Abstract
Background
Early life exposures impact immune system development and therefore the risk of immune-mediated diseases, including inflammatory bowel disease (IBD). We systematically reviewed the impact of pre-, peri‑, and postnatal exposures up to the age of five years on subsequent IBD diagnosis.
Methods
We identified case-control and cohort studies reporting on the association between early life environmental factors and Crohn's disease (CD), ulcerative colitis (UC), or IBD overall. Databases were search from their inception until May 24th, 2019 until July 14th, 2020. We conducted meta-analyses for quantitative review of relevant risk factors that were comparable across studies and qualitative synthesis of the literature for a wide range of early life exposures, including maternal health and exposures during pregnancy, perinatal factors, birth month and related-factors, breastfeeding, hygiene-related factors and social factors, immigration, antibiotics, offspring health, including infections, and passive smoking. PROSPERO registration: CRD42019134980.
Findings
Prenatal exposure to antibiotics (OR 1.8; 95% CI 1.2–2.5) and tobacco smoke (OR 1.5; 95% CI 1.2–1.9), and early life otitis media (OR 2.1; 95% CI 1.2–3.6) were associated with IBD. There was a trend towards an association between exposure to antibiotics in infancy and IBD (OR: 1.7, 95% CI 0.97, 2.9), supported by positive data on population-based data. Breastfeeding was protective against IBD. Other early life risk factors had no association with IBD, but data were limited and heterogenous.
Interpretation
Early life is an important period of susceptibility for IBD development later in life. Tobacco smoke, infections and antibiotics were associated positively, and breastfeeding was associated negatively with IBD. Our findings offer an opportunity to develop primary prevention strategies.
Funding
This study did not receive any funding.
Keywords: Inflammatory bowel disease, Crohn's disease, Ulcerative colitis, Early life, Risk factors, Environmental exposure, Non-genetic, Epidemiology
Research in context.
Evidence before this study
Several lines of evidence, such as the rapid rise of disease in developing countries, strongly implicate the environment in IBD risk modulation. Adequate microbial colonization in early life is an essential driver of immune maturation; subtle deviations in this process can impact susceptibility to diseases later in life, especially immune-mediated diseases. Several environmental risk factors have been implicated in IBD risk.
Added value of this study
We synthetize all available data on exposures operative between prenatal life to five years of age (early life), a period of susceptibility, and subsequent IBD risk. We report that prenatal exposure to tobacco smoke and antibiotics are associated with increased risk of developing IBD in the offspring. Early life otitis media increases the risk of IBD, early life exposure to antibiotics may be associated with IBD risk, while breastfeeding is protective against IBD. We highlight the heterogeneity in existing literature, as well the need for prospective studies.
Implications of all the available evidence
This study helps provides evidence-based guidance to mitigate exposures to potential early life IBD risk factors, and provides insights for development of primary prevention strategies.
Alt-text: Unlabelled box
1. Introduction
Inflammatory bowel disease (IBD) is a chronic progressive immune-mediated disease with significant long-term morbidity and disability. The pathophysiology of IBD involves a complex interaction between genetics and environmental exposures. Early life represents a critical window during which exposures may have short and long-term health consequences [1,2]. This hypothesis, collectively known as the Developmental Origins of Health and Disease (DOHaD), suggests that the developing foetus, and the growing child, will develop adaptive responses when exposed to various exposures that may lead to persistent alterations conferring increased resistance or susceptibility to diseases [3.] The recognition that the early life microbiome plays a central role in priming the immune system [4] has led to interest in characterizing the effects of pre-, peri‑ and postnatal exposures in immune-mediated diseases, especially IBD. While many studies have reported inconsistent associations for different early life exposures, no systematic appraisal of the evidence has yet been conducted. The aim of the present study was to critically review, appraise and synthesize the epidemiological evidence data linking exposures in early life (defined as from in utero up to 5 years of age) with the subsequent risk of developing IBD.
2. Methods
2.1. Literature search
We performed a rigorous literature search in collaboration with a qualified medical librarian using a predetermined and registered protocol (PROSPERO—CRD42019134980), and report results in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist [5]. We searched for relevant studies in Medline ALL, Embase Classic + Embase (Ovid), and Global Health (Ovid) databases from their inception until May 24th, 2019.We ran an update from January 2019 to July 14th, 2020. The search was designed using a combination of free-text terms and subject headings specific to each database. We did not apply any language or date restriction. We applied a filter for observational studies, adapted from the Observational Studies search filter developed by the Scottish Intercollegiate Guidelines Network (SIGN) [6]. The detailed protocol and search strategy are available in Supplementary Material.
2.2. Inclusion and exclusion criteria
We defined early life as extending from the prenatal period to 5 years of age. Exposures were categorized into (1) maternal health and exposures during pregnancy (2) perinatal factors, (3) birth month and related-factors, (4) breastfeeding, (5) hygiene-related factors and social factors, (6) immigration, (7) antibiotics and other medications, (8) offspring´s health, including infections, and (9) passive smoking. The outcomes of interest were IBD, Crohn´s disease (CD) and/or ulcerative colitis (UC) diagnosis. Case-control and cohort studies were included. Studies where the timing of exposure was not clearly defined as ≤5 years were excluded. Given the number of studies to be screened, and lack of relevant non-English studies, we excluded non-English studies during abstract screening.
2.3. Study selection
Studies were organized using the software Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia), which also automatically removes duplicates. Two of three independent reviewers (MA, JS, CG) screened the titles and abstracts, as well as full-texts that met inclusion criteria. Discrepancies were resolved by discussion and, if needed, resolution by a third arbitrator (JT).
2.4. Data extraction and quality assessment
The following variables were recorded using a standardized data extraction template: study title and year, region, study period, population, design, exposure(s), outcome(s), and measures of association (estimates), along with other relevant variables. Quality of studies was evaluated using the Newcastle-Ottawa Scale (NOS) [7].
2.5. Qualitative and quantitative data synthesis
We performed a qualitive analysis by synthesizing the literature for each category of all relevant exposures. When feasible, we also conducted random-effects meta-analyses (MA) to determine pooled estimates with 95% confidence intervals (CI) for homogenous exposures reported in ≥2 studies with comparable study design (cohort or case-control) and outcome (CD, UC or IBD). When relevant data were available from cohort studies, we calculated odds ratios (OR) and pooled with ORs from case-control studies for corresponding exposures. For such analyses, we also performed meta-regression to determine if there were significant differences based on study design. If so, we analyzed cohort and case control studies separately. Heterogeneity was measured using the I2 score. Publication bias was assessed using funnel plots, and when applicable, Egger's tests. MA were performed using the Comprehensive Meta-Analysis (version 2.0; Biostat, Englewood, NJ). For exposures with recent MA, we deferred quantitative analysis (mode of delivery and breastfeeding)[8 ,9].
2.6. Role of the funding source
The funding source had no role in the study design and data analysis/interpretation.
3. Results
3.1. Summary of literature search
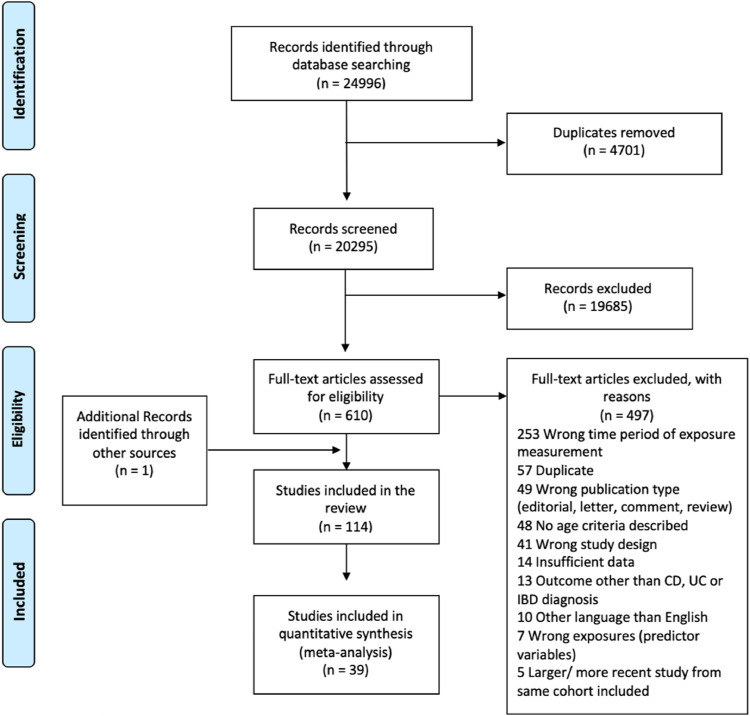
The search strategy yielded 24,996 unique citations. After excluding duplicates and screening abstracts, 20,295 full-text articles were assessed for eligibility. One-hundred and fourteen studies were included in the final qualitative systematic review, and 39 were appropriate for MA (Fig. 1). These studies pertained to the following categories (specific exposures): (1) maternal health and exposures during pregnancy (maternal age, smoking, and antibiotic exposure), (2) perinatal factors (premature birth and birthweight), (3) hygiene-related factors and social factors (rural vs urban living), (4) antibiotics (during infancy), (5) offspring´s health, including infections (measles vaccination), and (6) passive smoking. Table 1 summarizes the pooled estimates for each exposure, based on our MA. Supplementary Tables 1a-j summarize the extracted data, and Supplementary Tables 2a and 2b summarize the NOS grading for all studies. Supplementary Figures 1–33 present forest plots, meta-regression graphs and funnel plots for all MAs, unless otherwise stated.
Fig. 1.
PRISMA 2009 Flow Diagram for selection of studies in the meta-analyses and systematic review.
Table 1.
Summary data on the association between early life exposures and IBD on meta-analyses.
| Exposure category | Exposure analyzed | Number of studies meta-analyzed | Type of study | Outcome | Pooled estimate | 95% CI | I² (%) | P value for heterogeneity test |
|---|---|---|---|---|---|---|---|---|
| Maternal health and exposures during pregnancy | Maternal smoking | 9 | CC, cohort | IBD | 1.491 | 1.171–1.898 | 72.04 | <0.001 |
| Maternal smoking | 6 | CC | CD | 1.208 | 0.747–1.956 | 83.55 | <0.001 | |
| Maternal smoking | 4 | CC | UC | 1.511 | 0.990–2.306 | 68.05 | 0.025 | |
| Maternal age | 4 | CC, cohort | IBD | 0.863 | 0.452–1.648 | 87.05 | <0.001 | |
| Maternal age | 2 | Cohort | CD | 0.773 | 0.219–2.726 | 95.27 | <0.001 | |
| Antibiotics | 2 | Cohort | IBD | 1.751 | 1.222–2.510 | 0 | 0.62 | |
| Perinatal factors | Birth weight | 10 | CC, cohort | IBD | 0.92 | 0.727–1.164 | 76.75 | <0.001 |
| Birth weight | 6 | CC, cohort | CD | 0.875 | 0.624–1.225 | 70.72 | 0.004 | |
| Birth weight | 3 | CC, cohort | UC | 0.831 | 0.412–1.677 | 81.66 | 0.004 | |
| Premature birth | 8 | CC, cohort | IBD | 1.055 | 0.933–1.194 | 0 | 0.49 | |
| Premature birth | 5 | CC, cohort | CD | 1.073 | 0.890–1.293 | 5.33 | 0.38 | |
| Premature birth | 5 | CC, cohort | UC | 1.024 | 0.669–1.570 | 53.57 | 0.07 | |
| Hygiene factors | Rural vs. urban | 7 | CC | IBD | 1.033 | 0.814–1.311 | 52.76 | 0.048 |
| Rural vs. urban | 3 | CC | CD | 1.15 | 0.668–1.979 | 68.81 | 0.041 | |
| Rural vs. urban | 4 | CC | UC | 1.053 | 0.843–1.315 | 38.33 | 0.18 | |
| Antibiotics and other medications | Antibiotics in first year | 3 | CC, cohort | IBD | 1.683 | 0.965–2.936 | 55.38 | 0.11 |
| Antibiotics in first year | 2 | CC, cohort | CD | 1.19 | 0.512–2.765 | 49.29 | 0.16 | |
| Antibiotics in first year | 2 | CC, cohort | UC | 0.925 | 0.464–1.845 | 0 | 0.49 | |
| Child health and infections | Passive smoking | 3 | CC | CD | 1.084 | 0.951–1.207 | 0 | 0.64 |
| Measles vaccine | 12 | CC | IBD | 1.078 | 0.911–1.275 | 55.52 | 0.004 | |
| Measles vaccine | 9 | CC | CD | 1.06 | 0.822–1.366 | 64.69 | 0.004 | |
| Measles vaccine | 6 | CC | UC | 1.046 | 0.844–1.296 | 28.20 | 0.22 | |
| BCG vaccine | 4 | CC | IBD | 1.549 | 0.969–2.477 | 37.58 | 0.19 | |
| BCG vaccine | 3 | CC | CD | 1.773 | 0.846–3.714 | 53.17 | 0.12 | |
| Otitis media | 2 | CC | IBD | 2.105 | 1.224–3.619 | 36.85 | 0.21 | |
| Otitis media | 2 | CC | CD | 1.947 | 1.198–3.166 | 4.41 | 0.31 |
Abbreviations: CI = confidence interval; CC = case-control; IBD = inflammatory bowel disease; CD = Crohn's disease; UC = ulcerative colitis; BCG = Bacillus Calmette–Guérin.
3.2. Maternal health and exposures during pregnancy
3.2.1. Maternal tobacco smoking
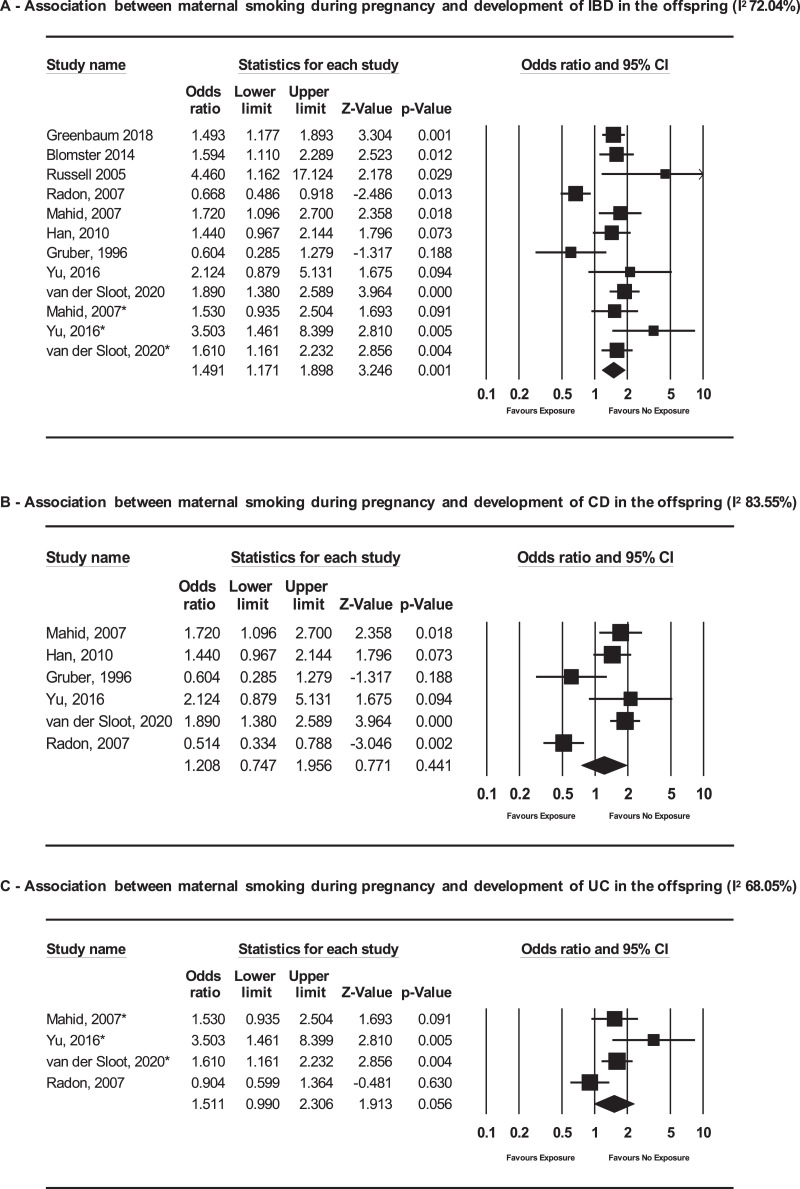
We included nine studies in the MA (Fig. 2A, Table 1, Supplementary Table 1a)[10], [11], [12], [13], [14], [15], [16], [17], [18]. The odds of developing IBD were higher among infants exposed to smoking during pregnancy compared to the unexposed [pooled OR (pOR) 1.49; 95% CI 1.17–1.90; I2 72.04%], but significant heterogeneity was identified (p value for heterogeneity test <0.001). However, when analyzed according to IBD subtype, the association was no longer significant (CD: pOR 1.21, 95% CI 0.75–1.96, I2 83.55%, based on 6 studies and UC: pOR 1.51, 95% CI 0.99–2.31, I2 68.05% based on 4 studies) (Fig. 2B and C).
Fig. 2.
Forest plots of the association between maternal smoking during pregnancy and subsequent diagnosis of (A) IBD, (B) CD or (C) UC in the offspring.
Abbreviations: IBD – inflammatory bowel disease; CD – Crohn's disease; UC – Ulcerative colitis.
3.2.2. Maternal age
We found no association between older maternal age (≥35 years) vs younger age and the odds of IBD (OR 0.86; 95% CI 0.45–1.65; I2 87.05%) or CD (OR 0.77; 95% CI 0.22–2.73; I2 95.27%) in the offspring (Table 1, Supplementary Table 1a, Supplementary Figures 3–5).19–22Lack of estimates for UC as the outcome precluded MA. There was significant heterogeneity between studies (p <0.001).
3.2.3. Maternal diseases during pregnancy
Four studies reported no association of diseases during pregnancy (preeclampsia, related disorders or others) and IBD on the offspring (Supplementary Table 1a)[20 ,[23], [24], [25]]. A population-based cohort study from Israel study reported higher odds of IBD in the offspring of mothers colonized with group B Streptococcus (OR 1.29; 95% CI 1.03–1.60)[26]. Overall, maternal infections during pregnancy (measles, respiratory or urinary tract infection, non-specific) were not associated with IBD risk in the offspring[25 ,[27], [28], [29], [30]].
3.2.4. Antibiotic exposure during pregnancy
Two high-quality studies, one cohort and another case-control, reported an association of intra-uterine exposure to antibiotics and subsequent diagnosis of IBD in the offspring (OR 1.75; 95% CI 1.22–2.51; I2 0) (Fig. 3A)[11,31]. In one study, the risk of IBD was significantly increased with antibiotic exposure in the third trimester (aHR 2.57; 95% CI 1.10–6.01), but not earlier in pregnancy (Supplementary Table 1a) [31]
Fig. 3.
Forest plots of the association between (A) prenatal exposure to antibiotics and (B) exposure to antibiotics during infancy and subsequent diagnosis of IBD.
Abbreviation: IBD – inflammatory bowel disease.
3.2.5. Exposure to pollutants
In a population-based cohort study, Elten et al. [32] reported that exposure to oxidant capacity (a measure of the oxidative potential of nitrogen dioxide and ozone) during the second trimester was associated with IBD in the offspring (aHR 1.21; 95% CI 1.03–1.42), but not when considering the overall duration of pregnancy (aHR 1.12; 95% CI 0.79–1.59) (Supplementary Table 1a).
3.3. Perinatal factors
3.3.1. Mode of delivery
Multiple studies, of mixed quality, evaluated the association of mode of delivery with IBD, CD or UC and yielded conflicting results, with predominant studies reporting no association (Supplementary Table 1b) [18 ,24 ,25 ,28 ,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42]].
3.3.2. Low birth weight (LBW)
Ten studies assessed the association of LBW (defined as ≤2500 gram or <5th centile of gestational age) with a subsequent diagnosis of IBD and reported mixed results (Supplementary Table 1b) [13 ,[18], [19], [20], [21], [22] ,25 ,28 ,43 ,44]. MA of these studies demonstrated no association between LBW and IBD (pOR 0.92; 95% CI 0.72–1.16; I2 76.75%), CD (pOR 0.86; 95% CI 0.62–1.23; I2 70.72%), or UC (pOR 0.83; 95% CI 0.41–1.68; I2 81.66%) (Table 1, Supplementary Figures 6–8). However, effect estimates differed by cohort vs case-control studies (meta-regression p<0.001) and on analyzing them separately, LBW was associated with IBD in cohort studies (pOR 1.2; 95% CI 1.06–1.36; I2 42.67%), but not in case-control studies (pOR 0.85; 95% CI 0.63–1.16; I2 73.39%, Supplementary Figures 9–11).
3.3.3. Premature birth
Eleven studies evaluated prematurity, which was variably defined as <35–37 weeks of gestational age (Supplementary Table 1b) [[19], [20], [21], [22] ,25 ,30 ,32 ,36 ,42 ,[44], [45], [46]]. On MA of eight eligible studies, prematurity was not associated with subsequent IBD (pOR 1.06; 95% CI 0.93–1.19, I2 0), CD (pOR 1.07; 95% CI 0.89–1.29, I2 5.33%), or UC (pOR 1.02; 95% CI 0.67–1.57; I2 53.57%) (Table 1, Supplementary Figures 13–15) [[20], [21], [22] ,24 ,25 ,30 ,44 ,46]. On meta-regression, there was a significant difference in effect estimates between cohort and case-control studies (p<0.001) (Table 1, Supplementary Figure 12).
3.3.4. Other perinatal factors
Apgar score >7 at one minute was associated with higher odds of CD (OR 1.42; 95% CI 1.02–1.96) but not UC in one study (Supplementary Table 1b).36In other studies, there was no association between Apgar score and IBD[19 ,21]. No significant associations were found between birth order[19], single versus multiple births[19 ,21], past spontaneous abortion, [19] or paternal age and IBD [47].
3.3.5. Birth month and related factors
Twelve studies from different regions reported on the association of seasonality or birth month and IBD (Supplementary Table 1c) [19 ,21 ,[48], [49], [50], [51], [52], [53], [54], [55], [56], [57]]. There was significant heterogeneity across studies with respect to exposure categorization and reference groups, precluding meaningful MA. Results were mixed and conflicting. Three studies analyzed latitude of residence, vitamin D, and sun exposure in early life and risk of subsequent IBD[22 ,58 ,59]. One study reported null association between latitude of residence in the United States at birth and subsequent IBD. [58] One population-based study from Denmark reported no association between perinatal vitamin D levels and risk of pediatric onset IBD[22]. Lastly, a study analyzing early life sun exposure and risk of IBD, reported no significant association [59]
3.3.6. Breastfeeding
Two cohort studies and 40 case-control studies reported the association of breastfeeding with IBD (Supplementary Table 1d) [13 ,15 ,16 ,18 ,23 ,24 ,29 ,30 ,33 ,39 ,40 ,[44], [45], [46] ,49 ,[60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84]]. Overall, most studies reported a significant protective effect of breastfeeding against IBD risk. In case-control studies, this protective effect was higher in studies from Asia, Australia and New Zealand, and when longer than 12 months, but not universally confirmed.
3.3.7. Hygiene-related and social factors
A total of 32 studies analyzed rural vs urban residence and hygiene-related factors meeting inclusion criteria (Supplementary Table 1e) [13 ,15 ,[18], [19], [20] ,23 ,29 ,32 ,34 ,36 ,[38], [39], [40] ,42 ,46 ,63 ,[65], [66], [67] ,69 ,72 ,[85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95]]. The studies were globally diverse, but no studies from East Asia met full inclusion criteria.
3.3.8. Rural versus urban living
In the only cohort study on rural vs urban residence in early life, Benchimol et al.[85] reported that the risk of IBD later in life was significantly lower in those who had spent early life in rural residence (IRR 0.76; 95% CI 0.51–1.00) (Supplementary Table 1e). Based on our MA of seven case-control studies, [18 ,36 ,46 ,63 ,66 ,91 ,92] there was no significant association between place of dwelling in early life and risk of IBD (pOR 1.03; 95% CI, 0.81–1.31; I2 52.76%), CD (pOR 1.15; 95% CI, 0.67–1.98; I2 68.81%), or UC (pOR 1.05; 95% CI, 0.84–1.32; I2 38.33%) (Table 1, Supplementary Figures 18–20). There was significant heterogeneity for the outcomes of IBD overall and CD (p = 0.04), but not for UC (p = 0.18).
3.3.9. Other hygiene-related and social factors
Studies on exposure to pets or farm animals early in life and risk of IBD offered mixed results (Supplementary Table 1e) [13 ,15 ,18 ,65 ,66 ,72 ,87 ,91 ,92]. Unpasteurized milk consumption was generally associated with an increased risk of IBD, particularly CD [66]. Regarding primary drinking water source and risk of IBD, studies were heterogeneous with respect to exposure assessment and definition of the reference group. A case-control study from South Africa reported that piped/bottled water consumption as the primary drinking water source between ages 0–5 years was associated with higher odds of CD compared to the reference group of outside tap and well/river/dam water consumption (OR 2.1; 95% CI 1.2–4.0)[66]. They also reported that neither helminth infection, nor treatment for helminth infection, in the age group 0–5 years were associated with risk of subsequent CD [66]. We found no study that reported on early life Helicobacter pylori infection and subsequent IBD risk.
Twelve studies examined household number and birth order (Supplementary Table 1e) [19 ,29 ,39 ,42 ,65 ,66 ,69 ,72 ,86 ,88 ,90 ,91]. Living in larger families seems to be protective against IBD[72 ,86 ,88]. In one case-control study, a higher number of household residents during early life was protective against CD (OR 0.90; 95% CI 0.83–0.97), but not UC[72]. Higher birth order, a proxy for family size, was associated with lower odds of IBD (OR 0.68; 95% CI 0.51–0.91) [88]. However, in a large cohort study, birth order was associated with subsequent IBD diagnosis (birth order of ≥5 vs 1, OR 2.35; 95% CI 1.47–3.77), after adjusting for family size[86]. In the same study, larger families were protective against IBD (1 sibling vs ≥5, OR 2.63; 95% CI 1.49–4.62)[86]. Only in one small study, family size was reported to have no association with IBD[29].
Twenty studies reported on socioeconomic factors such as socioeconomic status and parental education (Supplementary Table 1f) [15 ,[19], [20], [21] ,23 ,36 ,39 ,45 ,49 ,59 ,63 ,65 ,66 ,69 ,[88], [89], [90], [91], [92] ,96]. Significant heterogeneity in exposures precluded MA. In two large studies, highest socioeconomic quintile, compared to lower, was associated with higher odds of IBD[19 ,36], although this finding was not confirmed in other reports [63 ,91]. Additional studies found no associations between socioeconomic or household factors and IBD [15 ,39 ,65]. One study reported an inverse association between low vs high socioeconomic status at birth and UC diagnosis, as well as UC phenotype (extensive UC: OR 0.27; 95% CI 0.09–0.75; non-progressive: UC OR 6.13; 95% CI 1.08–34.53) [69]. One study analyzed parental education level for offspring with and without IBD, reporting that parents of individuals with IBD had lower education level compared to controls (p<0.001) [96]. In contrast, a case-control study reported that higher parental occupation (higher skill level) was associated with CD (OR 1.83; 95% CI 1.14–2.95) and UC (OR 2.04; 95% CI 1.31–3.17) in the offspring.49Other data on the impact of parental education were conflicting and inconclusive [20 ,21 ,23 ,45 ,59 ,65 ,90]. One study found that unmarried mothers had a higher risk of having children with CD (aHR 1.73; 95% CI 1.10–2.71) [19].
3.3.10. Immigration
Seven studies reported on IBD estimates among first-generation immigrants who immigrated to the host country at age ≤5 years, or among second-generation immigrants, who were born in the host country, in order to capture the impact of early life exposures (Supplementary Table 1 g) [18 ,72 ,[97], [98], [99], [100], [101]]. In these studies, host countries included countries in Europe and North America, where IBD prevalence is high [102]. IBD incidence in second-generation immigrants was either comparable or higher than that in host population for most immigrants, depending on the country of birth [18 ,[97], [98], [99], [100]]. Agrawal et al. [97] performed a subgroup analysis by age at immigration to Denmark and did not find an association of age at immigration with IBD risk, including among those who were born in a different country and had immigrated at age ≤5 years.
3.3.11. Antibiotics and other medications
In a population-based cohort, Kronman et al. [103] reported that exposure to antibiotics throughout childhood was associated with developing IBD (<1 year of age: aHR 5.51; 95% CI 1.66–18.28 and <5 year of age 2.62; 95% CI 1.61–4.25) (Supplementary Table 1 h) [103]. Receiving a greater number of antibiotics prescriptions was associated with higher IBD risk (>2 antibiotic courses: aHR 4.77; 95% CI 2.13–10.68 versus 1–2 antibiotic courses: aHR 3.33; 95% CI 1.69–6.5) [103]. Lack of raw data precluded inclusion of this study in the MA. Based on three studies eligible for MA, [21 ,31 ,104] there was a non-significant trend for the association between antibiotic exposure during infancy and IBD (pOR 1.68; 95% CI 0.97–2.94; I2 55.38%), CD (pOR 1.10; 95% CI 0.51–2.77; I2 49.29%), or UC (pOR 0.93; 95% CI 0.46–1.85; I2 0) (Table 1, Fig. 3B, Supplementary Figures 22–23).
3.4. Offspring health and infections
3.4.1. Vaccination
Results on vaccination were mixed, but mostly null (Supplementary Table 1i). Twelve studies reporting on measles vaccination and the risk for IBD were eligible for MA [15 ,63 ,64 ,67 ,68 ,71 ,73 ,[105], [106], [107], [108], [109]]. No association with IBD (OR 1.08; 95% CI 0.91–1.28; I2 55.52%), CD (OR 1.06; 95% CI 0.82–1.37; I2 64.69%) or UC (OR 1.05; 95% CI 0.84–1.30; I2 28.20%) was observed (Table 1, Supplementary Figures 25–27). The impact of the Bacillus Calmette–Guérin (BCG) vaccine on the risk for IBD was studied in six studies[45 ,64 ,68 ,71 ,72 ,110]. On MA of three eligible studies, there was no association of BCG vaccine with IBD (OR 1.55; 95% CI 0.97–2.48; I2 37.58%) or with CD (OR 1.77; 95% CI 0.85–3.71; I2 53.17%) [64 ,68 ,110]. (Table 1, Supplementary Figures 29 and 30).
3.4.2. Appendectomy and tonsillectomy
No study reported these exposures definitively at age ≤5 years.
3.4.3. Infections
In our MA, otitis media between 0-5 years of age was associated with subsequent IBD diagnosis (OR 2.1195% CI 1.22–3.62; I2 36.9%) and CD diagnosis (OR 1.95 95% CI 1.20-3.17, I2 4.41%)(Fig. 4A and B) [111 ,112]. No studies examined upper respiratory tract or gastrointestinal infection specifically within the first 5 years of life and subsequent IBD risk.
Fig. 4.
Forest plot of the association between otitis media between 0 and 5 years of age and subsequent diagnosis of IBD (A) and CD (B)
Abbreviation: IBD - inflammatory bowel disease; CD – Crohn's disease.
3.4.4. Passive exposure to tobacco smoke
Three case-control studies that analyzed the association between passive smoking during childhood and CD, with discordant conclusions, were included in our MA [15 ,46 ,66]. We found no association between passive smoking during childhood and CD (OR 1.08; 95% CI 0.95–1.2; I2 0) (Table 1, Supplementary Figure 32).
4. Discussion
Herein we have conducted a qualitative, and when applicable, quantitative appraisal of the evidence, supported by a comprehensive literature search, on early life exposures and risk for future IBD. Overall, 34 factors were studied and classified into 9 broader categories. Based on our analysis, we found that foetal exposure to tobacco smoke and antibiotics, and early life diagnosis of otitis media, were risk factors for IBD. A non-significant trend was observed for antibiotic exposure during the first year of life in our MA and a population-based cohort showed a clear effect more prominent in early life, suggesting that indeed antibiotic exposure in early life may also be an important risk factor for subsequent risk of IBD [103]. Finally, breastfeeding, especially of longer duration, was protective against CD or UC development. Based on our systematic review and MA, no consistent evidence was found to link mode of delivery, maternal age, passive smoking, perinatal factors, vaccination, socioeconomic factors and most hygiene-related factors to subsequent risk of developing IBD, although the heterogeneity and quality of studies may have limited analysis (Fig. 5).
Fig. 5.
Summary of prenatal and postnatal exposures that may modulate IBD risk.
Abbreviation: IBD – inflammatory bowel disease.
Mounting epidemiological evidence and experimental data have shown early life events may modulate the risk for certain chronic diseases, including immune-mediated diseases, later in life [3,113]. In IBD, the increasing incidence of disease in young children (≤5 years), [114] combined with reports pointing to a potential role for early life events, further supports the need for interrogating this time period. Moreover, recent data showing that healthy babies at-risk for IBD development (based on maternal history of IBD) present different gut microbiome, [115] and elevated fecal calprotectin, [116] as compared to their counterparts, additionally suggests that this sensitive time period may act as an important window of susceptibility.
Early variations in the pattern of immune response can influence the likelihood and dynamics of inflammation later in life [117,118]. Maturation of the foetal immune system starts during the first trimester of pregnancy, and maternal exposures during this period can have long-lasting consequences on the immune status of their offspring and resultant susceptibility to diseases [119]. In the postnatal, and possibly in the prenatal period, gastrointestinal microbiota drives maturation of the immune system, [4] and therefore, exposures affecting microbiota assemblage and colonization during this sensitive time period bear the potential to lead to long-lasting effects. Although a wide umbrella review of MA on environmental risk factors for IBD has been recently published, [120] ours is the first to specifically focus on early life exposures and early life environmental factors modulating IBD risk. We chose the five-year cut-off as it coincides with early life microbiota development.
Based on our results, maternal smoking during pregnancy was associated with 49% higher odds of subsequent IBD diagnosis. Foetal tobacco smoke exposure has been linked to LBW, childhood obesity and behavioural disorders, potentially through nicotine effects in foetal immune homeostasis and hypoxia, placental vasoconstriction, epigenetic modification and infant´s microbiome [121], [122], [123]. DNA methylation changes, due to epigenetic modification, are implicated in IBD [124]. While smoking is a well-established risk factor for CD, [120] our results, even if significant, were associated with considerable heterogeneity. No association was found for passive exposure to smoking; however, studies evaluating passive smoking during childhood (≤5 years of life) were sparse and of low quality, suggesting that further studies are needed to confirm our results.
Many studies have now shown that prenatal and perinatal factors may impact microbiome composition, with inconsistent epidemiological associations with chronic disease risk [125]. It is believed that a lack of exposure to "immune-tolerizing" microbial products and toll-like receptor-2 (TLR-2) tolerizing bacterial products, may result in poorly regulated immune systems and increased immune-mediated diseases [126]. Mode of delivery, antibiotic exposure, and feeding behavior are amongst the most important factors shaping early life microbiome colonization[127]. In a Danish population-based study, a modest increase in IBD risk was observed in offspring delivered by C-section[128]. However in a MA, C-section was not associated with a higher risk for IBD, UC or CD [8]. While C-section leads to different microbiota community structure and function, studies have shown that these differences disappear by 6 weeks of life [129]. Therefore, C-section may be an insufficient perinatal factor to increase the risk of IBD[130]. Similarly, other important perinatal factors such as prematurity, LBW or birth order did not consistently affect IBD risk in our analysis.
We found that prenatal exposure to antibiotics was associated with an increased risk for IBD development. Antibiotic exposure could influence microbial colonization in utero, [131] the offspring of antibiotic-exposed mothers has been shown to display reduced abundance of Bifidobacterium and Lactobacillus, [132] and positive associations have been reported with asthma/wheezing and eczema/atopic dermatitis, [133] pointing to biological plausibility in our findings. Our analysis for postnatal antibiotic exposure showed a trend toward increased risk of IBD. Taken together with population-based data pointing to a 5-fold increase in risk of IBD following exposure to antibiotics in the first year of life [103], we can conclude that there may be a link between antibiotic exposure in early childhood and risk for IBD. Further studies are needed to confirm this association. We may have been limited in our MA by the number of studies included and the inability to study dose-estimates associations. It is also plausible that frequent use of broad-spectrum antibiotics over a longer period of time, not only in early life, may be linked to IBD onset. A positive association with IBD risk was seen for early life and infections, specifically otitis media, a common childhood infection. Examining only two high-quality studies of otitis media, [111 ,112] an early life diagnosis was associated with the subsequent onset of IBD, and more specifically CD. In a Swedish cohort study, prior gastrointestinal infection and antibiotic therapy were associated with higher IBD risk than infection alone [134]. Long-term prospective and translational studies are needed to investigate how infections and antibiotics may permanently alter the gut microbiome, contributing to the pathogenesis of IBD, and mitigation strategies to reduce this risk.
A protective effect from breastfeeding has been observed for CD and UC development based on a prior MA, with greater protective effect in Asian cohorts and with breastfeeding for longer than 12 months, although with high heterogeneity across studies [9]. This heterogeneity might be explained by differences in definition (exclusive or nonexclusive) and duration of breastfeeding. Human breast milk is enriched with bioactive substances and immune cells which play crucial roles not only in providing passive immunity, but also in shaping neonatal immune system [135]. Furthermore, human milk oligosaccharides act as prebiotics, promoting healthy gut colonization [136]. Even based on low-quality evidence, taken into account all the putative benefits, [137] strategies directed at fostering breastfeeding could potentially contribute to primary prevention of IBD.
While some hygiene-related factors, such as having pets, living near farms, larger households, have been associated with reduced risk of IBD [120], our analysis restricted to the first 5 years of age offered mixed results, and the heterogeneity of results, mixed populations and reference population, precluded formal MA for most exposures. Based on our qualitative review, larger households were protective for IBD development, but quality of studies warrants caution in interpretation. In a recent population-based study by Benchimol et al., [85] rurality in the first 1–5 years of life was associated with lower risk of IBD (IRR range 0.75–0.78 depending on duration of rural residence). However, in our MA of case-control studies, no significant association between place of dwelling in early life and risk of IBD was found. In the single study looking into immigration and restricting to the age cut-off of ≤5 years of age in first-generation immigrants no increased risk was observed for IBD [97].
Our study represents the largest, most comprehensive and systematic investigation of associations between a broad spectrum of environmental exposures during early life and future risk of IBD. We used systematic methods, including registration of our protocol, systematic literature search in three different databases, and used using robust criteria for study selection, data extraction, and study quality assessment, conducting a large number of MA for exposures where data could be pooled. Acknowledging the increasing data on the early life dysbiosis and risk of immune-mediated diseases, we believe our study adds to the literature by focusing on a restricted time period of life when microbiome and immune system may be more sensitive to environmental incursions. However, our study is limited by the quality of the evidence that was analyzed. For most of the exposures, heterogeneity among studies, differences in adjusted variables, unmeasured confounding, variable follow-up duration and age at IBD diagnosis, and different comparison populations, limited the quality of the evidence and precludes strong conclusions. Nevertheless, for those exposures that were found to be protective or lead to higher risk of IBD, biological plausibility exists, making the association more credible, and prioritizing them for further investigations. We did not find publication bias on quantitative analyses. Importantly, based on our review, we cannot discount the possibility that there were unobserved events that could be important such as parity, antibiotic exposure during breastfeeding, and childcare attendance, that were not studied and that could theoretically modulate risk. For example, pre- and postnatal heavy metal exposures have been detected in deciduous teeth of individuals who developed IBD later in life [138]. Importantly, it is conceivable that some exposures may act synergistically or cumulatively, and be more deleterious or beneficial during restricted time periods, which could not be captured in our assessment of the literature.
Despite these limitations, we report that early childhood is an important susceptibility period for IBD risk modulation. Promoting breastfeeding, smoking cessation during pregnancy, and avoiding unnecessary courses of antibiotics during pregnancy and early life, could promote health, prevent microbiota deviation, and serve as primary prevention strategies. The ongoing MELODY (Modulating Early Life Microbiome through Dietary Intervention in Pregnancy) trial, aimed at modifying the maternal microbiome in pregnant women with CD, will determine the effectiveness of dietary intervention in balancing the microbiome during early life in the offspring, thereby promoting the priming of a healthy immune system [139]. Follow-up studies of birth cohorts like the MECONIUM (Exploring MEChanisms Of disease traNsmission In Utero through the Microbiome) study, [115] and new prospective cohorts, where sources of heterogeneity and confounding factors could be accounted for, should be developed.
Funding
MA receives research support from the Dickler Family Fund, New York Community Trust and the Leona M. and Harry B. Helmsley Charitable Trust for SECURE-IBD database; JS receives research support from Fonds Wetenschappelijk Onderzoek and the Helmsley Charitable Trust Fund; CMH receives support from the Leona M. and Harry B. Helmsley Charitable Trust for the MELODY trial; JEA receives research support from the Crohn's and Colitis Foundation, the Judith & Stewart Colton Center for Autoimmunity, Lyanne and Michael Saperstein, and the NIH NIDDK Diseases K23DK124570; SCS has received the 2019 American Gastroenterological Association Research Scholar Award, and Veterans Affairs Career Development Award ICX002027A. TL receives grant support from Digest Science Foundation (Lille, France); IP receives support from the Leona M. and Harry B. Helmsley Charitable Trust for the MELODY trial; NN holds a McMaster University Department of Medicine Internal Career Award.
Author contributions
MA: study concept and design, curation and analysis of data; interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content; JS: study concept and design, curation of data, interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content; CFG: curation and analysis of data; interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content; CMH: curation of data; interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content; JA: curation of data; interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content; SCS: curation of data; interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content; CS: curation of data; drafting of manuscript, critical revision of the manuscript for important intellectual content; TL: curation of data; FRM: curation of data, critical revision of the manuscript for important intellectual content; IP: critical revision of the manuscript for important intellectual content; JFC: study concept and design, critical revision of the manuscript for important intellectual content; NN: analysis of data; interpretation of data, critical revision of the manuscript for important intellectual content; JT: study concept and design, curation and analysis of data; interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material
Declaration of Competing Interest
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated. JS reports personal fees from Takeda, Abbvie, and Janssen, and grants from Helmsley Charity Trust Fund and Fonds voor Wetenschappelijk Onderzoek – Vlaanderen, outside the submitted work. TL reports grants from DigestScience foundation and received travel accommodation from Adacyte Therapeutics. JFC reports grants and consultancy and lectures from Abbvie; consultancy and lectures from Ferring Pharmaceutical; consultancy from Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Eli Lilly, Enterome, Geneva, and Genentech; grants and consultancy from Janssen Pharmaceuticals; consultancy from Landos, LimmaTech Biologics AG, Ipsen, Imedex, Merck, Novartis, O Mass, Ostuka, and Pfizer; consultancy and lectures from Shire; grants, consultancy and lectures from Takeda; consultancy from Tigenix; and is a stock holder with Intestinal Biotech Development and Genfit, outside the submitted work. NN has received honoraria from Janssen, Abbvie, Takeda, Pfizer, Merck, and Ferring for advisory boards. JT has received research grants from Janssen and Abbvie, speaker fees from Janssen, and Advisory Board fees from Janssen, Pfizer, Arena Pharmaceuticals, Pfizer, Gilead and Galapagos. MA reports grants from Dickler Family Fund, New York Community Trust, grants from Leona M. and Harry B. Helmsley Charitable Trust for SECURE-IBD database, outside the submitted work. All other authors have nothing to disclose.
Acknowledgment
We thank Jill Gregory, Certified Medical Illustrator, Icahn School of Medicine at Mount Sinai, for assistance with the illustration.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100884.
Appendix. Supplementary materials
References
- 1.Barker D.J. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [published Online First: 2007/04/21] [DOI] [PubMed] [Google Scholar]
- 2.Marques A.H., O'Connor T.G., Roth C. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci. 2013;7:120. doi: 10.3389/fnins.2013.00120. [published Online First: 2013/08/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluckman P.D., Hanson M.A., Cooper C. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [published Online First: 2008/07/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez de Aguero M., Ganal-Vonarburg S.C., Fuhrer T. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [published Online First: 2016/03/19] [DOI] [PubMed] [Google Scholar]
- 5.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. w64. [published Online First: 2009/07/23] [DOI] [PubMed] [Google Scholar]
- 6.Observational studies search filter Scottish intercollegiate guidelines network (SIGN) [Available from: https://www.sign.ac.uk/assets/search-filters-observational-studies.docx accessed January 8, 2021.
- 7.GA Wells B.S., D. O'Connell, J. Peterson, V. Welch, M. Losos, P. Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp accessed October 2018.
- 8.Frias Gomes C., Narula N., Morão B. Mode of delivery does not affect the risk of inflammatory bowel disease. Dig Dis Sci. 2020 doi: 10.1007/s10620-020-06204-7. [published Online First: 2020/03/23] [DOI] [PubMed] [Google Scholar]
- 9.Xu L., Lochhead P., Ko Y. Systematic review with meta-analysis: breastfeeding and the risk of Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;46(9):780–789. doi: 10.1111/apt.14291. doi: 10.1111/apt.14291 [published Online First: 2017/09/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.G S., W T., W A. Maternal smoking during pregnancy and longterm gastrointestinal morbidity in the offspring: results from a single-center population-based cohort study. Am J Obstet Gynecol. 2018;218:S439–S440. (1 Supplement 1) [Google Scholar]
- 11.B T.M., K O.-.P., K R. Smoking and use of antibiotics during pregnancy are risk factors for inflammatory bowel disease. United Eur Gastroenterol J. 2014;2 (1 SUPPL. 1):A520. [Google Scholar]
- 12.Russell R.K., Farhadi R., Wilson M. Perinatal passive smoke exposure may be more important than childhood exposure in the risk of developing childhood IBD. Gut. 2005;54(10):1500–1501. author reply 01. [published Online First: 2005/09/16] [PMC free article] [PubMed] [Google Scholar]
- 13.Radon K., Windstetter D., Poluda A.L. Contact with farm animals in early life and juvenile inflammatory bowel disease: a case-control study. Pediatrics. 2007;120(2):354–361. doi: 10.1542/peds.2006-3624. [published Online First: 2007/08/03] [DOI] [PubMed] [Google Scholar]
- 14.Mahid S.S., Minor K.S., Stromberg A.J. Active and passive smoking in childhood is related to the development of inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(4):431–438. doi: 10.1002/ibd.20070. [DOI] [PubMed] [Google Scholar]
- 15.Han D.Y., Fraser A.G., Dryland P. Environmental factors in the development of chronic inflammation: a case-control study on risk factors for Crohn's disease within New Zealand. Mutat Res. 2010;690(1–2):116–122. doi: 10.1016/j.mrfmmm.2009.09.002. [published Online First: 2009/09/12] [DOI] [PubMed] [Google Scholar]
- 16.Gruber M., Marshall J.R., Zielezny M. A case-control study to examine the influence of maternal perinatal behaviors on the incidence of Crohn's disease. Gastroenterol Nurs. 1996;19(2):53–59. doi: 10.1097/00001610-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Y I., ON E., S R. Aetiological risk factors for developing paediatric inflammatory bowel disease in a prospective cohort. J Crohn's Colitis. 2016;10(Supplement 1):S448. [Google Scholar]
- 18.van der Sloot K.W.J., Weersma R.K., Alizadeh B.Z. Identification of environmental risk factors associated with the development of Inflammatory Bowel Disease. J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa114. [published Online First: 2020/06/24] [DOI] [PubMed] [Google Scholar]
- 19.Ponsonby A.L., Catto-Smith A.G., Pezic A. Association between early-life factors and risk of child-onset Crohn's disease among Victorian children born 1983-1998: a birth cohort study. Inflamm Bowel Dis. 2009;15(6):858–866. doi: 10.1002/ibd.20842. [DOI] [PubMed] [Google Scholar]
- 20.Aspberg S., Dahlquist G., Kahan T. Fetal and perinatal risk factors for inflammatory bowel disease. Acta Paediatr. 2006;95(8):1001–1004. doi: 10.1080/08035250600573151. [DOI] [PubMed] [Google Scholar]
- 21.Canova C., Ludvigsson J.F., Di Domenicantonio R. Perinatal and antibiotic exposures and the risk of developing childhood-onset inflammatory bowel disease: a nested case-control study based on a population-based birth cohort. Int J Environ Res Public Health. 2020;17(7) doi: 10.3390/ijerph17072409. [published Online First: 2020/04/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorsen S.U., Jakobsen C., Cohen A. Perinatal vitamin D levels are not associated with later risk of developing pediatric-onset inflammatory bowel disease: a Danish case-cohort study. Scand J Gastroenterol. 2016;51(8):927–933. doi: 10.3109/00365521.2016.1144218. [published Online First: 2016/02/14] [DOI] [PubMed] [Google Scholar]
- 23.Strisciuglio C., Giugliano F., Martinelli M. Impact of environmental and familial factors in a cohort of pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;64(4):569–574. doi: 10.1097/MPG.0000000000001297. [DOI] [PubMed] [Google Scholar]
- 24.Sonntag B., Stolze B., Heinecke A. Preterm birth but not mode of delivery is associated with an increased risk of developing inflammatory bowel disease later in life. Inflamm Bowel Dis. 2007;13(11):1385–1390. doi: 10.1002/ibd.20206. [DOI] [PubMed] [Google Scholar]
- 25.Hutfless S., Li D.K., Heyman M.B. Prenatal and perinatal characteristics associated with pediatric-onset inflammatory bowel disease. Dig Dis Sci. 2012;57(8):2149–2156. doi: 10.1007/s10620-012-2128-1. [published Online First: 2012/03/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Y I., W T., S E. The association between maternal GroupB Streptococcus colonization and offspring Gastro-Intestinal morbidity. Am J Obstet Gynecol. 2018;218:S506–S507. (1 Supplement 1) [Google Scholar]
- 27.Bernstein C.N., Burchill C., Targownik L.E. Maternal infections that would warrant antibiotic use antepartum or peripartum are not a risk factor for the development of IBD: a population-based analysis. Inflamm Bowel Dis. 2017;23(4):635–640. doi: 10.1097/MIB.0000000000001042. [DOI] [PubMed] [Google Scholar]
- 28.Malmborg P., Bahmanyar S., Grahnquist L. Cesarean section and the risk of pediatric Crohn's disease. Inflamm Bowel Dis. 2012;18(4):703–708. doi: 10.1002/ibd.21741. [published Online First: 2011/04/27] [DOI] [PubMed] [Google Scholar]
- 29.Thompson N.P., Montgomery S.M., Wadsworth M.E. Early determinants of inflammatory bowel disease: use of two national longitudinal birth cohorts. Eur J Gastroenterol Hepatol. 2000;12(1):25–30. doi: 10.1097/00042737-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 30.Ekbom A., Adami H.O., Helmick C.G. Perinatal risk factors for inflammatory bowel disease: a case-control study. Am J Epidemiol. 1990;132(6):1111–1119. doi: 10.1093/oxfordjournals.aje.a115754. [DOI] [PubMed] [Google Scholar]
- 31.Ortqvist A.K., Lundholm C., Halfvarson J. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: a population-based study. Gut. 2019;68(2):218–225. doi: 10.1136/gutjnl-2017-314352. [DOI] [PubMed] [Google Scholar]
- 32.Elten M., Benchimol E.I., Fell D.B. Ambient air pollution and the risk of pediatric-onset inflammatory bowel disease: a population-based cohort study. Environ Int. 2020;138 doi: 10.1016/j.envint.2020.105676. [published Online First: 2020/03/29] [DOI] [PubMed] [Google Scholar]
- 33.Ananthakrishnan A.N., Sauk J., Nguyen D.D. Mo1754 early life environment interacts with genetic risk in inflammatory bowel diseases. Gastroenterology. 2015;148(4):S703–SS03. [Google Scholar]
- 34.Bager P., Simonsen J., Nielsen N.M. Cesarean section and offspring's risk of inflammatory bowel disease: a national cohort study. Inflamm Bowel Dis. 2012;18(5):857–862. doi: 10.1002/ibd.21805. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein C.N., Banerjee A., Targownik L.E. Cesarean section delivery is not a risk factor for development of inflammatory bowel disease: a population-based analysis. Clin Gastroenterol Hepatol. 2016;14(1):50–57. doi: 10.1016/j.cgh.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein C.N., Burchill C., Targownik L.E. Events within the first year of life, but not the neonatal period, affect risk for later development of inflammatory bowel diseases. Gastroenterology. 2019;156(8):2190–2197. doi: 10.1053/j.gastro.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black M., Bhattacharya S., Philip S., et al. Planned cesarean delivery at term and adverse outcomes in childhood health. 2019;314(21):2271–79. doi: 10.1001/jama.2015.16176 [DOI] [PMC free article] [PubMed]
- 38.Burnett D., Brown M.M., Otley A. The association between caesarean section and inflammatory bowel disease in childhood and young adulthood: findings from 2 retrospective cohort studies. JPGN. 2020;71(3):84–89. doi: 10.1097/MPG.0000000000002773. [DOI] [PubMed] [Google Scholar]
- 39.Doyle J.B., Hillemand C., Patterson C., et al. Tu:1808 Early life events and risk of inflammatory bowel disease and population-based study from a region of northern Ireland. 2017;152(5):S975–S75. doi: 10.1016/S0016-5085(17)33305-X
- 40.Levy L.C., Ramakrishna B.S., Peravali V. Tu1288 different environmental influences are associated with the development of IBD in India compared to the US: a case controlled study. AGA Abstracts. 2012;142(5):S793–S794. [Google Scholar]
- 41.Velosa M., Yerushalmi B., Asayag N. P799 Perinatal factors and development of IBD: a national case–control study with nearly 50 years of follow-up: report from the epiIIRN database. J Crohn's Colitis. 2019;13:S521–S522. [Google Scholar]
- 42.L A., F T., E R. Early life exposures and potential subsequent risk of Crohn's disease: a Danish national birth cohort. Gastroenterology. 2014;146:S212–S213. (5 SUPPL. 1) [Google Scholar]
- 43.Steiner N., Wainstock T., Sheiner E. Small for gestational age as an independent risk factor for long-term pediatric gastrointestinal morbidity of the offspring*. J Maternal-Fetal Neonatal Med. 2019;32(9):1407–1411. doi: 10.1080/14767058.2017.1406473. [DOI] [PubMed] [Google Scholar]
- 44.Khalili H., Ananthakrishnan A.N., Higuchi L.M. Early life factors and risk of inflammatory bowel disease in adulthood. Inflamm Bowel Dis. 2013;19(3):542–547. doi: 10.1097/MIB.0b013e31828132f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilat T., Hacohen D., Lilos P. Childhood factors in ulcerative colitis and Crohn's disease. An international cooperative study. Scand J Gastroenterol. 1987;22(8):1009–1024. doi: 10.3109/00365528708991950. [DOI] [PubMed] [Google Scholar]
- 46.Thompson N.P., Pounder R.E., Wakefield A.J. Perinatal and childhood risk factors for inflammatory bowel disease: a case-control study. Eur J Gastroenterol Hepatol. 1995;7(5):385–390. [PubMed] [Google Scholar]
- 47.Konijeti G., Khalili H., Ananthakrishnan A. Does parental age influence the risk of inflammatory bowel disease in off spring? Am J Gastroenterol. 2013;108(SUPPL. 1):S547. doi: 10.1038/ajg.2013.269. [DOI] [Google Scholar]
- 48.Shaw S.Y., Nugent Z., Targownik L.E. Association between spring season of birth and Crohn's disease. Clin Gastroenterol Hepatol. 2014;12(2):277–282. doi: 10.1016/j.cgh.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 49.López-Serrano P., Pérez-Calle J.L., Pérez-Fernández M.T. Environmental risk factors in inflammatory bowel diseases. Investigating the hygiene hypothesis: a Spanish case-control study. Scand J Gastroenterol. 2010;45(12):1464–1471. doi: 10.3109/00365521.2010.510575. [published Online First: 2010/08/12] [DOI] [PubMed] [Google Scholar]
- 50.Haslam N., Mayberry J.F., Hawthorne A.B. Measles, month of birth, and Crohn's disease. Gut. 2000;47(6):801–803. doi: 10.1136/gut.47.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Disanto G., Chaplin G., Morahan J.M. Month of birth, vitamin D and risk of immune-mediated disease: a case control study. BMC Med. 2012;10 doi: 10.1186/1741-7015-10-69. ((Ramagopalan) London School of Hygiene and Tropical Medicine, London, WC1E 7HT, United Kingdom):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ekbom A., Zack M., Adami H.O. Is there clustering of inflammatory bowel disease at birth? Am J Epidemiol. 1991;134(8):876–886. doi: 10.1093/oxfordjournals.aje.a116162. [DOI] [PubMed] [Google Scholar]
- 53.Van Ranst M., Joossens M., Joossens S. Crohn's disease and month of birth. Inflamm Bowel Dis. 2005;11(6):597–599. doi: 10.1097/01.MIB.0000163697.34592.d4. [DOI] [PubMed] [Google Scholar]
- 54.Chowers Y., Odes S., Bujanover Y. The month of birth is linked to the risk of Crohn's disease in the Israeli population. Am J Gastroenterol. 2004;99(10):1974–1976. doi: 10.1111/j.1572-0241.2004.40058.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee J., Kim J.H., Chung M.K. Effects of birth months on rheumatic diseases in South Korea: a nationwide case-control study. Clin Exp Rheumatol. 2020;38(3):411–419. [PubMed] [Google Scholar]
- 56.Card T.R., Sawczenko A., Sandhu B.K. No seasonality in month of birth of inflammatory bowel disease cases: a prospective population based study of British under 20 year olds. Gut. 2002;51(6):814–815. doi: 10.1136/gut.51.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonnenberg A. Date of birth in the occurrence of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(2):206–211. doi: 10.1002/ibd.20730. [DOI] [PubMed] [Google Scholar]
- 58.Khalili H., Huang E.S., Ananthakrishnan A.N. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61(12):1686–1692. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes E.A., Ponsonby A.L., Pezic A. Higher sun exposure is associated with lower risk of pediatric inflammatory bowel disease: a matched case-control study. J Pediatr Gastroenterol Nutr. 2019;69(2):182–188. doi: 10.1097/MPG.0000000000002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutfless S., Li D.K., Heyman M.B. Prenatal and perinatal characteristics associated with pediatric-onset inflammatory bowel disease. Dig. Dis. Sci. 2012;57(8):2149–2156. doi: 10.1007/s10620-012-2128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corrao G., Tragnone A., Caprilli R. Risk of inflammatory bowel disease attributable to smoking, oral contraception and breastfeeding in Italy: a nationwide case-control study. Int J Epidemiol. 1998;27(3):397–404. doi: 10.1093/ije/27.3.397. [DOI] [PubMed] [Google Scholar]
- 62.Burnett D., Kuhle S., Brown M. The association of caesarean section and breast feeding with pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;65:S40. doi: 10.1097/MPG.0000000000001805. (Supplement 2) [DOI] [PubMed] [Google Scholar]
- 63.Gearry R.B., Richardson A.K., Frampton C.M. Population-based cases control study of inflammatory bowel disease risk factors. J Gastroenterol Hepatol. 2010;25(2):325–333. doi: 10.1111/j.1440-1746.2009.06140.x. [published Online First: 2010/01/14] [DOI] [PubMed] [Google Scholar]
- 64.Baron S., Turck D., Leplat C. Environmental risk factors in paediatric inflammatory bowel diseases: a population based case control study. GutGut. 2005;54(3):357–363. doi: 10.1136/gut.2004.054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amre D.K., Lambrette P., Law L. Investigating the hygiene hypothesis as a risk factor in pediatric onset Crohn's disease: a case-control study. Am J Gastroenterol. 2006;101(5):1005–1011. doi: 10.1111/j.1572-0241.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 66.Basson A., Swart R., Jordaan E. The association between childhood environmental exposures and the subsequent development of Crohn's disease in the Western Cape, South Africa. PLoS ONE. 2014;9(12) doi: 10.1371/journal.pone.0115492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansen T.S., Jess T., Vind I. Environmental factors in inflammatory bowel disease: a case-control study based on a Danish inception cohort. J Crohns Colitis. 2011;5(6):577–584. doi: 10.1016/j.crohns.2011.05.010. [published Online First: 2011/06/28] [DOI] [PubMed] [Google Scholar]
- 68.Ng S.C., Tang W., Leong R.W. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut. 2015;64(7):1063–1071. doi: 10.1136/gutjnl-2014-307410. [published Online First: 2014/09/12] [DOI] [PubMed] [Google Scholar]
- 69.Samuelsson S.M., Ekbom A., Zack M. Risk factors for extensive ulcerative colitis and ulcerative proctitis: a population based case-control study. Gut. 1991;32(12):1526–1530. doi: 10.1136/gut.32.12.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.VL J., S H., F R. Epidemiological risk factors for childhood onset inflammatory bowel disease in Scotland: a case-control study. Gastroenterology. 2009;136 (5 SUPPL. 1):A358. [Google Scholar]
- 71.Niewiadomski O., Studd C., Wilson J. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern Med J. 2016;46(6):669–676. doi: 10.1111/imj.13094. [DOI] [PubMed] [Google Scholar]
- 72.Bernstein C.N., Rawsthorne P., Cheang M. A population-based case control study of potential risk factors for IBD. Am J Gastroenterol. 2006;101(5):993–1002. doi: 10.1111/j.1572-0241.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 73.Vahedi H., Chaharmahali M., Momtahen S. A case-control study on the risk factors of IBD in 258 Iranian patients. Govaresh. 2011;16(1):61–67. [Google Scholar]
- 74.Bergstrand O., Hellers G. Breast-feeding during infancy in patients who later develop Crohn's disease. Scand J Gastroenterol. 1983;18(7):903–906. doi: 10.3109/00365528309182113. [DOI] [PubMed] [Google Scholar]
- 75.Hlavaty T., Toth J., Koller T. Smoking, breastfeeding, physical inactivity, contact with animals, and size of the family influence the risk of inflammatory bowel disease: a Slovak case-control study. United Eur Gastroenterol J. 2013;1(2):109–119. doi: 10.1177/2050640613478011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jakobsen C., Paerregaard A., Munkholm P. Environmental factors and risk of developing paediatric inflammatory bowel disease - A population based study 2007-2009. J Crohn's Colitis. 2013;7(1):79–88. doi: 10.1016/j.crohns.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 77.Jiang L., Xia B., Li J. Risk factors for ulcerative colitis in a Chinese population: an age-matched and sex-matched case-control study. J Clin Gastroenterol. 2007;41(3):280–284. doi: 10.1097/01.mcg.0000225644.75651.f1. [DOI] [PubMed] [Google Scholar]
- 78.Klein I., Reif S., Farbstein H. Preillness non dietary factors and habits in inflammatory bowel disease. Ital J Gastroenterol Hepatol. 1998;30(3):247–251. [PubMed] [Google Scholar]
- 79.Decker E., Engelmann G., Findeisen A. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125(6):e1433–e1e40. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 80.Persson P.G., Leijonmarck C.E., Bernell O. Risk indicators for inflammatory bowel disease. Int J Epidemiol. 1993;22(2):268–272. doi: 10.1093/ije/22.2.268. [DOI] [PubMed] [Google Scholar]
- 81.Russel M.G., Engels L.G., Muris J.W. Modern life' in the epidemiology of inflammatory bowel disease: a case-control study with special emphasis on nutritional factors. Eur J Gastroenterol Hepatol. 1998;10(3):243–249. doi: 10.1097/00042737-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 82.Salgado V.C.L., Luiz R.R., Boechat N. Crohn's disease environmental factors in the developing world: a case-control study in a statewide catchment area in Brazil. World J Gastroenterol. 2017;23(30):5549–5556. doi: 10.3748/wjg.v23.i30.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanagapalli S., Ko Y., Gopikrishna S. Associations between oral contraceptive pill use and inflammatory bowel disease in an Australian cohort. J Gastroenterol Hepatol (Aust) 2015;30(SUPPL. 3):137. doi: 10.1111/jgh.13094. [DOI] [Google Scholar]
- 84.Urashima H., Ohmori I., Shiraki K. Epidemiological survey on chronic inflammatory bowel disease developed during childhood in Japan, and a case-control study on nutrition during infancy. Yonago Acta Med. 1999;42(1):95–102. [Google Scholar]
- 85.Benchimol E.I., Kaplan G.G., Otley A.R. Rural and urban residence during early life is associated with risk of inflammatory bowel disease: a population-based inception and birth cohort study. Am J Gastroenterol. 2017;112(9):1412–1422. doi: 10.1038/ajg.2017.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klement E., Lysy J., Hoshen M. Childhood hygiene is associated with the risk for inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2008;103(7):1775–1782. doi: 10.1111/j.1572-0241.2008.01905.x. [published Online First: 2008/06/28] [DOI] [PubMed] [Google Scholar]
- 87.Timm S., Svanes C., Janson C. Place of upbringing in early childhood as related to inflammatory bowel diseases in adulthood: a population-based cohort study in Northern Europe. Eur J Epidemiol. 2014;29(6):429–437. doi: 10.1007/s10654-014-9922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hampe J., Heymann K., Krawczak M. Association of inflammatory bowel disease with indicators for childhood antigen and infection exposure. Int J Colorectal Dis. 2003;18(5):413–417. doi: 10.1007/s00384-003-0484-1. [published Online First: 2003/04/10] [DOI] [PubMed] [Google Scholar]
- 89.Ramakrishna B.S., Levy L.C., Peravali V. Hygiene factors in India and the US in early childhood influence the subsequent development of crohn's disease but not ulcerative colitis: a large case controlled study in two countries. Gastroenterology. 2012;142(5 SUPPL. 1):S789. [Google Scholar]
- 90.Boneberger A., Weiss E.H., Calvo M. Environmental factors in infancy and ulcerative colitis in the Central South of Chile: a case-control study. J Crohn's Colitis. 2011;5(5):392–396. doi: 10.1016/j.crohns.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Feeney M.A., Murphy F., Clegg A.J. A case-control study of childhood environmental risk factors for the development of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2002;14(5):529–534. doi: 10.1097/00042737-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Ko Y., Kariyawasam V., Karnib M. Inflammatory bowel disease environmental risk factors: a population-based case-control study of middle eastern migration to Australia. Clin Gastroenterol Hepatol. 2015;13(8):1453. doi: 10.1016/j.cgh.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 93.Malekzadeh F., Alberti C., Nouraei M. Crohn's disease and early exposure to domestic refrigeration. PLoS ONE. 2009;4(1):e4288. doi: 10.1371/journal.pone.0004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gent A.E., Hellier M.D., Grace R.H. Inflammatory bowel disease and domestic hygiene in infancy. Lancet. 1994;343(8900):766–767. doi: 10.1016/s0140-6736(94)91841-4. [DOI] [PubMed] [Google Scholar]
- 95.Sahu M.K., Srinivasan P., Subramanian V. Childhood hygiene and IBD: a case control study from India. Gastroenterology. 2010;138:S201. (5 SUPPL. 1) [Google Scholar]
- 96.Eslahpazir J., Kumar R., Lalavi A. Parental occupations and risk for Crohn's disease in children. Scand J Gastroenterol. 2017;52(10):1093–1098. doi: 10.1080/00365521.2017.1339826. [published Online First: 2017/06/16] [DOI] [PubMed] [Google Scholar]
- 97.Agrawal M., Corn G., Shrestha S. Inflammatory bowel diseases among first-generation and second-generation immigrants in Denmark: a population-based cohort study. Gut. 2020 doi: 10.1136/gutjnl-2020-321798. [published Online First: 2020/09/09] [DOI] [PubMed] [Google Scholar]
- 98.Benchimol E.I., Mack D.R., Guttmann A. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol. 2015;110(4):553–563. doi: 10.1038/ajg.2015.52. [DOI] [PubMed] [Google Scholar]
- 99.Montgomery S.M., Morris D.L., Pounder R.E. Asian ethnic origin and the risk of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11(5):543–546. doi: 10.1097/00042737-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 100.Li X., Sundquist J., Hemminki K. Risk of inflammatory bowel disease in first- and second-generation immigrants in Sweden: a nationwide follow-up study. Inflamm. Bowel Dis. 2011;17(8):1784–1791. doi: 10.1002/ibd.21535. [DOI] [PubMed] [Google Scholar]
- 101.Ghersin I., Khteeb N., Katz L.H. Trends in the epidemiology of inflammatory bowel disease among Jewish Israeli adolescents: a population-based study. Aliment Pharmacol Ther. 2019;49(5):556–563. doi: 10.1111/apt.15160. [DOI] [PubMed] [Google Scholar]
- 102.Ng S.C., Shi H.Y., Hamidi N. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [published Online First: 2017/10/21] [DOI] [PubMed] [Google Scholar]
- 103.Kronman M.P., Zaoutis T.E., Haynes K. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130(4):e794–e803. doi: 10.1542/peds.2011-3886. [published Online First: 2012/09/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shaw S.Y., Blanchard J.F., Bernstein C.N. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2687–2692. doi: 10.1038/ajg.2010.398. [DOI] [PubMed] [Google Scholar]
- 105.Feeney M., Ciegg A., Winwood P. A case-control study of measles vaccination and inflammatory bowel disease. The East Dorset Gastroenterology Group. Lancet. 1997;350(9080):764–766. doi: 10.1016/s0140-6736(97)03192-9. [DOI] [PubMed] [Google Scholar]
- 106.Shaw S.Y., Blanchard J.F., Bernstein C.N. Early childhood measles vaccinations are not associated with paediatric IBD: a population-based analysis. J Crohns Colitis. 2015;9(4):334–338. doi: 10.1093/ecco-jcc/jjv029. [published Online First: 2015/02/03] [DOI] [PubMed] [Google Scholar]
- 107.Vcev A., Pezerovic D., Jovanovic Z. A retrospective, case-control study on traditional environmental risk factors in inflammatory bowel disease in Vukovar-Srijem County, north-eastern Croatia, 2010. Wien Klin Wochenschr. 2015;127(9–10):345–354. doi: 10.1007/s00508-015-0741-7. [published Online First: 2015/03/28] [DOI] [PubMed] [Google Scholar]
- 108.Davis R.L., Kramarz P., Bohlke K. Measles-mumps-rubella and other measles-containing vaccines do not increase the risk for inflammatory bowel disease: a case-control study from the vaccine safety datalink project. Arch Pediatr Adolesc Med. 2001;155(3):354–359. doi: 10.1001/archpedi.155.3.354. [DOI] [PubMed] [Google Scholar]
- 109.Lavy A., Broide E., Reif S. Measles is more prevalent in Crohn's disease patients. A multicentre Israeli study. Dig Liver Dis. 2001;33(6):472–476. doi: 10.1016/s1590-8658(01)80024-4. [DOI] [PubMed] [Google Scholar]
- 110.Villumsen M., Jess T., Sørup S. Risk of inflammatory bowel disease following Bacille Calmette-Guérin and smallpox vaccination: a population-based Danish case-cohort study. Inflamm Bowel Dis. 2013;19(8):1717–1724. doi: 10.1097/MIB.0b013e318281f34e. [DOI] [PubMed] [Google Scholar]
- 111.Shaw S.Y., Blanchard J.F., Bernstein C.N. Association between early childhood otitis media and pediatric inflammatory bowel disease: an exploratory population-based analysis. J Pediatr. 2013;162(3):510–514. doi: 10.1016/j.jpeds.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 112.Hildebrand H., Malmborg P., Askling J. Early-life exposures associated with antibiotic use and risk of subsequent Crohn's disease. Scand. J. Gastroenterol. 2008;43(8):961–966. doi: 10.1080/00365520801971736. [DOI] [PubMed] [Google Scholar]
- 113.Sbihi H., Boutin R.C., Cutler C. Thinking bigger: how early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy. 2019;74(11):2103–2115. doi: 10.1111/all.13812. [published Online First: 2019/04/10] [DOI] [PubMed] [Google Scholar]
- 114.Benchimol E.I., Bernstein C.N., Bitton A. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. 2017;112(7):1120–1134. doi: 10.1038/ajg.2017.97. [published Online First: 2017/04/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Torres J., Hu J., Seki A. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut. 2020;69(1):42–51. doi: 10.1136/gutjnl-2018-317855. [published Online First: 2019/05/01] [DOI] [PubMed] [Google Scholar]
- 116.Kim E.S., Tarassishin L., Eisele C. Longitudinal changes in fecal calprotectin levels among pregnant women with and without inflammatory bowel disease and their babies. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.11.050. [DOI] [PubMed] [Google Scholar]
- 117.Gensollen T., Blumberg R.S. Correlation between early-life regulation of the immune system by microbiota and allergy development. J Allergy Clin Immunol. 2017;139(4):1084–1091. doi: 10.1016/j.jaci.2017.02.011. [published Online First: 2017/04/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schaub B., Prescott S.L. 1 - the maturation of immune function in pregnancy and early childhood. In: Wahn U, Sampson HA, editors. Allergy, immunity and tolerance in early childhood. Academic Press; Amsterdam: 2016. pp. 1–17. [Google Scholar]
- 119.Renz H., Holt P.G., Inouye M. An exposome perspective: early-life events and immune development in a changing world. J Allergy Clin Immunol. 2017;140(1):24–40. doi: 10.1016/j.jaci.2017.05.015. [published Online First: 2017/07/05] [DOI] [PubMed] [Google Scholar]
- 120.Piovani D., Danese S., Peyrin-Biroulet L. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. 2019;157(3) doi: 10.1053/j.gastro.2019.04.016. 647-59 e4[published Online First: 2019/04/25] [DOI] [PubMed] [Google Scholar]
- 121.McLean C., Jun S., Kozyrskyj A. Impact of maternal smoking on the infant gut microbiota and its association with child overweight: a scoping review. World J Pediatr. 2019;15(4):341–349. doi: 10.1007/s12519-019-00278-8. [published Online First: 2019/07/11] [DOI] [PubMed] [Google Scholar]
- 122.Obel C., Zhu J.L., Olsen J. The risk of attention deficit hyperactivity disorder in children exposed to maternal smoking during pregnancy - a re-examination using a sibling design. J Child Psychol Psychiatry. 2016;57(4):532–537. doi: 10.1111/jcpp.12478. [published Online First: 2015/10/30] [DOI] [PubMed] [Google Scholar]
- 123.Wiklund P., Karhunen V., Richmond R.C. DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clin Epigenetics. 2019;11(1):97. doi: 10.1186/s13148-019-0683-4. [published Online First: 2019/07/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ventham N.T., Kennedy N.A., Adams A.T. Integrative epigenome-wide analysis demonstrates that DNA methylation may mediate genetic risk in inflammatory bowel disease. Nat Commun. 2016;7:13507. doi: 10.1038/ncomms13507. [published Online First: 2016/11/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aagaard K.M. Mode of delivery and pondering potential sources of the neonatal microbiome. EBioMedicine. 2020;51 doi: 10.1016/j.ebiom.2019.11.015. [published Online First: 2020/01/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wasko N.J., Nichols F., Clark R.B. Multiple sclerosis, the microbiome, TLR2, and the hygiene hypothesis. Autoimmun Rev. 2020;19(1) doi: 10.1016/j.autrev.2019.102430. [published Online First: 2019/11/18] [DOI] [PubMed] [Google Scholar]
- 127.Tamburini S., Shen N., Wu H.C. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22(7):713–722. doi: 10.1038/nm.4142. [published Online First: 2016/07/09] [DOI] [PubMed] [Google Scholar]
- 128.Sevelsted A., Stokholm J., Bonnelykke K. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1):e92–e98. doi: 10.1542/peds.2014-0596. [published Online First: 2014/12/03] [DOI] [PubMed] [Google Scholar]
- 129.Chu D.M., Ma J., Prince A.L. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–326. doi: 10.1038/nm.4272. [published Online First: 2017/01/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frias Gomes C., Narula N., Morão B. Mode of delivery does not affect the risk of inflammatory bowel disease. Dig Dis Sci. 2020 doi: 10.1007/s10620-020-06204-7. [DOI] [PubMed] [Google Scholar]
- 131.Liu C.-.J., Liang X., Niu Z.-.Y. Is the delivery mode a critical factor for the microbial communities in the meconium? EBioMedicine. 2019;49:354–363. doi: 10.1016/j.ebiom.2019.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milliken S., Allen R.M., Lamont R.F. The role of antimicrobial treatment during pregnancy on the neonatal gut microbiome and the development of atopy, asthma, allergy and obesity in childhood. Expert Opin Drug Saf. 2019;18(3):173–185. doi: 10.1080/14740338.2019.1579795. [published Online First: 2019/02/12] [DOI] [PubMed] [Google Scholar]
- 133.Zhong Y., Zhang Y., Wang Y. Maternal antibiotic exposure during pregnancy and the risk of allergic diseases in childhood: a meta-analysis. Pediatr Allergy Immunol. 2020 doi: 10.1111/pai.13411. [published Online First: 2020/11/16] [DOI] [PubMed] [Google Scholar]
- 134.Axelrad J.E., Olén O., Askling J. Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case-control study. Clin Gastroenterol Hepatol. 2019;17(7):1311–1322.e7. doi: 10.1016/j.cgh.2018.09.034. [published Online First: 2018/11/06] [DOI] [PubMed] [Google Scholar]
- 135.Bardanzellu F., Peroni D.G., Fanos V. Human breast milk: bioactive components, from stem cells to health outcomes. Curr Nutr Rep. 2020;9(1):1–13. doi: 10.1007/s13668-020-00303-7. [published Online First: 2020/01/14] [DOI] [PubMed] [Google Scholar]
- 136.Walsh C., Lane J.A., van Sinderen D. Human milk oligosaccharides: shaping the infant gut microbiota and supporting health. J Funct Foods. 2020;72 doi: 10.1016/j.jff.2020.104074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Victora C.G., Bahl R., Barros A.J. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi: 10.1016/S0140-6736(15)01024-7. [published Online First: 2016/02/13] [DOI] [PubMed] [Google Scholar]
- 138.Nair N., Austin C., Curtin P. Association between early-life exposures and inflammatory bowel diseases, based on analyses of deciduous teeth. Gastroenterology. 2020;159(1):383–385. doi: 10.1053/j.gastro.2020.03.040. [published Online First: 2020/04/01] [DOI] [PubMed] [Google Scholar]
- 139.Peter I., Maldonado-Contreras A., Eisele C. A dietary intervention to improve the microbiome composition of pregnant women with Crohn's disease and their offspring: the MELODY (Modulating early life microbiome through dietary intervention in pregnancy) trial design. Contemp Clin Trials Commun. 2020;18 doi: 10.1016/j.conctc.2020.100573. [published Online First: 2020/07/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material