PURPOSE:
National guidelines recommend genetic counseling and multigene germline testing (GC/MGT) for all patients with pancreatic ductal adenocarcinoma (PDAC). This study's aim was to assess real-world effectiveness of implementing systematic GC/MGT for all patients with PDAC at a high-volume academic institution.
METHODS:
An iterative process for systematizing GC/MGT was developed in which gastrointestinal oncology providers at the Dana-Farber Cancer Institute were recommended to refer all patients with PDAC for GC/MGT (clinician-directed referral). Workflows were subsequently changed such that patients with PDAC were automatically offered GC/MGT when scheduling their initial oncology consultation (automated referral). Clinical and germline data were collected on a consecutive cohort of patients with PDAC undergoing GC/MGT during a 25-month enrollment period (19-month clinician-directed referrals; 6-month automated referrals).
RESULTS:
One thousand two hundred fourteen patients with PDAC were seen for initial oncologic evaluation, 266 (21.9%) of whom underwent GC/MGT. Compared with baseline clinician-directed referrals, implementation of automated referrals led to a significant increase in patients with PDAC undergoing GC/MGT (16.5% v 38.0%, P < .001), including those undergoing multigene germline testing (MGT) ≤ 7 days of initial oncology evaluation (14.7% v 60.3%, P < .001), with preserved pathogenic variant detection rates (10.0% v 11.2%, P = 0.84). 16 of 28 (57.1%) pathogenic variant carriers had relatives who pursued cascade germline testing, and 13 of 26 (50.0%) carriers with incurable disease received targeted therapy based on MGT results.
CONCLUSION:
Implementation of systematic GC/MGT in patients with PDAC is feasible and leads to management changes for patients with PDAC and their families. GC/MGT workflows that bypass the need for clinician referral result in superior uptake and time to testing. Further investigation is needed to identify other barriers and facilitators of universal GC/MGT.
INTRODUCTION
Recent studies of unselected cohorts of patients with pancreatic ductal adenocarcinoma (PDAC) have demonstrated that 4%-10% harbor germline pathogenic variants (PVs) in high- or moderate-penetrance cancer susceptibility genes.1-7 Therefore, in mid-2018, National Comprehensive Cancer Network (NCCN) guidelines recommended testing all patients with PDAC with a multigene germline panel including breast cancer genes (ATM, BRCA1/2, and PALB2), Lynch syndrome genes (MLH1, MSH2, MSH6, and EPCAM), and others.8 Furthermore, recent developments in targeted therapies have shown promise for patients with PDAC with specific germline PVs.9-14
Since all the cancer susceptibility genes implicated in PDAC risk confer risk of other cancer types and have autosomal dominant inheritance patterns, there are considerable preventive implications for the healthy at-risk relatives of patients with PDAC found to harbor such germline PVs.15-17 Unfortunately, cascade testing (genetic testing for at-risk relatives of the proband with a known PV) has been notoriously difficult to routinely perform in cancer genetics,18 with various well-described barriers including inadequate access to genetics providers, poor communication regarding the implications of a hereditary susceptibility to probands and family members, financial concerns, and belief that cancer is inevitable.19
Despite the justifiable enthusiasm for systematically offering multigene germline testing (MGT) to all patients with PDAC, the question of how to effectively deliver high-quality genetic counseling and MGT (GC/MGT) on a large-scale basis has been vexing in other malignancies. Universal testing is likely to be a particular challenge in PDAC, where patients may have a short time frame for receiving GC/MGT given the disease's inherently poor prognosis. This study's aim was to study the real-world effectiveness and barriers of implementing systematic GC/MGT for all patients with PDAC at a single high-volume institution.
METHODS
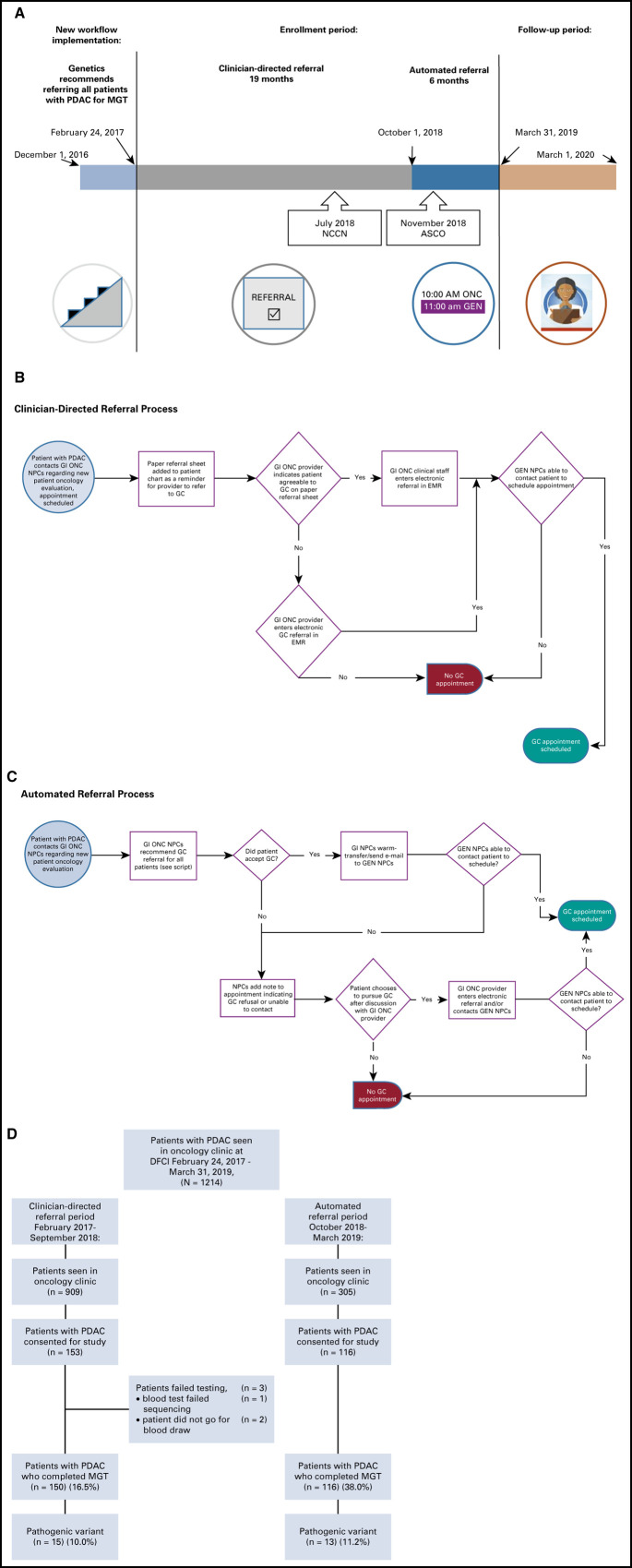
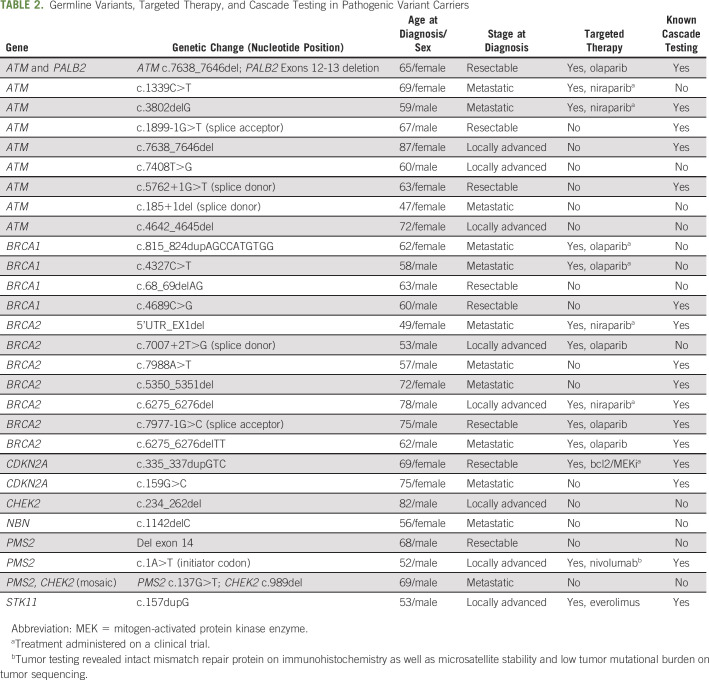
In late 2016, the Dana-Farber Cancer Institute (DFCI) Center for Cancer Genetics and Prevention implemented a new iterative workflow to facilitate GC/MGT for all individuals seen at DFCI for oncologic evaluation of a PDAC diagnosis based on growing data at our institution revealing frequent germline PVs.20 All oncology providers (medical oncologists, surgical oncologists, and radiation oncologists) in the DFCI Gastrointestinal Cancer Center were recommended to refer all patients with PDAC for GC/MGT beginning December 2016, regardless of clinical factors, such as age at diagnosis or personal or family history of cancer (clinician-directed referral; Figs 1A and 1B). The principal investigator (M.B.Y.) solicited feedback and educated providers about the rationale for universal GC/MGT at faculty meetings.
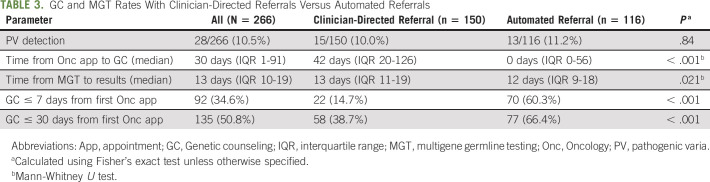
Fig 1.
(A) Study enrollment and follow-up timeline. July 2018 NCCN and November 2018 ASCO refer to published recommendations22,23 for genetic testing of patients with PDAC. (B) Schematic of the clinician-directed referral process. (C) Schematic of the automated referral process. See the script (Data Supplement). (D) Subject enrollment during the two referral periods. DFCI, Dana-Farber Cancer Institute; EMR, electronic medical record; GEN, cancer genetics department; GC, genetic counseling; MGT, multigene germline testing; ONC, oncology department; NPC, new patient coordinator; PDAC, pancreatic ductal adenocarcinoma.
After a lead-in period to refine workflows, all patients with PDAC (without prior MGT) who were referred for GC/MGT were offered enrollment in the current study beginning February 24, 2017. Three genetic counselors were affiliated with the study to ensure maximal flexibility in scheduling for patients with PDAC, and schedulers prioritized patients with PDAC for particularly prompt in-person genetic counseling (GC) appointments. In some cases, if enrolled patients were too ill to return for in-patient visits, GC was conducted via telephone with MGT kits sent to patients for saliva collection. As an iterative study, GC/MGT referral rates were continually assessed throughout the study period. Interim analysis21 found that < 20% of all patients with new PDAC were completing GC/MGT and that patients who returned to DFCI for > 1 oncologic visit (v a one-time second opinion) were significantly more likely to complete GC/MGT (odds ratio, 2.8; 95% CI, 1.7 to 4.7).
As such, in October 2018, workflows were changed such that patients with PDAC were automatically offered GC consultation at the time that they arranged their initial oncologic evaluation (automated referral), rather than requiring clinician referral. In the automated referral process (Fig 1C), gastrointestinal oncology new patient coordinators informed patients with PDAC that referral for GC/MGT was considered standard for all individuals with PDAC (see the script, Data Supplement, online only) at the time they were arranging initial oncologic consultation. For patients who agreed to GC referral, they were connected to the cancer genetics new patient coordinators via warm telephone transfer when possible. When this was not successful (eg, because of schedulers being away from their desk, patient preference, etc), the gastrointestinal oncology coordinators e-mailed the cancer genetics coordinators with the name and contact information of the patient(s) with PDAC who had agreed to GC. For patients with PDAC who declined GC or could not be contacted to arrange GC, a note was added to their new patient oncology visit in the electronic medical record to inform the oncology provider.
All patients with PDAC seen for GC were offered clinical MGT and enrollment in the study. Patients with nonadenocarcinoma histologies of malignant pancreatic neoplasia (eg, neuroendocrine carcinoma, acinar cell carcinoma, etc) were not eligible for study enrollment although individuals with poorly differentiated pancreatic carcinoma not otherwise specified were eligible. This study included all patients with PDAC without prior MGT who consented to enrollment from February 24, 2017, to March 31, 2019.
MGT was ordered as a clinical test through CLIA-certified laboratories, following informed consent. MGT results were provided directly to the participant (or a designee, if preferred by the study participant) by a genetic counselor or a genetics physician by telephone or in-person. All participants were offered in-person post-test consultation with a genetics physician, regardless of results. Germline variants classified as pathogenic or likely pathogenic by the clinical laboratory performing MGT were collectively defined as PVs for the purposes of this study, excluding specific low-penetrance germline variants (APC p.I1307K, CHEK2 p.I157T, CHEK2 p.S428F, or FH p.K477dup) and individuals found to have heterozygous germline variants in genes only associated with autosomal recessive forms of cancer susceptibility (eg, MSH3, MUTYH, NTHL1, and RECQL4). Individuals with germline variants of uncertain significance (VUS) who lacked PVs were included with noncarriers.
Clinical data collected included sex, age, PDAC stage or resectability status, treatment history, personal or family cancer history, race, ethnicity, timing of GC, date of death, and status of relatives' cascade testing (when applicable). The cutoff date for data analysis including treatment history and cascade testing was March 1, 2020. The primary end point was uptake of GC/MGT. Secondary end points included time to GC/MGT, germline PV detection, and uptake of targeted therapies (eg, PARP inhibitors), among those with PVs. Bivariate associations of PV status with clinical characteristics were analyzed using Fisher's exact test and Mann-Whitney U test for categorical and continuous variables, respectively. The primary and secondary end points were compared between the two eras of testing (clinician-directed and automated) using Fisher's exact test (for dichotomous outcomes) and Mann-Whitney U test (for continuous outcomes). All P-values were two-sided and considered statistically significant at < .05. Statistical analyses were performed using SPSS software (IBM Corp. released 2019. IBM SPSS Statistics for Macintosh, Version 26.0, Armonk, NY: IBM Corp.). This study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board.
RESULTS
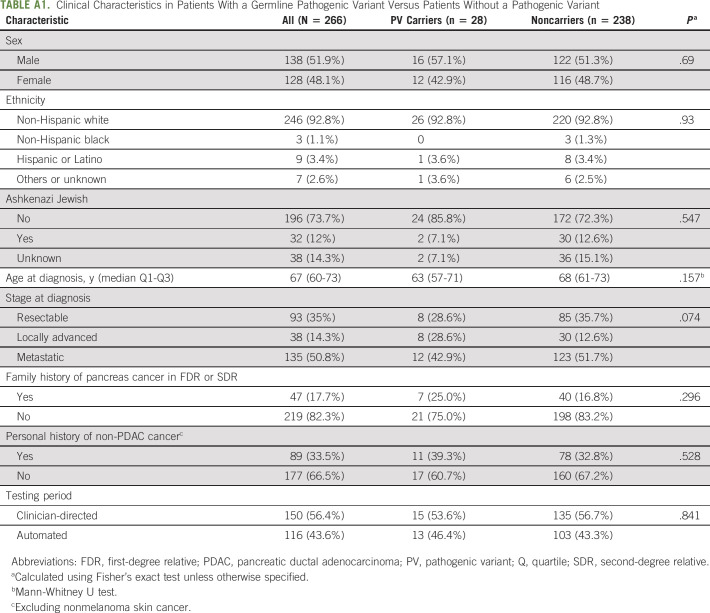
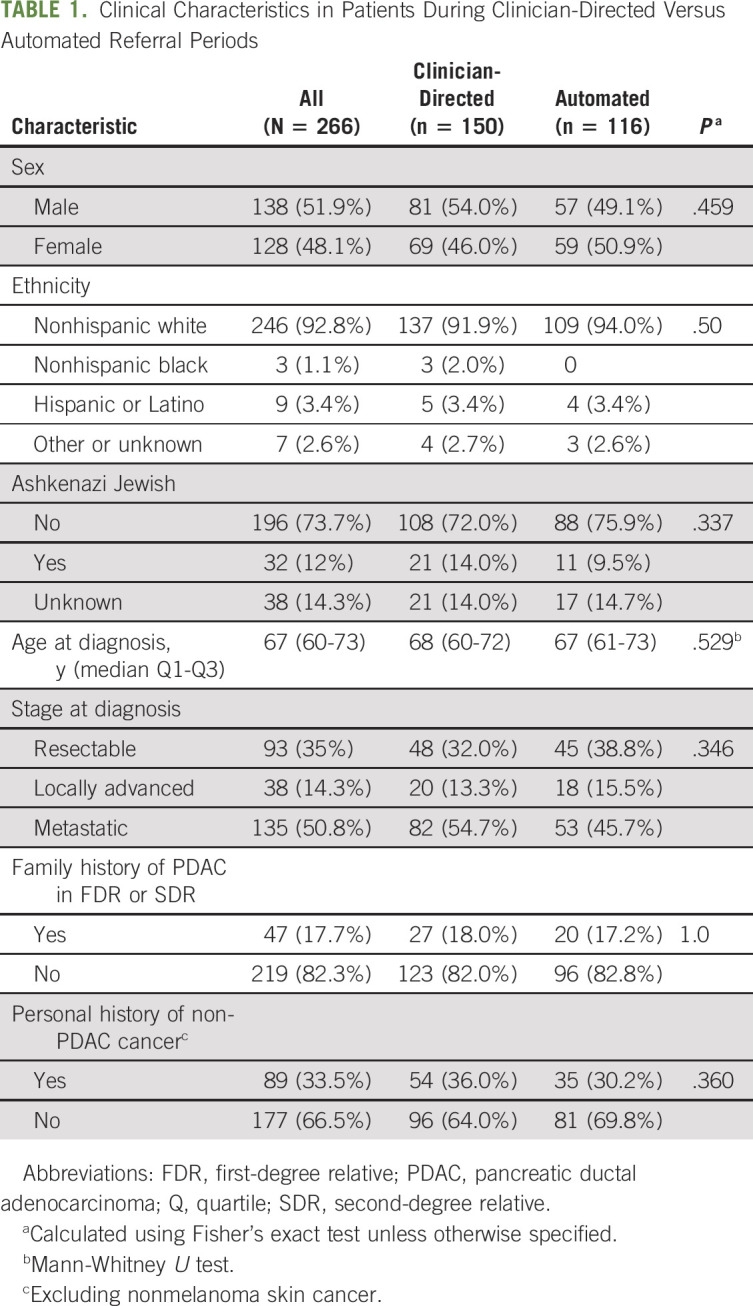
Over the 25-month enrollment period, 1,214 individuals were seen at DFCI for new outpatient oncologic consultation (medical, radiation, and/or surgical oncology) for a PDAC diagnosis. During the same span, 310 patients with PDAC were seen for GC, of whom 269 (86.8%) consented for the study with 266 (98.9%) undergoing MGT (2 patients did not have blood drawn, and 1 patient's sample failed testing; Table 1; Fig 1D).
TABLE 1.
Clinical Characteristics in Patients During Clinician-Directed Versus Automated Referral Periods
28 of 266 (10.5%) participants had ≥ 1 PV found on MGT (9 ATM [1 with concomitant PALB2], 4 BRCA1, 7 BRCA2, 2 CDKN2A, 1 CHEK2, 1 NBN, 3 PMS2, and 1 STK11; Table 2). Of note, the individual with the germline STK11 PV was known to harbor this variant from prior single-gene germline testing because of clinical features of Peutz-Jeghers syndrome, but had not had prior MGT. There was no significant difference between PV carriers and noncarriers with regard to sex, age at PDAC diagnosis, race, stage, personal cancer history, or family history of PDAC (Appendix Table A1, online only). An additional 13 of 266 (4.9%) participants were found to harbor low-penetrance germline variants or heterozygous germline variants in genes only associated with autosomal recessive forms of cancer susceptibility and were thus considered noncarriers for the purposes of this analysis. 96 of 266 (36.1%) participants had ≥ 1 germline VUS.
TABLE 2.
Germline Variants, Targeted Therapy, and Cascade Testing in Pathogenic Variant Carriers
During the clinician-directed referral period, 150 of 909 (16.5%) patients with PDAC seen for oncologic evaluation were enrolled in the study and underwent GC/MGT versus 116 of 305 (38.0%) patients with PDAC in the automated referral period (P < .001). There was no significant difference in the fraction of participants found to harbor PVs in the clinician-directed versus automated referral periods (15/150 [10.0%] v 13/116 [11.2%], P = .84). Time from initial oncologic appointment at DFCI to GC/MGT was significantly shorter in the automated referral period, with 70 of 116 (60.3%) undergoing GC/MGT ≤ 7 days from initial oncology appointment (v 22/150 [14.7%], P < .001 in the clinician-referral period; Table 3).
TABLE 3.
GC and MGT Rates With Clinician-Directed Referrals Versus Automated Referrals
Sixty-six patients (24.8%) were seen for their GC appointment immediately prior to (9 patients seen in GC 1-7 days prior to oncology) or on the same day as their oncologic appointment (with 60 of 66 [90.9%] such appointments occurring during the automated referral period). 133 of 266 (50%) participants had died as of data analysis, 9 of whom died 1-30 days from receiving MGT results and 6 of whom died prior to the return of MGT results.
Of the 28 participants with germline PVs, 26 were eligible for targeted therapy at the time of analysis based on having incurable disease and 13 (50%) of whom received targeted palliative systemic therapy based on their PV. 16 of 28 (57.1%) participants with germline PVs had ≥ 1 at-risk relative(s) who had undergone cascade testing at the time of analysis.
DISCUSSION
In this single-institution study of individuals undergoing routine PDAC oncologic care, we studied the implementation of systematic GC/MGT first through a workflow in which clinicians initiated a genetics referral and later through an automated process in which patients were directly offered genetics referral. The uptake of GC/MGT referrals in this study improved significantly with a change in workflow to an automated referral system, and time to GC was significantly shorter after automation with 25% of patients seen on the same day as their oncology appointment or shortly before the oncology appointment. Importantly, however, germline PV rates did not drop with the automated referral approach, indicating that overall PV detection was reliant primarily on the number of individuals referred for GC/MGT, rather than clinician discretion. Patients with PDAC, their families, and their clinicians commonly acted upon MGT findings through both cascade testing in at-risk relatives and also the uptake of PARP inhibitors and other germline-directed targeted PDAC therapies. These findings demonstrate both the feasibility and downstream benefits of systematic GC/MGT in individuals with PDAC.
The concept of screening all individuals with a particular cancer for inherited susceptibility has precedent in other cancer types, most notably ovarian cancer where guidelines from the NCCN and others have recommended germline BRCA1/2 testing for all patients since 2007, with updated guidelines now broadening this to MGT.22,23 Despite such recommendations, data have shown that < 30% of patients with ovarian cancer undergo germline evaluation in real-world practice.24 Now that clinical practice guidelines from the NCCN22 and ASCO23 endorse GC/MGT for all patients with PDAC, the lessons and failures from over a decade's worth of experience in ovarian cancer should provide valuable insight into devising strategies for successful real-world implementation in PDAC.
Our data demonstrate that simply directing providers to refer all patients with PDAC results in poor uptake of GC/MGT, mirroring such historical lessons from ovarian cancer.24 Although processes were designed so as to facilitate prompt and flexible GC scheduling once referral was initiated, our data suggest that a key barrier to GC/MGT uptake with a clinician-directed workflow may be the time from initial oncology evaluation to GC. PDAC may be a particularly challenging disease in which to implement universal GC/MGT because of the complex therapeutic, symptomatic, endoscopic, and psychosocial needs inherent to the disease,15 and the long time to GC in the clinician-directed referral arm of this study may reflect the prioritization of these other critical aspects of care over hereditary risk assessment. The lethality of PDAC further amplifies the importance of prompt GC/MGT, as illustrated by the observation that 15 of 266 (5.6%) enrollees in our study died ≤ 30 days of receiving MGT results, thereby precluding these individuals from having the opportunity to therapeutically benefit from the results.
By implementing an automated referral process for GC consultation, we achieved a significant increase in GC/MGT uptake and a reduction in time to GC consultation. Even with these improvements, however, the overall GC/MGT uptake was < 40%, highlighting the need to identify additional barriers and facilitators to universal germline evaluation. Several factors may have contributed to this low uptake, including the possibility that participants underwent GC/MGT at another institution, lack of patient understanding about the importance of GC/MGT, the potential of patients with PDAC to have been too symptomatic and/or overwhelmed to have undergone GC, and an inability of schedulers to make contact with each patient with PDAC to offer GC/MGT referral. None of these factors were directly measured in this study, and these remain important areas to investigate in future implementation analyses.
In the numerous recent studies,1-7 one consistent and critical finding has been that a large fraction of patients with PDAC with PVs lack obvious clinical features of inherited cancer risk (eg, young age and classic family history patterns) and would thus presumably go undiagnosed by phenotype-directed genetic testing practices. In our study, we likewise observed no significant differences in clinical characteristics between PV carriers and noncarriers nor a difference in PV detection rate among PDAC participants referred in the clinician-directed versus the automated referral period. Thus, performing germline testing on patients with PDAC in a more systematic and automated manner did not lead to a lower risk cohort of individuals undergoing evaluation, but rather, the 10%-12% PV detection rate was preserved with larger numbers of patients with PDAC undergoing MGT. Increasing the denominator of patients with PDAC undergoing MGT led directly to an increase in PV detection.
One key potential benefit of universal GC/MGT in patients with PDAC is the facilitation of cascade testing, in which at-risk relatives undergo their own testing for the familial PV identified in the PDAC proband. Given that the established PDAC susceptibility genes are all linked to risks of non-PDAC cancers for which there are evidence-based methods of prevention (eg, salpingo-oophorectomy and mastectomy in BRCA1/2), the familial benefits of universal MGT are potentially quite significant.15 Although no studies to date have examined the cost-effectiveness of universal MGT in PDAC, numerous analyses25-27 have consistently demonstrated that the cost-effectiveness of systematic screening for various inherited cancer syndromes is heavily reliant on the uptake of cascade testing among at-risk relatives. While the body of literature on cascade testing remains limited, data suggest that historical rates of cascade testing in Lynch syndrome and BRCA1/2 have been low (< 30%), indicating that this is a major barrier to achieving the full preventive impact from universal hereditary cancer risk assessment.28-32 Encouragingly, with relatively short follow-up, > 50% of participants with a PV in our study had at least one at-risk relative pursue cascade testing, demonstrating the potential for universal GC/MGT to facilitate downstream cancer prevention.
As targeted therapies are now becoming viable treatments for some PDACs,12,20,33 the other major potential benefit of universal GC/MGT is its ability to identify therapeutic opportunities for the PDAC proband themselves. The most notable example is the recent Food and Drug Administration approval of the PARP inhibitor olaparib as maintenance therapy in patients with advanced PDAC with germline BRCA1/BRCA2 PVs,12 with ongoing speculation34 that PARP inhibitors might also have benefit for patients with PDAC who harbor germline PVs in other homologous recombination genes (eg, PALB2 and ATM). One recent analysis33 demonstrated a median 12-month overall survival improvement for patients with PDAC with a somatic or germline abnormality who received therapy matched to their genomic findings versus those who received standard unmatched therapy (hazard ratio, 0.42; 95% CI, 0.26 to 0.68), highlighting the powerful therapeutic potential of such findings. With the high uptake of targeted therapies and cascade genetic testing among PV carriers and their relatives, respectively, our study's findings demonstrate that the theoretical rationale for universal GC/MGT in PDAC indeed translates into tangible real-world changes in management, with such downstream effects likely contributing to improved outcomes for the PDAC probands themselves, opportunities for cancer prevention in at-risk relatives, and a favorable cost-effectiveness profile for such efforts.
Our data also illustrate a number of barriers that threaten to limit the real-world effectiveness of universal GC/MGT. Previous publications discussing universal GC/MGT in PDAC have hypothesized that a major barrier to implementing such systematic testing will be the growing shortage of genetic counselors.2 Even with abundant genetic counselor availability, however, referral rates in this study were disappointingly low, indicating that other factors are also barriers to real-world implementation of universal MGT. Our findings suggest that workflow relying on oncology providers to initiate the genetics workup is likely to suffer from suboptimal referral rates and may miss the window of opportunity of testing patients who are still well enough to pursue GC and targeted therapies.
A key limitation is this study's single institution design, which limits generalizability to other settings, particularly those with more limited GC resources. Furthermore, the availability of research funding to help defray the costs of clinical MGT may have artificially enhanced participants' willingness to undergo testing. These potential limitations, however, would have biased our results toward higher GC/MGT usage, and our findings of low referral or testing rates and the potential underlying barriers are all likely to be generalizable to real-world settings. Another limitation is the study's inability to assess specific reasons why providers failed to refer patients with PDAC for GC/MGT and reasons why patients may have declined referral. Notably, the NCCN first recommended consideration of universal GC/MGT in all patients with PDAC in mid-20183 with ASCO providing a similar recommendation later the same year,23 both of which resulted in some patients with PDAC having already pursued GC/MGT prior to coming to DFCI. Finally, our cascade testing data may have been incomplete, since such data relied on report by the PDAC proband.
These data provide important insights into the effectiveness, downstream benefits, and barriers to systematically offering GC/MGT to all patients with PDAC in real-world practice. Changing from clinician-directed to automated referral workflows more than doubled the uptake of GC/MGT without any decline in PV detection rates. For those with PVs, there were tangible changes to management for the PDAC probands and their at-risk family members, demonstrating the real-world feasibility of systematic risk assessment. Even with an automated referral process, however, GC/MGT uptake was suboptimal, and additional data are needed to identify additional barriers to the real-world implementation of universal GC/MGT. Based on these data, however, we conclude that optimizing the real-world implementation of universal MGT will require workflows that directly offer GC/MGT to patients with PDAC, rather than relying on clinician referral.
ACKNOWLEDGMENT
We would like to acknowledge Eliana Talcove-Berko and Catherine Lafferty for their help with providing data abstraction and follow-up data for this paper.
APPENDIX
TABLE A1.
Clinical Characteristics in Patients With a Germline Pathogenic Variant Versus Patients Without a Pathogenic Variant
EQUAL CONTRIBUTION
A.C. and S.H. share first co-authorship.
PRIOR PRESENTATION
Preliminary data from this manuscript were presented as a poster abstract at the 21st Annual Meeting of the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer, Orlando, FL, October 20-21, 2017, and at the 2018 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018, and 23rd Annual Meeting of the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer, Salt Lake City, UT, November 3-5, 2019, and 2020 Gastrointestinal Meeting of the American Society of Clinical Oncology, San Francisco, January 23-25, 2020.
SUPPORT
Supported by a Stand Up To Cancer-Lustgarten Foundation Pancreatic Cancer Interception Translational Cancer Research Grant (Grant Number: SU2C-AACR-DT25-17). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. Also supported by Dana-Farber/Harvard Cancer Center SPORE in Gastrointestinal Cancer (P50 CA127003) Developmental Research Project Award (M.B.Y.) and Dana-Farber Cancer Institute Department of Medical Oncology Translational Research Grant (M.B.Y.). Supported by The Hale Family Center for Pancreatic Cancer Research, Lustgarten Foundation Dedicated Laboratory program, NIH grant U01 CA210171 and NIH grant P50 CA127003, Stand Up to Cancer, Pancreatic Cancer Action Network, Noble Effort Fund, Wexler Family Fund, and Promises for Purple (B.M.W.).
AUTHOR CONTRIBUTIONS
Conception and design: Sapna Syngal, Matthew B. Yurgelun
Financial support: Sapna Syngal, Matthew B. Yurgelun
Administrative support: Chinedu Ukaegbu, Sapna Syngal, Matthew B. Yurgelun
Provision of study materials or patients: Shraddha Gaonkar, Brian M. Wolpin, Matthew B. Yurgelun
Collection and assembly of data: Anu Chittenden, Sigurdis Haraldsdottir, Chinedu Ukaegbu, Shraddha Gaonkar, Lauren K. Brais, Kimberly Perez, Brian M. Wolpin, Matthew B. Yurgelun
Data analysis and interpretation: Anu Chittenden, Sigurdis Haraldsdottir, Meghan Underhill-Blazey, Hajime Uno, Sapna Syngal, Matthew B. Yurgelun
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Implementing Systematic Genetic Counseling and Multigene Germline Testing for Individuals With Pancreatic Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Anu Chittenden
Employment: Immunogen
Consulting or Advisory Role: Boston Pharmaceuticals
Shraddha Gaonkar
Travel, Accommodations, Expenses: Ambry Genetics
Hajime Uno
Consulting or Advisory Role: Roche
Kimberly Perez
Consulting or Advisory Role: Celgene, Eisai
Brian M. Wolpin
Honoraria: G1 Therapeutics, Celgene, Genentech, G1 Therapeutics, BioLineRx, GRAIL
Research Funding: Celgene, Lilly
Sapna Syngal
Consulting or Advisory Role: Myriad Genetics, DC Health, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Dana-Farber Cancer Institute has a registered service mark for the PREMM5 model and holds copyrights for the PREMM questionnaires, Myriad Genetics (through Dana-Farber Cancer Institute) paid an inventor share of the IP (license issue fee)
Travel, Accommodations, Expenses: Myriad Genetics
Matthew B. Yurgelun
Consulting or Advisory Role: Janssen
Other Relationship: UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1.Yurgelun MB, Chittenden AB, Morales-Oyarvide V, et al. : Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med 21:213-223, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shindo K, Yu J, Suenaga M, et al. : Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 35:3382-3390, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu C, Hart SN, Polley EC, et al. : Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 319:2401-2409, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RC, Selander I, Connor AA, et al. : Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology 148:556-564, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand R, Borazanci E, Speare V, et al. : Prospective study of germline genetic testing in incident cases of pancreatic adenocarcinoma. Cancer 124:3520-3527, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Lowery MA, Wong W, Jordan EJ, et al. : Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst 110:1067-1074, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johns AL, McKay SH, Humphris JL, et al. : Lost in translation: Returning germline genetic results in genome-scale cancer research. Genome Med 9:41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) genetic/familial high-risk assessment: Breast, ovarian, and pancreatic. Version 1.2021. nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [DOI] [PubMed]
- 9.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. : Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33:244-250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bono J, Ramanathan RK, Mina L, et al. : Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov 7:620-629, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shroff RT, Hendifar A, McWilliams RR, et al. : Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. JCO Precis Oncol 2018:PO.17.00316, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golan T, Hammel P, Reni M, et al. : Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 381:317-327, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klumpen HJ, Queiroz KC, Spek CA, et al. : mTOR inhibitor treatment of pancreatic cancer in a patient with Peutz-Jeghers syndrome. J Clin Oncol 29:e150-e153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemery S, Keegan P, Pazdur R: First FDA approval agnostic of cancer site—When a biomarker defines the indication. N Engl J Med 377:1409-1412, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Yurgelun MB: Germline testing for individuals with pancreatic cancer: the benefits and challenges to casting a wider net. J Clin Oncol 35:3375-3377, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Schwark AL, Stadler ZK: Should we lower our threshold for germline genetic assessment in pancreatic adenocarcinoma? JCO Precis Oncol 2, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Syngal S, Furniss CS: Germline genetic testing for pancreatic ductal adenocarcinoma at time of diagnosis. JAMA 319:2383-2385, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Lieberman S, Lahad A, Tomer A, et al. : Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med 20:1446-1454, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Landsbergen K, Verhaak C, Kraaimaat F, et al. : Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer 4:115-119, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Aguirre AJ, Nowak JA, Camarda ND, et al. : Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov 8:1096-1111, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yurgelun MB, Chittenden AB, Ukaegbu CI, et al. : Implementing universal genetic counseling (GC) and multigene germline testing (MGT) for pancreatic cancer (PC) patients (pts). J Clin Oncol 36:1512, 2018. (15 suppl) [Google Scholar]
- 22.Lowery MA, Kelsen DP, Capanu M, et al. : Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer 89:19-26, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoffel EM, McKernin SE, Brand R, et al. : Evaluating susceptibility to pancreatic cancer: ASCO provisional clinical opinion. J Clin Oncol 37:153-164, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Kurian AW, Ward KC, Howlader N, et al. : Genetic testing and results in a population-based cohort of breast cancer patients and ovarian cancer patients. J Clin Oncol 37:1305-1315, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuffaha HW, Mitchell A, Ward RL, et al. : Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet Med 20:985-994, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Eccleston A, Bentley A, Dyer M, et al. : A cost-effectiveness evaluation of germline BRCA1 and BRCA2 testing in UK women with ovarian cancer. Value Health 20:567-576, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladabaum U, Wang G, Terdiman J, et al. : Strategies to identify the Lynch syndrome among patients with colorectal cancer: A cost-effectiveness analysis. Ann Intern Med 155:69-79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suthers GK, Armstrong J, McCormack J, et al. : Letting the family know: Balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet 43:665-670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehniger J, Lin F, Beattie MS, et al. : Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns 22:603-612, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Frey MK, Kahn RM, Chapman-Davis E, et al. : Prospective feasibility trial of a novel strategy of facilitated cascade genetic testing using telephone counseling. J Clin Oncol 38:1389-1397, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blandy C, Chabal F, Stoppa-Lyonnet D, et al. : Testing participation in BRCA1/2-positive families: Initiator role of index cases. Genet Test 7:225-233, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Sharaf RN, Myer P, Stave CD, et al. : Uptake of genetic testing by relatives of lynch syndrome probands: A systematic review. Clin Gastroenterol Hepatol 11:1093-1100, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Pishvaian MJ, Blais EM, Brody JR, et al. : Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol 21:508-518, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel R, Fein D, Ramirez CB, et al. : PARP inhibitors in pancreatic cancer: From phase I to plenary session. Pancreas (Fairfax) 3:e5-e8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]