Abstract
Aging of population is characterized by multiple chronic conditions in the same individual. Health-related quality of life (HR-QOL) reflects the multidimensional impact of chronic disease on population and it is increasingly analysed as outcomes.
The aim of this study was the evaluation of the predictors of quality of life among elderly patients hospitalized in internal medicine ward, investigating the effect of comorbidities on health-related quality of life.
Data collected in this cross-sectional study were analysed. Socio-demographic, clinical characteristics, disease distribution and quality of life by the 12-Item Short Form Health Survey (SF-12) were evaluated.
Of 240 inpatients, subjects with Barthel Index (BI)≤40 were 23.7%, 55% had a Geriatric Depression Scale (GDS)≥2. After categorizing mental component score (MCS) and physical component score (PCS) in five classes, we found that diabetics and patients with cancer were more frequent in the first class of MCS while patients with NYHA III-IV are significantly more frequent in the first class of PCS. When we classified patients according to GDS≥2 or < 2, subjects with GDS≥2 had BI and MCS significantly lower. In the multivariate analysis GDS score ≥2 was independently associated with first MCS class [16.32 (3.77–70.68)] while NYHA III-IV class and claudicatio intermittents were strong predictors of the worst PCS class [9.54 (1.97–47.40), 2.53 (1.16–5.49), respectively]. Liver disease was independently associated with GDS≥2 [5.26 (1.13–24.39)].
Our study highlighted the impact of chronic diseases on health-related quality of life in elderly subjects hospitalized in an internal medicine ward pointing out the importance of taking into account patient's needs and perception and the setting up of a personalised health-care. Patients with diabetes and liver disease along with persons affected by cancer need psychological support to improve their quality of life. A GDS score ≥ 2 is a strong predictor of poor quality of life and should trigger an in-depth assessment of mental health in this kind of patients.
Keywords: depression, frail elders, multiple chronic conditions, quality of life
1. Introduction
Advances in public health and medical practice, have determined the worldwide increasing of life expectancy. According to the 2018 report by the Academy of Medical Sciences, globally, by 2050, people aged 60 years and older will reach 2.1 billion.[1] In Europe by 2060, people aged 65 and older will rise from 18% to 28% of the whole population and the share of people aged 80 and older will rise from 5% to 12% just like young person.[2] The same trend is expected in Italy, where by 2050, people aged 65 and older it is estimated to be 24% of the population and 15% of them is projected to be older than 80 years.[3] As a result of this demographic change, the impact of chronic diseases was most likely to occur[4] and many people are living with more than one conditions, and are more complex patient. In clinical practice patient complexity is defined by the interaction among medical issues, psychological and social characteristics and healthcare factors.[5] In view of this, a comprehensive assessment is necessary. Health related quality of life (HRQOL) is an instrument useful to characterize the impact of multiple chronic conditions on population by exploring patient's physical, emotional, and social functioning.[6] According to World Health Organization quality of life is defined as “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns”.[7] The association between chronic diseases and HRQOL has also been well studied[8] in particular the effect of multiple chronic conditions on HRQOL.[9,10] Although this piece of evidence, few data are available about the association between chronic conditions and HRQOL in patients admitted to internal medicine wards.[11] Given this background the aim of this investigation was to evaluate the predictors of quality of life in a cross-sectional study about elderly patients hospitalized in internal medicine ward focusing on the effect of comorbidities on health-related quality of life.
2. Patients and methods
Two hundred and forty elderly inpatients were consecutively enrolled. The patients were admitted to the Department of Internal Medicine, National Relevance and High Specialization Hospital Trust ARNAS Civico, Di Cristina, Benfratelli of Palermo (Italy). Each patient gave informed consent after receiving a detailed description of the study procedures. This cross-sectional study was approved by the Ethics Committee of our institution. Socio-demographic variables were considered. The following clinical characteristics were evaluated: disease distribution at hospital admission (classification was based on the International Classification of Diseases-Ninth Revision), performance in basic activities of daily living (measured by means of the Barthel Index), comorbidity and severity indexes (according to the Cumulative Illness rating Scale CIRS-c and CIRS-s), cognitive status according to the Short Blessed Test, SBT, and mood disorders using the Geriatric Depression Scale, GDS score.[12] Moreover, we subdivided subjects according to low risk and high risk of depression.
2.1. Quality of life (QOL)
Quality of life was measured by the 12-Item Short Form Health Survey (SF-12), developed for the Medical Outcomes Study (MOS), a multi-year study of patients with chronic conditions.[13,14] The SF-12 consists of 12 items from the larger SF-36 and it is used to measure health status in general population. The SF-12 measures physical functioning, role limitations due to physical health problems, bodily pain, general health, vitality (energy/fatigue), social functioning, role limitations due to emotional problems, and mental health (psychological distress and psychological well-being). Two composite scores— the physical component score (PCS) and the mental component score (MCS) are computed from all 12 items using a standard scoring algorithm.[15] The PCS score primarily focuses on physical functioning, role-physical, bodily pain, general health and vitality scales. The MCS focuses on vitality, social functioning, role-emotional, and emotional well-being scales. Both PCS and MCS scores range from 0 to 100, higher score indicates better health status.[16] All patients were categorized according to PCS and MCS. To simplify the analysis, we subdivided PCS and MCS score in five classes comparing the worst class to the other ones.
2.2. Statistical analysis
Data were reported as percentages for categorical variables and as means (95% confidence intervals) for quantitative variables. A Barthel index (BI) score of ≤ 40 was used to recognize patients with significant disability according to our population characteristics and it is the best cut-off value in prediction of mortality.[17] The comparison between groups was made using the exact Fisher test for contingency tables and the z test for comparison of proportions. The non-parametric Mann–Whitney U test was used for comparison of quantitative variables. Multivariate logistic analysis was used to explore the relationship between variables and outcomes. Odds ratios (ORs) and 95% confidence intervals (95%CIs) were computed. The choice of variables was performed according to the Hosmer–Lemeshow methodology:[18] after univariate analysis, only variables with a P < .20 were included in the final model; then, through a backward process, variables were excluded until a significance level of P < .05 was reached for each variable. A two-tailed P < .05 was considered statistically significant. Stata (StataCorp. 2016. Stata Statistical Software: Release 14.1. College Station, TX: StataCorp LP) was used for database management and analysis.
3. Results
Table 1 shows baseline characteristics of hospitalized inpatients. Among patients 52.1% were male, the mean age was 76.4 (75.3–77.4) years old and the mean BMI was 27.32 (26.63–28.02). Current smokers and ex-smokers were 43.3%, 50.4% of patients were diabetics. In-patients with clinically significant disability (Barthel index ≤ 40) were 23.7% and 12.1% had a Barthel index < 20. More than half of subjects had a probable depression (GDS score ≥ 2), 43.7% had a Short-Blessed Test > 10. Mean severity and co-morbidity index were 2.8 and 2.9, respectively. Mean MCS and PCS were 42.8 and 34.9 respectively. Overall, disease distribution showed that diabetes, arterial hypertension, acute infections, chronic renal failure, obesity, COPD and acute anaemia were more frequent (Fig. 1). After dividing the MCS and PCS score in five classes, we analyse the difference. A significantly higher proportion of diabetics was present in the first class of MCS (Table 2) in comparison with the other classes (P = .05) as well as patients with cancer (P = .03). Patients in the first class MCS had a Barthel Index score (P = .0138) and a physical component score (P = .0244) significantly lower, a GDS significantly higher (P < .0001) along with a higher rate of people with GDS ≥ 2. Taking into account the PCS (Table 3), the proportion of inpatients with NYHA III-IV class is significantly higher in the first class of PCS in comparison with the other ones (P = .0026). People in the first-class PCS were older (78.2 vs 75.2 years, P = .0110), 41.5% were male. They had a Barthel index score significantly lower (P < .0001) with a share of subjects with a Barthel Index Score < 40 and < 20 significantly higher in comparison with the other classes (P = .0060 and P = .0027 respectively). A Geriatric Depression Scale significantly higher (P = .0004) along with a percentage of patients with a GDS ≥ 2 (P = .0098) was present in the first class MCS. In view of the evidence that 55% of subjects had a GDS score ≥ 2 we divided population according to low risk of depression and high risk of depression (Table 4). Patients with a high risk for depression had a Barthel index and MCS significantly lower (P < .0001 for both comparisons) along with a PCS (P = .0347). In-patients with a GDS score ≥ 2 had a Short-Blessed Test > 10 significantly higher (.0012). A significantly higher proportion of patients with liver diseases was present in subjects with high risk of depression (P = .0004 and P = .0171), respectively. Regarding drug therapy, pump inhibitors, heparins, diuretics, antibiotics, antiaggregating agents and insulins were the most drugs taken in subjects with high risk of depression (89.8%, 55%, 50.7, 50.7%, 42.5%, 42% respectively). In patients with low risk of depression pump inhibitors, diuretics, antibiotics, heparins, beta blockers and antiaggregating agents (74.2%, 43.5%, 43.5%, 41.6%, 34.6%, 31.7% respectively) were given more frequent. When we assessed independent predictors of lower MCS class by a multivariate analysis (Fig. 1), GDS score ≥ 2 was independently associated with first MCS class I. Regarding first PCS class, heart failure NYHA III-IV class and claudicatio intermittents were strongly independently associated with the worst PCS class. Liver disease was the only predictor of probable depression.
Table 1.
Patient socio-demographic, laboratory and clinical baseline characteristics of hospitalized inpatients.
| N | 240 |
| Age (yrs)∗ | 76.4 (75.3–77.4) |
| Male (%) | 52.1 |
| Body Mass Index (BMI)∗ | 27.32 (26.63–28.02) |
| Current smokers (%) | 9.6 |
| Ex smokers (%) | 33.7 |
| Diabetes mellitus (%) | 50.4 |
| Type 1 diabetes (%) | 1.7 |
| Type 2 diabetes (%) | 48.3 |
| Systolic pressure (mmHg)∗ | 129.7 (127.3–132.1) |
| Diastolic pressure (mmHg)∗ | 73.8 (72.6–75.1) |
| Framingham risk > 10% (%) | 57.9 |
| ABI (right)∗ | 1.00 (0.97–1.02) |
| ABI (left)∗ | 1.02 (1.00–1.04) |
| Barthel index∗ | 65.6 (61.7–69.6) |
| Barthel index < 20 (%) | 12.1 |
| Barthel index < 40 (%) | 23.7 |
| Geriatric Depression Scale (GDS)∗ | 1.8 (1.6–2.0) |
| GDS ≥2 (%) | 55.0 |
| Short Blessed Test (SBT)∗ | 9.8 (8.8–10.7) |
| SBT > 10 (%) | 43.7 |
| Severity index (CIRS)∗ | 2.8 (2.7–2.9) |
| Comorbidity index (CIRS)∗ | 2.9 (2.6–3.2) |
| Mental Component Score (MCS)∗ | 42.8 (41.4–44.3) |
| Physical Component Score (PCS)∗ | 34.9 (33.8–36.0) |
| Glycemia (mg/dl)∗ | 119.12 (109.53–128.71) |
| Blood urea nitrogen (mg/dL)∗ | 27.10 (24.91–29.29) |
| Creatinine (mg/dL)∗ | 2.52 (0.84–4.20) |
| Total Cholesterol (mg/dL)∗ | 155.42 (149.22–161.63) |
| HDL (mg/dL)∗ | 38.31 (36.45 – 40.18) |
| Triglycerides (mg/dL)∗ | 127.02 (114.90–139.14) |
| Hemoglobin (g/dL)∗ | 11.3 (11.1–11.6) |
| Diabetes (%) | 50.4 |
| Hypertension (%) | 41.7 |
| Acute infections (%) | 26.7 |
| Chronic renal disease (%) | 19.2 |
| Obesity (%) | 17.1 |
| COPD (%) | 15.8 |
| Acute anemia (%) | 15.0 |
| Cancer (%) | 12.5 |
| Chronic anemia (%) | 11.2 |
| Atrial fibrillation (%) | 8.7 |
| Dilated cardiomyopathy (%) | 7.5 |
| Liver diseases (%) | 7.1 |
| Osteoporosis (%) | 6.2 |
| Heart failure NYHA I-II (%) | 5.4 |
| Heart failure NYHA III-IV (%) | 4.6 |
| Peripheral artery disease (%) | 4.2 |
| Ischemic stroke (%) | 1.2 |
| Myocardial infarction (%) | 0.4 |
Data are reported as mean (95% Confidence Interval).
ABI = Ankle-Brachial Index, BMI = Body Mass Index, CIRS = Cumulative Illness Rating Scale, COPD = Chronic obstructive pulmonary disease, GDS = Geriatric Depression Scale, HDL = High Density Lipoproteins, NYHA = New York Heart Association, SBT = Short Blessed Test.
Figure 1.
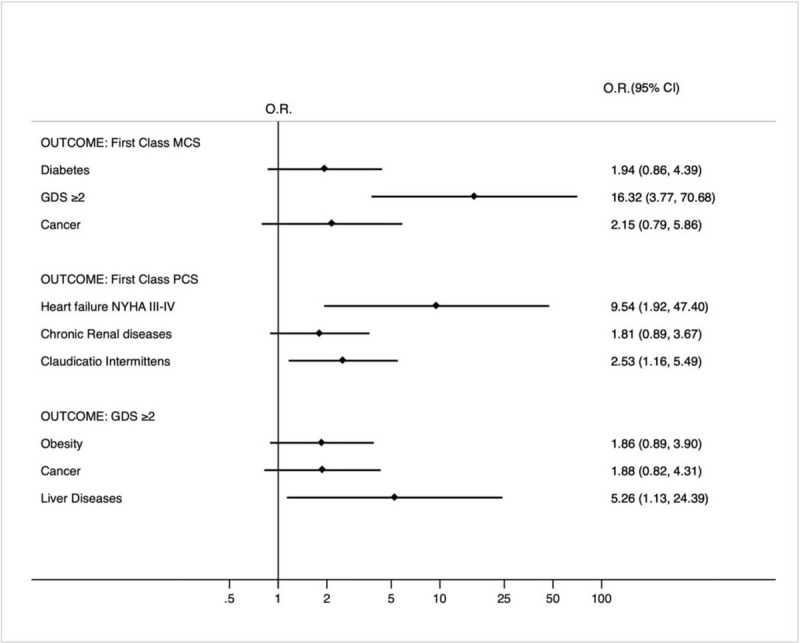
Multivariate analysis according to first class MCS, first class PCS and GDS ≥ 2 (high risk of depression) (OR = odds ratio; 95% CI = 95% confidence interval). The variables reported in the three models were selected by the Hosmer–Lemeshow methodology; only variables with a P < .20 are shown in the graph. ∗GDS = Geriatric depression score, MCS =Mental component score, PCS = Physical component score.
Table 2.
Patient socio-demographic, laboratory and clinical baseline characteristics of hospitalized inpatients according to first class in comparison to other classes of Mental Component Score (MCS).
| Variables | I Class N = 34 | II-V Classes N = 197 | P = |
| Age (yrs)∗ | 76.1 (73.8–78.4) | 76.3 (75.1–77.5) | .9235 |
| Male (%) | 52.9 | 50.8 | .8144 |
| Body Mass Index (BMI)∗ | 27.97 (26.30–29.63) | 27.17 (26.40–27.94) | .2477 |
| Current smokers (%) | 8.8 | 8.6 | .9704 |
| Ex smokers (%) | 38.2 | 33.5 | .5911 |
| Type 1 diabetes (%) | 2.9 | 1.5 | .5582 |
| Type 2 diabetes (%) | 61.8 | 44.7 | .0652 |
| Systolic pressure (mmHg)∗ | 134.2 (127.4–141.1) | 128.5 (125.9–131.2) | .1638 |
| Diastolic pressure (mmHg)∗ | 77.5 (73.7–81.2) | 72.9 (71.6–74.3) | .0251 |
| Framingham risk > 10% (%) | 55.9 | 58.9 | .7430 |
| ABI (right)∗ | 0.99 (0.94–1.05) | 1.00 (0.97–1.03) | .8070 |
| ABI (left)∗ | 0.99 (0.95–1.03) | 1.02 (1.00–1.05) | .3644 |
| Barthel index∗ | 55.7 (45.4–65.9) | 68.2 (63.9–72.4) | .0138 |
| Barthel index < 20 (%) | 20.6 | 10.1 | .0803 |
| Barthel index < 40 (%) | 29.4 | 21.8 | .3314 |
| Geriatric Depression Scale (GDS)∗ | 3.03 (2.72–3.33) | 1.59 (1.40–1.78) | <.0001 |
| GDS ≥2 (%) | 93.9 | 48.2 | <.0001 |
| Short Blessed Test (SBT)∗ | 11.7 (9.0–14.3) | 9.4 (8.3–10.5) | .1049 |
| SBT > 10 (%) | 41.2 | 59.2 | .0513 |
| Severity index (CIRS)∗ | 2.59 (2.20–2.99) | 2.82 (2.67–2.97) | .1223 |
| Comorbidity index (CIRS)∗ | 2.58 (1.81–3.36) | 2.95 (2.64–3.25) | .3510 |
| Physical Component Score (PCS)∗ | 32.13 (29.34–34.92) | 35.34 (34.16–36.52) | .0244 |
| Glycemia (mg/dl)∗ | 126.2 (103.8–148.5) | 118.0 (107.5–128.5) | .3057 |
| Blood urea nitrogen (mg/dL)∗ | 25.1 (19.3–31.0) | 27.8 (25.4–30.3) | .2027 |
| Creatinine (mg/dL)∗ | 1.23 (0.85–1.62) | 2.82 (0.77–4.86) | .4721 |
| Hemoglobin (g/dL)∗ | 10.8 (10.2–11.4) | 11.3 (11.0–11.7) | .2771 |
| Diabetes (%) | 64.7 | 46.7 | .05 |
| Hypertension (%) | 32.3 | 42.6 | .2603 |
| COPD (%) | 26.5 | 14.7 | .0879 |
| Cancer (%) | 23.5 | 10.7 | .0365 |
| Obesity (%) | 20.6 | 15.2 | .4313 |
| Atrial fibrillation (%) | 20.6 | 16.7 | .585 |
| Chronic renal disease (%) | 11.8 | 20.8 | .2187 |
| Chronic anemia (%) | 8.8 | 10.6 | .7459 |
| Acute anemia (%) | 8.8 | 16.2 | .2651 |
| Liver diseases (%) | 8.8 | 7.1 | .7233 |
| Acute infections (%) | 2.9 | 11.7 | .1232 |
| Heart failure NYHA III-IV (%) | 2.9 | 4.6 | .6668 |
| Ischemic stroke (%) | 2.9 | 1.0 | .3597 |
| Myocardial infarction (%) | 0.0 | 0.5 | .6772 |
| Dilated cardiomyopathy (%) | 0.0 | 8.1 | .085 |
| Osteoporosis (%) | 0.0 | 7.6 | .0961 |
| Heart failure NYHA I-II (%) | 0.0 | 6.6 | .1231 |
| Peripheral artery disease (%) | 0.0 | 4.6 | .2036 |
Data are reported as mean (95% Confidence Interval).
ABI = Ankle-Brachial Index, BMI = Body Mass Index, CIRS = Cumulative Illness Rating Scale, COPD = Chronic obstructive pulmonary disease, GDS = Geriatric Depression Scale, NYHA = New York Heart Association, SBT = Short Blessed Test.
Table 3.
Patient socio-demographic, laboratory and clinical baseline characteristics of hospitalized inpatients according to first class in comparison to other classes of Physical Component Score (PCS).
| Variables | I Class N = 82 | II-V Classes N = 149 | P = |
| Age (yrs)∗ | 78.2 (76.5–80.0) | 75.2 (73.9–76.5) | .0110 |
| Male (%) | 41.5 | 56.4 | .0300 |
| Body Mass Index (BMI)∗ | 27.20 (26.11–28.29) | 27.33 (26.42–28.25) | .7428 |
| Current smokers (%) | 6.1 | 10.1 | .3046 |
| Ex smokers (%) | 23.2 | 40.3 | .0088 |
| Type 1 diabetes (%) | 1.2 | 2.0 | .6580 |
| Type 2 diabetes (%) | 48.8 | 46.3 | .7188 |
| Systolic pressure (mmHg)∗ | 130.7 (126.8–134.6) | 128.6 (125.4–131.8) | .5928 |
| Diastolic pressure (mmHg)∗ | 74.2 (72.6–75.8) | 72.5 (70.2–74.7) | .1727 |
| Framingham risk > 10% (%) | 52.4 | 61.7 | .1697 |
| ABI (right)∗ | 1.01 (0.97–1.04) | 0.99 (0.96–1.03) | .8234 |
| ABI (left)∗ | 1.00 (0.97–1.03) | 1.03 (1.00–1.06) | .2853 |
| Barthel index∗ | 54.15 (47.38–60.92) | 73.09 (68.51–77.66) | <.0001 |
| Barthel index < 20 (%) | 19.5 | 7.4 | .0060 |
| Barthel index < 40 (%) | 34.1 | 16.8 | .0027 |
| Geriatric Depression Scale (GDS)∗ | 2.25 (1.94–2.56) | 1.56 (1.35–1.78) | .0004 |
| GDS ≥2 (%) | 66.7 | 48.6 | .0098 |
| Short Blessed Test (SBT)∗ | 10.46 (8.91–12.00) | 9.44 (8.16–10.71) | .1019 |
| SBT > 10 (%) | 51.3 | 59.2 | .2553 |
| Severity index (CIRS)∗ | 2.66 (2.44–2.88) | 2.84 (2.67–3.02) | .3704 |
| Comorbidity index (CIRS)∗ | 2.86 (2.29–3.43) | 2.92 (2.59–3.25) | .9057 |
| Mental Component Score (MCS)∗ | 41.19 (38.30–44.08) | 43.74 (42.19–45.29) | .0858 |
| Glycemia (mg/dl)∗ | 108.4 (97.3–119.5) | 123.0 (110.6–135.5) | .4792 |
| Blood urea nitrogen (mg/dL)∗ | 27.9 (24.3–31.4) | 27.2 (24.3–30.1) | .4449 |
| Creatinine (mg/dL)∗ | 1.21 (1.01–1.41) | 3.33 (0.65–6.01) | .9405 |
| Hemoglobin (g/dL)∗ | 11.2 (10.8–11.7) | 11.3 (10.9–11.7) | .9287 |
| Diabetes (%) | 51.2 | 48.3 | .6734 |
| Hypertension (%) | 40.2 | 41.6 | .8399 |
| Chronic renal disease (%) | 24.4 | 16.8 | .1622 |
| Atrial fibrillation (%) | 20.7 | 15.4 | .3088 |
| Obesity (%) | 20.6 | 15.2 | .4313 |
| COPD (%) | 17.1 | 16.1 | .8497 |
| Acute anemia (%) | 15.8 | 14.8 | .8252 |
| Cancer (%) | 14.6 | 11.4 | .4791 |
| Chronic anemia (%) | 11.0 | 10.1 | .8286 |
| Heart failure NYHA III-IV (%) | 9.8 | 1.3 | .0026 |
| Acute infections (%) | 8.5 | 11.4 | .4935 |
| Liver diseases (%) | 8.5 | 6.7 | .6112 |
| Dilated cardiomyopathy (%) | 7.3 | 6.7 | .8623 |
| Osteoporosis (%) | 4.9 | 7.4 | .4598 |
| Heart failure NYHA I-II (%) | 2.4 | 7.4 | .1187 |
| Peripheral artery disease (%) | 1.2 | 5.4 | .1189 |
| Myocardial infarction (%) | 0.0 | 0.7 | .4572 |
| Ischemic stroke (%) | 0.0 | 2.0 | .1959 |
Data are reported as mean (95% Confidence Interval).
ABI = Ankle-Brachial Index, BMI = Body Mass Index, CIRS = Cumulative Illness Rating Scale, COPD = Chronic obstructive pulmonary disease, GDS = Geriatric Depression Scale, NYHA = New York Heart Association, SBT = Short Blessed Test.
Table 4.
Patient socio-demographic, laboratory and clinical baseline characteristics of hospitalized inpatients according to the risk of depression.
| Variables | High risk for depression (GDS ≥ 2) N = 127 | Low risk for depression (GDS <2) N = 104 | P |
| Age (yrs)∗ | 76.3 (74.9–77.7) | 76.4 (74.7–78.1) | .9502 |
| Male (%) | 50.4 | 51.9 | .8170 |
| Body Mass Index (BMI)∗ | 27.37 (26.51–28.24) | 27.52 (26.37–28.67) | .4910 |
| Current smoking (%) | 11.8 | 7.7 | .2983 |
| Ex smokers (%) | 36.2 | 33.6 | .6842 |
| Diabetes mellitus (%) | 52.0 | 48.1 | .5562 |
| Type 1 diabetes (%) | 3.1 | 0.0 | .0679 |
| Type 2 diabetes (%) | 48.0 | 48.1 | .9945 |
| Systolic pressure (mmHg)∗ | 130.9 (127.8–133.9) | 127.9 (123.9–131.9) | .6011 |
| Diastolic pressure (mmHg)∗ | 74.8 (73.2–76.5) | 72.5 (70.5–74.5) | .0566 |
| Framingham risk > 10% (%) | 55.9 | 63.5 | .2448 |
| ABI (right)∗ | 1.00 (0.96–1.04) | 1.01 (0.98–1.04) | .5231 |
| ABI (left)∗ | 1.01 (0.98–1.05) | 1.04 (1.01–1.07) | .1424 |
| Barthel index∗ | 60.3 (55.3–65.4) | 74.9 (69.4–80.3) | <.0001 |
| Barthel index < 20 (%) | 11.8 | 7.7 | .2983 |
| Barthel index < 40 (%) | 26.0 | 17.3 | .1137 |
| Short Blessed Test (SBT)∗ | 10.8 (9.5–12.1) | 8.1 (6.8–9.5) | .0031 |
| SBT > 10 (%) | 52.8 | 31.4 | .0012 |
| Severity index (CIRS)∗ | 2.9 (2.7–3.1) | 2.7 (2.5–2.8) | .1128 |
| Comorbidity index (CIRS)∗ | 3.2 (2.7–3.6) | 2.6 (2.2–3.0) | .0690 |
| Mental Component Score (MCS)∗ | 39.6 (37.6–41.5) | 46.8 (44.8–48.7) | <.0001 |
| First Class MCS (%) | 25.2 | 2.0 | <.0001 |
| Physical Component Score (PCS)∗ | 33.8 (32.4–35.3) | 36.3 (34.6–37.9) | .0347 |
| First Class PCS (%) | 42.3 | 25.7 | .0098 |
| Glycemia (mg/dl)∗ | 119.7 (104.1–135.3) | 118.4 (107.3–129.5) | .7044 |
| Blood urea nitrogen (mg/dL)∗ | 26.9 (24.0–29.9) | 27.7 (24.2–31.1) | .5739 |
| Creatinine (mg/dL)∗ | 1.22 (1.06–1.39) | 4.27 (0.40–8.14) | .7220 |
| Hemoglobin (g/dL)∗ | 11.1 (10.8–11.4) | 11.3 (10.9–11.8) | .6361 |
| Diabetes (%) | 52.0 | 48.1 | .5562 |
| Hypertension (%) | 46.5 | 37.5 | .1706 |
| Acute infections (%) | 27.6 | 27.9 | .9561 |
| Chronic renal disease (%) | 22.0 | 13.5 | .0923 |
| Obesity (%) | 20.5 | 12.5 | .1076 |
| Acute anemia (%) | 16.5 | 12.5 | .3891 |
| COPD (%) | 15.7 | 17.3 | .7504 |
| Cancer (%) | 15.7 | 9.6 | .1677 |
| Atrial fibrillation (%) | 10.2 | 7.7 | .5034 |
| Liver diseases (%) | 9.4 | 1.9 | .0171 |
| Chronic anemia (%) | 8.7 | 11.5 | .4675 |
| Dilated cardiomyopathy (%) | 8.7 | 6.7 | .5860 |
| Osteoporosis (%) | 6.3 | 6.7 | .8946 |
| Heart failure NYHA III-IV (%) | 6.3 | 2.9 | .2253 |
| Heart failure NYHA I-II (%) | 5.5 | 5.8 | .9327 |
| Peripheral artery disease (%) | 3.1 | 5.8 | .3304 |
| Ischemic stroke (%) | 0.8 | 1.9 | .4482 |
| Myocardial infarction (%) | 0.8 | 0.0 | .3645 |
Data are reported as mean (95% Confidence Interval).
ABI = Ankle-Brachial Index, BMI = Body Mass Index, CIRS = Cumulative Illness Rating Scale, COPD = Chronic obstructive pulmonary disease, GDS = Geriatric Depression Scale, MCS = Mental Component Score, NYHA = New York Heart Association, PCS = Physical Component Score, SBT = Short Blessed Test.
3.1. Discussion
The present study is one of very few performed in internal medicine ward. Because of the demographic change due to the aging of the population, the management of multiple chronic conditions are becoming the main challenge in clinical practice. Along with this issue, in subjects admitted to internal medicine wards the assessment of the impact of chronic diseases on health-related quality of life is important in order to identify subjects at higher risk of poor health and quality of life and to improve the quality of health-care. To date few data are available about the perception of health from in-patients admitted to internal medicine wards.[11] Different studies showed that chronic medical conditions affect physical functioning and reduced quality of life.[19,20] Particularly, gastrointestinal diseases, cerebrovascular conditions, musculoskeletal and renal diseases determined poorer quality of life.[20]
One of the main finding of our analysis concerned the impact of heart failure and intermittent claudication on physical component of HRQOL. This is likely to be closed linked to the functional limitation with a progressive reduction of daily activities.[21] In fact, heart failure had a negative impact on quality of life with increasing functional class, by a significant association with physical functioning and psychosocial function. Our results are consistent with previous studies that showed that cardiovascular diseases had a significant impact on physical component in elderly patients.[22] In addition, intermittent claudication has a significant reduction in health-related quality of life (HRQoL) caused by impaired mobility and by cardiovascular morbidity. Patients with peripheral artery disease had a significant impairment of physical functional score in comparison with controls[23,24] and even worse scores than individuals with coronary artery disease and congestive heart failure.[25]
Our findings indicate that GDS ≥ 2 was an independent strong predictor of first class MCS. It is well known that depression is an important determinant of quality of life. Mood and anxiety disorders are the most prevalent among elderly patients.[26] As shown by Beladon et al[22] a strong relationship between depression symptoms and mental quality of life is observed in older primary care patients both on men and women. Mood disorders had the greatest impact on HRQoL among the elderly.[22,27,28] Mainly, depression affects emotion, motivation, and cognition that are closely related to vitality, role function, or social functioning, some of the major components of HRQoL measures.[29] Data of community-dwelling elderly subjects, showed that GDS scores above 9/30 or 5/15 were able to predict subjects in whom treatment could likely improve quality of life.[30]
A recent analysis of the DIAREG registry, a nationwide German general medicine practice database showed that patients with <2 years diabetes duration had a significantly decreased of mental component score.[31] Moreover, data from the longitudinal Living with Diabetes Study showed that depression and anxiety were associated with poorer diabetes-specific quality of life.[32] Wong et al showed that insulin treatment was associated with lower MCS-12.[33] In the present study diabetes did not enter the multivariate analysis as independent predictor of MCS, although diabetes was suggestive of worst mental component score. A possible explanation for the apparent discrepancy lies in the lack of information about the diabetic disease duration, the limited sample size and type of drugs taken.
Cancer diagnosis often results in negative impacts on health-related quality of life[34] because of alteration of mental health, anxiety, fear of cancer recurrence,[35] therefore the risk of depression is more likely among subjects with cancer.[36] Depression in people affected by cancer is negatively associated with health-related quality of life.[37,38] Ehus et al showed that the diagnosis of small breast cancer affects health related quality of life including both physical and mental component score.[39] Our data are in accordance with this piece of evidence even if a cancer did not represent a strong predictor of lower mental component score and GDS ≥ 2 probably due to the limited sample size.
It is well known that depression and cognitive impairment lead to progressive disability,[40,41] especially in oldest-old subjects[42] with implications on short and long-term outcomes.
Globally, depression represents the leading cause of disability.[43] Depression combined with other chronic diseases result in greater reduction of health-related quality of life (HRQoL).[44] The present analysis highlighted the role of liver diseases that significantly increased the risk of depression. According to the Global Burden of Diseases, Injuries, and Risk Factors Study, the combination of depression and alcohol abuse lead to lifespan disability and premature mortality rates.[45,46] Subjects with NAFLD and HCV have a higher prevalence of depression in comparison with HBV patients and the general population.[47] Recent data showed that in subjects with major depressive syndrome a significantly higher prevalence and incidence of chronic liver disease than the general population is detectable.[48] In this population-based database, an older age, the male sex, diabetes, hyperlipidemia and first-generation antipsychotic use were associated with chronic liver disease.
In this study some limitations have to be outlined. First of all, this is a single centre analysis. No history data were available about timing of comorbidities to assess possible relationship with both quality of life measures and depression. No other specific instrument for assessing symptoms of depression except for geriatric depression scale was used. No investigation about any correspondence between different type of diseases and outcomes by specific treatment was made. The population study consists only of oldest old patients and the sample size is limited. On the contrary, the major strength of our study lies in the rigorous collection of data in a standardized fashion. Although the sample size is limited the results are quite robust to support further research. However, our data may not be generalizable. Our observations need to be verified by later studies to avoid population bias due to the single centre study. Finally, our analysis revealed the importance of GDS that is a quick and simple tool that might be utilized in every day clinical practice of internal medicine wards.
4. Conclusion
This analysis showed the impact of chronic diseases on health-related quality of life in the real-world scenario of an internal medicine ward. This is the first study that pointed out the association between GDS score and chronic conditions in elderly inpatients. Thus, GDS score might be used as a screening tool to select elderly patients that must be assessed for psychological and therapeutic support in internal medicine ward. Our study strongly supports a different approach to subjects admitted to internal medicine ward that take into account of patient's needs and perception. This could be the first step in order to reduce health care costs, the burden of hospitalization and to establish a personalized care. Further studies are necessary to confirm our data in multicentre reliability and a bigger sample size.
Author contributions
Conceptualization: Salvatore Corrao, Christiano Argano, Nicola Catalano.
Data curation: Giuseppe Natoli.
Formal analysis: Salvatore Corrao, Christiano Argano, Nicola Catalano.
Investigation: Salvatore Corrao, Nicola Catalano, Giuseppe Natoli, Marika Lo Monaco.
Methodology: Salvatore Corrao, Christiano Argano, Giuseppe Natoli.
Project administration: Salvatore Corrao, Christiano Argano, Giuseppe Natoli.
Resources: Salvatore Corrao.
Software: Giuseppe Natoli.
Supervision: Salvatore Corrao, Christiano Argano.
Validation: Salvatore Corrao, Christiano Argano, Nicola Catalano, Giuseppe Natoli, Marika Lo Monaco.
Visualization: Salvatore Corrao, Christiano Argano, Nicola Catalano, Giuseppe Natoli, Marika Lo Monaco.
Writing – original draft: Christiano Argano.
Writing – review & editing: Salvatore Corrao, Giuseppe Natoli.
Footnotes
Abbreviations: BI = Barthel Index, BMI = Body Mass Index, CIRS = Cumulative Illness Rating Scale, COPD = Chronic obstructive pulmonary disease, GDS = Geriatric Depression Scale, HBV = Hepatitis B Virus, HCV = Hepatitis C Virus, HR-QOL = Health-related quality of life, MCS = Mental Component Score, MOS = Medical Outcomes Study, NAFLD = Nonalcoholic Fatty Liver Disease, NYHA = New York Heart Association, OR = Odds ratio, PCS = Physical Component Score, QOL = Quality of Life, SBT = Short Blessed Test.
How to cite this article: Argano C, Catalano N, Natoli G, Monaco ML, Corrao S. GDS score as screening tool to assess the risk of impact of chronic conditions and depression on quality of life in hospitalized elderly patients in internal medicine wards. Medicine. 2021;100:26(e26346).
The authors have no funding to disclose.
The authors have no conflicts of interest to disclose.
All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Available at: https://acmedsci.ac.uk/file-download/82222577 [access date February 1, 2019, English version]. [Google Scholar]
- [2].The 2015 Aging Report European Commission. Available at: http://ec.europa.eu/economy_finance/publications/european_economy/2015/ee3_en.htm [access date December 13, 2018, English version]. [Google Scholar]
- [3].Mannucci PM, Nobili A, Pasina L. REPOSI Collaborators (REPOSI is the acronym of REgistro POliterapie SIMI, Società Italiana di Medicina Interna). Polypharmacy in older people: lessons from 10 years of experience with the REPOSI register. Intern Emerg Med 2018;13:1191–200. [DOI] [PubMed] [Google Scholar]
- [4].Cohen JE. Human population: the next half century. Science 2003;302:1172–5. 14. [DOI] [PubMed] [Google Scholar]
- [5].Corrao S, Natoli G, Nobili A, et al. RePoSI Investigators. Comorbidity does not mean clinical complexity: evidence from the RePoSI register. Intern Emerg Med 2019;doi: doi:10.1007/s11739-019-02211-3. [DOI] [PubMed] [Google Scholar]
- [6].Alonso JF, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res 2004;13:283–98. [DOI] [PubMed] [Google Scholar]
- [7].Available at: https://www.who.int/healthinfo/survey/whoqol-qualityoflife/en/accessed [access date April 4, 2019, English version]. [Google Scholar]
- [8].Fortin M, Bravo G, Hudon C, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res 2006;15:83–91. [DOI] [PubMed] [Google Scholar]
- [9].Tooth L, Hockey R, Byles J, Dobson A. Weighted multimorbidity indexes predicted mortality, health service use, and health-related quality of life in older women. J Clin Epidemiol 2008;61:151–9. [DOI] [PubMed] [Google Scholar]
- [10].Ford ES, Mannini DM, Redd SC, Moriarty DG, Mokdad AH. Determinants of quality of life among people with asthma: findings from the behavioral risk factor surveillance system. J Asthma 2004;41:327–36. [DOI] [PubMed] [Google Scholar]
- [11].Jakobsson S, Jakobsson Ung E, Lindström M, Eliasson B, Ringström G. Health status and most distressing concerns at admission and discharge reported by patients cared for at an internal medical care ward. Scand J Caring Sci 2018;32:1168–78. [DOI] [PubMed] [Google Scholar]
- [12].Corrao S, Argano C, Nobili A, et al. REPOSI Investigators. Brain and kidney, victims of atrial microembolism in elderly hospitalized patients? data from the REPOSI study. Eur J Intern Med 2015;26:243–9. [DOI] [PubMed] [Google Scholar]
- [13].Hurst NP, Ruta DA, Kind P. Comparison of the MOS Short Form-12 (SF12) health status questionnaire with the SF36 in patients with rheumatoid arthritis. Br J Rheumatol 1998;37:862–9. [DOI] [PubMed] [Google Scholar]
- [14].McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-items Short Form Health Survey (SF-36): III: tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66. [DOI] [PubMed] [Google Scholar]
- [15].Ware J, Kosinski M, Keller SD. SF-36 physical and mental health summary scales: a user's manual. Boston, MA: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- [16].Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- [17].Corrao S, Santalucia P, Argano C, et al. REPOSI Investigators. Gender-differences in disease distribution and outcome in hospitalized elderly: data from the REPOSI study. Eur J Intern Med 2014;25:617–23. [DOI] [PubMed] [Google Scholar]
- [18].Hosmer DW, Lemeshow S. Applied logistic regression. New York: J Wiley; 2002. [Google Scholar]
- [19].Corrao S, Argano C, Natoli G, et al. REPOSI Investigators. Disability, and not diabetes, is a strong predictor of mortality in oldest old patients hospitalized with pneumonia. Eur J Intern Med 2018;54:53–9. [DOI] [PubMed] [Google Scholar]
- [20].Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA 1989;262:907–13. Erratum in: JAMA 1989 Nov 10;262(18):2542. [PubMed] [Google Scholar]
- [21].López Castro J, Cid Conde L, Fernández Rodríguez V, et al. Analysis of quality of life using the generic SF-36 questionnaire in patients with heart failure. Rev Calid Asist 2013;28:355–60. [DOI] [PubMed] [Google Scholar]
- [22].Baladón L, Rubio-Valera M, Serrano-Blanco A, Palao DJ, Fernández A. Gender differences in the impact of mental disorders and chronic physical conditions on health-related quality of life among non-demented primary care elderly patients. Qual Life Res 2016;25:1461–74. [DOI] [PubMed] [Google Scholar]
- [23].Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med 2008;13:15–24. [DOI] [PubMed] [Google Scholar]
- [24].Dumville JC, Lee AJ, Smith FB, et al. The healthrelated quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br J Gen Pract 2004;54:826–31. [PMC free article] [PubMed] [Google Scholar]
- [25].Ware JE, Jr. The status of health assessment 1994. Annu Rev Public Health 1995;16:327–54. [DOI] [PubMed] [Google Scholar]
- [26].Baladón L, Fernández A, Rubio-Valera M, et al. Prevalence of mental disorders in non-demented elderly people in primary care [corrected]. Int Psychogeriatr 2015;27:757–68. [DOI] [PubMed] [Google Scholar]
- [27].Pinto-Meza A, Ferna’ndez A, Fullana MA, et al. Impact of mental disorders and chronic physical conditions in health-related quality of life among primary care patients: results from an epidemiological study. Qual Life Res 2009;18:1011–8. [DOI] [PubMed] [Google Scholar]
- [28].Subramaniam M, Abdin E, Vaingankar JA, et al. Impact of psychiatric disorders and chronic physical conditions on health-related quality of life: Singapore Mental Health Study. J Affect Disord 2013;147:325–30. [DOI] [PubMed] [Google Scholar]
- [29].Alonso J, Angermeyer MC, Bernert S, et al. Disability and quality of life impact of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatrica Scandinavica: Supplementum 2004;420:38–46. [DOI] [PubMed] [Google Scholar]
- [30].Laudisio A, Antonelli Incalzi R, Gemma A, et al. Definition of a geriatric depression scale cutoff based upon quality of life: a population-based study. Int J Geriatr Psychiatry 2018;33:e58–64. [DOI] [PubMed] [Google Scholar]
- [31].Rathmann W, Kuß O, Anderson D, et al. Increased depression symptom score in newly diagnosed type 2 diabetes patients. Psychiatry Res 2018. 259–63. [DOI] [PubMed] [Google Scholar]
- [32].Donald M, Dower J, Coll JR, Baker P, Mukandi B, Doi SA. Mental health issues decrease diabetes-specific quality of life independent of glycaemic control and complications: findings from Australia's living with diabetes cohort study. Health Qual Life Outcomes 2013;11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wong CK, Lo YY, Wong WH, et al. The associations of body mass index with physical and mental aspects of health-related quality of life in Chinese patients with type 2 diabetes mellitus: results from a cross-sectional survey. Health Qual Life Outcomes 2013;21:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA Cancer J Clinicians 2007;57:278–300. [DOI] [PubMed] [Google Scholar]
- [35].American Cancer Society. Anxiety, Fear, and Depression. Available at: https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/changes-in-mood-or-thinking/anxiety-andfear. Html [access date June 15, 2017, English version]. [Google Scholar]
- [36].Chochinov HM. Depression in cancer patients. Lancet Oncol 2001;2:499–505. [DOI] [PubMed] [Google Scholar]
- [37].Fann JR, Thomas-Rich AM, Katon WJ, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry 2008;30:112–26. [DOI] [PubMed] [Google Scholar]
- [38].Faller H, Brahler E, Harter M, et al. Performance status and depressive symptoms as predictors of quality of life in cancer patients. A structural equation modelling analysis. Psychooncology 2015;24:1456–62. [DOI] [PubMed] [Google Scholar]
- [39].Euhus DM, Addae JK, Snyder CF, Canner JK. Change in health-related quality of life in older women after diagnosis of a small breast cancer. Cancer 2019. [DOI] [PubMed] [Google Scholar]
- [40].Corrao S, Argano C, Natoli G, et al. REPOSI Investigators. Sex-Differences in the pattern of comorbidities, functional independence, and mortality in elderly inpatients: evidence from the RePoSI Register. J Clin Med 2019;8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the women's health and aging study. J Clin Epidemiol 1999;52:27–37. [DOI] [PubMed] [Google Scholar]
- [42].García-Pérez L, Linertová R, Lorenzo-Riera A, Vázquez-Díaz JR, Duque-González B, Sarría-Santamera A. Risk factors for hospital readmissions in elderly patients: a systematic review. QJM 2011;104:639–51. [DOI] [PubMed] [Google Scholar]
- [43].Friedrich MJ. Depression is the leading cause of disability around the world. JAMA 2017;317:1517. [DOI] [PubMed] [Google Scholar]
- [44].Baumeister H, Hutter N, Bengel J, Härter M. Quality of life in medically ill persons with comorbid mental disorders: a systematic review and meta-analysis. Psychother Psychosom 2011;80:275–86. [DOI] [PubMed] [Google Scholar]
- [45].Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2010;382:1575–86. [DOI] [PubMed] [Google Scholar]
- [46].Le Strat Y, Le Foll B, Dubertret C. Major depression and suicide attempts in patients with liver disease in the United States. Liver Int 2015;35:1910–6. [DOI] [PubMed] [Google Scholar]
- [47].Weinstein AA, Kallman Price J, Stepanova M, et al. Depression in patients with non-alcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics 2011;52:127–32. [DOI] [PubMed] [Google Scholar]
- [48].Hsu JH, Chien IC, Lin CH. Increased risk of chronic liver disease in patients with major depressive disorder: a population-based study. J Affect Disord 2019;251:180–5. [DOI] [PubMed] [Google Scholar]


