Abstract
Kaposi’s sarcoma (KS) originates from vascular endothelial cells, with KS-associated herpesvirus (KSHV) as the etiological agent. SRY-box transcription factor 5 (SOX5) plays different roles in various types of cancer, although its role in KS remains poorly understood. In this study, we identified the role of SOX5 in KS tissues and KSHV-infected cells and elucidated the molecular mechanism. Thirty-two KS patients were enrolled in this study. Measurement of SOX5 mRNA and protein levels in human KS tissues and adjacent control tissues revealed lower levels in KS tissues, with KS patients having higher SOX5 level in the early stages of the disease compared to the later stages. And SOX5 mRNA and protein was also lower in KSHV-infected cells (iSLK-219 and iSLK-BAC) than normal cells (iSLK-Puro). Additionally, SOX5 overexpression inhibited cell proliferation and promoted apoptosis and decreased KSHV-infected cell migration and invasion. Moreover, we found that SOX5 overexpression suppressed the epithelial-to-mesenchymal transition of KSHV-infected cells. These results suggest SOX5 is a suppressor factor during KS development and a potential target for KS treatment.
Keywords: SRY-box transcription factor 5 (SOX5), Kaposi sarcoma (KS), Proliferation, Apoptosis, Epithelial-to-mesenchymal transition (EMT)
Introduction
Kaposi’s sarcoma (KS) is an invasive tumor of vascular endothelial cells and is possibly associated with KS-associated herpesvirus (KSHV; also identified as human herpesvirus-8). Four KS variants exist: classical KS (CKS), endemic KS, organ-transplant KS, and epidemic autoimmune deficiency syndrome-related KS (Boshoff and Weiss 2001; Cook-Mozaffari et al. 1998; Pelser et al. 2009). Typical skin lesions of CKS present in the lower and upper extremities and generally affect elderly men of Mediterranean or Eastern European origin (Jackson et al. 2016; Mesri et al. 2010). In China, it has been shown that the highest incidence of classic KS is found mostly among people of Uygur ethnicity in Xinjiang (Dilnur et al. 2001; Zheng et al. 2017) while the lowest incidence was found in Guangdong (Zhang et al. 2012). KSHV is a malignant human oncovirus belonging to the Gammaherpesvirinae family, with KS believed to have originated due to KSHV invasion and multiple pro-angiogenic stimuli. Notably, the invasive properties and formation of KS tumors can be reversed by clearance of KSHV from infected cells (Dubich et al. 2019; Guedes et al. 2008). Given recent increases in cases of KS, it is important to elucidate its associated molecular mechanisms in order to improve diagnostic and therapeutic methods.
SRY-related high-mobility-group box5 (SOX5) is an important member of the SOX family and is capable of regulating the target gene expression in combination with DNA or regulatory proteins. Physiologically, SOX5 regulates embryonic development and differentiation (Baroti et al. 2016), and recent studies report that SOX5 mediates cell metastasis, epithelial-to-mesenchymal transition (EMT) (Lamouille et al. 2014; Xu et al. 2015), and angiogenesis, with elevated SOX5 levels promoting the migration and invasion of cells related to hepatocellular carcinoma, breast cancer, gastric cancer, and prostate cancer (Wang et al. 2015; Sun et al. 2019; You et al. 2019; Yang et al. 2019). Ephithelial to mesenchymal transition is a process responsible for tumor metastasis which ephithelial cells gradually change into mesenchymal-like cells, and lose the epithelial functionality and characteristic molecular features (Lamouille et al. 2014). EMT closely related with various kinds of cancer and in hepatoma cell line, SOX5 regulated EMT through affecting the expression of E-cadherin and vimentin (Wang et al. 2015). A study reported low levels of SOX5 mRNA in colorectal cancer according to high levels of methylation in the SOX5 promoter, suggesting a possible role as a tumor suppressor (Moon et al. 2014). These findings suggest different functions of SOX5 in various cancer types; however, there are currently no studies focusing on the relationship between SOX5 and KS. In our previous study, we found SOX5 expression decreased in KS tissues from analysis of a tissue mRNA profile chip (unpublished data). Therefore, in the present study, we measured SOX5 expression in KS tissues and paired non-tumor controls and identified SOX5 function in KSHV-infected cell lines during proliferation, apoptosis, invasion, and migration. Furthermore, we analyzed SOX5-specific molecular mechanisms involved in KS development.
Materials and Methods
Patients
KS tissues and adjacent control tissues were obtained from 32 patients with CKS at the First Affiliated Hospital of the Medical College, Shihezi University, and the Sixth People’s Hospital of Xinjiang Uygur Autonomous Region. The patients were aged from 18 to 68 years, and five were women. Classic KS can be classified into four stages according to the lesion distribution and disease progression (Brambilla et al. 2003). The characteristics of the patients are summarized in Table 1.
Table 1.
Association between Clinical Characteristics and SOX5 expression in KS Tissues.
| Variable | Case (n) | SOX5 | P | |
|---|---|---|---|---|
| Positive (%) | Negative (%) | |||
| Ethnicity | ||||
| Uygur | 7 | 2 (28.6) | 5 (71.4) | 0.590 |
| Kazakh | 25 | 4 (16.0) | 21 (84.0) | |
| Age | ||||
| <50 | 8 | 3 (37.5) | 5 (62.5) | 0.148 |
| ≥50 | 24 | 3 (12.5) | 21 (87.5) | |
| Clinical stages | ||||
| I/II | 11 | 5 (45.4) | 6 (54.5) | 0.011 |
| III/IV | 21 | 1 (4.8) | 20 (95.2) | |
| Skin lesion form | ||||
| Nodule and Plaque | 10 | 3 (30.0) | 7 (70.0) | 0.346 |
| Patch | 22 | 3 (13.6) | 19 (86.4) | |
| Skin lesion distribution | ||||
| Limbs | 13 | 3 (23.1) | 10 (76.9) | 0.666 |
| Upper limbs/lower limbs | 19 | 3 (15.8) | 16 (84.2) | |
Immunohistochemistry (IHC)
IHC analysis was performed to detect SOX5 levels in CKS tissues and adjacent control tissues. The paraffin-embedded slides were deparaffinized, rehydrated, and heat-treated for antigen retrieval, followed by blocking with hydrogen peroxide and blocking serum and incubation with a primary antibody at 4 °C overnight. The primary antibody against SOX5 was rabbit polyclonal (ab26041, 1:50) and obtained from Abcam (Cambridge, UK). Proteins were then stained with biotin-conjugated anti-IgG serum and a DAB working solution until development. Slides were observed under a microscope (Olympus Corporation, Tokyo, Japan) in a blinded manner.
Cell Culture and Transfection
iSLK-219 and iSLK-BAC cells infected with KSHV were maintained in Dulbecco’s modified Eagle medium (DMEM) containing 20% fetal bovine serum (FBS), 4 μg/mL puromycin, 100 μg/mL G418 (Life Technologies, Carlsbad, CA, USA), and 100 μg/mL hygromycin. The iSLK-Puro cell line as the negative control was maintained in DMEM containing 20% fetal bovine serum, 4 μg/mL puromycin, and 100 μg/mL G418. Cells were incubated at 37 °C with 5% CO2. For SOX5 overexpression, plasmids constructed by Genechem Company (Shanghai, China) harboring the SOX5 gene were transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Cells were seeded in 24-well plates and grown to 70%–90% confluence for transfection. Plasmid complexes were prepared (1 μg plasmid and 2.5 μL reagent per well), incubated at room temperature for 20 min, and then cell transfections were performed. After 48 h, cells were collected for experiments. Western blot and qRT-PCR were used to confirm transfection efficiency.
RNA Isolation and Quantitative Reverse Transcription PCR (RT-PCR)
Total RNA was extracted from CKS tissues, adjacent control tissues, and cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA were generated using the Titanium One-Step RT-PCR kit (Takara, Dalian, China). The following primers were used for PCR: SOX forward (5′-AACAAGCACAGATCCCCATTG-3′) and reverse (5′-ACACCGTAAGTGCTCTGGATA-3′); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward (5′-GCACCGTCAAGGCTGAGAAC-3′) and (5′-ATGGTGGTGAAGACGCCAGT-3′). GAPDH was used as an internal control, and data were presented as the relative quantity of targets normalized against the internal control or relative to a calibrator control sample.
Western Blot Analysis
Rabbit polyclonal anti-SOX5 was purchased from Abcam (Cambridge, UK, ab26041, 1:500), and rabbit anti-GADPH was purchased from Boster Biological Technology (BBT; Wuhan, China; A00227-1, 1:500). For Western blot analysis, total cellular protein was extracted from sodium dodecyl sulfate (SDS) sample buffer twice and heated for 5 min at 95 °C. The proteins were separated by 4%–12% SDS polyacrylamide gel electrophoresis and transferred to polyvinylidenefluoride membranes, blocked with 5% nonfat milk in Tris-buffered saline with Tween 20, and incubated with the appropriate primary and secondary antibodies (Zhongshan Golden Bridge Bio-technology, Beijing, China). The blots were developed in ECL reagent and visualized by Imager.
Cell Apoptosis Assay
iSLK-BAC and iSLK-219 cells were transfected with pEGFP-SOX5. iSLK-Puro cells were used as controls. After a 24-h incubation, cells were harvested with trypsin, centrifuged at 200 g for 5 min, and washed twice with phosphate-buffered saline. The cell pellet was suspended in binding buffer from the AnnexinV-fluorescein isothiocyanate (FITC) kit (BD Biosciences, San Jose, CA, USA), followed by addition of 5 μL of Annexin V FITC solution to the mixture. Cells were then incubated for 5 min at 25 °C and analyzed by flow cytometry (Millipore, Billerica, MA, USA).
Proliferation Assay
Following transfection, cell lines were seeded in six-well plates, and at the indicated time, 10 μL of Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) solution was added to each well and cultured for another 100 min. The absorbance was measured at 450 nm to determine growth rates according to manufacturer instructions.
Colony Formation Assay
iSLK-BAC, iSLK-219, and iSLK-Puro cells were digested and seeded into six-well plates (2 × 103 cells/well) in 2 mL of complete medium. After incubating for 2 weeks, the colonies were washed and fixed with 4% paraformaldehyde for 30 min at room temperature, followed by staining with 0.1% Crystal Violet.
Cell Migration and Invasion Assays
For the migration assay, transfected cells were seeded into the top chamber of a 24-well 8-μm pore Transwell plate (Corning Incorporated, Corning, NY, USA). For the invasion assay, cells were seeded into a Matrigel-coated chamber (BD Biosciences), after which medium supplemented with 20% FBS was added as the chemoattractant. After 24 h, cells on the lower surface of the filters were fixed with 4% paraformaldehyde and stained with 0.05% Crystal Violet solution. The average number of cells in five random fields was counted under an inverted light microscope (Olympus Corporation).
Wound-Healing Assay
Transfected iSLK-BAC and iSLK-219 cells were seeded in serum-free medium in six-well plates and grown until confluence. An artificial linear wound was created using a sterile 10-μL plastic tip, followed by washing to remove detached cells. Photomicrographs were obtained at 0 h and 48 h to assess wound healing.
Immunofluorescence Staining
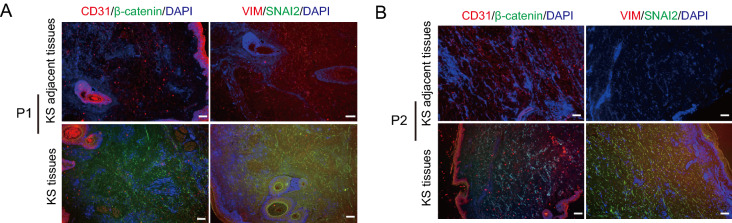
Immunofluorescence staining was performed to detect CD31/β-catenin and Vim/Snai2 expression in KS tissues and adjacent control tissues. The paraffin-embedded slides were deparaffinized, rehydrated, and heat-treated for antigen retrieval, followed by blocking with hydrogen peroxide and bovine serum albumin and incubation with a primary antibody (CD31, BBT, PB9094, 1:200; β-catenin, BBT, PA1212, 1:200; Vim, BBT, BM0135,1:200; Snai2, BBT, PB9439, 1:200) at 4 °C overnight. Slides were then incubated with a FITC-labeled secondary antibody (AAT Bioquest, CA, USA, Catalogue#16,448, goat anti-mouse IgG(H + L), 1:2000; Catalogue#16,220, goat anti-rabbit IgG (H + L), 1:2000) and stained with 4′, 6-diamidino-2-phenylindole for another 10 min. Images were captured under a fluorescence microscope (Olympus Corporation).
Statistical Analysis
SOX5 expression and clinicopathological features were compared using the chi-squared test. Other data are presented as the mean ± standard deviation and were analyzed using Student’s t test. All statistical analyses were performed using SPSS software (v.19.0; IBM Corp, Armonk, NY, USA). A value of P < 0.05 was considered statistically significant.
Results
SOX5 Expression is Lower in KS Tissues and KSHV-Infected Cells Relative to Control Tissues and Cells
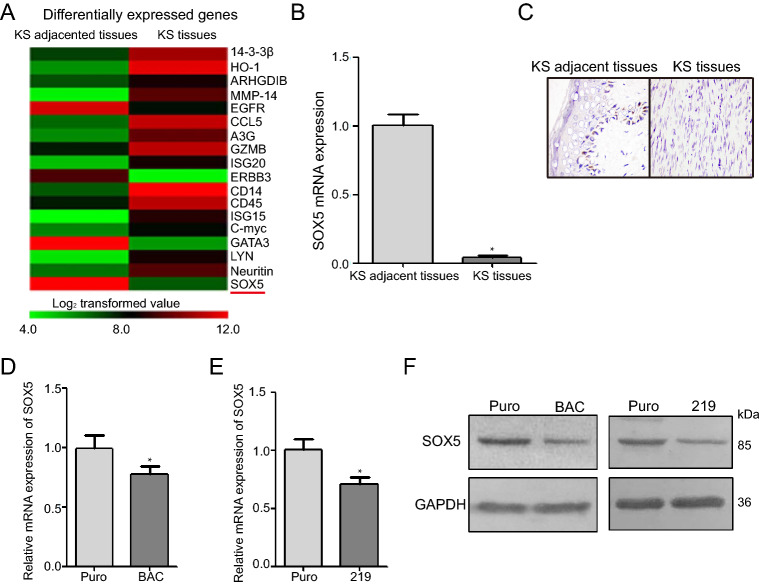
We analyzed the relevance between SOX5 expression and clinical parameters, such as disease stage and skin-lesion formation and distribution (Table 1). The positive rate of SOX5 in KS tissues (9.09%) was drastically lower than that in normal tissues (95.45%; P < 0.05), and collectively, the positive rate of SOX5 was higher in stages I and II of disease cases than in III and IV (P < 0.05). Additionally, SOX5 expression was lower in KS tissues than that in adjacent control tissues from a tissue mRNA profile chip (Fig. 1A). qRT-PCR analysis confirming decreased SOX5 mRNA levels in KS tissues relative to normal tissues (Fig. 1B), with IHC staining showed lower SOX5 expression in KS tissues relative to adjacent normal tissues (P < 0.01; Fig. 1C). Moreover, we found that SOX5 mRNA and protein levels were lower in KSHV-infected iSLK-219 and iSLK-BAC cells relative to control iSLK-Puro cells (Fig. 1D, 1E and 1F; P < 0.05). These findings indicated that SOX5 levels decreased in KS tissues and KSHV-infected cells.
Fig. 1.
SOX5 expression is attenuated in KS tissues and KSHV-infected cells. A cDNA microarray analysis revealed lower expression of SOX5 in KS tissues relative to adjacent normal tissues. B SOX5 mRNA levels were lower in KS tissues than in adjacent normal tissues. C Immunohistochemical analysis of SOX5 levels indicating a decrease in KS tissues relative to adjacent tissues. D, E and F SOX5 mRNA and protein levels were higher in control cells (iSLK-Puro) than in KSHV-infected cells (iSLK-BAC and iSLK-219). *P < 0.05.
SOX5 Inhibits Cell Proliferation and Promotes Apoptosis in KSHV-Infected Cells
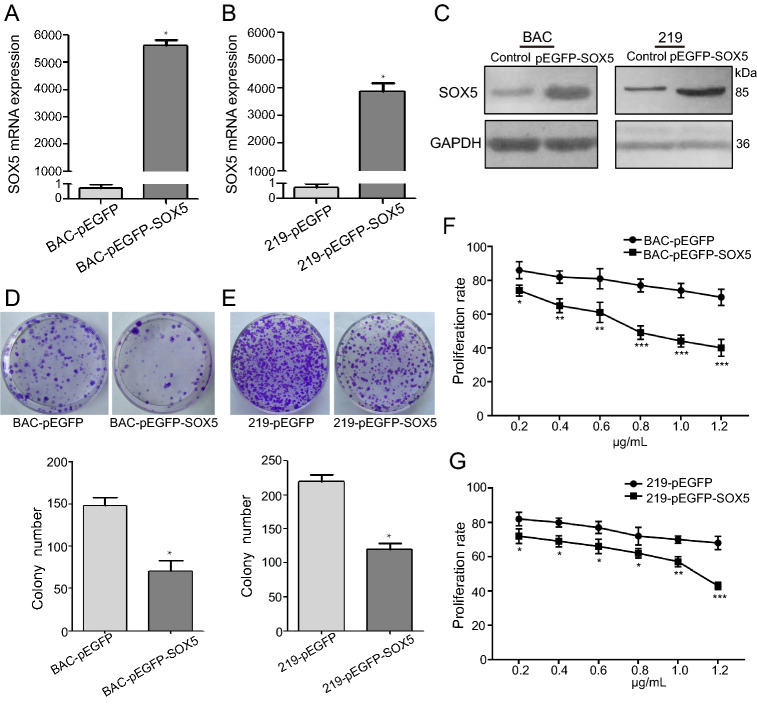
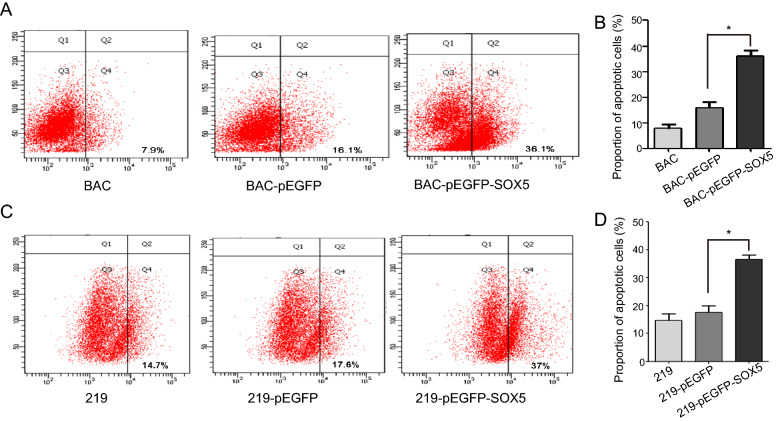
To determine the function of SOX5 in KS development, pEGFP-SOX5 plasmids were transfected into KSHV-infected cells, and cell proliferation and apoptosis were evaluated. We confirmed increases in SOX5 mRNA and protein levels in iSLK-219 and iSLK-BAC cells according to qRT-PCR and Western blot, respectively (Fig. 2A−2C; P < 0.05). Additionally, colony formation assays demonstrated fewer colonies of transfected cells relative to controls (Fig. 2D, 2E), and the CCK8 assay revealed dose-dependent decreases in proliferation according to the amount of transfected SOX5 plasmid (Fig. 2F, 2G). Moreover, the proportion of cells undergoing apoptosis increased in transfected cells according to flow cytometry in iSLK-BAC (Fig. 3A, 3B) and iSLK-219 cells (Fig. 3C, 3D; P < 0.05). These data suggested that SOX5 overexpression inhibited cell growth and promoted apoptosis, suggesting its potential efficacy as a tumor suppressor in KS development.
Fig. 2.
SOX5 inhibits cell proliferation of KSHV-infected cells. A, B and C Elevated SOX5 mRNA and protein levels following transfection of the pEGFP-SOX5 plasmid into KSHV-infected cells (iSLK-BAC and iSLK-219). D and E Colony formation decreased in iSLK-BAC (P < 0.05) and iSLK-219 (P < 0.01) transfected with the pEGFP-SOX5 plasmid. F and G Proliferation rates decreased in iSLK-BAC and iSLK-219 cells following SOX5 overexpression. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 3.
SOX5 promotes apoptosis of KSHV-infected cells. A and B SOX5 overexpression increased apoptosis of in iSLK-BAC cells. C and D SOX5 overexpression increased apoptosis of in iSLK-219 cells. *P < 0.05.
SOX5 Inhibits the Migration and Invasion of KSHV-Infected Cells
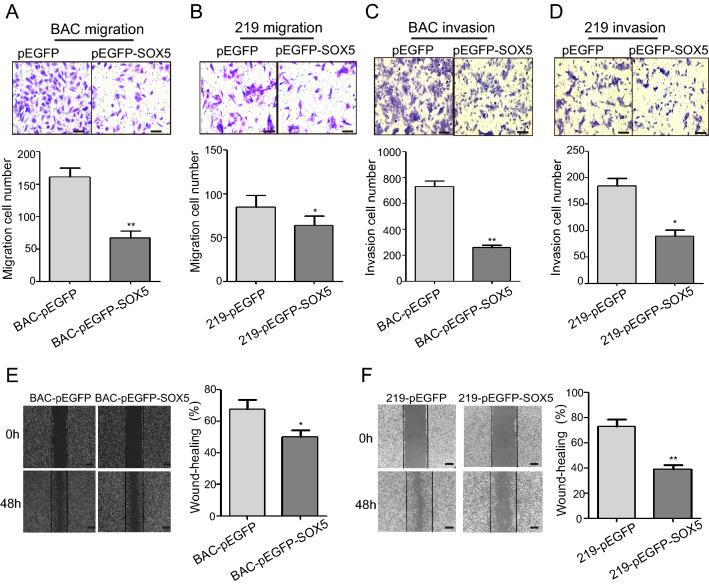
We then performed cell migration assay to detect cell mobility using KSHV-infected cells transfected with pEGFP-SOX5 plasmids. The results indicated decreased migration in both iSLK-BAC and iSLK-219 cells following SOX5 overexpression relative to controls (Fig. 4A, 4B), and transwell invasion assays indicated that SOX5 reduced cell invasion (Fig. 4C, 4D). Additionally, wound-healing assays suggested that both KSHV-infected cell lines exhibited weaker migration capability relative to their respective controls (Fig. 4E, 4F). These findings showed that SOX5 depressed cell migration and invasion in vitro.
Fig. 4.
SOX5 inhibits the migration and invasion of KSHV-infected cells. A and B SOX5 overexpression decreased the migratory in iSLK-BAC and iSLK-219. C and D SOX5 overexpression decreased the invasive capabilities of iSLK-BAC and iSLK-219 according to transwell assays. E and F SOX5 overexpression decreased cell migration in iSLK-BAC and iSLK-219 according to wound-healing assays. *P < 0.05; **P < 0.01. Scale bar = 50 μm.
SOX5 Inhibits KS Cancer-Cell Migration and Invasion by Regulating EMT
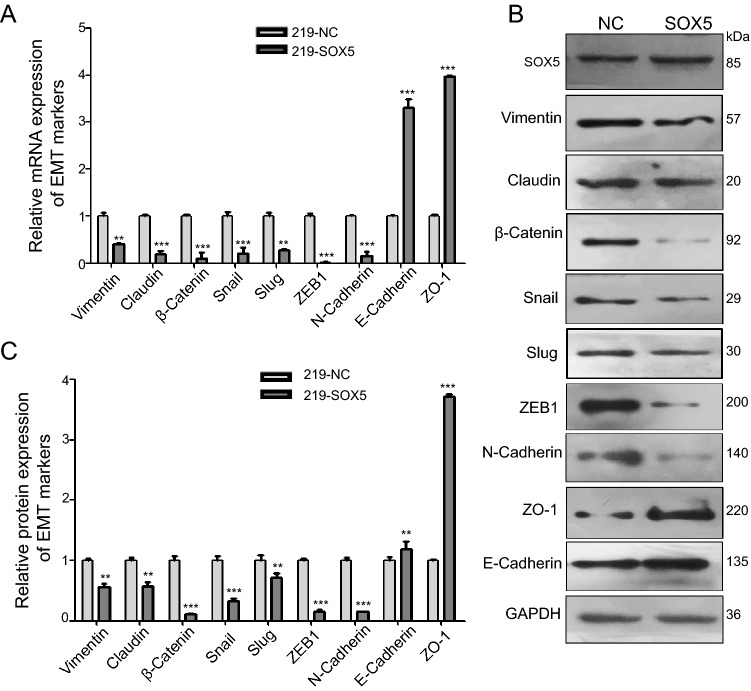
Because SOX5 reportedly promotes cancer-cell metastasis in lung adenocarcinoma, osteosarcoma, and prostate cancer via the EMT (Chen et al. 2018; Hu et al. 2017; Zhang and Liu 2017), we investigated an association between SOX5 expression and EMT in KS tissues and KSHV-infected cells. Immunofluorescence assays using KS tissues indicated lower levels of vimentin, β-catenin, and SNAI2 relative to that in normal tissues (Fig. 5A, 5B). Additionally, in iSLK-219 cells, upregulated SOX5 levels resulted in decreased mRNA and protein levels of vimentin, β-catenin, and SNAI2, whereas those of E-cadherin and zonula occludens-1 (ZO-1) increased (Fig. 6A–6C). These data indicated that SOX5 inhibited KS migration and invasion by inhibiting EMT.
Fig. 5.
The lower level expression of epithelial-to-mesenchymal transition (EMT) is in the KS tissues than that of normal tissues. A and B Immunofluorescence of KS tissues from two patients revealing increases in the expression of CD31 (red), vimentin (red), β-catenin (red), and SNAI2 (green). Scale bar = 100 μm.
Fig. 6.
SOX5 attenuates the migration and invasion of KSHV-infected cells by inhibiting EMT. A Decreases in the mRNA levels of vimentin, claudin, β-Catenin, Snai2, Slug, ZEB1 and N-cadherin accompanied by increases in E-cadherin and ZO-1 levels in iSLK-219 cells transfected with the pEGFP-SOX5 plasmid. B and C Western blot analysis detects the expression of EMT Markers. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Recent studies demonstrate that SOX5 is upregulated in many human malignancies, including hepatocellular carcinoma, prostate cancer, glioma, and nasopharyngeal carcinoma (Wang et al. 2015; Ma et al. 2009; Ueda et al. 2007; Huang et al. 2008), however, there is little evidence demonstrating a role for SOX5 in KS. Here, our transcriptome chip results showed lower SOX5 expression in KS tissues relative to normal tissues, as well as in 32 KS tissues relative to paired adjacent tissues, according to quantitative RT-PCR and immunohistochemical analysis. Moreover, qRT-PCR and western blot analysis confirmed these findings in vitro using KSHV-infected cells. Previous studies have reported varying levels of SOX5 expression according to cancer type. In colon cancer, SOX5 expression decreased due to promoter hypermethylation (Moon et al. 2014), whereas in nasopharyngeal carcinoma, SOX5 expression elevated (Huang et al. 2008). Additionally, posttranscriptional modification of SOX5 produces five subtypes that might play different roles in cancer development (Wunderle et al. 1996). While in the present study, we did not examine the different subtypes in the SOX5 overexpression samples, we plan to analyze the different subtypes of SOX5 in KSHV-infected cells and KS tissues in further studies.
Transcription factors Sox5 and Sox6 exert direct and indirect influences on oligodendroglial migration in the spinal cord and forebrain. SOX5 plays an important role in cancer progression and is closely related to prognosis. Elevated SOX5 expression in nasopharyngeal carcinoma is possibly related to poor survival rates (Huang et al. 2008), and in lung cancer, SOX5 expression is higher in tumor tissue than in non-tumor tissues and correlates with poor prognosis (Chen et al. 2018). By contrast, upregulated SOX5 expression inhibits glioma progression and improves patient prognosis (Ueda et al. 2007). In the present study, we found that SOX5 expression was unrelated to patient age, ethnicity and skin-lesion form, whereas it was related to clinical stage (SOX5 expression was relatively higher in the early stages of KS than in later stages). This suggests that SOX5 expression might be a marker of KS progression.
There are few reports about the relationship between SOX5 expression and KS development. To clarify its role, we upregulated SOX5 expression in KSHV-infected cells and evaluated changes in cell proliferation and colony formation. The results showed that SOX5 overexpression inhibited cell proliferation and colony formation and promoted apoptosis. A previous report showed that SOX5 upregulation promotes the proliferation of lung adenocarcinoma cells (Chen et al. 2018), and RNA interference experiments revealed that downregulated SOX5 expression suppressed the proliferation of pituitary tumor cells (Wang et al. 2015). Additionally, in a melanoma cell line, SOX5 inhibited cell proliferation by decreasing SOX10 expression (Stolt et al. 2008). The differential expression of SOX5 in various tumors indicates its diverse functions according to cancer type. As a transcriptional factor, SOX5 levels can be regulated in multiple ways in different kinds of cancer (Hu et al. 2017; Liu et al. 2018), supporting its varied roles in cancer progression. In prostate cancer, SOX5 can be induced by TGF-β-Smad3, which then promotes Twist1 expression to accelerate the tumor metastasis by inducing EMT.
Previous studies investigated SOX5 function in cell motility according to cancer type. In breast cancer, SOX5 overexpression eliminated the effects of miR-146 s-5p mimics and increased levels of the epithelial marker (E-cadherin) in triple-negative breast cancer cells (Si et al. 2018). Another report indicated that SOX5 overexpression promotes breast cancer metastasis by increasing levels of TWIST and regulating EMT (Pei et al. 2014). In osteosarcoma, SOX5 overexpression promotes EMT by regulating Snail (Zhang and Liu 2017) and enhances cell migration and invasion by increasing levels of key EMT markers in gastric cancer and lung cancer (You et al. 2019; Chen et al. 2018). In the present study, upregulated SOX5 levels in KSHV-infected cells decreased the migration and invasion functions of the cells. Additionally, investigation of SOX5-mediated effects on EMT during KS development revealed decreased vimentin, β-catenin, N-cadherin, and SNAI2 levels in both KS tissue and KSHV-infected cells following SOX5 overexpression, whereas those of E-cadherin and ZO-1 were increased. These findings suggest that SOX5 regulates cell migration and invasion by suppressing EMT during KS development and in KHSV-infected cells. Furthermore, the results indicate that SOX5 represents a tumor suppressor in KS; however, further studies are needed to determine the mechanism associated with how SOX5 regulates EMT during KS development. In conclusion, our data indicate that SOX5 expression is lower in KS tissues and KSHV-infected cells relative to normal tissues and controls, and is related to clinical stage in KS patients. Additionally, we showed that SOX5 overexpression inhibited cell proliferation, migration, and invasion and promoted apoptosis in KSHV-infected cells. Moreover, SOX5 suppressed EMT in KSHV-infected cells. These findings suggest that SOX5 might play a role as a tumor suppressor in KS and represents a valuable therapeutic candidate for controlling KS metastasis.
Acknowledgements
We thank Prof. Ke Lan (State Key Laboratory of Virology, College of Life Sciences, Wuhan University, Wuhan, China) for generously sharing iSLK-PURO, iSLK-219 and iSLK-BAC cell lines. This work was supported by the National Natural Science Foundation of China (U1603117; 81560473), Xinjiang Production and Construction Corps Key Areas Innovation Team Project (2018CB002) and a grant from Shihezi University International Cooperation Project (GJHZ201901).
Author Contributions
YZ and JZ designed the experiments. WMY, YGF, MC, TL and JZ carried out the experiments. YEW, ZJS, and JZ acquired the clinical samples, YGF and WMY analyzed the data. WMY, YGF, JZ and YZ wrote the manuscript. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
All patients provided written informed consent, the procedures used were in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki), and the study was approved by the Ethics Committee of the First Affiliated Hospital of the Medical College, Shihezi University, and Xinjiang Uygur Autonomous Region Sixth People’s Hospital.
Footnotes
Wu-Mei Yuan and Ya-Ge Fan have contributed equally to this work.
Contributor Information
Jun Zheng, Email: haohaoay@yeah.net.
Yan Zeng, Email: zengyan910@gmail.com.
References
- Baroti T, Zimmermann Y, Schillinger A, Liu L, Lommes P, Wegner M, Claus Stolt C. Transcription factors Sox5 and Sox6 exert direct and indirect influences on oligodendroglial migration in spinal cord and forebrain. Glia. 2016;64:122–138. doi: 10.1002/glia.22919. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Weiss RA. Epidemiology and pathogenesis of Kaposi’s sarcoma-associated herpesvirus. Philos Trans R SocLond B BiolSci. 2001;356:517–534. doi: 10.1098/rstb.2000.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla L, Boneschi V, Taglioni M, Ferrucci S. Staging of classic Kaposi's sarcoma: a useful tool for therapeutic choices. Eur J Dermatol. 2003;13:83–86. [PubMed] [Google Scholar]
- Chen X, Fu Y, Xu H, Teng P, Xie Q, Zhang Y, Yan C, Xu Y, Li C, Zhou J, Ni Y, Li W. SOX5 predicts poor prognosis in lung adenocarcinoma and promotes tumor metastasis through epithelial-mesenchymal transition. Oncotarget. 2018;9:10891–10904. doi: 10.18632/oncotarget.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook-Mozaffari P, Newton R, Beral V, Burkitt DP. The geographical distribution of Kaposi’ s sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78:1521–1528. doi: 10.1038/bjc.1998.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilnur P, Katano H, Wang ZH, Osakabe Y, Kudo M, Sata T, Ebihara Y. Classic type of Kaposi’s sarcoma and human herpesvirus 8 infection in Xinjiang, China. PatholInt. 2001;51:845–852. doi: 10.1046/j.1440-1827.2001.01293.x. [DOI] [PubMed] [Google Scholar]
- Dubich T, Lieske A, Santag S, Beauclair G, Rückert J, Herrmann J, Gorges J, Büsche G, Kazmaier U, Hauser H, Stadler M, Schulz TF, Wirth D (2019) An endothelial cell line infected by Kaposi’s sarcoma–associated herpes virus (KSHV) allows the investigation of Kaposi’s sarcoma and the validation of novel viral inhibitors in vitro and in vivo. J Mol Med 97:311–324 [DOI] [PubMed]
- Guedes F, de Andrade HF, Fernandes ER, Tuon FF, Brasil RA, Pagliari C, Duarte MI. The effects of human herpesvirus 8 infection and interferon-gamma response in cutaneous lesions of Kaposi sarcoma differ among human immunodeficiency virus-infected and uninfected individuals. Br J Dermatol. 2008;159:839–846. doi: 10.1111/j.1365-2133.2008.08755.x. [DOI] [PubMed] [Google Scholar]
- Huang DY, Lin YT, Jan PS, Hwang YC, Liang ST, Peng Y, Huang C, Wu H, Lin C. Transcription factor SOX-5 enhances nasopharyngeal carcinoma progression by down-regulating SPARC gene expression. J Pathol. 2008;214:445–455. doi: 10.1002/path.2299. [DOI] [PubMed] [Google Scholar]
- Hu J, Tian J, Zhu S, Sun L, Yu J, Tian H, Dong Q, Luo Q, Jiang N, Niu Y, Shang Z. Sox5 contributes to prostate cancer metastasis and is a master regulator of TGF-β-induced epithelial mesenchymal transition through controlling Twist1 expression. Br J Cancer. 2017;118:88–97. doi: 10.1038/bjc.2017.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CC, Dickson MA, Sadjadi M, Gessain A, Abel L, Jouanguy E, Casanova JL. Kaposi sarcoma of childhood: inborn or acquired immunodefiency to oncogenic HHV-8. Pediatr Blood Cancer. 2016;63:392–397. doi: 10.1002/pbc.25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zheng J, Xue Y, Qu C, Chen J, Wang Z, Li Z, Zhang L, Liu Y. Inhibition of TDP43-mediated SNHG12-miR-195-SOX5 feedback loop impeded malignant biological behaviors of glioma cells. MolTher Nucleic Acids. 2018;10:142–158. doi: 10.1016/j.omtn.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Chan YP, Woolcock B, Hu L, Wong KY, Ling MT, Brainbridge T, Webber D, Chan T, Guan X, Lam W, Vielkind J, Chan K. DNA fingerprinting tags novel altered chromosomal regions and identifies the involvement of SOX5 in the progression of prostate cancer. Int J Cancer. 2009;124:2323–1332. doi: 10.1002/ijc.24243. [DOI] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JW, Lee SK, Lee JO, Kim N, Lee YW, Kim SJ, Kang HJ, Kim J, Kim HS, Park SH. Identification of novel hypermethylated genes and demethylating effect of vincristine in colorectal cancer. J ExpClin Cancer Res. 2014;33:4. doi: 10.1186/1756-9966-33-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei XH, Lv XQ, Li HX. Sox5 induces epithelial to mesenchymal transition by transactivation of Twist1. BiochemBiophys Res Commun. 2014;446:322–327. doi: 10.1016/j.bbrc.2014.02.109. [DOI] [PubMed] [Google Scholar]
- Pelser C, Dazzi C, Graubard BI, Lauria C, Vitale F, Goedert JJ. Risk of classic Kaposi sarcoma with residential exposure to volcanic and related soils in sicily. Ann Epidemiol. 2009;19:597–601. doi: 10.1016/j.annepidem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si C, Yu Q, Yao Y. Effect of miR-146a-5p on proliferation and metastasis of triple-negative breast cancer via regulation of SOX5. ExpTher Med. 2018;15:4515–4521. doi: 10.3892/etm.2018.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Hillgärtner S, Wegner M. The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res. 2008;36:5427–5440. doi: 10.1093/nar/gkn527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Ban Y, Wang K, Sun Y, Zhao Z. SOX5 promotes breast cancer proliferation and invasion by transactivation of EZH2. OncolLett. 2019;17:2754–2762. doi: 10.3892/ol.2019.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda R, Yoshida K, Kawase T, Kawakami Y, Toda M. Preferential expression and frequent IgG responses of a tumor antigen, SOX5, in glioma patients. Int J Cancer. 2007;120:1704–2171. doi: 10.1002/ijc.22472. [DOI] [PubMed] [Google Scholar]
- Wang D, Han S, Wang X, Peng R, Li X. SOX5 promotes epithelial-mesenchymal transition and cell invasion via regulation of Twist1 in hepatocellular carcinoma. Med Oncol. 2015;32:461. doi: 10.1007/s12032-014-0461-2. [DOI] [PubMed] [Google Scholar]
- WunderleCritcher VMR, Ashworth A, Goodfellow PN. Cloning and characterization of SOX5, a new member of the human SOX gene family. Genomics. 1996;36:354–358. doi: 10.1006/geno.1996.0474. [DOI] [PubMed] [Google Scholar]
- Xu W, Yang Z, Lu N. A new role for the PI3K/Aktsignaling pathway in the epithelial-mesenchymal transition. Cell AdhMigr. 2015;9:317–324. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Zhao Q, Fan X, Wang J. SOX5 promotes cell invasion and metastasis via activation of Twist-mediated epithelial-mesenchymal transition in gastric cancer. Onco Targets Ther. 2019;12:2465–2476. doi: 10.2147/OTT.S197087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Zhang W, Sun D, Wei X, Ding Y, Ma Y, Wang Z. Downregulation of miR-139-5p promotes prostate cancer progression through regulation of SOX5. Biomed Pharmacother. 2019;109:2128–2135. doi: 10.1016/j.biopha.2018.09.029. [DOI] [PubMed] [Google Scholar]
- Zhang D, Liu S. SOX5 promotes epithelial-mesenchymal transition in osteosarcoma via regulation of Snail. J BUON. 2017;22:258–264. [PubMed] [Google Scholar]
- Zhang T, Shao X, Chen Y, Zhang T, Minhas V, Wood C, He N. Human herpesvirus 8 seroprevalence, China. Emerg Infect. 2012;18:150–152. doi: 10.3201/eid1801.102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Yang Y, Cui M, Shu ZJ, Han LL, Liu ZQ, Wood C, Zhang T, Zeng Y (2017) Prevalence of Kaposi’s sarcoma-associated herpesvirus in Uygur and Han populations from the Urumqi and Kashgar regions of Xinjiang, China. Virol Sin 32:396–403 [DOI] [PMC free article] [PubMed]