PURPOSE:
Performance status (PS) is assessed during cancer treatment to determine clinical trial eligibility, appropriateness for treatment, and need for supportive care. There is rising interest for patients to report this information directly. We determined whether clinician- and patient-reported PS were equally associated with mortality and service utilization in patients with cancer.
METHODS:
A secondary analysis was conducted using data from an radiotherapy plus chemotherapy in which 441 patients with advanced cancer and clinicians reported PS using the Eastern Cooperative Oncology Group scale. Simple kappa statistics measured agreement between clinician-reported performance status (cPS) and patient-reported performance status (pPS). Associations of cPS and pPS with emergency department (ED) and hospital visits and overall survival were evaluated via Cox regression, competing risk regression, and Fisher's exact tests.
RESULTS:
cPS and pPS correlated weakly (kappa = 0.27). Both pPS and cPS were associated with survival, ED visits, and hospitalizations, but only cPS remained associated after adjustment (survival: HR, 1.75; P < .0001). The first available cPS predicted mortality more strongly than the first available pPS (HR for death, comparing PS ≥ 1 v 0: 2.05 for cPS and 1.41 for pPS). When pPS questionnaires were repeated over time and averaged, associations with outcomes were stronger as measured by AIC model fit. Both pPS and cPS were associated with EQ-5D subcomponents (eg, 75%-77% with no usual activity deficits for PS 0, v 42%-51% for PS ≥ 1).
CONCLUSION:
Both clinician-reported PS and patient-reported PS provide useful information and can be considered for clinical trials and routine care.
INTRODUCTION
Physical function, a measure of how capably an individual can perform physical activities, is frequently assessed in oncology to evaluate appropriateness for treatment, need for supportive care, and clinical trial eligibility.1-4 From a care perspective, physical function is associated with symptom burden5 and is meaningful to patients and caregivers.6 From a research perspective, virtually all cancer clinical trials require baseline and serial assessments of performance status (PS).2 Physical function is also recognized by the US Food and Drug Administration as an important component of patient-focused drug development, along with disease-related symptoms and treatment-related symptomatic toxicities.1
Conventional practice is for physical function to be assessed by clinicians at office visits, using a single-item clinician-assessed outcome measure referred to as performance status (clinician-reported performance status [cPS]). cPS is prognostic of survival, underscoring its relationship to meaningful downstream events.7-10
However, cPS is limited by clinician interpretation and the requirement for assessment to occur in the clinical setting. Like many measurements in medicine, the current clinician-reporting approach developed historically and did not incorporate the patient voice, nor was it compared with patient-reporting as an alternative. Increasingly, patient self-reporting is being successfully employed in closely related areas, such as symptom assessment and adverse event monitoring.11,12 Patient reported performance status (pPS) could be one way to overcome limitations in cPS by obtaining similar information directly from patients, without the constraints of eliciting this measure only during office visits and without the risk of clinician biases. Moreover, in routine care, pPS might identify and mitigate decrements in physical function earlier than routine cPS assessment. Changes in physical function may result from intercurrent acute medical issues, and thus, earlier recognition of these changes may also identify opportunities for intervention on acute and reversible concerns that would otherwise lead to avoidable emergency department (ED) visits or hospitalizations. pPS might also be useful in clinical trials for eligibility and ongoing evaluation, either as a trial outcome or to assist in clinical management.
However, the degree to which cPS and pPS are associated with morbidity and mortality is unclear. This was assessed via secondary data analysis of a randomized controlled trial in which cPS and pPS were collected within 7 days of each other.
METHODS
Randomized Trial Dataset
A secondary analysis was performed using data from a randomized controlled trial conducted at Memorial Sloan Kettering from 2012 to 2016 (ClinicalTrials.gov: NCT00578006). Patients initiating treatment for metastatic breast, genitourinary, gynecologic, or lung cancer were randomly assigned either to serially self-reported symptoms and PS or usual care, to evaluate impact on clinical outcomes.13,14 This is an ideal trial for the current analysis, as 441 patients and their paired clinicians reported PS within 7 days of each other at baseline and throughout the study.
Measures
Clinicians and patients reported PS using analogous versions of the well-established Eastern Cooperative Oncology Group (ECOG) scale,15 which ranges from 0 (fully active, able to carry out all predisease performance without restriction) to 4 (completely disabled, cannot carry on any self-care and totally confined to bed or chair). The patient version was adapted from the clinician version and has been administered in numerous multicenter clinical trials.12,16,17 PS could be reported by patients as often as weekly. Patients also completed the EuroQoL EQ-5D, a validated five-item questionnaire measuring mobility, self-care, usual activities, pain or discomfort, and anxiety or depression.18 Individual items are asked on a three-point scale (no problems, some problems, and unable or extreme) that produces a composite score between 0 and 1, representing general health status. The EQ-5D was completed every 2 months.
Statistical Methods
Patient- and clinician-reported ECOG data were evaluated in several ways using SAS statistical software v9.4 (Cary, NC). Clinician-reported and patient-reported ECOG data over a 1-year period of treatment are summarized using descriptive statistics including median and interquartile range (IQR).
We then compared differences in the number of reports by patient characteristics using Kruskal-Wallis tests. Many patients and clinicians provided more than 1 report, and so for comparative analyses, reports were linked when they were within 7 days of each other to ensure that both reports were referencing the same time period. In cases where there was more than one report in a 7-day period, the closest proximate clinician and patient reports to each other were used. The 7-day interval was chosen as a pragmatic compromise to try to capture as many paired reports as possible while recognizing that longer periods between clinician and patient reports might have resulted in changes in underlying PS. Nonetheless, 85% of paired reports were on the same day or within 1 day.
A simple kappa statistic evaluated the agreement between patient- and clinician-reported PS, using a dichotomization between 0 and ≤ 1 and using all possible pairs of reports. Kappa ranging from 0.01-0.20 indicates slight agreement, 0.21-0.40 fair agreement, 0.41-0.60 moderate agreement, and ≥ 0.61 substantial agreement. The Kaplan-Meier method was used to estimate the relationship of first report with overall survival (OS), and Cox regression modeling was used to estimate hazard ratios (HRs). OS time started at first report and ended at death or censoring at last known date alive. Analyses were adjusted for age, sex, race or ethnicity, education, cancer type, and home internet access because of their known association with survival outcomes.19
For the combined end point of ED visits or hospitalization usage, time started at first report and ended at the first visit to either the ED or hospital. Cumulative incidence functions were calculated with death treated as a competing event,20 and competing risk regression was used to model risk with and without adjustment for baseline covariates. For the analysis of whether repeated pPS over time prognosticated downstream outcomes, a landmark analysis was performed. Patients were included if they did not have an event within 2 months of the first pPS report. A landmark analysis was performed, excluding any patients with events before 2 months, to investigate the benefit of repeated pPS. Akaike's Information Criterion (AIC) was used to compare model fit,21 with large differences providing justification for one model over another.
We examined associations between PS ratings and EQ-5D quality-of-life subdomains using Fisher's exact tests; all pairs of reports were used for these associations. We looked at dichotomized PS and also dichotomized each subdomain of the EQ-5D as no problem versus problem identified (ie, 0 v ≥ 0).
RESULTS
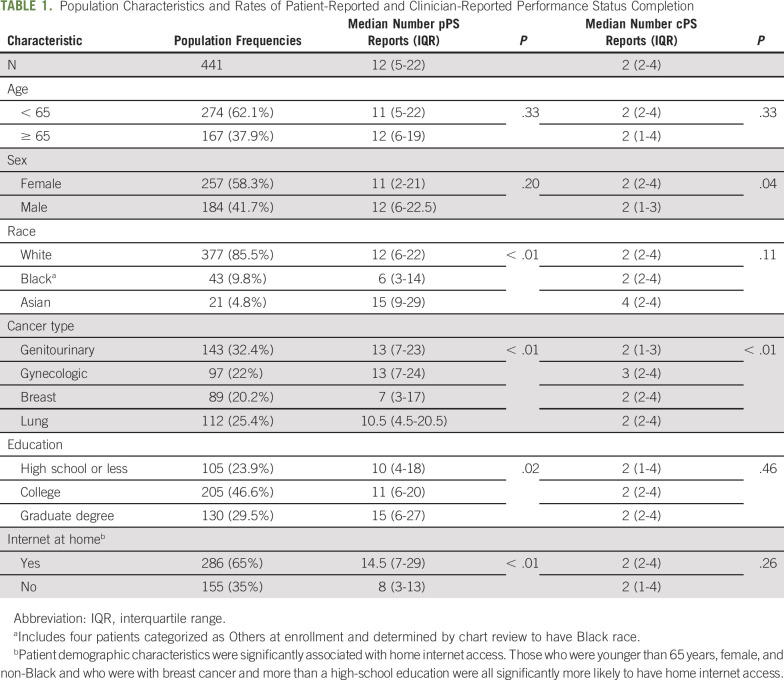
Table 1 shows population characteristics and rates of patient-reported and clinician-reported ECOG PS completion. Of the 441 patient-clinician dyads, 11 had no cPS available and 15 had no pPS, leaving 415 dyads with at least 1 cPS and 1 pPS ECOG report. Clinicians provided a median of 2 PS reports (IQR, 2-4), whereas patients provided a median of 12 (IQR, 5-22). This difference was partly attributable to clinicians reporting PS only at office visits, whereas patients reported from home between visits as well. The number of patient reports was significantly higher for those with home internet access, but was still high even for those without home access (median, 8; IQR, 3-13). Differences were also noted by race, cancer type, and education for patient reports and by sex and cancer type for clinician reports. The median duration of reporting was 138 days, with an IQR of 73-286 days.
TABLE 1.
Population Characteristics and Rates of Patient-Reported and Clinician-Reported Performance Status Completion
Abbreviation: IQR, interquartile range.
Includes four patients categorized as Others at enrollment and determined by chart review to have Black race.
Patient demographic characteristics were significantly associated with home internet access. Those who were younger than 65 years, female, and non-Black and who were with breast cancer and more than a high-school education were all significantly more likely to have home internet access.
Clinician-assessed PS only weakly agreed with patient-reported PS (Data Supplement, online only). We examined 380 dyads who had a cPS and pPS within a week of each other, which represented a total of 839 pairs of cPS and pPS assessments. Of all clinician reports, only 23 PS ratings were > 1, so all further analyses dichotomized PS as 0 or ≥ 1. When comparing cPS of 0 or ≥ 1 with pPS of 0 or ≥ 1, the correlation was weak (kappa = 0.27). Concordance was 64%, with 16% of clinicians rating a patient ≥ 1 when patients rated themselves a 0 and 20% of patients rating themselves ≥ 1 when the clinician rated them a 0.
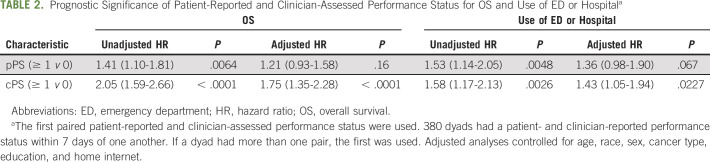
Both pPS and cPS were prognostic for OS and use of the ED and/or hospital in unadjusted analyses (Table 2). There was a larger difference in risk of death between patients rated ≥ 1 and 0 on the basis of the clinician report compared with the patient report. Table 2 shows that the HR for death was 2.05 when clinicians rated a patient ≥ 1 versus 0 compared with 1.41 when patients reported themselves a ≥ 1 versus 0. After adjusting for age, sex, race, education, cancer type, and home internet, cPS retained its prognostic significance for OS (HR, 1.75; P < .0001) and use of ED or hospital (HR, 1.43; P = .02) although pPS was no longer statistically significantly associated with either outcome. AIC model fit criteria demonstrated better model fit for cPS compared with pPS.
TABLE 2.
Prognostic Significance of Patient-Reported and Clinician-Assessed Performance Status for OS and Use of ED or Hospitala
For the analysis of whether repeated pPS over time prognosticated downstream outcomes, a landmark analysis was performed. Patients were included if they did not have an event within 2 months of the first pPS report. Data for 422 patients were available for the survival analysis and 413 for the use of ED and/or hospital analysis. In the first 2 months of reporting, the median number of repeated pPS reports was 5 (range, 1-23; IQR, 3-10). We evaluated the first reported scores, maximum reported scores, total number of reported scores, range of reported scores, median reported scores, and mean reported scores and compared model fit using AIC (Data Supplement). Lower AIC values represent better fits, with an AIC difference of two considered significant. Although most of these measures showed significant associations with outcomes, model fit was the best for mean scores and was significantly better than first report alone.21 For OS, the risk of death increased by 50% for each 1 point increase in mean pPS (HR, 1.51; P < .001). For the use of ED and/or hospital, the risk of event increased by 68% (HR, 1.68; P < .001) when patients reported more decrements in PS.
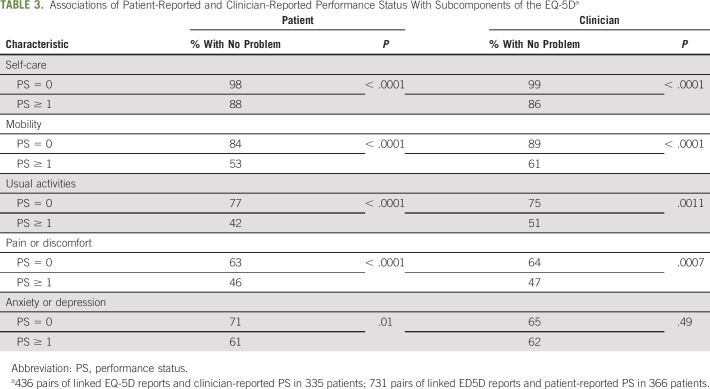
To explore associations between PS ratings and quality-of-life domains, we looked at the association of PS with EQ-5D subdomains using all available pairs of reports, with EQ-5D dichotomized as reporting any problems (some or unable or extreme) versus no problems. Both cPS and pPS discriminated between patients with and without any deficit in particular EQ-5D subdomains. These differences were apparent in all subdomains except anxiety or depression. For example, for usual activity, 77% of patients with pPS = 0 had no deficits versus 42% of patients with pPS ≥ 1; 75% of patients with cPS = 0 had no deficits in usual activity versus 51% of those with cPS ≥ 1. For anxiety or depression, 71% of patients with pPS = 0 had no problems versus 61% of patients with pPS ≥ 1, which was a significant difference. However, there was no difference in patient reported anxiety or depression between patients with cPS ≥ 1 and 0 (P = .49). These results are shown in Table 3.
TABLE 3.
Associations of Patient-Reported and Clinician-Reported Performance Status With Subcomponents of the EQ-5Da
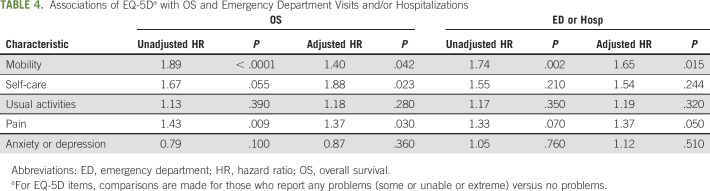
In terms of prognostic significance for EQ-5D subdomains, the mobility item was significantly associated with both OS and use of ED and/or hospital after adjustment. Those who reported any mobility problems had 1.4 higher risk of death (P = .04) and 1.65 higher risk of ED and/or hospital visit (P = .015). Both pain (HR, 1.37; P = .03) and self-care (HR, 1.88; P = .02) were significantly associated with OS, but not with the use of ED or hospital. These results, based solely on the first EQ-5D report, are shown in Table 4.
TABLE 4.
Associations of EQ-5Da with OS and Emergency Department Visits and/or Hospitalizations
DISCUSSION
We conducted a secondary data analysis of a randomized trial to evaluate if clinician-assessed PS and patient-reported PS are equally associated with morbidity and mortality in patients receiving chemotherapy. Consistent with previous reports, we found poor correlation between the cPS and pPS.5As noted, 20% of patients rated themselves with a PS > 1 when clinicians rated them a 0. This shows that patients often reported a poor PS when clinicians did not report their PS as such.
We found that the first obtained cPS was significantly associated with ED visit, hospitalizations, and survival, whereas the first obtained pPS was not, after adjusting for other relevant factors. However, we found that repeated pPS measures over time improved the strength of associations with morbidity and mortality, supporting the hypothesis that repeated home assessments may enable pPS to become a more useful measure by showing change over time. We believe that the benefit of longitudinal data is a particularly important finding of this analysis. Intuitively, it makes sense that clinical deterioration would be associated with worsening self-reported PS over time and thus associated with higher subsequent risk of ED visits, hospitalizations, and survival. Our findings support this explanation and provide rationale for further study of longitudinal self-reported PS data. We also found that both cPS and pPS discriminated among deficits in subcomponents of the EQ-5D, demonstrating that the construct of PS is patient-centered, as it is associated with each component of quality of life on this instrument. Interestingly, specific EQ-5D subcomponents attained prognostic significance for morbidity and mortality that approached cPS.
Thus, we conclude that pPS is a useful measure of physical function during routine care that provides information complementary to cPS. pPS is attractive because it is a single measure of physical function with minimal additional respondent burden when added to symptom questionnaires. pPS also overcomes assessment limitations of cPS and can be obtained multiple times from home throughout the trajectory of advanced cancer. The EQ-5D data suggest that an optimally selected pPS measure may perform particularly well. This observation is consistent with previous literature indicating that patient-reported physical function, using well-validated patient-reported outcome (PRO) measures, provides independent prognostic information for survival.22 The ease of home-based patient assessments would need to be balanced with any added clinician or staff burden incurred by following and responding to these reports. Ongoing studies are addressing feasibility and acceptability considerations associated with home-based physical function reporting.
However, our analysis also suggests limitations to pPS and does not suggest that pPS, in its current form, can replace cPS in routine cancer care. If we assume that pPS is a representation of patient PS, it is possible that cPS may perform differently because it may incorporate clinical information beyond PS. If clinicians do not actually ask patients a direct question about PS, we do not know if their inference about an assumed answer is based on other factors. Patients with biologically aggressive malignancies might be assigned a low cPS,23-25 because clinicians might subconsciously incorporate other biologically prognostic factors into their assessments. Disentangling these various issues might require cognitive interviewing of clinicians and patients.
A second hypothesis is that the pPS measure tested in this study may have suboptimal psychometric characteristics. Patients might not have understood or interpreted the wording of the measure appropriately. For instance, response options on the ECOG PRO are lengthy and may be more difficult to read and interpret for patients with lower literacy. Intriguingly, the EQ-5D subcomponent findings suggest that other single-item patient-reported measures may perform better. To this end, a third hypothesis is that other attributes of physical function, such as mobility, may be more prognostically relevant than the more global patient-reported PS measure.
There are other potential approaches to ascertain physical function of patients with cancer (Data Supplement). Multiple types of measure assessments (eg, clinician-assessed, patient-reported, performance-based, and passively generated) may provide different and potentially orthogonal information to provide a complete picture of physical function. One approach could be to identify a better patient-reported measure of physical function. A single-item measure like an improved patient-reported performance measure or an EQ-5D subcomponent could be a candidate, but so could a multi-item measure like the PROMIS Physical Function measure available in short forms of 4-8 items26 or the EORTC QLQ-C30.27 Another approach might be to incorporate a testing-based measure of physical function. Examples include 6-minute walk distance testing,28,29 gait speed,30 a combination (eg, the short physical performance battery31), or cardiopulmonary exercise testing.32-35 These tests all have advantages and disadvantages, and many have only been formally studied in the clinical setting. Some, such as gait speed, may be amenable to home-based capture, although research is ongoing.36
A third approach could involve passive capture of home-based data with a wearable sensor. Some early work has been done looking at the ability of home-based activity and physiologic metrics from sensors to associate with or approximate self-reported physical function, although this work is in early development.37,38 A fourth approach could be to develop hybrid measures with data from multiple sources. A geriatric assessment, for example, is an approach that includes data from clinician-assessed, patient-reported, and performance-based measures to provide information about frailty and function.39 Although a complete geriatric assessment requires clinical evaluation, other types of hybrid measures, such as passive data collection plus self-report, may be possible in the home-based setting. Additional work is needed to determine the extent to which different sources of physical function-related data complement and add value to one another and whether additional benefit is worth additional complexity in implementation of multiple measures in usual care.
There are also several important limitations to our work. We did not have information on time since diagnosis, which may have been an important predictor of PS and prognosis. This was a single institution, retrospective analysis, limiting generalizability. As noted, the design of the patient-reported instrument and the method of ensuring adherence to reporting could be further optimized to better understand the potential of patient-reported functional status to predict outcomes.
In summary, our study represents a proof of concept for acquisition of patient-reported PS at home among patients with advanced cancer. Future work is needed to test other types of patient-reported physical function measures for validation and/or feasibility in routine care and research and to further develop performance-based or passively generated measures for use in the home setting. For now, though, we can say that patient-reported and clinician-assessed PS provide unique and complementary information for predicting morbidity and mortality in adults with advanced cancer receiving chemotherapy. Both should be considered for future clinical trial use and routine care.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: William A. Wood, Allison M. Deal
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comparing Clinician-Assessed and Patient-Reported Performance Status for Predicting Morbidity and Mortality in Patients With Advanced Cancer Receiving Chemotherapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
William A. Wood
Stock and Other Ownership Interests: Koneksa Health, Elektra Labs
Consulting or Advisory Role: Koneksa, Teledoc, Inc
Research Funding: Genentech/Roche, Pfizer
Angela M. Stover
Honoraria: Genentech
Ethan Basch
Consulting or Advisory Role: Sivan, Carevive Systems, Navigating Cancer, AstraZeneca
Other Relationship: Centers for Medicare and Medicaid Services, National Cancer Institute, American Society of Clinical Oncology, Journal of the American Medical Association, Patient-Centered Outcomes Research Institute (PCORI)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kluetz PG, Slagle A, Papadopoulos EJ, et al. : Focusing on core patient-reported outcomes in cancer clinical trials: Symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res 22:1553-1558, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Abi Jaoude J, Kouzy R, Mainwaring W, et al. : Performance status restriction in phase III cancer clinical trials. J Natl Compr Canc Netw 18:1322-1326, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates JW, Chalmer B, McKegney FP: Evaluation of patients with advanced cancer using the karnofsky performance status. Cancer 45:2220-2224, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Presley CJ, Krok-Schoen JL, Wall SA, et al. : Implementing a multidisciplinary approach for older adults with cancer: geriatric oncology in practice. BMC Geriatr 20:231, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson TM, Andreotti CF, Roberts KE, et al. : The level of association between functional performance status measures and patient-reported outcomes in cancer patients: A systematic review. Support Care Cancer 23:3645-3652, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness KK, Wall MM, Oakes JM, et al. : Physical performance limitations and participation restrictions among cancer survivors: A population-based study. Ann Epidemiol 16:197-205, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Artz AS, Pollyea DA, Kocherginsky M, et al. : Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 12:954-964, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Erdogan B, Turkmen E, Uzunoglu S, et al. : Performance status is an important prognostic factor in second line treatment of pancreaticobiliary adenocarcinoma. Hepatogastroenterology 60:1479-1483, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Evers PD, Logan JE, Sills V, et al. : Karnofsky performance status predicts overall survival, cancer-specific survival, and progression-free survival following radical cystectomy for urothelial carcinoma. World J Urol 32:385-391, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Firat S, Bousamra M, Gore E, et al. : Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 52:1047-1057, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Bennett AV, Jensen RE, Basch E: Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin 62:337-347, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Basch E, Artz D, Dulko D, et al. : Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol 23:3552-3561, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Basch E, Deal AM, Kris MG, et al. : Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 34:557-565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basch E, Deal AM, Dueck AC, et al. : Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318:197-198, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, et al. : Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-655, 1982 [PubMed] [Google Scholar]
- 16.Basch E, Dueck AC, Rogak LJ, et al. : Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol 3:1043-1050, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ottery FD: Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 12:S15-S19, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Group E: EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 16:199-208, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Nipp RD, Horick NK, Deal AM, et al. : Differential effects of an electronic symptom monitoring intervention based on the age of patients with advanced cancer. Ann Oncol 31:8, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 21.Burnham K, Anderson D, Huyvaert K: AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav Ecol Sociobiol 65:3, 2011 [Google Scholar]
- 22.Quinten C, Martinelli F, Coens C, et al. : A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 120:302-311, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Wen X, Song YS, et al. : Associations and prognostic implications of Eastern Cooperative Oncology Group performance status and tumoral LINE-1 methylation status in stage III colon cancer patients. Clin Epigenetics 8:36, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YH, Goto K, Yoh K, et al. : Performance status and sensitivity to first-line chemotherapy are significant prognostic factors in patients with recurrent small cell lung cancer receiving second-line chemotherapy. Cancer 113:2518-2523, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi Y, Tamiya A, Isa SI, et al. : Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res 37:5857-5862, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Jensen RE, Potosky AL, Reeve BB, et al. : Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res 24:2333-2344, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aaronson NK, Ahmedzai S, Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Enright PL: The six-minute walk test. Respir Care 48:783-785, 2003 [PubMed] [Google Scholar]
- 29.Galiano-Castillo N, Arroyo-Morales M, Ariza-Garcia A, et al. : The six-minute walk test as a measure of health in breast cancer patients. J Aging Phys Act 24:508-515, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Liu MA, DuMontier C, Murillo A, et al. : Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 134:374-382, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klepin HD, Geiger AM, Tooze JA, et al. : Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 121:4287-4294, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood WA, Deal AM, Reeve BB, et al. : Cardiopulmonary fitness in patients undergoing hematopoietic SCT: A pilot study. Bone Marrow Transplant 48:1342-1349, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Kelsey CR, Scott JM, Lane A, et al. : Cardiopulmonary exercise testing prior to myeloablative allo-SCT: A feasibility study. Bone Marrow Transplant 49:1330-1336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones LW, Eves ND, Haykowsky M, et al. : Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol 9:757-765, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Jones LW, Watson D, Herndon JE, II, et al. : Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer 116:4825-4832, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takayanagi N, Sudo M, Yamashiro Y, et al. : Relationship between daily and in-laboratory gait speed among healthy community-dwelling older adults. Sci Rep 9:3496, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng Y, Speier W, Shufelt C, et al. : A machine learning approach to classifying self-reported health status in a cohort of patients with heart disease using activity tracker data. IEEE J Biomed Health Inform 24:878-884, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowlands AV, Edwardson CL, Davies MJ, et al. : Beyond cut points: Accelerometer metrics that capture the physical activity profile. Med Sci Sports Exerc 50:1323-1332, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Hurria A, Cirrincione CT, Muss HB, et al. : Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 29:1290-1296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]