Abstract
We aimed to determine the clinical characteristics and prognosis factors of young patients with gastric cancer (GC).
A total of 101 young patients with GC referred to Zhengzhou University People's Hospital, Henan province, China between January 1st, 2003 and June 1st, 2015 were retrospectively reviewed. The medical records included ages, genders, marital status, family history of tumors, comorbidity, Helicobacter pylori (H.pylori) infection, fibrinogen, prealbumin, alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), tumor location, tumor size, TNM stage, differentiation of the tumor, WHO type, treatment method and prognostic factors effect were further assessed.
The mean age of GC patients in our group was 26.0 years. The incidence was slightly higher in females (female: male = 1.1:1). Some patients had the family history of tumor and H.pylori infection (2.0%, 6.9%). The tumor sizes were mainly under 5 cm (52.4%) and the most locations were in the antrum (43.5%) and body (42.5%). A large number of patients were diagnosed as adenocarcinomas (66.3%) and the main histological of GC was poor differentiated (72.3%). Moreover, a high proportion of patients were diagnosed at the stages III-IV (61.4%), and most patients received surgery combined chemotherapy (63.4%), however, the survival outcome was poor. In univariate Cox analysis, tumor sizes, TNM stage were significantly associated with overall survival (OS) and the multivariate Cox analysis demonstrated that TNM stage was significantly associated with OS.
GC in young patients has its unique biological and clinical features. A large majority of young patients were diagnosed at their advanced stages with poor prognostic. TNM stage was an independent prognostic factor for young patients with GC, early GC screening in young people should be actively promoted.
Keywords: clinical characteristics, gastric cancer, prognosis factors, young patients
1. Introductions
Gastric cancer (GC), which has a unique substantial geographical heterogeneity, is a highly aggressive cancer and the second most frequent cause of cancer-related death worldwide.[1] Between high-risk and low-risk countries, its incidence may vary 5-fold to 10-fold.[2] The incidence of GC is decreasing in high-income countries, whereas it remains high in Asia, particularly, in China.[3,4] GC has different age distributions in different regions; for most countries, GC is considered to be the disease of the elderly and its prevalence increases with aging.[4] However, in the USA, the incidence of GC in younger people (i.e., <50 years) might be increasing.[5]
The clinical and pathological characteristics of young patients were distinct from their older counterparts. Previous studies have shown that GC patients under 40 years have a more aggressive cancer patterns and higher recurrence rate in the gastric remnant than elderly patients, while the 5-year survival rate better than the elderly.[6] Zhao et al found that GC patients 18 to 40 years had a larger number of retrieved lymph nodes, higher proportion of undifferentiated histology type, and more commonly located in the middle or lower third of the stomach than older patients.[7] However, the prognosis is similar. Braga-Neto et al showed that the young patients (≤ 40 years) have a higher frequency of diffuse type of Lauren and less differentiated of GC than elderly patients.[8] The survival was lower in the advanced cases. Similarly, Sandeep et al found that GC patients under 40 years had a higher proportion of poorly differentiated and undifferentiated type of tumors compared with those older than 40 years. Moreover, patients younger than 35 years had a poor prognosis.[9] Overall, the characteristics and prognosis in the young GC patients remain controversial. Most of the previous studies involves patients who were under 40 years, few customized studies on younger groups of GC patients are currently available.
Therefore, we conducted a retrospective study that focused on describing and analyzing the clinical features and prognosis of GC aged 30 and younger. To the best of our knowledge, this is the first study to characterize young GC patients aged 30 or younger in China. We expected that by studying the clinic characteristics and prognostic factors of this special population, we may provide constructive suggestions for the prevention and treatment of young GC patients in the future.
2. Materials and methods
This single-center retrospective cohort study included a total of 85,037 patients diagnosed with GC and treated at Zhengzhou University People's Hospital, Henan province, China between January 1st, 2003 and June 1st, 2015. The inclusion criteria included:
-
1.
histologically confirmed GC;
-
2.
aged 30 years and younger;
-
3.
underwent surgical treatment;
-
4.
no history of malignancy before treatment.
The exclusion criteria included:
-
1.
age above 30 years;
-
2.
pathological information not available.
One hundred one patients were eventually included in this study (Fig. 1).
Figure 1.
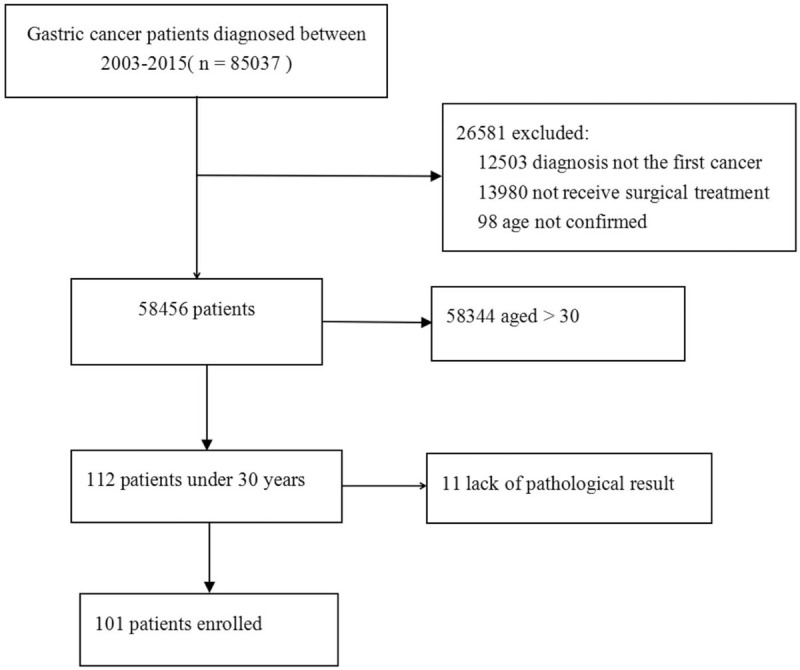
Flow chart of exclusion criteria and study design.
The parameters of the GC patients, including genders, ages at diagnosis, marital status, family history of tumors, comorbidities, H.pylori infection, tumor sizes and locations, histology types, tumor stages, treatment methods, and biochemical tests prior to treatment and diagnosis were reviewed and recorded. This information was retrieved from the electronic record system at our center.
GC may locate in the cardia, body, antrum and cardia + body + antrum. All histological data were reviewed and reclassified according to the 7th edition of the TNM Classification of Malignant Tumors for gastric cancer of the Union for International Cancer Control.[10] Histological types included well-differentiated, moderately-differentiated or poorly-differentiated based the hall-markers of each lesion. Patients were followed up until May 31st, 2020 or their death date. The return visit information was obtained mainly by reviewing the outpatient records or contacting patients or family members by telephone.
This study was conducted by following the Declaration of Helsinki, and was approved by the the Ethics Committee of Zhengzhou University People's Hospital, Henan province, China. All patients were provided with written informed consent before the surgery, and medical records of patients were anonymized.
2.1. Statistical analysis
All analyses were performed by SPSS 25.0 (IBM Corp., Armonk, NY, USA) software. The base distribution in the study used descriptive statistical methods. Survival curve of each patient was obtained according to the Kaplan–Meier method and compared using the log-rank test. The prognostic factors were identified by univariate and multivariate Cox analyses. Variables that reached significance with P < .05 (two-tailed) indicates that the difference was statistically significant.
3. Results
3.1. Patient characteristics
A total of 101 young GC patients, aged ≤30 years were identified from 2003 to 2015. The clinical information of these was shown in Table 1. The patients in this study had a mean age of 26.0 years, among all the young patients with GC, and the majority of patients were female (52.5%) and married (77.2%). In addition, family history of tumor, comorbidity account for 2.0% and 5.9%, respectively. The H.pylori infection status was recorded in only 87 patients, most of whom were diagnosed after 2013, of whom 7 (8.0% in patients with recorded H.pylori status) were H.pylori positive. Mean fibrinogen was 3.13 g/L (range: 1.45–17.6 g/L, reference value: 2–4 g/L), mean prealbumin was 205.0 mg/L (range:41.0–390.0 mg/L, reference value: 200–400 mg/L), mean AFP was 2.65 ng/mL (range: 0.5–219.86 ng/mL, reference value: ≤7 ng/mL), and mean CEA was 2.06 ng/mL (range: 0.2–213.22 ng/mL, reference value: ≤5 ng/mL).
Table 1.
Baseline characteristics of young GC patients in the study.
| Variables | N (%) | |
| Patient-related | Number | 101 |
| Age (y) | ||
| Median | 26.0 | |
| Range | 17–30 | |
| Gender | ||
| Male | 48 (47.5) | |
| Female | 53 (52.5) | |
| Marital status | ||
| Married | 78 (77.2) | |
| Unmarried | 23 (22.8) | |
| Family history of tumor | ||
| Yes | 2 (2.0) | |
| No | 99 (98.0) | |
| Comorbidity | ||
| Yes | 6 (5.9) | |
| No | 95 (94.1) | |
| H.pylori | ||
| + | 7 (6.9) | |
| − | 80 (79.2) | |
| unknown | 14 (13.9) | |
| Fibrinogen (g/L) | ||
| Median | 3.13 | |
| Range | 1.45–17.6 | |
| Prealbumin (mg/L) | ||
| Median | 205.0 | |
| Range | 41.0–390.0 | |
| AFP (ng/mL) | ||
| Median | 2.65 | |
| Range | 0.5–219.86 | |
| CEA (ng/mL) | ||
| Median | 2.06 | |
| Range | 0.2–213.22 | |
| Tumor-related | Location | |
| Cardia | 7 (6.9) | |
| Body | 43 (42.5) | |
| Antrum | 44 (43.5) | |
| Cardia + Body + Antrum | 2 (2.0) | |
| Others | 5 (5.0) | |
| Tumor size (cm) | ||
| 0–2 | 12 (11.9) | |
| 2–5 | 41 (40.5) | |
| 5–7 | 24 (23.8) | |
| >7 | 24 (23.8) | |
| T stage | ||
| 1a | 4 (4.0) | |
| 1b | 3 (3.0) | |
| 2 | 15 (14.9) | |
| 3 | 18 (17.8) | |
| 4a | 46 (45.4) | |
| 4b | 15 (14.9) | |
| N stage | ||
| 0 | 29 (28.7) | |
| 1 | 47 (46.5) | |
| 2 | 19 (18.8) | |
| 3 | 6 (6.0) | |
| M stage | ||
| 0 | 80 (79.3) | |
| 1 | 31 (30.7) | |
| TNM | ||
| IA | 7 (6.9) | |
| IB | 4 (4.0) | |
| IIA | 11 (10.9) | |
| IIB | 17 (16.8) | |
| IIIA | 21 (20.8) | |
| IIIB | 7 (6.9) | |
| IIIC | 3 (3.0) | |
| IV | 31 (30.7) | |
| Differentiation of the tumor | ||
| Well differentiated | 10 (9.9) | |
| Moderately differentiated | 18 (17.8) | |
| Poorly differentiated | 73 (72.3) | |
| WHO type | ||
| adenocarcinoma | 67 (66.3) | |
| Signet ring | 7 (7.0) | |
| Adenocarcinoma & Signet ring | 18 (17.8) | |
| Others | 9 (8.9) | |
| Treatment-related | Surgical appoach | |
| Laparotomy | 38 (37.6) | |
| Laparoscope | 63 (62.4) | |
| Surgical treatment | ||
| Curative | 64 (63.4) | |
| Palliative | 37 (36.6) | |
| Treatment | ||
| Surgery | 35 (34.6) | |
| Surgery + chemotherapy | 64 (63.4) | |
| Lost to follow-up after surgery | 2 (2.0) |
AFP = alpha-fetoprotein, CEA = carcinoembryonic antigen, H.pylori = Helicobacter pylori.
3.2. Lesion characteristics
The tumors of 7 patients (6.9%) were located at the cardia, 43 patients (42.5%) were located at the body, 44 patients (43.5%) were located at the antrum, 2 patients (2.0%) were located at the cardia + body + antrum, 5 patients (5.0%) were located at the others.
The tumors were divided into 5 grades according to their maximum diameters. Among them, 12 cases (11.9%) had a maximum diameter of 0 to 2 cm, 41 cases (40.5%) had a maximum diameter of 2 to 5 cm, 24 cases (23.8%) had a maximum diameter of 5 to 7 cm, 24 cases (23.8%) had a maximum diameter of more than 7 cm.
According to TNM staging criteria, there were 7 patients (6.9%) at stage IA, 4 patients (4.0%) at stage IB, 11 patients (10.9%) at stage IIA, 17 patients (16.8%) at stage IIB, 21 patients (20.8%) at stage IIIA, 7 patients (6.9%) at stage IIIB, 3 patients (3.0%) at stage IIIC, and 31 patients (30.7%) at stage IV.
According to the WHO classification criteria for GC, 67 cases (66.3%) were diagnosed as adenocarcinomas, 7 cases (7.0%) were signet ring carcinoma, 18 cases (17.8%) were adenocarcinoma & signet ring carcinoma, 9 cases (8.9%) were others. In terms of histological grading, the main histological of GC in China is poorly-differentiated, this type of case was represented in up to 72.3% in the cohort, while 10 cases (9.9%) were well-differentiated, 18 cases (17.8%) were moderately-differentiated.
3.3. Treatment characteristics
All patients were treated surgically. Among them, 38 patients (37.6%) received laparotomy, and 63 patients (62.4%) received laparoscope. On the surgical methods, the gastrectomy was divided into curative or pallative resections, more than half of the patients (64, 63.4%) underwent curative operation, while 37 patients (36.6%) underwent palliative resection. In addition, 64 patients (63.4%) received chemotherapy after surgery.
3.4. Type of distant metastasis
The information on distant metastasis was shown in Table 2. There were 31 patients diagnosed stage IV GC, including the involvements in the following locations: peritoneum (3, 9.6%), liver (6, 19.4%), distant lymph nodes (7, 22.6%), lung (4, 12.9%), bone (6, 19.4%), brain (3, 9.6%), and kidney (2, 6.5%).
Table 2.
Distant metastasis type of patients who were diagnosed with stage IV gastric cancer in the cohort.
| Distant metastasis organ involved | Patient number (N = 31) |
| Peritoneum | 3 (9.6) |
| Liver | 6 (19.4) |
| Distant lymph nodes | 7 (22.6) |
| Lung | 4 (12.9) |
| Bone | 6 (19.4) |
| Brain | 3 (9.6) |
| Kidney | 2 (6.5) |
3.5. Symtoms
Of these patients, 13 patients (8 patient at stage I, 3 patients at stage II, 1 patients at stage III, 1 patents at stage IV) were found on physical examination, 88 patients (3 patients at stage I, 25 patients at stage II, 30 patients at stage III, 30 patents at stage IV) were undertaken examination because of symptoms. Respect to symptoms, 81.8% (72 patients) presented with pain, 42.0% (37 patients) presented with a clinical profile of gastric obstruction or early gastric fullness, 30.7% (27 patients) presented with loss of weight.
3.6. Survival outcomes
A total of 99 patients were followed up successfully, the median follow-up time was 26 months (range, 1–60), 85 patients (85.9%) died and 14 patients (14.1%) were still alive. The 1-year and 3-year survival rates for the 99 cases were 67.7% and 24.2%, respectively (Fig. 2). During the follow-up period, 76 patients had gastric cancer recurrence. In univariate Cox analysis, tumor sizes, TNM staging were significantly associated with overall survival (OS) (P < .05). The multivariate Cox analysis demonstrated that TNM staging (HR:0.460, 95% CI: 0.259–0.817, P = .008) was significantly associated with OS (Table 3).
Figure 2.
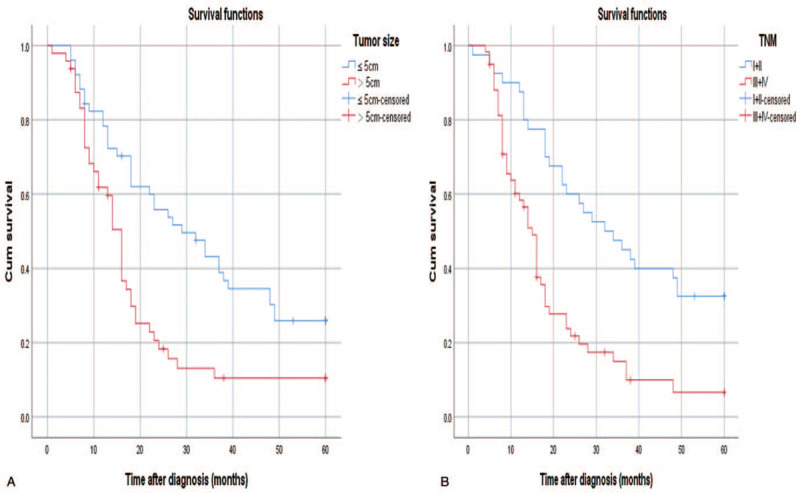
Kaplan–Meier curve depicting overall survival according to tumor size (A), TNM stage (B).
Table 3.
Univariate and multivariate Cox proportional hazard model for GC overall survival (OS) in 99 cases.
| Univariate analysis | Multivariate analysis | ||||
| Variables | N (%) | HR(95% CI) | P value | HR(95% CI) | P value |
| Gender | |||||
| male | 47 (47.5) | Reference | |||
| female | 52 (52.5) | 1.064 (0.677–1.672) | 0.789 | ||
| Maritual | |||||
| No | 31 (31.3) | Reference | |||
| Yes | 68 (68.7) | 1.169 (0.727–1.880) | 0.519 | ||
| Fibrinogen (g/L) | |||||
| ≤ 4 | 67 (67.7) | Reference | |||
| >4 | 32 (32.3) | 0.937 (0.573–1.530) | 0.794 | ||
| Prealbumin (mg/L) | |||||
| >200 | 51 (51.5) | Reference | |||
| ≤200 | 48 (48.5) | 1.279 (0.813–2.010) | 0.287 | ||
| AFP (ng/mL) | |||||
| ≤7 | 86 (86.9) | Reference | |||
| >7 | 13 (13.1) | 1.004 (0.500–2.017) | 0.991 | ||
| CEA (ng/mL) | |||||
| ≤5 | 72 (72.7) | Reference | |||
| >5 | 27 (27.3) | 0.893 (0.544–1.467) | 0.656 | ||
| Tumor size (cm) | |||||
| ≤5 | 74 (74.7) | Reference | |||
| >5 | 25 (25.3) | 0.465 (0.291–0.741) | 0.001 | 1.428 (0.828–2.462) | 0.200 |
| TNM | |||||
| I + II | 40 (40.4) | Reference | |||
| III + IV | 59 (59.6) | 0.380 (0.232–0.621) | <0.001 | 0.460 (0.259–0.817) | 0.008 |
4. Discussion
The incidence of young GC patients has increased, while the definition of young age of GC is inconsistent. Some studies defined early onset as established diagnosis younger than 40,[6,9] while other studies included all patients diagnosed before 45.[8] However, there is no official or recognized cancer organization to give a clear definition of the age limit of young GC patients. Most of the previous studies enrolled patients younger than 40 years. There is a scarcity of data regarding the clinical characteristics and prognosis factors of GC in patients under the age of 30 years. In addition, young people under 30 years old (including 30 years old) represent the next generation and potential future social contributors, and studying the characteristics and prognosis of GC patients in the younger population may have significant implications. For these reasons and for consistency and simplicity, we defined the age as under 30 years.
This study clarified the clinical characteristics and prognosis factors of GC in young patients aged ≤30 years. First, the mean age of GC was 26.0 years, the young female patients (52.5%) accounted for a higher proportion than male patients (47.5%), a few patients had the family history of tumor (2.0%) and H.pylori infection (6.9%). Second, the tumor sizes were usually under 5 cm (52.4%) and a high proportion of tumor location were in the antrum (43.5%) and body (42.5%), a large majority of patients were diagnosed as adenocarcinomas (66.3%) and the main histological type was poor differentiated (72.3%). Third, more than half of young GC patients (59.6%) were diagnosed at the stage III-IV, and 72 patients (71.3%) received chemotherapy after surgery. However, the 5-year survival was poor. Fourth, TNM stage was an independent prognostic factor for young patients with GC.
In the current study, the proportion of young female patients (52.5%) was higher than their male counterparts (47.5%), with a male-to-female ratio of 1:1.1. This trend is mostly consistent with the results of previous studies and demonstrated that a similar female predominance pattern in the young GC.[11,12] To date, the cause of GC is still inexplicable. Previous studies suggest that hereditary and H.pylori infection are critical for its pathogenesis.[13–20]H. pylori may synthesize many different virulence factors that can disrupt the balance between cell proliferation and apoptosis, leading to GC formation.[13,14] Koshida has reported that approximately 73% of antral biopsy specimens obtained from cancerous conditions under the 40 years are H.pylori positive.[15] WHO reported that more than 85% of noncardia gastric cancer cases, accounting for 78% of all gastric cancer cases, may be caused by chronic H.pylori infection.[16] On the contrary, in our study, the proportion of H.pylori infection was very low (7.0%). However, the improvement of socio-economic status, hygienic practices, and the application of widespread antibiotic, the risk of most infections has been reduced, resulting in a reduction in the infection rate.[17] Our result proved the effectiveness of the comprehensive anti-H. pylori infection.
Familial gastric cancer means that ≥3 first- or second-degree relativesrespectively with GC or ≥2 first- or second-degree relatives with GC (at least 1 diagnosed <50 years of age)[18] Approximately 10% of all GC cases show familial aggregation and 1% to 3% of patients with GC have germline mutations, or, in the absence of a germline variant, familial aggregation of these tumors is observed (i.e., familial cancer).[19,20] Besides, in the study by Braga-Neto et al the proportion of family history of GC was 7.3%.[8] However, in our study, the proportion of family history was only 2%, which is inconsistent with previous study. This difference may be due to the H.pylori infection, since several studies have demonstrated that a synergistic interaction between H. pylori infection and family history.[21] The H.pylori infection cluster within families, and it may often be transmitted from parents to their children in early childhood as well as between siblings.[22] Previously studies have reported that the prevalence of H.pylori infection among the first degree relatives of gastric cancer is similar to dyspeptic patient from the same economic level.[23] However, in our study, the proportion of H.pylori infection was very low.
Abnormal biochemical tests are frequently detected in patients with malignant tumors. Previous studies have demonstrated that elevated pre-operative fibrinogen, pre-albumin, AFP, and CEA are associated with the progression and prognosis of GC.[24–28] Inconsistent with these studies, our data showed that the proportions of abnormal fibrinogen, pre-albumin, AFP, and CEA are 32.3%, 48.5%, 13.1%, and 27.3%. In addition, no association has been found between these biochemical tests and outcomes of GC. One explanation is that our study only enrolled patients under 30 years old (including 30 years old), and other studies included patients of all ages.
In our study, tumors were predominant located at the gastric antrum (43.5%), followed by the gastric body (42.5%), which was consistent with the observations made by other groups.[7] The young GC was more likely to have tumor size at 2 to 5 cm (40.5%). In relation to histological types, our study found that 72.3% had poorly differentiated and 66.3% of patients had adenocarcinoma, which was consistent with previous studies.[29] Some studies showed that poorly differentiated is commonly found in younger women patients,[30] and well-differentiated GC predominately found in individuals of an older age, >70 years, who are mostly male and patients present with larger tumor sizes.[31] This may be attributed to the fact that the proportion of men and women is different in different age groups. Among younger patients, female patients account for a larger proportion and younger patients are more likely to have distant metastasis, while among older patients, male patients account for a larger proportion and older patients are more frequent and prolonged exposure to environmental carcinogens.[31] Surprisingly, numerous previous studies showed that undifferentiated was observed more frequently in the young GC.[9] While in our present study, histological type of undifferentiated were not seen in young GC. The reason why undifferentiated was not found may be that undifferentiated type carcinoma often caused by H. pylori infection,[32,33] while, in our study, the percentage of H.pylori infection was low.
According to previous studies, most young patients are already in the advanced level of TNM stages when they are diagnosed.[34,35] When we examined a total of 101 cases, we found that a large percentage of patients were in T4 invasion (60.3%), M1 involvement (30.7%), which was the reason why the patient was in stage IV. Of these patients, 28 patients (1 patient at stage I, 5 patients at stage II, 13 patients at stage III, 9 patents at stage IV) were found on physical examination, 73 patients (10 patients at stage I, 23 patients at stage II, 18 patients at stage III, 22 patents at stage IV) were undertaken examination because of symptoms. The symptoms included: pain (59 patients, 80.8%), gastric obstruction or early gastric fullness (15 patients, 20.5%), weight loss (23 patients, 31.5%). Delayed diagnosis may explain why some of our patients were already in their advanced stage.[36] Therefore, an early diagnosis is crucial in successfully completing a curative resection that provides a better prognosis.[37]
The pathological morphology of GC can be divided into differentiated and undifferentiated types. The overall prognosis of differentiated GC patients is better than that of undifferentiated gastric cancer patients.[38] Based on the survival rate, nutritional status and quality of life of the patients, regardless of the type of GC patients, surgery is the treatment of choice.[39,40] Surgical treatments includes curative or palliative resection. Curative surgery is to complete removal of the primary tumor, regional lymph nodes and invaded tissues and organs without residual tumors. Palliative resection refers to patients with gastric cancer whose primary or metastatic lesions cannot be completely removed.[41] Young GC patients have a good compensatory ability in organ function and a strong ability to resist surgical trauma, surgical treatment is a favorable option for this group of patients. However, it is still controversial whether the metastatic patients are treated surgically. The Dutch Gastric Cancer Group reported that palliative surgical treatment may increase the survival rate in patients with incurable GC, especially for patients under the age of 70 with only 1 metastasis sites.[42] A retrospective cohort study of gastric cancer patients in including 162 stage IV gastric cancer also showed that the median overall survival rates were 22 months vs 9.0 months for patients with and without gastrectomy.[43] This analysis showed that patients with IV GC could benefit from gastrectomy. In our study, all patients received surgical treatment though 31 patients with metastasis. 63.4% patients underwent curative surgical and 36.6% patients underwent palliative surgical. In addition, combined chemotherapy is a major strategy to improve the long-term survival rate of patients with GC.[44,45] Our study found that surgical combination chemotherapy significantly improved the prognosis of young GC patients (P < .001).
In our study, the 5 year survival of young patients was very poor (14.1%), the 1-year and 3-year survival rates for the 99 cases were 67.7% and 24.2%, respectively. Young GC patients showed poor prognosis, which was consistent with the results of Puhr et al.[46] Their study showed that the 5 year survival of patients under 45 years was 17%. Our study found that TNM stage was significantly associated with the prognosis of young GC (P < .05), which was also consistent with previous studies,[9] suggesting a pivotal importance of early diagnosis.
Based on the results, specific measures should be taken to reduce GC in the younger generation. Firstly, reduce risk factors and promote protective factors. Although our study showed that the proportion of H.pylori infection was 7%, the H. pylori infection is a recognized carcinogen for GC and eradication of H. pylori is considered the best strategy to reduce the risk of developing GC.[14] In addition to H. pylori infection, other potential risk factors include smoking, alcohol consumption, coffee and meat consumption, long term PPIs use, etc[47–49] High salt diet may increase the risk of H. pylori infection and may synergistically promote the development of GC.[3] Others seem to have a protective effect, especially the intake of fruits, vegetables and vitamins. Briefly, healthy life styles may decrease the incidence of GC, and these include smoking cessation, Mediterranean diet and normal body mass index.[50] Secondly, close screening and monitoring should be done in young people with respect to the associated common symptoms which could indicate the presence of early GC. The development of gastric mucosa is a multistep process, and timely diagnosis and appropriate management of preneoplastic conditions can reduce GC-related mortality.[51] In addition, the younger generation should also be educated to understand the signs and symptoms of the disease in order to detect and prevent the deterioration of the disease as soon as possible, thereby reducing the mortality rate of the younger generation.
Our study has some limitations. Firstly, it is a retrospective study in a single center, which may lead to bias in patient selection. Therefore, these results might not apply to other populations. Further multicenter prospective studies are thus recommended. Secondly, the sample size is limited, which cannot give sufficient power to the observed results. Further large sample studies are needed. Third, we were unable to collect data from other generations of gastric cancer patients treated in our center, so we did not compare the characteristics of younger generation with other generations. Despite these limitations, to the best of our knowledge, it is the largest published cohort that clarifies the clinical characteristics and prognosis factors of GC in young patients aged ≤30 years, so the results obtained could be a good estimate of the real situation.
5. Conclusions
In summary, our study shows that the clinical and pathological characteristics of gastric cancer in young patients aged 30 years and younger. In our studies, hereditary and H.pylori infection were not the main reasons for young GC. The histology type was mainly adenocarcinoma, and most pathologies were poorly differentiated. Most of the young GC were already in advanced stage at the time of diagnosis, and the 5-year survival was poor. TNM stage was an independent prognostic factor for young patients with GC. Improving the prognosis of young GC patients is critical.
Author contributions
Conceptualization: Liyun Zhou, Zhenhua Jiang.
Data curation: Liyun Zhou, Wenhui Gu.
Formal analysis: Zhenhua Jiang.
Methodology: Wenhui Gu.
Project administration: Shuangyin Han.
Resources: Shuangyin Han.
Writing – original draft: Liyun Zhou, Zhenhua Jiang.
Writing – review & editing: Shuangyin Han.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, CEA = carcinoembryonic antigen, GC = gastric cancer, H.pylori = Helicobacter pylori, OS = overall survival.
How to cite this article: Zhou L, Jiang Z, Gu W, Han S. STROBE-clinical characteristics and prognosis factors of gastric cancer in young patients aged ≤30 years. Medicine. 2021;100:26(e26336).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Białek A, Kulig J. Analysis of clinicopathologic characteristics of gastric cancer in patients ≤40 and ≥40 years of age. Scand J Gastroenterol 2020;55:62–6. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Laversanne M, Brewster DH, Mbalawa CG, Kohler B. Cancer incidence in five continents: inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer 2015;137:2060–71. [DOI] [PubMed] [Google Scholar]
- [3].Smyth EC, Nilsson M, Grabsch H I, Grieken NC, Lordick F. Gastric cancer. Lancet 2020;396:635–48. [DOI] [PubMed] [Google Scholar]
- [4].GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 2020;5:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anderson WF, Rabkin CS, Turner N, Fraumeni JF, Jr, Rosenberg PS, Camargo MC. The changing face of noncardia gastric cancer incidence among US non-Hispanic Whites. J Natl Cancer Inst 2018;110:608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Z, Xu J, Shi Z, Shen X, Luo T, Bi J. Clinicopathologic characteristics and prognostic of gastric cancer in young patients. Scand J Gastroenterol 2016;51:1043–9. [DOI] [PubMed] [Google Scholar]
- [7].Zhao B, Mei D, Lv W, Lu H, Bao S, Lin J. Clinicopathologic features, survival outcome, and prognostic factors in gastric cancer patients 18–40 years of age. J Adolesc Young Adult Oncol 2020;9:514–21. [DOI] [PubMed] [Google Scholar]
- [8].Braga-Neto MB, Carneiro JG, de Castro Barbosa AM, et al. Clinical characteristics of distal gastric cancer in young adults from Northeastern Brazil. BMC Cancer 2018;18:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sandeep B, Huang X, Li Y, Mao L, Gao K, Xiao Z. Gastric carcinoma in young patients and its clinicopathological characteristics and prognosis. Gastroenterol Res Pract 2020. eCollection7378215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7th edOxford (UK): Wiley-Blackwell; 2009. [Google Scholar]
- [11].Mori M, Sugimachi K, Ohiwa T, Okamura T, Tamura S, Inokuchi K. Early gastric carcinoma in Japanese patients under 30 years of age. Br J Surg 1985;72:289–91. [DOI] [PubMed] [Google Scholar]
- [12].Ferro A, Peleteiro B, Malvezzi M. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 2014;50:1330–44. [DOI] [PubMed] [Google Scholar]
- [13].Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202. [DOI] [PubMed] [Google Scholar]
- [14].Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 2018;378:1085–95. [DOI] [PubMed] [Google Scholar]
- [15].Koshida Y, Koizumi W, Sasabe M, Katoh Y, Okayasu I. Association of Helicobacter pylori-dependent gastritis with gastric carcinomas in young Japanese patients: histopathological comparison of diffuse and intestinal type cancer cases. Histopathology 2000;37:124–30. [DOI] [PubMed] [Google Scholar]
- [16].International Agency for Research on Cancer, World Health Organization. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. In: IARC Working Group Report. Volume 8.2014. http://www.gastrohealth-now.org/wp/wp-content/uploads/2014/09/WHO-IARCReport-2014.pdf. [Google Scholar]
- [17].Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009;7:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Llach J, Moreno L, Sánchez A, et al. Genetic counseling for hereditary gastric and pancreatic cancer in high-risk gastrointestinal cancer clinics: an effective strategy. Cancers (Basel) 2020;12:E2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kluijt I, Sijmons RH, Hoogerbrugge N, et al. Familial gastric cancer: Guidelines for diagnosis, treatment and periodic surveillance. Fam Cancer 2012;11:363–9. [DOI] [PubMed] [Google Scholar]
- [21].Shin CM, Kim N, Yang HJ, et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol 2010;44:e34–9. [DOI] [PubMed] [Google Scholar]
- [22].Fialho AM, Braga AB, Braga Neto MB, et al. Younger siblings play a major role in Helicobacter pylori transmission among children from a low-income community in the Northeast of Brazil. Helicobacter 2010;15:491–6. [DOI] [PubMed] [Google Scholar]
- [23].Motta CR, Cunha MP, Queiroz DM, et al. Gastric precancerous lesions and Helicobacter pylori infection in relatives of gastric cancer patients from Northeastern Brazil. Digestion 2008;78:03–8. [DOI] [PubMed] [Google Scholar]
- [24].Ding P, Zheng C, Cao G, et al. Combination of preoperative plasma fibrinogen and AJCC staging improves the accuracy of survival prediction for patients with stage I-II gastric cancer after curative gastrectomy. Cancer Med 2019;8:2919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shen Q, Liu W, Quan H, et al. Prealbumin and lymphocyte-based prognostic score, a new tool for predicting long-term survival after curative resection of stage II/III gastric cancer. Br J Nutr 2018;120:1359–69. [DOI] [PubMed] [Google Scholar]
- [26].Liu X, Cheng Y, Sheng W, et al. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol 2010;102:249–55. [DOI] [PubMed] [Google Scholar]
- [27].Park SH, Ku KB, Chung HY, et al. Prognostic significance of serum and tissue carcinoembryonic antigen in patients with gastric adenocarcinomas. Cancer Res Treat 2008;40:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen Y, Qu H, Jian M, et al. High level of serum AFP is an independent negative prognostic factor in gastric cancer. Int J Biol Markers 2015;30:e387–93. [DOI] [PubMed] [Google Scholar]
- [29].Bloss RS, Miller TA, Copeland EM. Carcinoma of the stomach in the young adult. Surg Gynecol Obstet 1980;150:883–6. [PubMed] [Google Scholar]
- [30].Petrelli F, Berenato R, Turati L, et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2017;8:148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liang YX, Deng JY, Guo HH, et al. Characteristics and prognosis of gastric cancer in patients aged ≥70 years. World J Gastroenterol 2013;19:6568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- [33].Asaka M, Takeda H, Sugiyama T, Kato M. What role does Helicobacter pylori play in gastric cancer? Gastroenterology 1997;113:S56–60. [DOI] [PubMed] [Google Scholar]
- [34].Kunisaki C, Akiyama H, Nomura M, et al. Clinicopathological features of gastric carcinoma in younger and middle-aged patients: a comparative study. J Gastrointest Surg 2006;10:1023–32. [DOI] [PubMed] [Google Scholar]
- [35].Santoro R, Carboni F, Lepiane P, Ettorre GM, Santoro E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg 2007;94:737–42. [DOI] [PubMed] [Google Scholar]
- [36].López-Basave HN, Morales-Vásquez F, Ruiz-Molina JM. Ñamendys-Silva SA, Vela-Sarmiento I, Ruan JM, et al. Gastric cancer in young people under 30 years of age: worse prognosis, or delay in diagnosis? Cancer Manag Res 2013;5:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang Y, Li T. Differential diagnosis of gastric lymphoma, gastric stromal tumor and advanced gastric cancer by MSCT three-phase enhanced scan. Chin J CT and MRI 2019;10:120–3. [Google Scholar]
- [38].Feng H, Wang Y, Cao L, et al. Lymph node metastasis in differentiated-type early gastric cancer: a single-center retrospective analysis of surgically resected cases. Scand J Gastroenterol 2016;51:48–54. [DOI] [PubMed] [Google Scholar]
- [39].National Comprehensive Cancer Network Gastric Cancer.(Version 2.2020). [Google Scholar]
- [40].Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (Ver. 4). Gastric Cancer 2017;20:01–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schünemann HJ, Oxman AD, Brozek J, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008;336:1106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hartgrink HH, Putter H, Klein Kranenbarg E, et al. Value of palliative resection in gastric cancer. Br J Surg 2002;89:1438–43. [DOI] [PubMed] [Google Scholar]
- [43].Li SC, Zang L. The effectiveness of Gastrectomy with chemoradioththerapy Among stage IV gastric adenocarcinoma: a population-based analysis. Front Oncol 2020;10:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315–21. [DOI] [PubMed] [Google Scholar]
- [45].Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810–20. [DOI] [PubMed] [Google Scholar]
- [46].Puhr HC, Karner A, Taghizadeh H, Jomrich G, Schoppmann SF, Preusser M. Clinical characteristics and comparison of the outcome in young versus older patients with upper gastrointestinal carcinoma. J Cancer Res Clin Oncol 2020;146:3313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lai HT, Koriyama C, Tokudome S, et al. Waterpipe tobacco smoking and gastric cancer risk among vietnamese men. PLoS One 2016;11:e0165587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dong J, Thrift AP. Alcohol, smoking and risk of oesophago-gastric cancer. Best Pract Res Clin Gastroenterol 2017;31:509–17. [DOI] [PubMed] [Google Scholar]
- [49].Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 2018;67:28–35. [DOI] [PubMed] [Google Scholar]
- [50].Buckland G, Travier N, Huerta JM, et al. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int J Cancer 2015;137:598–606. [DOI] [PubMed] [Google Scholar]
- [51].Eusebi LH, Telese A, Marasco G, Bazzoli F, Zagari RM. Gastric cancer prevention strategies: a global perspective. J Gastroenterol Hepatol 2020;35:1495–502. [DOI] [PubMed] [Google Scholar]


