Abstract
Background:
This meta-analysis aimed to estimate the association of human immunodeficiency virus (HIV) infection and risk of coronavirus disease 2019 (COVID-19) mortality.
Methods:
We systematically retrieved articles published on HIV infection and risk of COVID-19 mortality through PubMed, EMBase, China National Knowledge Infrastructure, WanFang, and Chongqing VIP databases using a predefined search strategy from December 1, 2019 to January 31, 2021. Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included studies. Cochran Q test and I2 statistics were quantified to measure heterogeneity. Odds ratio (OR) and 95% confidence intervals (CI) were computed and displayed in the form of forest plots. Subgroup analysis was performed to explore the source of heterogeneity. Funnel plot, Begg test, and Egger test were used to assess potential publication bias. Stata software version 11.0 was used to analyze all the statistical data.
Results:
We included 10 studies with 18,122,370 COVID-19 patients, of whom 41,113 were with HIV infection and 18,081,257 were without HIV infection. The pooled overall results suggested that people living with HIV infection had a higher risk of mortality from COVID-19 than those without HIV infection (OR = 1.252, 95% CI 1.027–1.524). Subgroup analysis showed that people living with HIV infection had a higher risk of COVID-19 mortality than those without HIV infection in the United States (OR = 1.520, 95% CI 1.252–1.845) and in South Africa (OR = 1.122, 95% CI 1.032–1.220); however, no significant association was found in the United Kingdom (OR = 0.878, 95% CI 0.657–1.174).
Conclusion:
Patients with HIV infection should be the emphasis population to prevent the risk of mortality during the clinical treatment of COVID-19 patients.
Keywords: COVID-19, HIV, meta-analysis, mortality, SARS-CoV-2
1. Introduction
As of January 31, 2021, 103 million people from 223 countries had been confirmed to be infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since its emergence in late 2019, and this virus had caused more than 2.4 million people death.[1] The global pandemic of coronavirus disease 2019 (COVID-19) is having a dramatic impact on social, economic, and population health all over the world.[2–5] It is not clear when the outbreak will stop.
A large number of studies have shown that comorbidities,[6–12] such as hypertension, diabetes, cardiovascular disease, hepatic, pulmonary disease, etc can increase the risk of COVID-19 mortality. Could COVID-19 patients also have an increased risk of death after suffering from human immunodeficiency virus (HIV) co-infection? So far, some studies[13–16] have reported the relationship between HIV co-infection and COVID-19, but the results are inconsistent. Now, there were 2 meta-analyses[17,18] that showed no association between HIV co-infection and the risk of COVID-19 mortality. However, there have been many new studies in this field to reveal the relationship between them since the 2 meta-analyses were performed, and the pooled results of these studies need to be further updated. In addition, some studies included in these 2 meta-analysis studies were with small sample sizes. Studies with a small sample size were more likely to produce negative results due to their insufficient efficacy. Also, the 2 meta-analyses point out that further researches with a larger sample size are needed. Recently, a large-scale population-based study[19] with over 17 million individuals was performed in England to investigate the association of HIV co-infection and the risk of COVID-19 mortality, and found that people living with HIV had a higher risk of COVID-19 mortality than those without HIV after adjusting for age and sex (HR = 2.90, 95% CI 1.96–4.30). If we combined the COVID-19 epidemic information around the world, would this relationship hold up as well as the England study? Are there differences among different countries? So far there is no clear answer.
In view of this, our study intends to adopt the meta-analysis method by selecting literature studies with a relatively large sample size to explore whether HIV co-infection increases the risk of death due to COVID-19.
2. Materials and methods
2.1. Information source and search strategy
This present study was being reported according to the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement. All the studies revealing the relationship between HIV co-infection and the risk of COVID-19 mortality were included. In this study, we searched PubMed (December 1, 2019 to January 31, 2021), EMBase (December 1, 2019 to January 31, 2021), China National Knowledge Infrastructure (December 1, 2019 to January 31, 2021), WanFang Data (December 1, 2019 to January 31, 2021), and Chongqing VIP (December 1, 2019 to January 31, 2021) using a predefined search strategy (Fig. 1). The keywords included the following items: (“COVID-19” OR “2019-nCoV” OR “severe acute respiratory syndrome coronavirus 2” OR “SARS-CoV-2”) AND (“HIV” OR “human immunodeficiency virus infection” OR “AIDS” OR “acquired immune deficiency syndrome”). Two reviewers independently screened the titles and abstracts of the studies. No limitations were applied to language and study design. Additionally, reference lists in this present review were screened to identify the additional relevant studies.
Figure 1.
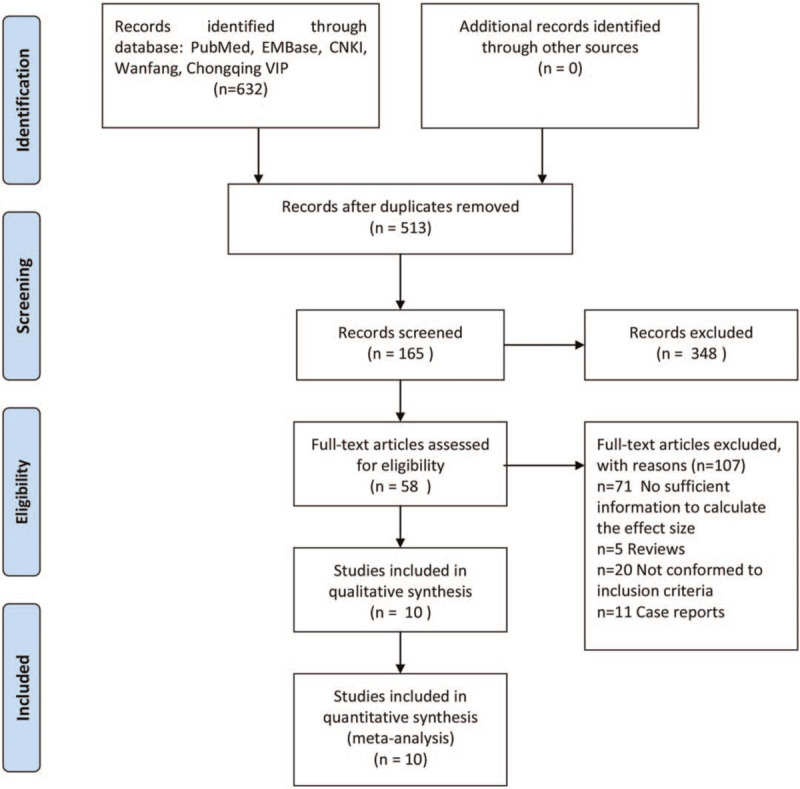
Flowchart of literature retrieval.
2.2. Eligibility criteria
All the included studies should be selected if they met the following criteria: (1) the participants (population) were positive/confirmed cases of COVID-19; (2) the participants were divided into 2 groups: HIV-positive group and HIV-negative group; (3) outcomes of mortality due to COVID-19 were provided; (4) the sample size for each of the 2 groups should not be less than 100; and (5) the study design was a randomized control trial, cohort study, and case-control study. Those articles, such as review articles, case reports, commentaries, and letters, were excluded. If 2 or more articles were published based on the same population, the study with larger samples was included.
2.3. Data extraction
The information of the articles included the first author, publication year, age, country, study design, the number of participants with HIV who died and did not die, or the effect size (the OR or relative risk (RR)), etc, were independently extracted by the same researchers.
2.4. Quality assessment of the studies
According to the Newcastle–Ottawa Scale,[20] 2 reviewers independently evaluated the quality of the included articles. The evaluation criteria mainly included 3 aspects, such as the selection, comparability, and outcomes, and the total score of the Newcastle–Ottawa Scale checklist ranged from 0 to 9. In this review, studies with scores ≥7 were considered good quality, 4to 6 scores were considered moderate quality, and ≤3 scores were considered low quality.
2.5. Ethical approval
There was no need for ethical approval for this review because all the data were extracted from previously published articles.
2.6. Statistical analyses
In this study, OR along with the 95% CI was set as the outcome for each study. Cochran Q test and I2 statistics were quantified to measure heterogeneity. When I2 < 50% was observed, low heterogeneity among studies was considered, and DerSimonian and Laird random-effect method was used to pool the effect size. Otherwise, the fixed-effects methods were used. Potential ascertainment bias was assessed with the funnel plots. While the Begg and Egger tests were considered an objected measure of publication bias statistically. Subgroup analysis and sensitivity analysis were conducted to investigate the source of heterogeneity. A P value ≤.05 was considered to be statistically significant. All statistical analyses were analyzed using the Stata software version 11.0 (Stata Corporation, College Station, TX, USA).
3. Results
3.1. Characteristics of the included studies
A total of 632 records were obtained through electronic searches. After screening through strict literature retrieval procedures, a total of 10 literature were finally included in this review (see Fig. 1 and Table 1). The 10 studies[19,21–29] included 41,113 COVID-19 patients with HIV infection and 18,081,257 COVID-19 patients without HIV infection. Among the 10 included studies, 6 studies[21,24–26,28,29] were carried out in United States, 2 studies[19,23] were in United Kingdom, and 2 studies[22,27] were in South Africa. Eight studies[19,21,22,25–29] were designed as a retrospective cohort study and 2 studies[23,24] were designed as a prospective cohort study. All the included studies were written in English. Detailed data source information for each study was listed in Table 1.
Table 1.
Characteristics of articles included in this meta-analysis.
| HIV(+) | HIV(−) | ||||||||||
| ID | Author | Country | Study design | Data source | Age (years of age) | Death | Alive | Death | Alive | Mortality time | Quality of literature |
| 1 | Hadi (2020) | United States | Retrospective cohort | Multicentre research network TriNETX (Cambridge, Massachusetts, USA) | >10 | 20 | 384 | 1585 | 48,178 | Mortality within 30 days | H |
| 2 | Harrison (2020) | United States | Retrospective cohort | Adults with COVID-19 were from 24 healthcare organizations in United States between January 20, 2020 and May 26, 2020 | Median: 50 (35–63) | 17 | 209 | 1279 | 29,956 | Mortality within 30 days | H |
| 3 | Braunstein (2020) | United States | Retrospective cohort | Data sources included the New York City (NYC) Department of Health and Mental Hygiene (DOHMH) HIV surveillance registry and the NYC DOHMH COVID-19 surveillance system | 0- | 312 | 2098 | 16,160 | 185,852 | NA | H |
| 4 | Jassat (2020) | South Africa | Retrospective cohort | The national surveillance system for laboratory-confirmed COVID-19 hospital admissions (DATCOV) during March 5, 2020 and August 11, 2020 | Median: 52(40–63) | 644 | 2433 | 6122 | 26,351 | NA | H |
| 5 | Gudipati (2020) | United States | Prospective cohort | Patients living with HIV in Michigan as of June 30, 2020 | Median: 52 | 23 | 255 | 5919 | 59,074 | NA | H |
| 6 | Tesoriero (2020) | United States | Retrospective cohort | Patients were diagnosed between March 1, 2020 and June 7, 2020 in New York State | NA | 207 | 2781 | 14,522 | 360,738 | NA | H |
| 7 | Bhaskaran (2021) | United Kingdom | Retrospective cohort | Data came from the OpenSAFELY platform on behalf of NHS England | ≥18 years, median: 48 (40–55) | 25 | 27,455 | 14,857 | 17,240,568 | NA | H |
| 8 | Geretti (2020) | United Kingdom | Prospective cohort | All data were captured by the International Severe Acute Respiratory and Emerging Infection Consortium WHO Clinical Characterization Protocol study | ≥18 years | 30 | 81 | 14,555 | 28,460 | Mortality within 28 days | H |
| 9 | Miyashita (2021) | United States | Retrospective cohort | All the data came from the electronic medical records of the Mount Sinai Health System in New York between March 1, 2020 and April 30, 2020 | NA | 23 | 138 | 1235 | 7516 | NA | H |
| 10 | Boulle (2020) | South Africa | Retrospective cohort | Data came from adults attending public sector health facilities in the Western Cape, South Africa | ≥20 | 115 | 3863 | 510 | 17,820 | NA | H |
NA = not applicable, H = high quality of literature, COVID-19 = coronavirus disease 2019, HIV = human immunodeficiency virus.
3.2. Overall results
Among the COVID-19 patients with HIV infection, the mortality rate due to COVID-19 was 3.44% (1416/41,113), and among the COVID-19 patients without HIV infection, the mortality rate due to COVID-19 was 0.42% (76,744/18,081,257).
Five studies resulted that people living with HIV infection had a higher risk of COVID-19 mortality than those without HIV infection. Five studies reported that there was no association between HIV infection and the mortality risk of COVID-19. The OR effect size among the various studies ranged from 0.724 to 1.905. Among the 10 studies, significant heterogeneity was found (Q = 71.29, P < .001; I2 = 87.4%; see Fig. 2). In this study, the pooled overall effect size (OR) was 1.252 (95% CI 1.027–1.524, Z = 2.22, P = .026 < .05).
Figure 2.
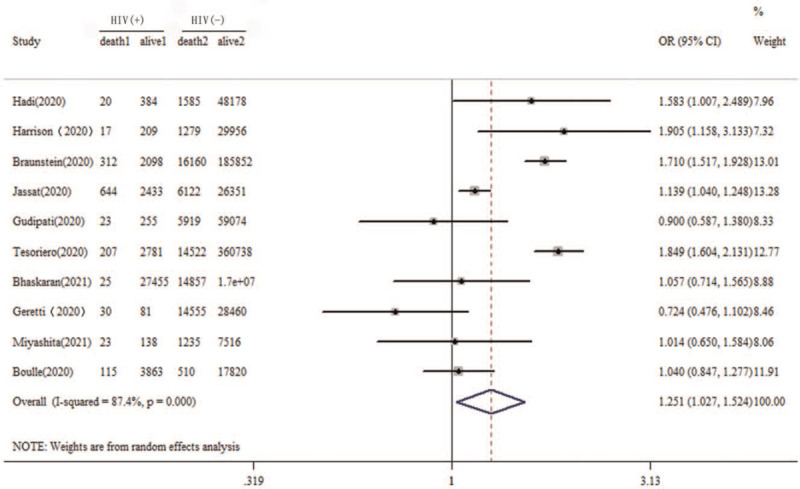
Forest plot of association of HIV coinfection and the risk of mortality due to COVID-19. COVID-19 = coronavirus disease 2019, HIV = human immunodeficiency virus.
3.3. Subgroup analyses
For the countries variables, subgroup analysis was performed in this meta-analysis (Fig. 3). In the United States, people living with HIV infection had a higher risk of COVID-19 mortality than those without HIV infection (OR = 1.520, 95% CI 1.252–1.845). In South Africa, people living with HIV infection also had a higher risk of COVID-19 mortality than those without HIV infection (OR = 1.122, 95% CI 1.032–1.220). However, there was no significant association between HIV infection and the mortality risk of COVID-19 in the United Kingdom (OR = 0.878, 95% CI 0.657–1.174).
Figure 3.
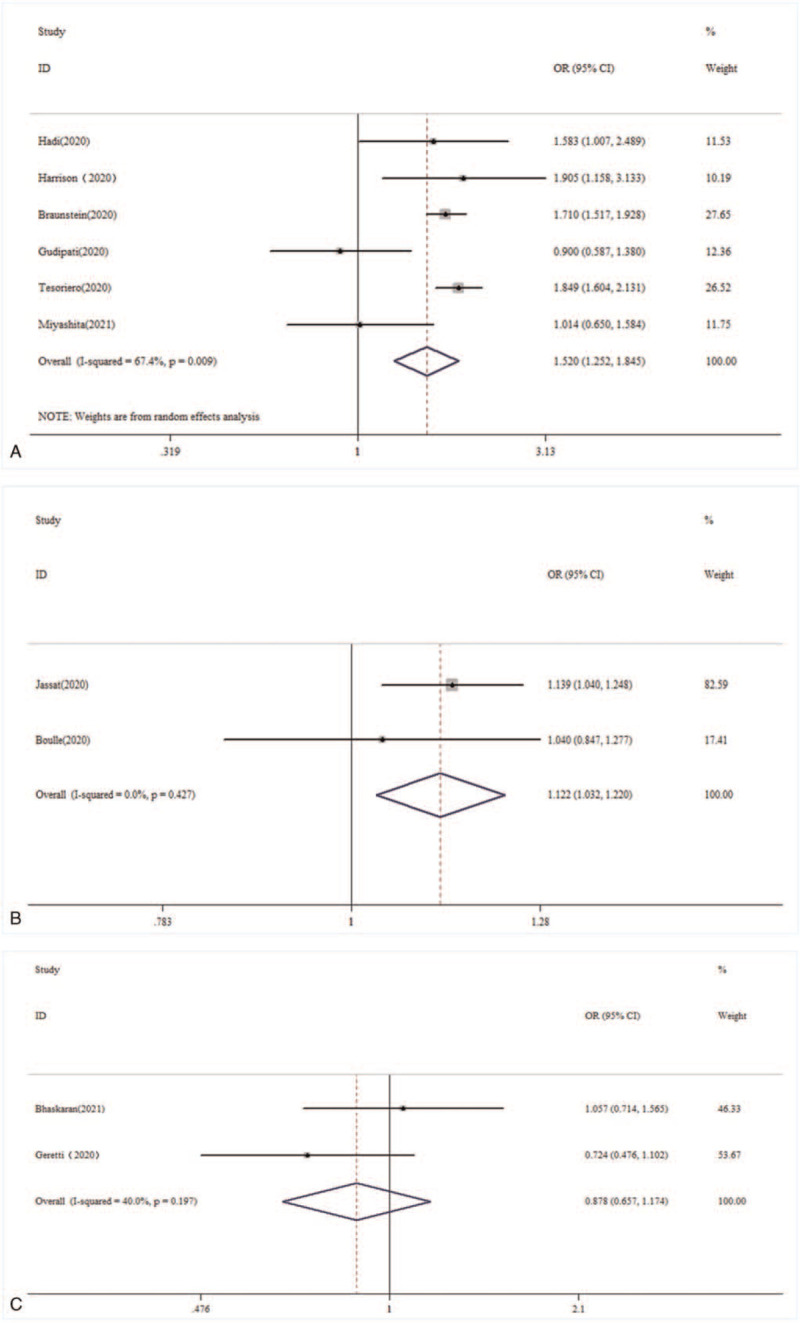
Subgroup analysis of the association between HIV coinfection and the mortality risk from COVID-19 in different countries. (A) United States; (B) South Africa; and (C) United Kingdom. COVID-19 = coronavirus disease 2019, HIV = human immunodeficiency virus.
3.4. Publication bias
Subjectively, the funnel plot analysis showed symmetry among the included studies (Fig. 4). Statistically, the Begg test and Egger test revealed that there was no publication bias for the publications (Z = 1.07, P = .283; t = 0.37, P = .718).
Figure 4.
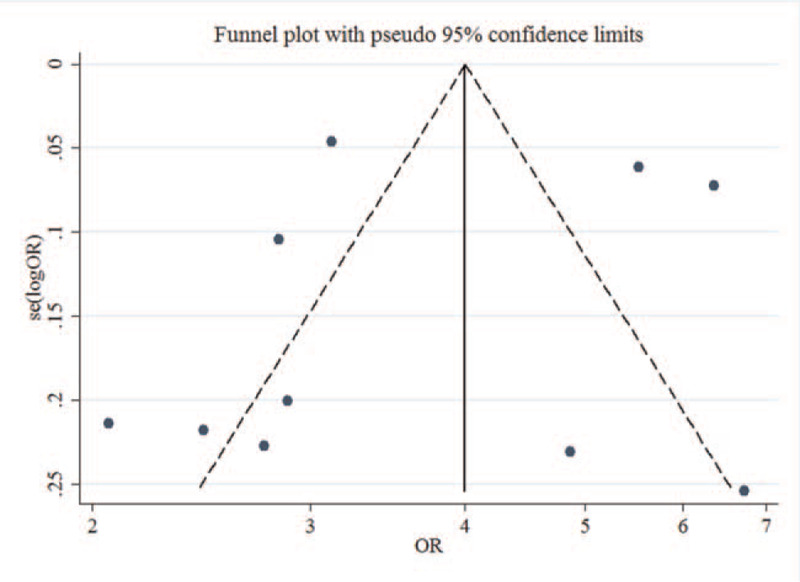
Funnel plot of the included studies in this meta-analysis.
4. Discussion
This is the first meta-analysis to analyze the association between HIV infection and risk of COVID-19 mortality which includes a larger sample size in each selected study. The key findings of our present study suggested that people living with HIV infection had a higher risk of death from COVID-19 than those without HIV infection (OR = 1.252, 95% CI 1.027–1.524).
The larger the sample size, the smaller the error range will be and the more reflective of the overall characteristics. Therefore, the inclusion of research literature in this study ensured that the sample size of the HIV-positive population should be relatively large, that is, the sample size should be more than 100 subjects so that the calculated results are more representative and can better reflect the overall characteristics. Ssentongo et al[17] searched 3 articles published from December 1, 2019 to July 9, 2020, and performed a meta-analysis resulting that HIV/AIDS comorbidity was not significantly associated with a greater risk of COVID-19 mortality (RR = 0.88, 95% CI 0.34–2.31). But 1 study had a very small sample size in the included studies, while the other 2 studies had more than 100 times that size. Therefore, there was a huge difference in the sample size and the effectiveness of the research results. Sarkar et al[18] screened electronic databases up to September 3, 2020, and pooled the results of 7 articles that reported the association of HIV and risk of COVID-19 mortality, and they reported that no significant relationship was resulted (RR = 0.99, 95% CI 0.82–1.19). The weakness of this meta-analysis is the same as that of the previous study, 4 of the 7 included studies had very small sample sizes for the HIV-positive group. However, we found that the CI for the pooled effect in this study had been narrowing as the number of the included studies increased. Our current review abandoned the shortcomings of the previous meta-analysis and only selected studies with a large sample size, which made the research results more reliable.
In addition, we also analyzed HIV infection to the risk of COVID-19 mortality in different countries. Based on the data with 0.74 million individuals from 6 surveys, people living with HIV infection had a higher risk of COVID-19 mortality than those without HIV infection in the United States (OR = 1.520, 95% CI 1.252–1.845). A similar risk was also achieved in South Africa from 2 studies, our subgroup analysis found that people living with HIV infection also had a higher risk of COVID-19 mortality than those without HIV infection (OR = 1.122, 95% CI 1.032–1.220). However, no significant association between HIV infection and the mortality risk of COVID-19 was found in the United Kingdom (OR = 0.878, 95% CI 0.657–1.174). The reason for this difference might be related to SARS-CoV-2 virus mutations in different countries.[30–32] In addition, the affordability of health services in different countries could also affect it.[33–35]
This present meta-analysis had several limitations. Firstly, of the literature that met the inclusion criteria, only 3 countries were covered. At present, the epidemic of COVID-19 is sweeping almost all countries in the world, but the vast majority of countries have not reported the research results in this field. Therefore, the association of HIV infection and the mortality risk of COVID-19 will need to be updated in the future through the synthesis of more homogeneous studies. Secondly, the mortality of COVID-19 was also linked to the severity of the COVID-19. Despite the immunocompromised status, HIV patients are more likely to suffer from SARS-CoV-2 infection than ordinary beings.[36–38] Laracy et al[39] carried out a retrospective cohort study of COVID-19 and found people with HIV were more likely to be admitted from the emergency department than patients without HIV (91% vs 71%; P = .001). However, among hospitalized patients, patients living with HIV did not differ from HIV-uninfected controls by the rate of mechanical ventilation or death/discharge to hospice. Patel et al[40] systematically compiled 63 reports of HIV-1 and SARS-CoV-2 co-infection, and found the presence of comorbidities was associated with a poorer prognosis in HIV/SARS-CoV-2 patients, despite cART and viral suppression. Some studies[41–44] also reported that HIV co-infection could influence the severity of patients with COVID-19. However, it was not possible to analyze the effect of HIV infection on the severity of COVID-19 patients, as there were no available data in our current studies. Thirdly, how frequently people living with HIV mount the intense cytokine response leading to severe COVID-19 was unknown. Finally, some factors, such as age and sex, could influence the accuracy of the results if they were not adjusted. For example, Bhaskaran et al[19] revealed that people living with HIV had a higher risk of COVID-19 mortality than those without HIV after adjusting for age and sex, but this relationship was not significant if the result was not adjusted. Given that we could only get the crude raw data for most studies, our conclusion needed to be treated with caution.
To sum up, during the clinical treatment of COVID-19 patients, those people with HIV co-infection should be regarded as the key crowd to prevent the risk of mortality. However, the association of HIV co-infection and the mortality risk of COVID-19 still need to be updated in the future.
Acknowledgments
We acknowledge all those who help us during the various stages of this study.
Author contributions
Conceptualization: Yonghai Dong, Jiahong Liu, Yun Liu.
Data curation: Yonghai Dong, Zhongjian Li, Sheng Ding.
Formal analysis: Zhongjian Li, Sheng Ding.
Investigation: Shulong Liu, Zhiyuan Tang.
Methodology: Lina Jia.
Resources: Zhiyuan Tang.
Software: Shulong Liu, Zhiyuan Tang, Lina Jia.
Supervision: Lina Jia.
Writing – original draft: Yonghai Dong, Jiahong Liu, Yun Liu.
Writing – review & editing: Yonghai Dong, Yun Liu.
Footnotes
Abbreviations: AIDS = acquired immune deficiency syndrome, CI = confidence intervals, COVID-19 = corona virus disease 2019, HIV = human immunodeficiency virus, OR = odds ratio, RR = relative risk, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
How to cite this article: Dong Y, Li Z, Ding S, Liu S, Tang Z, Jia L, Liu J, Liu Y. HIV infection and risk of COVID-19 mortality: a meta-analysis. Medicine. 2021;100:26(e26573).
This research was funded by Science and Technology Planning Project of Health Commission of Jiangxi Province, China (Nos: 202130994 and 20162001).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/. Accessed on: January 31, 2021. [Google Scholar]
- [2].Jardine R, Wright J, Samad Z, Bhutta ZA. Analysis of COVID-19 burden, epidemiology and mitigation strategies in Muslim majority countries. East Mediterr Health J 2020;26:1173–83. [DOI] [PubMed] [Google Scholar]
- [3].Jones DL, Rodriguez VJ, Salazar AS, et al. Sex differences in the association between stress, loneliness, and COVID-19 burden among people with HIV in the United States. AIDS Res Hum Retroviruses 2021;37:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Labgold K, Hamid S, Shah S, et al. Estimating the unknown: greater racial and ethnic disparities in COVID-19 burden after accounting for missing race and ethnicity data. Epidemiology 2021;32:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schneider M, Altersberger M, Binder C, Hengstenberg C, Binder T. The COVID-19 burden for health care professionals: results of a global survey. Eur J Intern Med 2021;83:96–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eleni M, Evangelia M, Eleftheria K, et al. Clinical features and outcomes of hospitalized COVID-19 patients in a low burden region. Pathog Glob Health 2021;01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ayinbuomwan SA, Mokogwu N, Akoria OA, Okwara BU, Omuemu CE, Obaseki DE. Arterial oxygen saturation and other clinical predictors of survival in patients with covid-19: a review of cases in a tertiary care hospital in Nigeria. West Afr J Med 2021;38:109–13. [PubMed] [Google Scholar]
- [8].Kumar N, Shahul HS, Babu GR, et al. Descriptive epidemiology of SARS-CoV-2 infection in Karnataka state, South India: transmission dynamics of symptomatic vs. asymptomatic infections. EClinicalMedicine 2021;32:100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thiabaud A, Iten A, Balmelli C, et al. Cohort profile: SARS-CoV-2/COVID-19 hospitalised patients in Switzerland. Swiss Med Wkly 2021;151:w20475. [DOI] [PubMed] [Google Scholar]
- [10].Gacche RN, Gacche RA, Chen J, Li H, Li G. Predictors of morbidity and mortality in COVID-19. Eur Rev Med Pharmacol Sci 2021;25:1684–707. [DOI] [PubMed] [Google Scholar]
- [11].El-Jawahri A, Bohossian HB, Paasche-Orlow MK, et al. Clinical outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19) in Boston. J Gen Intern Med 2021;01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Elezkurtaj S, Greuel S, Ihlow J, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep 2021;11:4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bell N, Bracchi M, Dalla PA, Nelson M, Bofitto M. Indirect HIV morbidity and mortality due to COVID-19. Clin Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mehta SA, Rana MM, Motter JD, et al. Incidence and outcomes of COVID-19 in kidney and liver transplant recipients with HIV: report from the national HOPE in action consortium. Transplantation 2021;105:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nagarakanti SR, Okoh AK, Grinberg S, Bishburg E. Clinical outcomes of patients with COVID-19 and HIV coinfection. J Med Virol 2021;93:1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Waters LJ, Pozniak AL. COVID-19 death in people with HIV: interpret cautiously. Lancet HIV 2021;8:e2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ssentongo P, Ssentongo AE, Heilbrunn ES, et al. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One 2020;15:e238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sarkar S, Khanna P, Singh AK. Impact of COVID-19 in patients with concurrent co-infections: a systematic review and meta-analyses. J Med Virol 2021;93:2385–95. [DOI] [PubMed] [Google Scholar]
- [19].Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021;8:e24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [21].Braunstein SL, Lazar R, Wahnich A, Daskalakis DC, Blackstock OJ. COVID-19 infection among people with HIV in New York City: a population-level analysis of linked surveillance data. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boulle A, Davies M, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Geretti AM, Stockdale AJ, Kelly SH, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) clinical characterization protocol (UK): a prospective observational study. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gudipati S, Brar I, Murray S, et al. Descriptive analysis of patients living with HIV affected by COVID-19. J Acquir Immune Defic Syndr (1999) 2020;85:123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hadi YB, Naqvi SFZ, Kupec JT, Sarvari AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS 2020;34:F3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med 2020;17:e1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jassat W, Cohen C, Masha M, et al. COVID-19 in-hospital mortality in South Africa: the intersection of communicable and non-communicable chronic diseases in a high HIV prevalence setting. medRxiv 2020;doi:10.2139/ssrn.3783089. [Google Scholar]
- [28].Miyashita H, Kuno T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV Med 2021;22:e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tesoriero JM, Swain CE, Pierce JL, et al. Elevated COVID-19 outcomes among persons living with diagnosed HIV infection in New York State: results from a population-level match of HIV, COVID-19, and hospitalization databases. medRxiv 2020;doi:10.1101/2020.11.04.20226118. [Google Scholar]
- [30].Arif TB. The 501.V2 and B 1. 1. 7 variants of coronavirus disease 2019 (COVID-19): a new time-bomb in the making? Infect Control Hosp Epidemiol 2021;01–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Galloway SE, Paul P, MacCannell DR, et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage – United States, December 29, 2020–January 12, 2021. MMWR Morb Mortal Wkly Rep 2021;70:95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kostaki EG, Tseti I, Tsiodras S, Pavlakis GN, Sfikakis PP, Paraskevis D. Temporal dominance of B.1.1. 7 over B. 1. 354 SARS-CoV-2 variant: a hypothesis based on areas of variant co-circulation. Life (Basel) 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Singh DR, Sunuwar DR, Shah SK, et al. Impact of COVID-19 on health services utilization in Province-2 of Nepal: a qualitative study among community members and stakeholders. BMC Health Serv Res 2021;21:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Amimo F, Lambert B, Magit A, Hashizume M. A review of prospective pathways and impacts of COVID-19 on the accessibility, safety, quality, and affordability of essential medicines and vaccines for universal health coverage in Africa. Global Health 2021;17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kogut SJ. Racial disparities in medication use: imperatives for managed care pharmacy. J Manag Care Spec Pharm 2020;26:1468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morani Z, Patel S, Ghosh S, et al. COVID-19 in HIV: a review of published case reports. SN Compr Clin Med 2020;01–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang JC, Yu XH, Ding XH, et al. New HIV diagnoses in patients with COVID-19: two case reports and a brief literature review. BMC Infect Dis 2020;20:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tian C, Tang L, Wu J, et al. An HIV-infected patient with coronavirus disease 2019 has a favourable prognosis: a case report. Ann Palliat Med 2020;9:03. [DOI] [PubMed] [Google Scholar]
- [39].Laracy J, Zucker J, Castor D, et al. HIV-1 infection does not change disease course or inflammatory pattern of SARS-CoV-2-infected patients presenting at a large urban medical center in New York City. Open Forum Infect Dis 2021;8:b29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Patel RH, Acharya A, Chand HS, Mohan M, Byrareddy SN. Human immunodeficiency virus and severe acute respiratory syndrome coronavirus 2 coinfection: a systematic review of the literature and challenges. AIDS Res Hum Retroviruses 2021;37:266–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cabello A, Zamarro B, Nistal S, et al. COVID-19 in people living with HIV: a multicenter case-series study. Int J Infect Dis 2021;102:310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Del AJ, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med 2020;173:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hoffmann C, Casado JL, Harter G, et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med 2021;22:372–8. [DOI] [PubMed] [Google Scholar]
- [44].Kowalska JD, Kase K, Vassilenko A, et al. The characteristics of HIV-positive patients with mild/asymptomatic and moderate/severe course of COVID-19 disease—a report from Central and Eastern Europe. Int J Infect Dis 2021;104:293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


