PURPOSE:
Human papilloma virus–positive (HPV+) oropharyngeal squamous cell carcinoma (OPSCC), diagnosed with p16 immunohistochemistry, is associated with favorable prognosis; however, this connection was established using European American (EA)–skewed populations. The impact of p16/human papillomavirus status on outcomes in African American (AA) OPSCC patients remains to be settled. In this study, we determine the association between cancer disparity and p16 status in an OPSCC cohort controlling for time to treatment initiation (TTI), a surrogate for medical care access.
MATERIALS AND METHODS:
We analyzed data from all patients diagnosed with OPSCC (N = 440) between 2010 and 2017, who received treatment at our academic medical center. Associations between age, disease stage, sex, p16 status, race, TTI, and overall survival (OS) were investigated.
RESULTS:
TTI was similar between AA and EA OPSCC patients in our p16+ (P = .291) or p16− (P = .715) cohorts. Among p16+ OPSCC patients, the median OS was > 8.65 years for EA patients compared with 5.038 years (95% CI, 2.019 to 5.30; P = .003, log-rank) for AA patients. For p16− patients, the median OS was 5.74 years (95% CI, 3.32 to 6.99) for EA patients and 1.85 years (95% CI, 0.978 to 4.50; P = .03, log-rank) for AA patients. Multivariate Cox regression analysis showed that race was an independent prognostic biomarker and the most impactful co-variate for OS (hazard ratio, 0.40; 95% CI, 0.00 to 0.69; P = .001).
CONCLUSION:
Our work showed that AAs with p16+ OPSCC have surprisingly poor clinical outcomes and are thus poor candidates for treatment de-escalation regimens. Caution should be exercised when extending clinical guidelines based on EA-majority studies to non-EA populations.
INTRODUCTION
In the United States, African Americans (AAs) bear a disproportionate burden of cancer incidence and mortality across most cancer types.1-4 The reasons for these disparities are not fully understood and may vary by disease site, but a range of factors is believed to contribute, including socioeconomic and cultural factors, such as medical care access, diet, tobacco and alcohol use, exposure to environmental carcinogens, and biologic factors.1,5-8
The assessment of racial disparity in head and neck squamous cell carcinoma (HNSCC) is complicated by the role of human papillomaviruses (HPVs), which drive an increasing proportion of oropharyngeal squamous cell carcinomas (OPSCCs).9 HPV+ OPSCC, diagnosed clinically with p16 immunohistochemistry (IHC), tends to respond better to standard-of-care treatment and is associated with much better prognosis.10,11 There is accumulating evidence that the frequency of HPV+ OPSCC is lower in the AA population.12-15 Several groups have shown that racial disparity in OPSCC outcomes is minimized after adjusting for p16/HPV status.15,16 However, in direct contrast, Goodman and colleagues found that AA patients with OPSCC had a 2.6-fold greater risk of death at 5 years, after controlling for various clinical co-variates, including p16/HPV status.12 Reasons for the discrepancies between these studies are unclear but may be due to the failure to include socioeconomic factors in their analyses. Race-based inequities to health care access are a well-recognized issue and known to directly affect cancer outcomes. At this time, the impact of p16/HPV status on cancer disparity in OPSCC controlling for medical care access remains to be settled.
In this study, we identified and analyzed a cohort of patients with OPSCC, p16+ and p16−, diagnosed between 2010 and 2017 at University Hospitals Seidman Cancer Center. Our work showed that time to treatment initiation (TTI) was statistically similar between AA and European American (EA) patients in our OPSCC cohort, indicating that both populations had equal access to medical care. AA patients with OPSCC had poorer overall survival (OS) compared with EA patients in both p16+ and p16− cohorts, and multivariate analysis revealed race as an independent predictor of OS in our OPSCC cohort. This disparity was not fully explained by any of the clinical covariates we examined, including TTI, and thus suggests that tumor biology differences exist between AA and EA OPSCC patients. Moreover, our work argues that AA patients with p16+ OPSCC are poor candidates for treatment de-escalation regimens.
MATERIALS AND METHODS
Study Cohort
Our study (IRB# 20191051) was approved by our Institutional Review Board at University Hospitals Cleveland Medical Center. We queried the University Hospitals Seidman Cancer Center databases and identified all patients diagnosed with OPSCC between 2010 and 2017, receiving treatment at our academic medical center. p16 IHC is a standard-of-care assay for OPSCC primaries at our institution and was defined as p16+ if there was strong and diffuse nuclear and cytoplasmic staining in ≥ 70% of tumor cells. TTI is defined as the time between pathologic diagnosis and the onset of first treatment modality. Follow-up time was defined as the time between pathologic diagnosis and date of last contact or date of death.
Statistical Analysis
All statistical analyses were performed using XLSTAT.17 Continuous data were analyzed using two-tailed t-tests. Categorical variables were compared using the chi-squared test for contingency tables. Survival analysis was performed with Kaplan-Meier curves and log-rank tests. Multivariate Cox proportional hazard models were used to evaluate the relationship among known risk factors associated with HNSCC outcomes, including age, disease stage, sex, p16 status, and TTI. A P value < .05 was considered statistically significant.
RESULTS
Incidence Rates of p16+ and p16− OPSCC in the EA and AA Patients
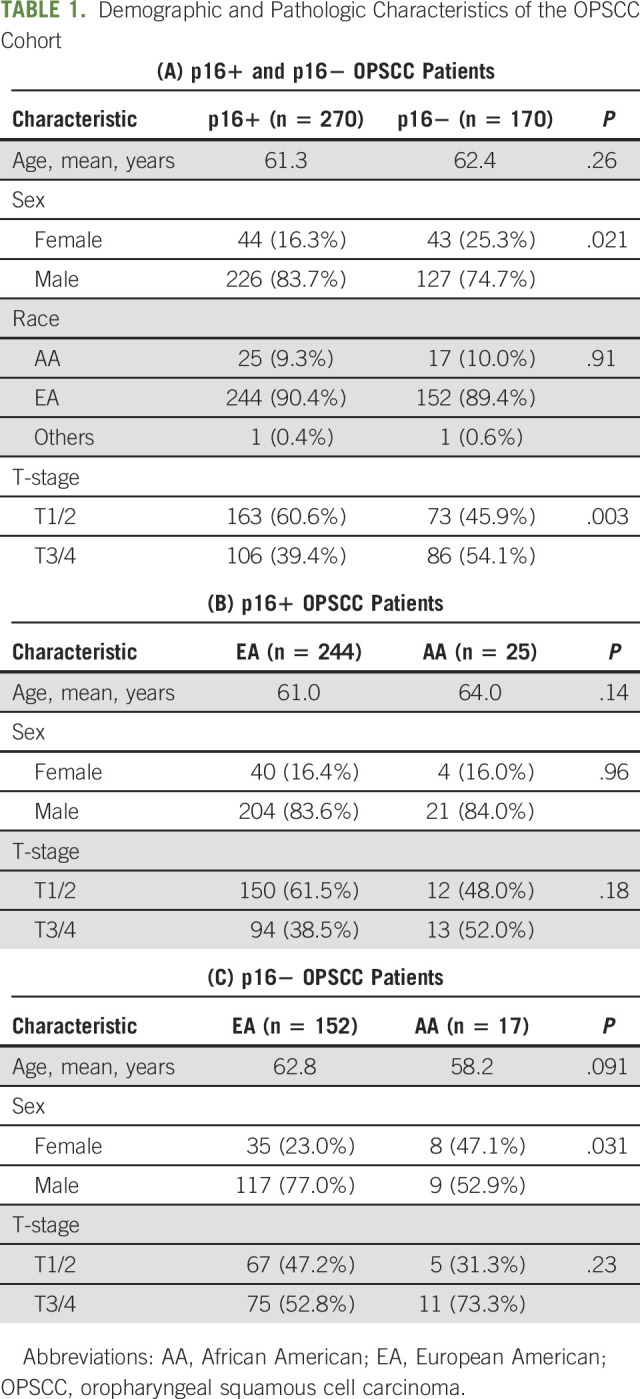
Our study cohort included 440 patients with OPSCC who received treatment at our academic medical center with an extensive satellite network between 2010 and 2017 (Table 1A). Our p16+ cohort consisted of 270 patients, with 25 AA (9.3%), 244 EA (90.4%), and one patient with race recorded as other (Table 1B). Our p16− cohort included 170 patients; 152 EA (89.4%), 17 AA (10%), and one other (Table 1C). Although p16+ OPSCC is often associated with younger patient age, mean age was similar (P = .264) for our p16+ and p16− patients; means of 61.3 and 62.4 were observed for p16+ and p16− OPSCC patients, respectively. p16+ and p16− AA patients tend to present with advanced stage; however, this association did not reach significance. Two intriguing findings are that the proportion of AA patients in our p16+ and p16− cohorts was similar (P = .91), and although females comprised a minority of both OPSCC cohorts, they were represented to a greater extent in our p16− cohort than in our p16+ cohort (16.3% of p16+ OPSCC and 25.3% of p16− OPSCC; P = .021).
TABLE 1.
Demographic and Pathologic Characteristics of the OPSCC Cohort
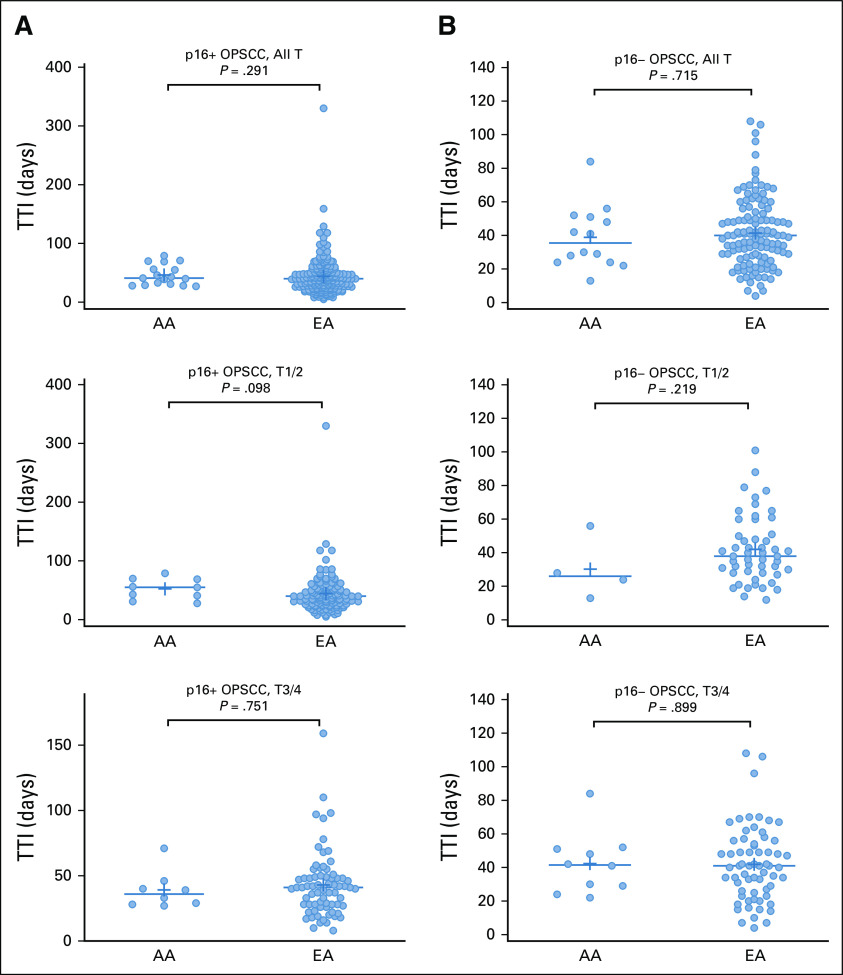
TTI for the AA and EA OPSCC Patients
Socioeconomic factors, known to influence treatment outcomes, are multifactorial, and in the context of patients with cancer, access to medical care and timely treatment are recognized variables. We used TTI, defined as the number of days between pathologic diagnosis and onset of initial treatment, as a surrogate for medical care access. For patients with p16+ OPSCC, TTI did not differ significantly between EA and AA patients (mean of 43.81 days and 46.18 days, respectively; P = .29). Among patients with early-stage p16+ disease, EA patients had a mean TTI of 44.4 days, compared with 52.4 days for AA (P = .098). For patients with late-stage p16+ disease, the mean TTI was 42.9 days for EAs compared with 39.1 days for AAs (P = .899) (Fig 1A). The same analyses were performed for our p16− cohort and revealed similar TTIs between AA and EA patients, 38.9 days for AAs and 41.4 days for EAs (P = .715) (Fig 1B). We did not find evidence of TTI disparity looking separately at patients with early-stage (the mean TTI for EAs was 42.1 compared with 30.3 for AAs; P = .219) or late-stage (the mean TTI was 41.8 for EAs and 42.3 for AAs, P = .751) p16− OPSCC.
FIG 1.
TTI for AA and EA OPSCC patients: (A) p16+ cohort and (B) p16− cohort. For each cohort, comparisons are shown for all patients and for early-stage and late-stage patients separately. AA, African American; EA, European American; OPSCC, oropharyngeal squamous cell carcinoma; TTI, time to treatment initiation.
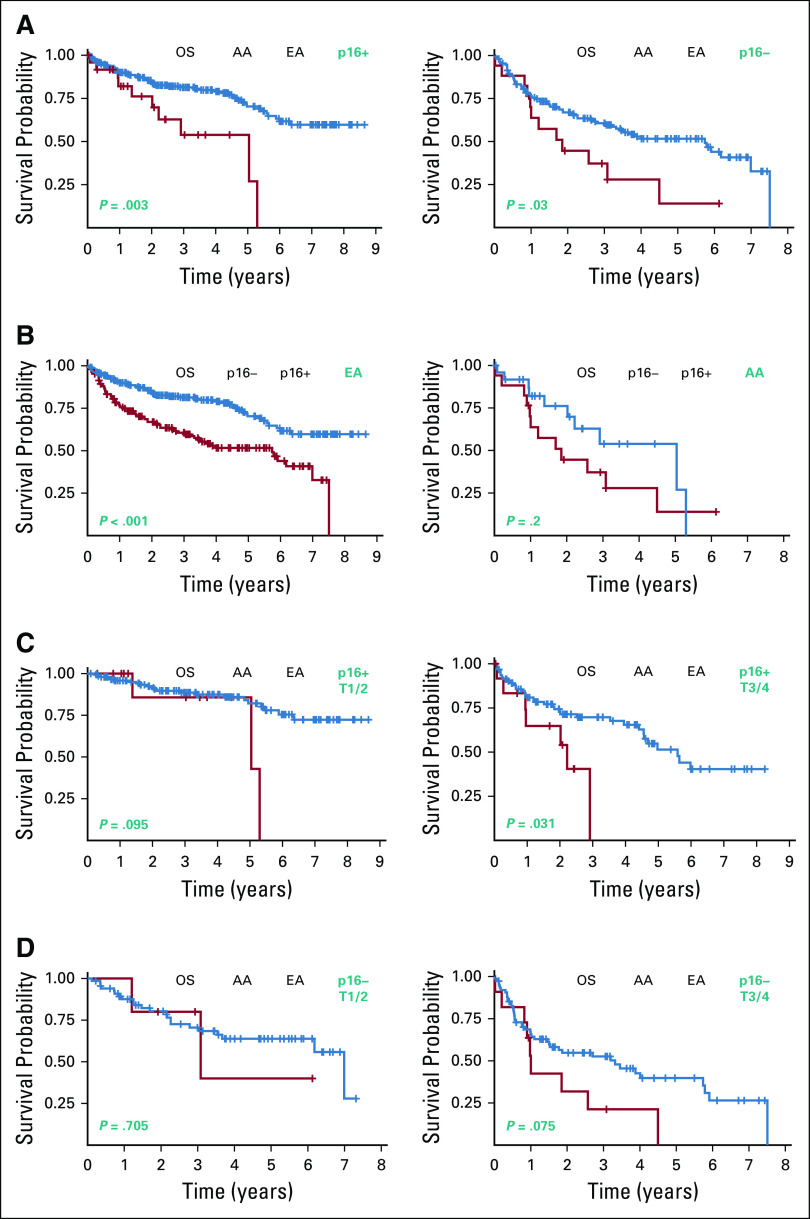
Race Is an Independent Prognostic Biomarker in OPSCC
Kaplan-Meier curves for p16+ and p16− OPSCC showed a significant difference in OS between AA and EA patients (Fig 2A). Among patients with p16+ OPSCC, the median OS for EA patients was > 8.65 years, compared with 5.038 years (95% CI, 2.019 to 5.30; P = .003, log-rank) for AA patients. In our p16− cohort, the median OS was 5.74 years (95% CI, 3.32 to 6.99) for EA patients and 1.85 years for AA patients (95% CI, 0.978 to 4.50; P = .03, log-rank).
FIG 2.
Kaplan-Meier curves for p16+ and p16− OPSCC patients. (A) Prognostic impact of race for p16+ and p16− OPSCC cohorts. (B) Prognostic impact of p16 status for EA and AA OPSCC patients. (C) Prognostic impact of race for early-stage and late-stage p16+ OPSCC patients. (D) Prognostic impact of race for early-stage and late-stage p16− OPSCC patients. AA, African American; EA, European American; OPSCC, oropharyngeal squamous cell carcinoma; OS, overall survival.
Since p16+ status is widely reported to have a favorable prognostic impact in EA-majority studies, we were interested to see if p16+ status would confer a similar survival benefit for our AA patients. We first assessed the impact of p16 status among our EA patients and found that those with p16+ disease had a median OS of > 8.65 years, compared with 5.74 (95% CI, 3.32 to 6.99) for p16− patients (Fig 2B). Consistent with other reports, p16+ status conferred a survival advantage (P < .001, log-rank) in EA patients. However, among AA patients, the survival benefit associated with p16+ status was not statistically significant (P = .200, log-rank). The median OS was 5.038 years (95% CI, 2.019 to 5.30) for p16+ AA patients, compared with 1.85 (95% CI, 0.978 to 4.50) for p16− AA patients.
We further explored the impact of disease stage at time of diagnosis on OS in our EA and AA OPSCC cohorts. The median OS for early-stage AA patients with p16+ disease was 5.038 years (95% CI, 5.038 to 5.30) compared with more than 8.65 years for their EA counterparts (P = .095, log-rank) (Fig 2C). Among late-stage patients with p16+ disease, however, a statistically significant difference in OS was identified. For p16+ AA patients, the median OS was 2.22 years (95% CI, 0.945 to 2.91) compared with 5.59 years (95% CI, 4.55 to upper limit not reached [NR]) for p16+ EA patients (P = .031, log-rank). Analysis of our p16− cohort did not reveal significant disparity for patients with either early-stage or late-stage diagnoses (Fig 2D). For early-stage p16− patients, the median OS for AAs was 3.079 years (95% CI, 1.21 to NR) compared with 6.99 years (95% CI, 6.18 to NR) for p16− EA patients (P = .71). For those diagnosed at a later stage, the median OS was 1.003 years (95% CI, 0.822 to 2.57) for p16− AA patients compared with 3.32 years (95% CI, 1.504 to 5.74) for p16− EAs (P = .075).
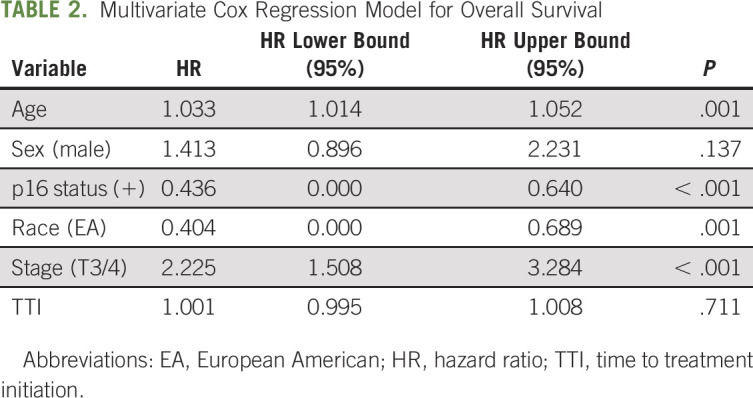
Multivariate modeling, adjusting for age, sex, p16 status, race, stage, and TTI, showed age (hazard ratio [HR], 1.03; 95% CI, 1.01 to 1.05; P = .001), stage (HR, 2.23; 95% CI, 1.51 to 3.28; P = .001), race (HR, 0.4; 95% CI, 0.00 to 0.69; P = .001), and p16 status (HR, 0.44; 95% CI, 0.00 to 0.64; P < .001) to be independent predictors of OS (Table 2). HR of race was, impressively, similar to p16 status, further demonstrating the high clinical impact of race as a prognostic biomarker in OPSCC, independent of p16 status.
TABLE 2.
Multivariate Cox Regression Model for Overall Survival
DISCUSSION
Our analysis revealed racial disparities in OS in OPSCC, in both p16+ and p16− settings. This finding was unexpected in light of evidence in the literature attributing racial disparities in OPSCC to lower rates of p16/HPV+ cases in the AA population. In our study, however, similar proportion of AA patients were shown in our p16+ and p16− cohorts (9.3% of p16+ OPSCC and 10% of p16− OPSCC). Moreover, in a multivariate model including age, sex, p16 status, race, stage, and TTI, race remained a significant determinant of OS. In fact, the clinical impact of race was as strong as p16 status; HRs of 0.404 and 0.436 were observed for race and p16 status, respectively. In contrast to the findings of other groups, the racial disparity in outcomes identified in our cohort is independent of p16 status.
Socioeconomic disadvantage is known to influence access to medical care and treatment outcomes, and we anticipated that it would have some impact on the OS disparity observed in our OPSCC study cohort. Surprisingly, we did not observe a difference in TTI between EA and AA patients. Additionally, in our multivariate model, TTI was not an independent prognostic biomarker in this cohort. Although socioeconomic disadvantage may contribute to disparity in OPSCC, our data showed that medical care access as measured by TTI was not a factor in our cohort. Although TTI may reflect obstacles to timely treatment, we recognize that it is not a measure of socioeconomic disadvantage and may miss important socioeconomic contributors to disparate disease outcomes. Another limitation of our study is that treatment details were incomplete, and thus, we were unable to confirm that patients were treated according to established guidelines and completed the full regimen at the recommended dose.
There is conflicting literature regarding disparity in OS between AA and EA OPSCC patients, and this variability may be partly attributable to the methods used to determine HPV status, which often involve p16 IHC or PCR-based assays. In the context of racially diverse populations, each of these two methods may have significant limitations. For PCR-based tests, probes specific to HPV16 are often used since this genotype accounts for the majority of cases of HPV-driven OPSCC. However, our knowledge of the frequencies of HPV genotypes in OPSCC is based largely on studies of primarily EA patients, and these findings may not be generalizable to other racial groups. Indeed, a number of studies provide evidence that this is not the case. A meta-analysis by Ragin and colleagues identified a 15-fold higher frequency of HPV18 in AA patients compared with EA patients with OPSCC.18 Therefore, PCR-based assays using HPV16-specific probes are likely to be problematic because of the high number of false-negative results in the non-EA OPSCC patients. Moreover, there is accumulating evidence that non-HPV16 genotypes are associated with poor treatment outcomes in HPV+ OPSCC.12,19 Thus, significant differences in the distribution of high-risk HPV genotypes between EA and AA patients could contribute to racial disparity in OPSCC outcomes.
IHC for p16 overexpression is routinely used in the clinical setting as a surrogate marker for HPV+ status, and this approach, too, may be problematic in the setting of a diverse patient population. p16 IHC has gained wide acceptance as a surrogate marker for HPV+ OPSCC, since excellent concordance between HPV and p16 status has consistently been observed for OPSCC. AA patients comprise a minority of participants in most of these studies, and several studies of AA-majority cohorts have raised serious concerns regarding the accuracy of p16 IHC as a surrogate for HPV status in this group.20 Interestingly, Liu and colleagues found the discordant HPV+/p16− status to be the most common scenario among AA patients, and these patients had less favorable outcomes compared with AA patients with HPV+/p16+ OPSCC.20 In addition to compromising diagnostic and prognostic accuracy, p16/HPV discordance in AA patients may suggest important differences in OPSCC tumor biology. Although there remains no consensus on the extent of racial disparity in OPSCC or its potential causes, the current body of literature provides evidence that findings of EA-majority studies of OPSCC do not generalize to AA patients. Improved understanding of the frequency of p16/HPV discordance and HPV genotype distribution in AAs will be critical to the development of evidence-based guidelines for this patient group.
Growing evidence reveals that genetic variants linked to disease susceptibility may differ in frequency among different racial populations. For example, a G/C polymorphism at nucleotide −174 within the promoter region of the interleukin-6 (IL-6) gene modulates circulating levels of this pro-inflammatory cytokine.21 In non-EA groups, the frequency of the G allele ranges from 0.87 to 1.0, compared with 0.54 to 0.62 in EA individuals.22,23 It has been proposed that this IL-6 gene polymorphism contributes significantly to higher breast cancer mortality among AA women.22 IL-6 plays a well-established prognostic role in HNSCC,24,25 and the extent of the contributions of this and other genetic variants to racial disparity in OPSCC remains to be elucidated.
The most important finding in our study is that p16+ status was not associated with favorable prognosis compared with p16− status among AA OPSCC patients. The five-year OS in p16+ AA patients was 53.9%, which is similar to that for p16− EA patients with a 5-year OS of 51.6%. Current interest in treatment de-escalation strategies for p16+ OPSCC patients is justified by the outstanding clinical outcomes of EA patients with p16+ OPSCC, 5-year OS of 72.8%. Our findings argue that AA patients with p16+ OPSCC are poor candidates for treatment de-escalation and add to the growing evidence that data from EA-majority studies should not be used to guide clinical management of diverse patient populations. It is becoming increasingly clear that a one-size-fits-all approach to the management of OPSCC does a disservice to our AA patients. Research needs to be prioritized to better understand the distinct biology and clinical needs of the AA OPSCC population.
Rod Rezaee
Honoraria: Zimmer Biomet
Speakers' Bureau: Zimmer Biomet
Patents, Royalties, Other Intellectual Property: Zimmer/Biomet trauma fixation device
Travel, Accommodations, Expenses: Zimmer BioMet
Quintin Pan
Research Funding: Bristol Myers Squibb Foundation
Patents, Royalties, Other Intellectual Property: I have patents on p53 reactivation therapeutics for HPV+ HNSCC
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by the National Institute of Craniofacial and Dental Research and the National Cancer Institute of the National Institutes of Health under award numbers R01DE023555 and R01CA193590, and University Hospitals Seidman Cancer Center.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI https://doi.org/10.1200/OP.20.01105.
AUTHOR CONTRIBUTIONS
Conception and design: W. Quinn O'Neill, Quintin Pan
Financial support: Quintin Pan
Administrative support: Lizabeth Lukesic, John Shanahan, Theodoros N. Teknos
Provision of study materials or patients: W. Quinn O'Neill, John Shanahan, Pierre Lavertu, Rod Rezaee, Theodoros N. Teknos, Quintin Pan
Collection and assembly of data: W. Quinn O'Neill, Jay Wasman, Lizabeth Lukesic, Ravi Kyasram, John Shanahan, Blake Szelesety, Brandon Vu, Theodoros N. Teknos
Data analysis and interpretation: W. Quinn O'Neill, Jason Thuener, Kate Chatfield-Reed, John Shanahan, Brandon Vu, Pierre Lavertu, Rod Rezaee, Shawn Li, Nicole Fowler, Theodoros N. Teknos, Quintin Pan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
African Americans With p16+ and p16− Oropharyngeal Squamous Cell Carcinomas Have Distinctly Poor Treatment Outcomes Independent of Medical Care Access
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rod Rezaee
Honoraria: Zimmer Biomet
Speakers' Bureau: Zimmer Biomet
Patents, Royalties, Other Intellectual Property: Zimmer/Biomet trauma fixation device
Travel, Accommodations, Expenses: Zimmer BioMet
Quintin Pan
Research Funding: Bristol Myers Squibb Foundation
Patents, Royalties, Other Intellectual Property: I have patents on p53 reactivation therapeutics for HPV+ HNSCC
No other potential conflicts of interest were reported.
REFERENCES
- 1.Singh GK, Jemal A: Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: Over six decades of changing patterns and widening inequalities. J Environ Public Health 2017:2819372, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel MI, Wang A, Kapphahn K, et al. : Racial and ethnic variations in lung cancer incidence and mortality: Results from the Women's Health Initiative. J Clin Oncol 34:360-368, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson CS, Oman M, Patel AM, et al. : Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol 7:S32-S43, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Chen Z, Anderson GL, et al. : Ethnicity and breast cancer: Factors influencing differences in incidence and outcome. J Natl Cancer Inst 97:439-448, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Auslander BA, Biro FM, Succop PA, et al. : Racial/ethnic differences in patterns of sexual behavior and STI risk among sexually experienced adolescent girls. J Pediatr Adolesc Gynecol 22:33-39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson EH, Jackson LA, Hinkle Y, et al. : What is the significance of black-white differences in risky sexual behavior? J Natl Med Assoc 86:745-759, 1994 [PMC free article] [PubMed] [Google Scholar]
- 7.Özdemir BC, Dotto GP: Racial differences in cancer susceptibility and survival: More than the color of the skin? Trends Cancer 3:181-197, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaubatz ME, Bukatko AR, Simpson MC, et al. : Racial and socioeconomic disparities associated with 90-day mortality among patients with head and neck cancer in the United States. Oral Oncol 89:95-101, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Gillison ML, Chaturvedi AK, Anderson WF, et al. : Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 33:3235-3242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ang KK, Harris J, Wheeler R, et al. : Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24-35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fakhry C, Gillison ML: Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol 24:2606-2611, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman MT, Saraiya M, Thompson TD, et al. : Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur J Cancer 51:2759-2767, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Settle K, Posner MR, Schumaker LM, et al. : Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2:776-781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chernock RD, Zhang Q, El-Mofty SK, et al. : Human papillomavirus-related squamous cell carcinoma of the oropharynx: A comparative study in whites and African Americans. Arch Otolaryngol Head Neck Surg 137:163-169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen KM, Stephen JK, Havard S, et al. : IGSF4 methylation as an independent marker of human papillomavirus-positive oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg 141:257-263, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Fakhry C, Westra WH, Wang SJ, et al. : The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer 123:1566-1575, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Addinsoft : XLSTAT Statistical and Data Analysis Solution. New York, NY, 2020. http://www.xlstat.com [Google Scholar]
- 18.Ragin C, Liu JC, Jones G, et al. : Prevalence of HPV infection in racial-ethnic subgroups of head and neck cancer patients. Carcinogenesis 38:218-229, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatfield-Reed K, Gui S, O'Neill WQ, et al. : HPV33+ HNSCC is associated with poor prognosis and has unique genomic and immunologic landscapes. Oral Oncol 100:2020, 104488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JC, Parajuli S, Blackman E, et al. : High prevalence of discordant human papillomavirus and p16 oropharyngeal squamous cell carcinomas in an African American cohort. Head Neck 38:E867-E872, 2016. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isayeva T, Xu J, Dai Q, et al. : African Americans with oropharyngeal carcinoma have significantly poorer outcomes despite similar rates of human papillomavirus-mediated carcinogenesis. Hum Pathol 45:310-319, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Berger FG: The interleukin-6 gene: A susceptibility factor that may contribute to racial and ethnic disparities in breast cancer mortality. Breast Cancer Res Treat 88:281-285, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Cox ED, Hoffmann SC, DiMercurio BS, et al. : Cytokine polymorphic analyses indicate ethnic differences in the allelic distribution of interleukin-2 and interleukin-6. Transplantation 72:720-726, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Kumar B, Yadav A, Brown NV, et al. : Nuclear PRMT5, cyclin D1 and IL-6 are associated with poor outcome in oropharyngeal squamous cell carcinoma patients and is inversely associated with p16-status. Oncotarget 8:14847-14859, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesinski GB, Nannapaneni S, Griffith CC, et al. : Interleukin-6/STAT3 signaling is prominent and associated with reduced overall survival in p16 negative oropharyngeal squamous cell carcinoma. Head Neck Pathol 13:304-312, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]