Abstract
Post-acute care (PAC) is a type of transitional care for poststroke patients after the acute medical stage; it offers a relatively intensive rehabilitative program. Under Taiwan's National Health Insurance guidelines, the only patients who can transfer to PAC institutions are those who have had an acute stroke in the previous month, are in a relatively stable medical condition, and have the potential for improvement after aggressive rehabilitation. Poststroke patients receive physical, occupational, and speech therapy in PAC facility. However, few studies have evaluated the effects of PAC in poststroke patients since PAC's initiation in Taiwan. Thus, this study aims to investigate whether the length of stay in a PAC institution correlates with patients’ improvements.
This retrospective and single-center study in Taiwan enrolled 193 poststroke patients who had received acute care at Chi-Mei Medical Center, Taiwan, at any period between 2014 and 2017. Data on their length of stay in the PAC institution were collected. Poststroke patients’ functional ability—such as activities of daily living (ADL) function and swallowing ability—as well as their corresponding scales were assessed on the first and last day of PAC stay. Statistical analysis was conducted by SPSS version 21.0.
The average duration of PAC stay was 35.01 ± 16.373 days. Duration of PAC stay was significantly positively correlated with the Barthel index (P < .001), Berg balance test score (P < .001), gait speed (P = .002), and upper sensory function and upper motor function within the Fugl–Meyer Assessment (both P < .001).
Poststroke patients with longer stay in a PAC institution had superior ADL function, balance and coordination, walking speed, and upper-limb dexterity and sensory function.
Keywords: activities of daily living, length of stay, stroke, subacute care
1. Introduction
Post-acute care (PAC) is a type of transitional care for patients with stroke after the acute medical stage; it offers a relatively intensive rehabilitation program. Because people have longer lifespan in current societies and decreasing 30-days stroke-related mortality,[1] the need for rehabilitating poststroke patients is growing. In the United States, 62.6% to 74.5% of poststroke patients were noted to require further rehabilitative PAC programs after being discharged from the hospital.[2]
In Taiwan, stroke is the major cause of disability and the third-leading cause of death.[3] According to Taiwan's National Health Insurance (NHI) guidelines, the only patients who can be transfer to PAC institutions are those who have had an acute stroke within the preceding month, are in a relative stable medical condition, and have the potential for favorable recovery. PAC institutions are mainly situated in local or district hospitals in Taiwan. Poststroke patients receive physical, occupational, and speech therapy in their daily 1-hour therapy session during weekdays in PAC. The rehabilitative programs were designed according to the patients’ functional ability and included range of motion exercise, facilitation, stretching and strengthening, balance training, bed/mattress mobility training, coordination training, postural training, ambulation training, daily activities training, electrical stimulation, speech, and swallowing training. In addition to physiatrist and therapists, the care team comprises of neurologist, case manager, nurse, nutritionist, pharmacist, and social worker. In Taiwan, the NHI pays for a maximum of 12 weeks of PAC.
Epidemiologic data in Taiwan's current PAC programs shows the patients were generally over 60 years old, male predominant, with ischemic stroke.[4] PAC programs have been noted to significantly improve activities of daily living (ADL), oral intake, nutrition, balance, walking speed, cardiorespiratory endurance, sensation, motor function, cognition, mobility, and language.[4–6] A previous study also reported that poststroke patients undergoing PAC are less likely to visit the emergency department or be readmitted within 90 days for stroke-related reasons or otherwise.[4]
Medical cost and stroke-related expenditure are a burden to society, for instance, yearly stroke-related medical expenditure was estimated to be US$375 million[7] in Taiwan. Furthermore, a French study showed stroke rehabilitation is one of the 3 largest contributions to this cost.[8] PAC was thus established to alleviate this financial burden[5]: poststroke patients can be discharged earlier to undergo PAC in place of a lengthier and more expensive hospital stay.[6]
In the past literature, the average length of stay for poststroke inpatient rehabilitation has been noted to be 29.4 days in Thailand, 31.2 days in Switzerland, and 37.1 days in Singapore,[9] all longer than that in the United States.[2] For Taiwan in particular, the mean hospital stay in a rehabilitative ward is 34.7 days according to one study[10] and 25.3 days (after 23.3 days in an acute ward) according to another.[11]
The ability of PAC programs to improve the overall well-being, the mortality rate, the readmission rate, the likelihood of an emergency department visit, the medical costs, and different types of PAC institutions, has been discussed in the literature. However, no study has evaluated the effects of PAC inpatient stay for poststroke patients in Taiwan since PAC's introduction in Taiwan in 2014. Therefore, this Taiwanese study investigated whether the duration of hospitalization in PAC is associated with improvements in poststroke patients.
2. Methods
2.1. Design
This was a retrospective and single-center study conducted in Taiwan. We included 305 patients with stroke who had received acute care at our hospital in any period during 2014 to 2017. Length of PAC stay for each patient was recorded after discharging from acute stroke unit in our hospital. Data on the participants’ health condition were collected by the PAC case manager from the respective PAC institution. The study design was approved by an Institutional Review Board of Chi Mei Medical Center (10711-J02) and has been listed on ClinicalTrials.gov (identification number: NCT03778905).
2.2. Study sample and enrollment
Patients were included if they had a modified Rankin scale (MRS) score of 2–4, had acute onset of stroke in the previous 30 days, and were >18 years old. Among the 305 poststroke patients eligible for PAC admission who were recruited, 3 refused PAC admission, and 6 had a second stroke during the acute phase. Among the remaining 296 patients who successfully transferred to a PAC institution, 8 patients ended their PAC hospitalization early due to stroke or to non-stroke disease progression. We excluded a further 95 patients due to incomplete data on their functional ability during PAC stay. Therefore, 193 poststroke patients who completed their PAC stay and for whom complete data on functional ability were available were enrolled in our study (Fig. 1).
Figure 1.
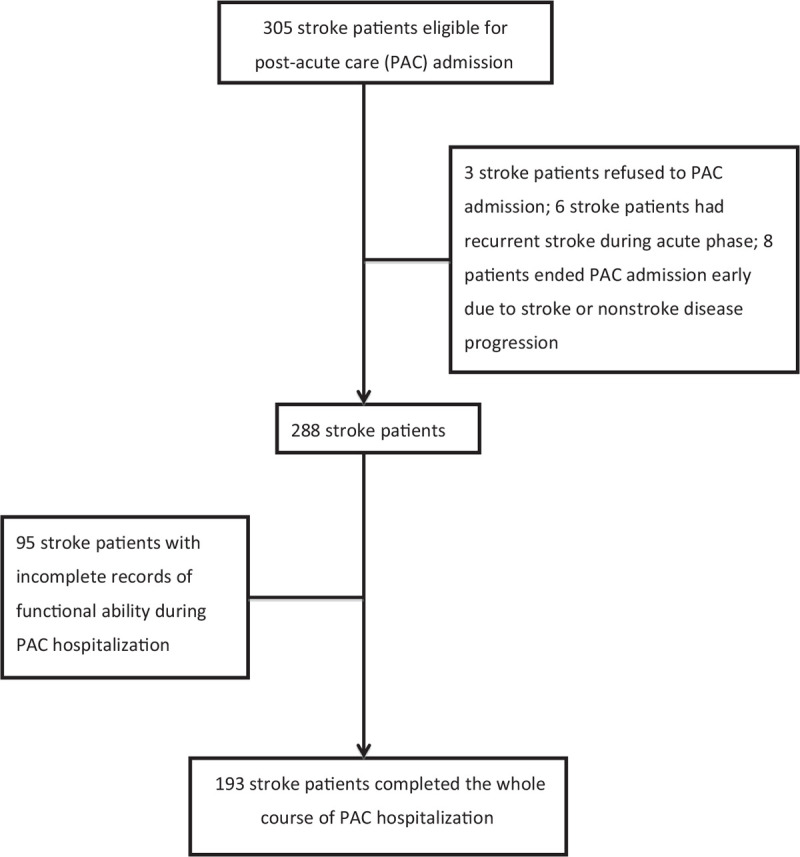
Enrollment procedure.
2.3. Functional ability and variables
The following functional domains of poststroke patients were analyzed: ADL function, swallowing ability, nutritional status, quality of life, instrumental ADL function, balance and coordination, walking speed, cardiopulmonary capability, sensory function of upper extremities, motor function of upper extremities, cognitive function, occupational mobility,[4] and linguistic ability. Data on these domains were collected at the beginning and end of admission to PAC by the patients’ physiatrists and therapists.
2.4. Measurements
We used various measurements to evaluate the status of the patients. The MRS reflects a poststroke patient's general condition and degree of handicap,[12] and it is also used to determine whether a poststroke patient is eligible for PAC. The MRS score ranges from 0 to 6, from better to worse.[13] The Barthel index (BI) describes a patient's mobility and ability for self-care. It contains 10 domains of activities (feeding, transfer, ambulation, stair climbing, dressing oneself, bowel and bladder control, self-hygiene, toilet use, and showering). The BI score ranges from 0 to 100, from least to most independent.[14] The Functional Oral Intake Scale (FOIS) describes the seriousness of dysphagia in poststroke patients in 7 levels, from mild to severe.[15] The mini nutritional assessment (MNA) comprises of 4 parts: anthropometric, general, self and dietary evaluations with the lower score indicates more likely to be malnutrition. The Taiwanese version of the EuroQoL EQ-5D (abbreviated as QoL hereafter), with each aspect is given a score from 1 to 3 from best to worst quality of life[16–18] by patients’ personal perception, indicates 5 aspects related to life quality—ambulation, self-care, conduct of common activities, level of pain and discomfort, and level of anxiety and depression. The Lawton–Brody Instrumental ADL Scale (referred to as IADL hereafter) describes how patients engage in advanced everyday activities; it contains 8 items; making phone calls, shopping, preparing food, housekeeping, doing laundry, taking pills, and managing finances.[19] The Berg balance test (BAL) comprises of 14 tasks, which are used to evaluate a patient's static and dynamic balance. These tasks comprise of sitting unsupported, standing unsupported, standing with eyes closed, standing with feet together, standing on 1 foot, turning to look behind, retrieving an object from the floor, tandem standing, reaching forward with an outstretched arm, sitting to standing, standing to sitting, transferring, and turning 360° and then stepping on a stool. For each task, a score from 0 to 4 is given for a maximum score of 56 in total,[20] where a higher total score indicates better balance. The usual gait speed measure (referred to hereafter as gait speed) was used and is calculated as how long a patient takes to finish a 5-m walk as quickly as they can. It is a crucial prognostic factor for older adults.[21] Higher gait speed indicates better walking ability. The 6-Minute Walk Test (6MWT) is scored as how far a patient can walk in 6 minutes, where a greater distance indicates greater cardiopulmonary capability.[22]
The Fugl–Meyer Assessment (FMA) validly indicates sensorimotor function in poststroke patients.[23] It comprises the domains of upper and lower limb motor function, sensory function, balance, range of motion, and degree of joint pain. These domains are assigned scores,[24] where a higher score indicates better sensorimotor ability. The mini-mental state examination (MMSE) indicates a patient's cognitive function by testing their faculties of orientation, attention, calculation, memory, language, and visuospatial ability. Cognitive impairment is suggested by scores <24.[25,26] The motor activity log (MAL) of the quantity and quality of motor activity indicates occupational mobility and performance. In the MAL, a patient is asked how often and how well they use their affected upper limb in daily activities. Each question is scored from 0 to 5, the MAL comprises of 2 sections, and the mean score for each section is considered.[27] The Concise Chinese Aphasia Test (CCAT) assesses speech function. The CCAT comprises the 9 domains of simple response, expository speech, matching, auditory and reading comprehension, naming, repetition, copying, and spontaneous writing. Each domain is scored from 1 to 12 from lowest to highest performance.[28] Functional improvement in each scale is defined as the score after PAC minus the score before PAC.
2.5. Analysis
Statistical analysis was conducted by SPSS version 21.0 (IBM Corp, Armonk, NY, USA). Continuous variables, such as length of stay in PAC, were expressed in terms of their mean and standard deviation. Bivariate correlations, indicating improvements in functional ability in relation to the length of stay, were represented by the Pearson product-moment correlation coefficient. The distribution of each scale was analyzed using the Kolmogorov–Smirnov test, and the significance of each improvement was analyzed using the Wilcoxon signed-rank test or Wilcoxon rank-sum test if the value distributed non-normally. Effect size was indicated by Cohen d.[29] Statistical significance was indicated if P < .05.
3. Results
The 193 enrolled patients had a mean age (±standard deviation) of 63.07 (±14.135; range: 26–91) years. The mean length of stay in PAC was 35.01 (±16.373; range: 4–84) days. Among the patients enrolled, 76.7% (n = 148) and 23.3% (n = 45) had ischemic and hemorrhagic stroke, respectively, and 60.6% (n = 117) and 39.4% (n = 76) were men and women, respectively (Table 1); only 51.8% (n = 100) received speech therapy during their PAC stay.
Table 1.
Demographics of enrolled stroke patients.
| Variables | n (%) or mean ± standard deviation |
| Gender | |
| Male | 117 (60.6%) |
| Female | 76 (39.3%) |
| Age (year-old) | 63.07 ± 14.135 |
| Length of stay in PAC, d | 35.01 ± 16.373 |
| Stroke types | |
| Hemorrhagic | 45 (23.3%) |
| Ischemic | 148 (76.7%) |
The pre- and post-PAC scores for each scale, and the corresponding improvement, are described by their mean ± standard deviation, median, and maximum and minimum (Table 2). In the patients enrolled, PAC significantly improved general function—specifically, the patients’ general condition, ADL, IADL, oral intake, nutrition, balance, quality of life, gait speed, cardiopulmonary endurance, sensation, motor function, occupational performance and mobility, and linguistic ability (Table 2); these findings are consistent with those of a previous study.[4]Table 2 presents the effect sizes of the scales with their Cohen d values.
Table 2.
Functional ability before and after post-acute care (PAC) training program.
| Before PAC program | After PAC program | Improvements | ||||||
| Variables | Mean ± SD | Median (rangeb) | Mean ± SD | Median (rangeb) | Mean ± SD | Median (rangeb) | Pa | dc |
| General function | ||||||||
| MRS | 3.69 ± 0.57 | 4 (2–4) | 3.13 ± 0.87 | 3 (1–5) | –0.554 ± 0.68 | 0 (–3∼1) | <.001∗∗∗ | 0.76 |
| BI | 42.64 ± 22.87 | 40 (0–100) | 68.48 ± 25.44 | 70 (0–100) | 25.839 ± 18.59 | 25 (–25–75) | <.001∗∗∗ | 1.07 |
| Balance | ||||||||
| BAL | 20.26 ± 16.66 | 16 (0–56) | 35.80 ± 17.32 | 41 (0–56 | 15.534 ± 11.97 | 14 (–21–47) | <.001∗∗∗ | 0.91 |
| MAL (amount use) | 0.87 ± 1.26 | 0.20 (0–5) | 1.55 ± 1.93 | 1.08 (0–19) | 0.679 ± 1.47 | 0.3 (–0.50–18.10) | <.001∗∗∗ | 0.42 |
| MAL (quality) | 1.51 ± 7.50 | 0.20 (0–101) | 1.79 ± 3.17 | 1.10 (0–40) | 0.278 ± 7.92 | 0.3 (–99.30–39.10) | <.001∗∗∗ | 0.05 |
| Lower extremity | ||||||||
| Gait speed | 7.28 ± 17.92 | 0 (0–110) | 12.00 ± 16.92 | 6.76 (0–103) | 4.726 ± 23.41 | 0 (–89.54–103.21) | .001∗∗ | 0.27 |
| 6MWT | 55.30 ± 106.36 | 0 (0–490) | 146.74 ± 153.33 | 90 (0–603) | 91.443 ± 99.42 | 62 (–33.00–511.30) | <.001∗∗∗ | 0.69 |
| Sensor-motor function | ||||||||
| FMA sensory | 28.08 ± 15.52 | 29 (0–97) | 37.44 ± 12.62 | 42 (0–103) | 9.358 ± 11.79 | 6 (–36–44) | <.001∗∗∗ | 0.66 |
| FMA motor | 27.56 ± 22.29 | 28 (0–97) | 39.32 ± 22.71 | 46 (0–103) | 11.757 ± 11.72 | 9 (–42–45) | <.001∗∗∗ | 0.52 |
| Swallowing | ||||||||
| FOIS | 6.03 ± 1.53 | 7 (1–7) | 6.53 ± 1.02 | 7 (2–7) | 0.495 ± 1.04 | 0 (0–6) | <.001∗∗∗ | 0.38 |
| Nutrition | ||||||||
| MNA | 19.65 ± 5.31 | 21 (2–29) | 21.48 ± 4.89 | 22.5 (8–29) | 1.832 ± 3.58 | 1.50 (–15–13) | <.001∗∗∗ | 0.36 |
| Cognition | ||||||||
| MMSE | 18.78 ± 8.67 | 20.5 (0–30) | 21.92 ± 7.70 | 24 (0–30) | 3.156 ± 4.83 | 2 (–14–28) | <.001∗∗∗ | 0.38 |
| Language | ||||||||
| CCAT | 9.84 ± 2.39 | 11 (2–12) | 10.42 ± 2.03 | 11 (3–12) | 0.680 ± 1.07 | 0.07 (–1.25–5.20) | <.001∗∗∗ | 0.26 |
| Advanced function | ||||||||
| IADL | 1.59 ± 1.50 | 1 (0–8) | 2.94 ± 1.86 | 3 (0–8) | 1.352 ± 1.35 | 1 (–1–5) | <.001∗∗∗ | 0.80 |
| Life quality | ||||||||
| QoL | 10.47 ± 2.26 | 10 (5–15) | 8.55 ± 2.25 | 8 (0–15) | –1.922 ± 2.17 | –2 (–12–6) | <.001∗∗∗ | 0.85 |
6MWT = 6-Min Walk Test, BAL = Berg balance test, BI = Barthel index, CCAT = Concise Chinese Aphasia Test, FMA = Fugl–Meyer assessment, FOIS = functional oral intake scale, gait speed = usual gait speed, IADL = Lawton–Brody IADL Scale, MAL = motor activity log, MMSE = mini-mental state examination, MNA = mini nutritional assessment, MRS = modified rankin scale, QoL = EuroQoL-5D, SD = standard deviation.
Compared with Wilcoxon signed-rank test.
Upper and lower limits.
Effect size presented with Cohen d.
P < .01.
P < .001.
Regarding the length of stay's correlation with functional improvements, Table 3 indicates significant functional improvements with longer length of stay in BI, BAL, gait speed, upper sensory function in FMA, and upper motor function in FMA. Meanwhile, the following functions did not significantly improve with longer length of stay: MRS, FOIS, MNA, QoL, IADL, 6MWT, MMSE, MAL occupational mobility (quantity and quality of use), and CCAT (Table 3).
Table 3.
Improvements in functional domains after PAC program and correlations with the PAC length of stay.
| Variables | P | R |
| ΔMRS | .071 | –0.130 |
| ΔBI | <.001∗∗∗ | 0.330 |
| ΔBAL | <.001∗∗∗ | 0.461 |
| ΔMAL (amount use) | .714 | –0.027 |
| ΔMAL (quality) | .859 | –0.013 |
| ΔGait Speed | .002∗∗ | 0.218 |
| Δ6MWT | .208 | 0.091 |
| ΔFMA sensory | <.001∗∗∗ | 0.263 |
| ΔFMA motor | <.001∗∗∗ | 0.276 |
| ΔFOIS | .175 | 0.099 |
| ΔMNA | .408 | 0.060 |
| ΔMMSE | .066 | 0.133 |
| ΔCCAT | .060 | 0.189 |
| ΔIADL | .052 | 0.140 |
| ΔQoL | .219 | –0.089 |
6MWT = 6-Min Walk Test, BAL = Berg Balance test, BI = Barthel index, CCAT = Concise Chinese Aphasia Test, FMA = Fugl–Meyer Assessment, FOIS = Functional Oral Intake Scale, gait speed = usual gait speed, IADL = Lawton–Brody IADL Scale, MAL = motor activity log, MMSE = mini-mental state examination, MNA = mini nutritional assessment, MRS = modified rankin scale, QoL = EuroQoL-5D, SD = standard deviation.
P < .01.
P < .001.
4. Discussion
PAC has been recognized as an economical[6,17] and a more favorable recovery strategy than its counterparts.[30] Improvement of ADL function, balance and coordination, walking speed, and motor and sensory function of the upper extremities were noted in a previous study.[4,31] In our study, the average PAC length of stay was 35.01 ± 16.373 days and we found a longer PAC stay was noted to greatly improve BI, BAL, gait speed, and FMA sensory and motor function for the upper limbs. Contrary to existing findings indicating otherwise, our study noted no greater improvement with increased PAC length of stay, in MRS, FOIS, MNA, QoL, IADL, 6MWT, MMSE, MAL occupational mobility (quantity and quality of use), and CCAT[31] scores, but the relationship of longer PAC stay is nearly positively correlating to improvements in cognition (MMSE, P = .066) and linguistic ability (CCAT P = .066). Demographics of our study indicated 76.7% (n = 148) and 23.3% (n = 45) of patients with ischemic and hemorrhagic stroke, respectively; these proportions are comparable with the 80% and 20% epidemiological prevalence for these 2 types of stroke, respectively.[32] 60.6% (n = 117) male patients and 39.4% (n = 76) female patients were enrolled in our study and the sex predilection could also be noted in general incidence and prevalence of stroke among men, surpass women by 33% and 47%, respectively.[33]
Previous study showed 82.37% stroke patients had a speech disorder.[34] But among the recruited participants in our study, only 51.8% (n = 100) had speech problem and underwent speech therapy. This may be due to speech disorders resolution after acute phase of stroke and less patients requiring speech therapy in the subacute phase of stoke during PAC. Longer PAC stay did not result in significantly greater improvements in CCAT score in our report. This might indicate CCAT evaluate an area of speech that needs longer length of time to show improvement. In addition to speech disorder, 23% to 50% of poststroke patients were found to have dysphagia,[35] but almost all poststroke participants 99% (N = 191) underwent swallowing training in our study. Dysphagia may lead to poor oral feeding and contribute to malnutrition. Improvements in FOIS and MNA scores, representing swallowing function and nutritional status, were noted in poststroke patients[31] after PAC program but the improvements in these 2 scales did not increase with PAC length of stay.
In our study, gait speed greatly improved with PAC length of stay instead of the 6MWT score. Conventionally, gait speed is measured as the time it takes to finish a walk in the regular distance (5 m of valid testing, 1 m for acceleration, and 1 m for deceleration) in another word the walking velocity.[36] In contrast, the 6MWT result is how far the patient can walk in 6 minutes.[37] Gait speed is viewed as the sixth vital sign in assessing the general function of a patient[36]; among patients with severe chronic lung disease, gait speed was noted to be significantly and independently associated with the 6MWT result.[38] Gait speed tests and the 6MWT allow the aid of walking devices but not the aid of other people. Both tests are based on autonomous ambulation, but the 6MWT relies more heavily on cardiopulmonary capacity. Thus, 6MWT result was less likely to be positively correlated with PAC length of stay than the gait speed; this was because high cardiopulmonary capacity is difficult to establish in the early subacute phase of stroke patients.
ADL function is recorded by the MRS and BI in acute clinical and PAC settings. Findings on the relationship between MRS and BI in poststroke patients are numerous but conflicting. Poor outcomes of stroke are defined as MRS >3 and BI <60.[39] Improvements in BI, but not MRS, were more significant with the longer PAC length of stay. However, this finding was most likely due to the different categories used in these 2 scales. The MRS focuses on ambulation and prestroke ability to accomplish a given activity on a scale of 0 to 6, whereas the BI focuses on the ability to accomplish everyday tasks on a scale of 0 to 100. A previous study noted that the MRS is useful for assessing overall disability rather than competence in everyday tasks specifically.[40] Thus, because the BI is sensitive to small improvements, the score is more likely to show significant greater improvements with longer PAC length of stay.
Upper-extremity function was evaluated using the MAL (quantity and quality of use) and FMA in our study. In recent study, the FMA was more commonly used than the MAL. In general, there is no international consensus on a standard measurement.[41] Improvements in FMA but not MAL significantly increased with PAC length of stay in our study. This finding is attributable to the FMA's and MAL's divergent methods of examining the upper extremities. Specifically, in the FMA, evaluate joint movement—for example, wrist extension and coordination in the finger-to-nose test[24]—are examined, whereas the MAL focuses on whether the patient can use their upper extremities to accomplish a given task—for example, opening a door with a key—through self-reporting.[42] A study noted that the FMA's applicability in evaluating multiple upper-extremity impairments and the MAL's potential for inaccuracy due to patient lapses in recall and cognition.[43] Because greater coordination and muscle power is required to execute the tasks assessed in the MAL, more rehabilitation is required after joint-movement training. In the subacute phase of poststroke patients in our PAC program, the FMA, in measuring motor function, is more sensitive to improvements than the MAL. This explains why improvements in FMA, but not MAL, motor function were significantly greater for greater PAC length of stay.
There are various dispositions after discharged from the acute stroke unit. Around 45% patients go home or attends outpatient rehabilitation, 24% choosing inpatient rehabilitation facilities, 31% staying in skilled nursing facilities[44,45] and home-based model.[46] Subacute rehabilitation for stroke patients were mainly allocated in outpatient and inpatient PAC setting in Taiwan. Patient who attends outpatient stroke rehabilitation had better functional independence measure (FIM) score, socialization, and self-esteem when compared with patients who didn’t receive any rehabilitation.[47] Another study showed significant improvements in physical function, mobility, and balance after hospital-based interdisciplinary outpatient stroke rehabilitation program.[48] Currently, many researches comparing inpatient PAC and non-PAC group during subacute phase of stroke and revealed patients in PAC group had a lower 14-day, 30-day, and 90-day readmission rates, lower emergency department visits and lower mortality rate.[4,5] However, these studies didn’t clarify whether patients in non-PAC groups had undergo any outpatient stroke rehabilitations or additional rehabilitative programs. Few studies had compared inpatient and outpatient stroke rehabilitation and found similar improvements in ADL and life quality in both group, with the outpatient rehabilitative model averaging lasting longer than 8 days.[49] Both outpatient and inpatient rehabilitative models had positive impacts on stroke functional recovery and inpatient PAC model offers an alternative for motivated patients for intensive therapy.
4.1. Limitations
Our study has the following limitations. First, its retrospective and single-center research design limits the generalizability of its results to the whole Taiwanese or global population. Subgroup analyses by subtype of stroke, sex, and age should be conducted in a future study. Second, bias may have been introduced; because many PAC institutions were involved in this study, the data were recorded by different personnel, and the efficacy of training will have been non-uniform between the participants. Finally, the scales used in this study have inherent limitations. For example, the 6MWT cannot represent cardiopulmonary capacity in poststroke patients who are bedridden. Future studies can address these limitations.
5. Conclusions
In poststroke patients, longer PAC stay more greatly improves ADL function, balance and coordination, walking speed, and upper-limb dexterity and sensory function. Clinicians should consider this result when determining discharge times and predicting improvements in a patient's condition.
Acknowledgments
This manuscript has been released as a preprint at Research Square.[50]
Author contributions
Conceptualization: Willy Chou.
Data curation: Yu-Ju Tung, Chin-Tsan Huang, Hsin Han Cheng, Wen-Chih Lin, Chung-Han Ho.
Formal analysis: Yu-Ju Tung, Chin-Tsan Huang, Hsin Han Cheng, Wen-Chih Lin, Chung-Han Ho, Willy Chou.
Investigation: Yu-Ju Tung.
Methodology: Yu-Ju Tung, Willy Chou.
Project administration: Willy Chou.
Resources: Willy Chou.
Software: Chung-Han Ho.
Supervision: Willy Chou.
Validation: Chin-Tsan Huang, Hsin Han Cheng.
Writing – original draft: Yu-Ju Tung.
Writing – review & editing: Chin-Tsan Huang, Wen-Chih Lin, Julie Chi Chow, Willy Chou.
Footnotes
Abbreviations: 6MWT = 6-Min walk test, ADL = activities of daily living, BAL = Berg balance test, BI = Barthel index, CCAT = Concise Chinese aphasia test, FMA = Fugl–Meyer assessment, FOIS = Functional oral intake scale, IADL = Lawton–Brody instrumental activities of daily living scale, MAL = motor activity log, MMSE = mini-mental state examination, MNA = mini nutritional assessment, MRS = modified rankin scale, PAC = post-acute care, QoL = quality of life.
How to cite this article: Tung YJ, Huang CT, Lin WC, Cheng HH, Chow JC, Ho CH, Chou W. Longer length of post-acute care stay causes greater functional improvements in poststroke patients. Medicine. 2021;100:26(e26564).
YJT and CTH contributed equally to the work.
Ethics approval and consent to participate: The study design was approved by an institutional review board of Chi Mei Medical Center (10711-J02) and has been listed on ClinicalTrials.gov (identification number: NCT03778905).
Consent for Publication: Not applicable.
Availability of data and material: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
There's no funding regarding this researches.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Taiwan's Ministry of Health and Welfare. National healthcare quality and disparities report 2013. First ed. No.36, Tacheng St. Datong District, Taipei City 10341, R.O.C. (Taiwan): the Ministry of Health and Welfare, R.O.C. (Taiwan); 2014. [Google Scholar]
- [2].Kane RL, Lin WC, Blewett LA. Geographic variation in the use of post-acute care. Health Serv Res 2002;37:667–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hsieh FI, Chiou HY. Stroke: morbidity, risk factors, and care in taiwan. J Stroke 2014;16:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peng LN, Lu WH, Liang CK, et al. Functional outcomes, subsequent healthcare utilization, and mortality of stroke postacute care patients in Taiwan: a Nationwide Propensity Score-matched Study. J Am Med Dir Assoc 2017;18:990.e7–12. [DOI] [PubMed] [Google Scholar]
- [5].Hsieh CY, Tsao WC, Lin RT, Chao AC. Three years of the nationwide post-acute stroke care program in Taiwan. J Chin Med Assoc 2018;81:87–8. [DOI] [PubMed] [Google Scholar]
- [6].Wang CY, Chen YR, Hong JP, Chan CC, Chang LC, Shi HY. Rehabilitative post-acute care for stroke patients delivered by per-diem payment system in different hospitalization paths: a Taiwan pilot study. Int J Qual Health Care 2017;29:779–84. [DOI] [PubMed] [Google Scholar]
- [7].Lee M, Wu YL, Ovbiagele B. Trends in incident and recurrent rates of first-ever ischemic stroke in Taiwan between 2000 and 2011. J Stroke 2016;18:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cotté FE, Chaize G, Kachaner I, Gaudin AF, Vainchtock A, Durand-Zaleski I. Incidence and cost of stroke and hemorrhage in patients diagnosed with atrial fibrillation in France. J Stroke Cerebrovasc Dis 2014;23:e73–83. [DOI] [PubMed] [Google Scholar]
- [9].Kuptniratsaikul V, Kovindha A, Massakulpan P, Permsirivanich W, Kuptniratsaikul PS. Inpatient rehabilitation services for patients after stroke in Thailand: a multi-centre study. J Rehabil Med 2009;41:684–6. [DOI] [PubMed] [Google Scholar]
- [10].Lin JH, Hsiao SF, Liu CK, Lin YT. Rehabilitation fees, length of stay and efficiency for hospitalized stroke patients: a preliminary study based on function-related groups. Kaohsiung J Med Sci 2001;17:475–83. [PubMed] [Google Scholar]
- [11].Chen CC, Cheng SH. Does pay-for-performance benefit patients with multiple chronic conditions? Evidence from a universal coverage health care system. Health Policy Plan 2016;31:83–90. [DOI] [PubMed] [Google Scholar]
- [12].van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. [DOI] [PubMed] [Google Scholar]
- [13].Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991;54:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. MD State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- [15].Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005;86:1516–20. [DOI] [PubMed] [Google Scholar]
- [16].Mizrahi EH, Fleissig Y, Arad M, Adunsky A. Functional gain following rehabilitation of recurrent ischemic stroke in the elderly: experience of a post-acute care rehabilitation setting. Arch Gerontol Geriatr 2015;60:108–11. [DOI] [PubMed] [Google Scholar]
- [17].Chan L, Sandel ME, Jette AM, et al. Does postacute care site matter? A longitudinal study assessing functional recovery after a stroke. Arch Phys Med Rehabil 2013;94:622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Postacute stroke care: same standards as acute care? Lancet 2015;386:2366. [DOI] [PubMed] [Google Scholar]
- [19].Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- [20].Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health 1992;83: suppl: S7–11. [PubMed] [Google Scholar]
- [21].Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci 2013;68:39–46. [DOI] [PubMed] [Google Scholar]
- [22].Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am J Respir Crit Care Med 2003;167:1287. [DOI] [PubMed] [Google Scholar]
- [23].Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther 1993;73:447–54. [DOI] [PubMed] [Google Scholar]
- [24].Sullivan KJ, Tilson JK, Cen SY, et al. Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke 2011;42:427–32. [DOI] [PubMed] [Google Scholar]
- [25].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [26].Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–35. [DOI] [PubMed] [Google Scholar]
- [27].Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology 2006;67:1189–94. [DOI] [PubMed] [Google Scholar]
- [28].Zhong Y, Li S, Zhang M. Concise Chinese Aphasia Test. Taipei: Psychological Publishing Co., Ltd.; 2003. [Google Scholar]
- [29].Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- [30].Wang G, Zhang Z, Ayala C, Dunet DO, Fang J, George MG. Costs of hospitalization for stroke patients aged 18-64 years in the United States. J Stroke Cerebrovasc Dis 2014;23:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lai CL, Tsai MM, Luo JY, Liao WC, Hsu PS, Chen HY. Post-acute care for stroke - a retrospective cohort study in Taiwan. Patient Prefer Adherence 2017;11:1309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ojaghihaghighi S, Vahdati SS, Mikaeilpour A, Ramouz A. Comparison of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World J Emerg Med 2017;8:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke 2009;40:1082–90. [DOI] [PubMed] [Google Scholar]
- [34].Vidovic M, Sinanovic O, Sabaskic L, Haticic A, Brkic E. Incidence and types of speech disorders in stroke patients. Acta Clin Croatica 2011;50:491–4. [PubMed] [Google Scholar]
- [35].Singh S, Hamdy S. Dysphagia in stroke patients. Postgrad Med J 2006;82:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act 2015;23:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Erratum: ATS statement: guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med 2016;193:1185. [DOI] [PubMed] [Google Scholar]
- [38].DePew ZS, Karpman C, Novotny PJ, Benzo RP. Correlations between gait speed, 6-minute walk distance, physical activity, and self-efficacy in patients with severe chronic lung disease. Respir Care 2013;58:2113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538–41. [DOI] [PubMed] [Google Scholar]
- [40].Uyttenboogaart M, Luijckx GJ, Vroomen PC, Stewart RE, De Keyser J. Measuring disability in stroke: relationship between the modified Rankin scale and the Barthel index. J Neurol 2007;254:1113–7. [DOI] [PubMed] [Google Scholar]
- [41].Santisteban L, Térémetz M, Bleton J-P, Baron J-C, Maier MA, Lindberg PG. Upper limb outcome measures used in stroke rehabilitation studies: a systematic literature review. PLoS One 2016;11:e0154792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Taub E, McCulloch K, Uswatte G, et al. Motor activity log (mal) manual. UAB Train CI Ther 2011;1:01–18. [Google Scholar]
- [43].Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier RL. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther 2013;26:104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Buntin MB, Colla CH, Deb P, Sood N, Escarce JJ. Medicare spending and outcomes after postacute care for stroke and hip fracture. Med Care 2010;48:776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang Q, Yang Y, Saver JL. Discharge destination after acute hospitalization strongly predicts three month disability outcome in ischemic stroke. Restor Neurol Neurosci 2015;33:771–5. [DOI] [PubMed] [Google Scholar]
- [46].Tung Y-J, Lin W-C, Lee L-F, Lin H-M, Ho C-H, Chou W. Comparison of cost-effectiveness between inpatient and home-based post-acute care models for stroke rehabilitation in Taiwan. Int J Environ Res Public Health 2021;18:4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Werner RA, Kessler S. Effectiveness of an intensive outpatient rehabilitation program for postacute stroke patients. Am J Phys Med Rehabil 1996;75:114–20. [DOI] [PubMed] [Google Scholar]
- [48].Rice D, Janzen S, McIntyre A, Vermeer J, Britt E, Teasell R. Comprehensive outpatient rehabilitation program: hospital-based stroke outpatient rehabilitation. J Stroke Cerebrovasc Dis 2016;25:1158–64. [DOI] [PubMed] [Google Scholar]
- [49].Bölsche F, Hasenbein U, Reissberg H, Lotz-Rambaldi W, Wallesch CW. [Short term results of outpatient vs. inpatient rehabilitation after stroke]. Die Rehabil 2002;41:175–82. [DOI] [PubMed] [Google Scholar]
- [50].Tung Y-J, Cheng H-H, Chou W, et al. The correlation between the length of stay in post-acute care(PAC) and general and improvements in stroke patients. Res Square [Preprint]; 2019. Available at: 10.21203/rs.2.1813/v1. Accessed June 24, 2021. [DOI] [Google Scholar]


