impact differed by trauma severity; specifically, infection after trauma increased in-hospital mortality to a greater degree in patients with mild or moderate trauma than in patients with severe trauma.
Among patients with mild or moderate trauma, infection complications were associated with increased in-hospital mortality. Our results were consistent with previous studies using a national database1 and a statewide database16 in the United States. Although other studies10,14,19 did not show the association between infection and in-hospital mortality among trauma, their results have limited generalizability because they were small and single-center studies. Infectious complications in trauma, similar to in postoperative patients20 and patients with non-infectious internal diseases21, would have a worse impact on their prognosis.
Results of the present study revealed little association between infection complications and increased in-hospital mortality in patients with severe trauma. The results in previous studies1,16 were partially inconsistent with our study. They noted that infection complications were associated with increased in-hospital mortality although it was a little effect among patients with severe trauma compared with patients with mild trauma. We believe our study is more accurate because we excluded early trauma death and we adjusted for more important confounders such as transfusion, which were not included in previous studies1,16. The lower impact of infection in severely injured patients might have occurred because non-infectious complications had a greater impact on mortality than infectious complications17. Infection is a common complication among trauma patients regardless of the injury severity. On the other hand, complications associated with high mortality, including acute respiratory destress syndrome or disseminated intravascular coagulation, rarely occur in mild trauma patients, as shown in Table 2. Infection complication alone might not affect mortality in severely injured patients.
Previous studies10,17,22 have reported that the risk for developing infection was high in patients with severe trauma. However, our findings emphasize the importance of paying more attention to the complications of infection, even if the severity of trauma is mild. Patients with less severe disease usually receive less monitoring23. Thus, the early recognition of infection plays a key role in managing patients with trauma.
Limitations
There are some limitations in the present study. First, infection was diagnosed by the physician in charge, which might have resulted in misclassification. Our study included not only septic patients but also patients with non-septic infections. The incidence of infection in patients with trauma in the present study of 6.8%. This incidence may be lower than some previous studies, which included incidences of sepsis from 2 to 15%1,16,22. The variety of the study population and study design may have contributed to the different incidence of infection. Second, some complications might have been under-reported, as discussed in our previous study2, potentially leading to the overestimation or underestimation of the impact of infection on mortality. Third, we did lacked data on the treatments for both trauma and infection, which might have affected the outcomes. Since 2002, guidelines for trauma care called the Japan Advanced Trauma Evaluation and Care that was created with reference to the Advanced Trauma Life Support24 were introduced in Japan. Furthermore, a previous study showed high compliance with the sepsis bundle in Japan25. Therefore, we believe that most patients received appropriate treatments. Fourth, immortal time bias may have affected our results because the onset of infection was unknown. To address immortal bias, we excluded patients who died < 7 days from hospital admission. In addition, a subgroup analysis of patients with hospital stays longer than 14 days demonstrated similar findings. Therefore, the effect of immortal bias was not strong enough to change our results. Fifth, a number of concomitant complications were significantly associated with decreased in-hospital mortality (Supplementary Table 2), which might be inconsistent clinically. We could not determine whether the complications had a positive or negative impact on in-hospital mortality because we had no data on the time of the onset of complications. However, we kept these variables in the logistic regression analysis because of their clinical importance. Sixth, infection as the primary cause of death could not be verified because of data limitations. This limitation may have affected our results. Finally, data on the level of consciousness after admission were unavailable. Prolonged disturbance of consciousness might have affected the outcomes.
Conclusion
Infection complications after trauma affected in-hospital mortality differently according to injury severity. Greater attention to infection complications is necessary among patients with trauma, even if their severity is mild.
Supplementary Information
Acknowledgements
We thank Enago (https://www.enago.jp) for the final English language editing. We also thank the Japan Trauma Data Bank and all personnel at the participating institutions who contributed the data.
Author contributions
A.K. conceived of and designed this study, analyzed and interpreted the patient data, and was a major contributor in writing the manuscript. H.I. contributed to data interpretation and revised the manuscript for important intellectual content. T.K. contributed to data interpretation. M.A. contributed to the acquisition of data. T.N. revised the manuscript for important intellectual content. T.A. contributed to the acquisition of data, conceived of and designed this study, interpreted the data, and revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Data availability
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-93314-5.
References
- 1.Eguia E, et al. Trends, cost, and mortality from sepsis after trauma in the United States: An evaluation of the national inpatient sample of hospitalizations, 2012–2016. Crit. Care Med. 2020;48:1296–1303. doi: 10.1097/CCM.0000000000004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe T, et al. Trauma complications and in-hospital mortality: Failure-to-rescue. Crit. Care. 2020;24:223. doi: 10.1186/s13054-020-02951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Saverio S, et al. Changes in the outcomes of severe trauma patients from 15-year experience in a Western European trauma ICU of Emilia Romagna region (1996–2010). A population cross-sectional survey study. Langenbecks Arch. Surg. 2014;399:109–126. doi: 10.1007/s00423-013-1143-9. [DOI] [PubMed] [Google Scholar]
- 4.Nagata I, Abe T, Uchida M, Saitoh D, Tamiya N. Ten-year inhospital mortality trends for patients with trauma in Japan: A multicentre observational study. BMJ Open. 2018;8:e018635. doi: 10.1136/bmjopen-2017-018635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar G, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 6.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, et al. Comparison of European ICU patients in 2012 (ICON) versus 2002 (SOAP) Intensive Care Med. 2018;44:337–344. doi: 10.1007/s00134-017-5043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy MM, et al. Surviving Sepsis Campaign: Association between performance metrics and outcomes in a 7.5-year study. Crit. Care Med. 2015;43:3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 9.Kempker JA, Martin GS. The changing epidemiology and definitions of sepsis. Clin. Chest Med. 2016;37:165–179. doi: 10.1016/j.ccm.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eguia E, et al. Risk factors for infection and evaluation of Sepsis-3 in patients with trauma. Am. J. Surg. 2019;218:851–857. doi: 10.1016/j.amjsurg.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japan trauma care and Research, Japan trauma data bank report Japan trauma data bank report 2018 (2013–2017). https://www.jtcr-jatec.org/traumabank/dataroom/data/JTDB2018e.pdf (2018).
- 12.Almpani M, et al. Denver and Marshall scores successfully predict susceptibility to multiple independent infections in trauma patients. PLoS ONE. 2020;15:e0232175. doi: 10.1371/journal.pone.0232175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung S, et al. Timing and associated factors for Sepsis-3 in severe trauma patients: A 3-year single trauma Center Experience. Acute Crit. Care. 2018;33:130–134. doi: 10.4266/acc.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole E, Davenport R, Willett K, Brohi K. The burden of infection in severely injured trauma patients and the relationship with admission shock severity. J. Trauma Acute Care Surg. 2014;76:730–735. doi: 10.1097/TA.0b013e31829fdbd7. [DOI] [PubMed] [Google Scholar]
- 15.Levy MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 16.Osborn TM, Tracy JK, Dunne JR, Pasquale M, Napolitano LM. Epidemiology of sepsis in patients with traumatic injury. Crit. Care Med. 2004;32:2234–2340. doi: 10.1097/01.CCM.0000145586.23276.0F. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman EJ, Earl-Royal E, Barie PS, Holena DN. Failure to rescue after infectious complications in a statewide trauma system. Surg. Infect. Larchmt. 2017;18:89–98. doi: 10.1089/sur.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prin M, Li G. Complications and in-hospital mortality in trauma patients treated in intensive care units in the United States, 2013. Int. J. Epidemiol. 2016;3:18. doi: 10.1186/s40621-016-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazarus HM, et al. Trauma patient hospital-associated infections: Risks and outcomes. J. Trauma. 2005;59:188–194. doi: 10.1097/01.TA.0000171535.75484.DF. [DOI] [PubMed] [Google Scholar]
- 20.Rogers MA, et al. Contribution of infection to increased mortality in women after cardiac surgery. Arch. Intern. Med. 2006;166:437–443. doi: 10.1001/archinte.166.4.437. [DOI] [PubMed] [Google Scholar]
- 21.Renaud B, Brun-Buisson C, ICU-Bacteremia Study Group Outcomes of primary and catheter-related bacteremia. A cohort and case–control study in critically ill patients. Am. J. Respir. Crit. Care Med. 2001;163:1584–1590. doi: 10.1164/ajrccm.163.7.9912080. [DOI] [PubMed] [Google Scholar]
- 22.Wafaisade A, et al. Epidemiology and risk factors of sepsis after multiple trauma: An analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit. Care Med. 2011;39:621–628. doi: 10.1097/CCM.0b013e318206d3df. [DOI] [PubMed] [Google Scholar]
- 23.van Galen LS, et al. Delayed recognition of deterioration of patients in General Wards is mostly caused by human related monitoring failures: A root cause analysis of unplanned ICU admissions. PLoS ONE. 2016;11:e0161393. doi: 10.1371/journal.pone.0161393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammad A, Branicki F, Abu-Zidan FM. Educational and clinical impact of Advanced Trauma Life Support (ATLS) courses: A systematic review. World J. Surg. 2014;38:322–329. doi: 10.1007/s00268-013-2294-0. [DOI] [PubMed] [Google Scholar]
- 25.Abe T, et al. Characteristics, management, and in-hospital mortality among patients with severe sepsis in intensive care units in Japan: The FORECAST study. Crit. Care. 2018;22:322. doi: 10.1186/s13054-018-2186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.