PURPOSE:
Financial distress (FD) among older adults with cancer is not well studied. We sought to characterize prevalence and factors associated with FD among older adults with cancer and the association of FD with geriatric assessment (GA) –identified deficits.
PATIENTS AND METHODS:
We included adults age ≥ 60 years with cancer in the University of Alabama at Birmingham Cancer and Aging Resilience Evaluation Registry who underwent GA during initial consultation with a medical oncologist before starting systemic therapy. We captured FD using a single-item question: “Do you have to pay for more medical care than you can afford?” We built multivariable models to study the impact of sociodemographic/clinical factors on FD as well as the association of FD with GA impairments.
RESULTS:
We identified 447 older adults with a median age of 69 years; 60% were men, 75% were White, and colorectal (26%) and pancreatic (19%) cancers were the most common. Overall, 27% (n = 121) reported having FD. Factors associated with FD included being Black (v White; odds ratio [OR], 2.26; 95% CI, 1.35 to 3.81; P = .002), being disabled/unemployed (v employed; OR, 2.60; 95% CI, 1.17 to 5.76; P = .019), and having an advanced degree (v less than high school; OR, 0.13; 95% CI, 0.03 to 0.65; P = .012). Patients with FD were more likely to report several GA impairments, including depression (OR, 2.10; 95% CI, 1.06 to 4.18; P = .034) and impaired health-related quality of life in physical (β = −2.82; P = .014) and mental health domains (β = −3.31; P = .002).
CONCLUSION:
More than a quarter of older adults with cancer reported FD at the time of initial presentation to an oncologist. Several demographic factors and GA impairments were associated with FD.
INTRODUCTION
In 2020, it is estimated that roughly 1.8 million people in the United States will be diagnosed with cancer.1 According to the Agency for Healthcare Research and Quality, total health care expenditures for cancer in the United States were $80.2 billion in 2015, with 52% of those costs attributable to hospital outpatient or provider office visits and 38% to inpatient hospital stays.2 Along with advancements in therapies and diagnostic tools, patients with cancer in the United States are also paying more for their treatments and medications.3 As a result, patients with cancer experience financial hardship, with many unable to afford their medical copayments, prescriptions, and other health services, such as mental health or dental health care, which might be perceived as being nonessential.4 The term financial distress (FD) is used to describe these financial pressures related to health care that can lead to significant delays in seeking medical care for conditions, abandoning treatment, and forgoing other health care services.4-6
Cancer is predominantly a disease of aging. Given changing demographics, by 2030, nearly 70% of new cancer diagnoses are expected to occur in patients age > 65 years.7 As a result, most cancer costs will be taken on by Medicare, which provides insurance coverage for a majority of older adults age ≥ 65 years in the United States. Although prior studies have suggested that older age is associated with a lower risk of FD,8-10 many older adults are on fixed incomes, and many Medicare beneficiaries have significant out-of-pocket costs.11 However, there are limited data on FD specifically among older patients with cancer. Given the aging population, the burden and potential consequences of FD in this population warrant further exploration. Identification of demographic and clinical characteristics associated with FD may allow early identification of a subgroup of patients at the greatest risk of having FD, thereby creating opportunities for targeted screening and early intervention. Furthermore, certain geriatric domain impairments may additionally affect an older adult’s ability to obtain high-quality cancer care (eg, cognitive impairment may limit navigating complex patient assistance programs, functional impairment may lead to lack of transportation to the clinic), additionally compromising cancer care among those already distressed financially. Therefore, it is important to study the coexistence and relationship between FD and GA domain impairments. To fill these gaps, we used a prospective registry of older adults age ≥ 60 years with cancer to understand the prevalence and predictors of FD in older adults with cancer.
PATIENTS AND METHODS
Study Population
We used participants from the Cancer and Aging Resilience Evaluation (CARE) study, a prospective registry of older adults age ≥ 60 years receiving cancer care at University of Alabama at Birmingham (UAB) hospitals and clinics.12 For this study, we limited our cohort to older adults age ≥ 60 years diagnosed with a solid or hematologic malignancy seen for initial consultation at the UAB medical oncology clinic, before starting any systemic therapy, who underwent a patient-reported geriatric assessment (GA) at their initial visit. We chose 60 years of age as a criterion for enrollment in this registry, recognizing the uncertainty of the right age cutoff and recent evidence suggesting significant geriatric domain impairments even among patients age < 65 years.13,14 Patients newly diagnosed with cancer are given the GA as part of routine medical care. The UAB Institutional Review Board approved this study (IRB-300000092).
Primary Outcome
The primary outcome of interest was presence of FD. FD was measured using a single-item screening question obtained from the Patient Satisfaction Questionnaire (PSQ-18)15: “Do you have to pay for more medical care than you can afford?” Participants picked one of five possible answers on a Likert scale: strongly agree, agree, uncertain, disagree, and strongly disagree. Those responding as strongly agree or agree were considered to have FD, as previously described.16 FD captured using this single-item question has been previously shown to be associated with increased risk of medication noncompliance and forgoing of mental health care, physician visits, and medical tests because of cost.16
GA
All patients in the CARE registry undergo a patient-reported GA that has been adapted from the Cancer and Aging Research Group geriatric assessment tool, originally developed by Hurria et al.12,17 Briefly, this tool captures the following GA domains18: Older Americans Resources and Services (OARS) Activities of Daily Living (ADL) and instrumental ADL Scale (IADL), number of falls, self-reported Eastern Cooperative Oncology Group performance status (ECOG PS), cognition, malnutrition, social activities, comorbidities, medications, hearing/vision impairments, social support, mental health, and ability to walk one block. The Patient-Reported Outcomes Measurement Information System (PROMIS) 10-item Global Health measured health-related quality of life (HRQOL) outcomes, including Physical Health and Mental Health subscores.19,20 Malnutrition was measured using a shortened Patient Generated–Subjective Global Assessment (PG-SGA), which assesses weight loss, food intake, symptoms related to nutrition, and a general activities/function question.21,22 Psychological morbidity was measured using the four-item PROMIS Anxiety and Depression Short Form. The OARS comorbidity scale was used to assess comorbidities (dichotomized as ≥ three or < three comorbid conditions) and presence of visual or hearing impairments, along with the number of daily medications (dichotomized as ≥ nine or < nine medications).23,24 Cognitive impairments were assessed using the PROMIS Cognitive Function Short Form 4a, which has been determined to be a reliable instrument to capture self-reported cognitive complaints.25,26
Definitions of Frailty Index and GA-Identified Impairments
We constructed a frailty index using Rockwood’s principle of deficit accumulation,15,27,28 following procedures outlined by Searle et al.29 We selected 44 GA variables, each of which captured a health deficit, and categorized patients as robust (0-0.2), prefrail (0.2-0.35), and frail (> 0.35), as previously described.1
On the basis of GA, we defined patients with domain-specific GA-identified impairment as follows: > one fall in the last 6 months, self-reported ECOG PS ≥ 2, significant limitation in walking one block (those responding as limited a lot), impairment of any IADL, and impairment of any ADL. Using established cutoffs, we defined anxiety (PROMIS T score ≥ 60), depression (T score ≥ 60), and cognitive impairment (T score ≤ 40).30 We defined presence of multimorbidity as presence of ≥ three comorbidities and polypharmacy as use of ≥ nine medications on a daily basis. Meanwhile, we identified malnutrition based on abbreviated PG-SGA score ≥ 6 and noted the presence of significant fatigue and hearing and vision loss in the population.31,32
Covariates
Covariates in this analysis included age at survey completion, sex, race/ethnicity, educational level, marital status, and employment status. Insurance status was captured from the electronic health record at the time of initial appointment. Lastly, we captured data on cancer type, cancer stage, and date of diagnosis from the electronic health record.
Statistical Analyses
We used descriptive statistics to characterize the demographic, clinical, and geriatric assessment domains of participants at baseline. We compared the distribution of characteristics between patients with and without FD using bivariate tests (χ2 and Mann-Whitney U test/independent sample t tests where applicable). We built a multivariable logistic regression model to study the impact of demographic and clinical variables on presence of FD. Covariates in this model included age, sex, race, educational level, marital status, employment status, insurance status, cancer type, and cancer stage. These covariates were chosen based on a review of prior literature supporting their impact on FD; all variables were entered into the model at once. We constructed separate multivariable logistic and linear regression models to measure the strength of association between FD and each domain-specific geriatric impairment; all of these models additionally adjusted for age, sex, race, educational level, marital status, employment status, insurance status, cancer type, and cancer stage. Odds ratios (ORs) and 95% CIs were calculated and reported. All multivariable models were thoroughly assessed for multicollinearity by examining the variance-covariance matrix of independent variables as well as by calculating the variation inflation factor (VIF), where a value > 5 was considered problematic. All hypotheses were two sided, and α < 0.05 was considered statistically significant. Statistical analyses were conducted using STATA MP 13.0 (StataCorp, College Station, TX).
RESULTS
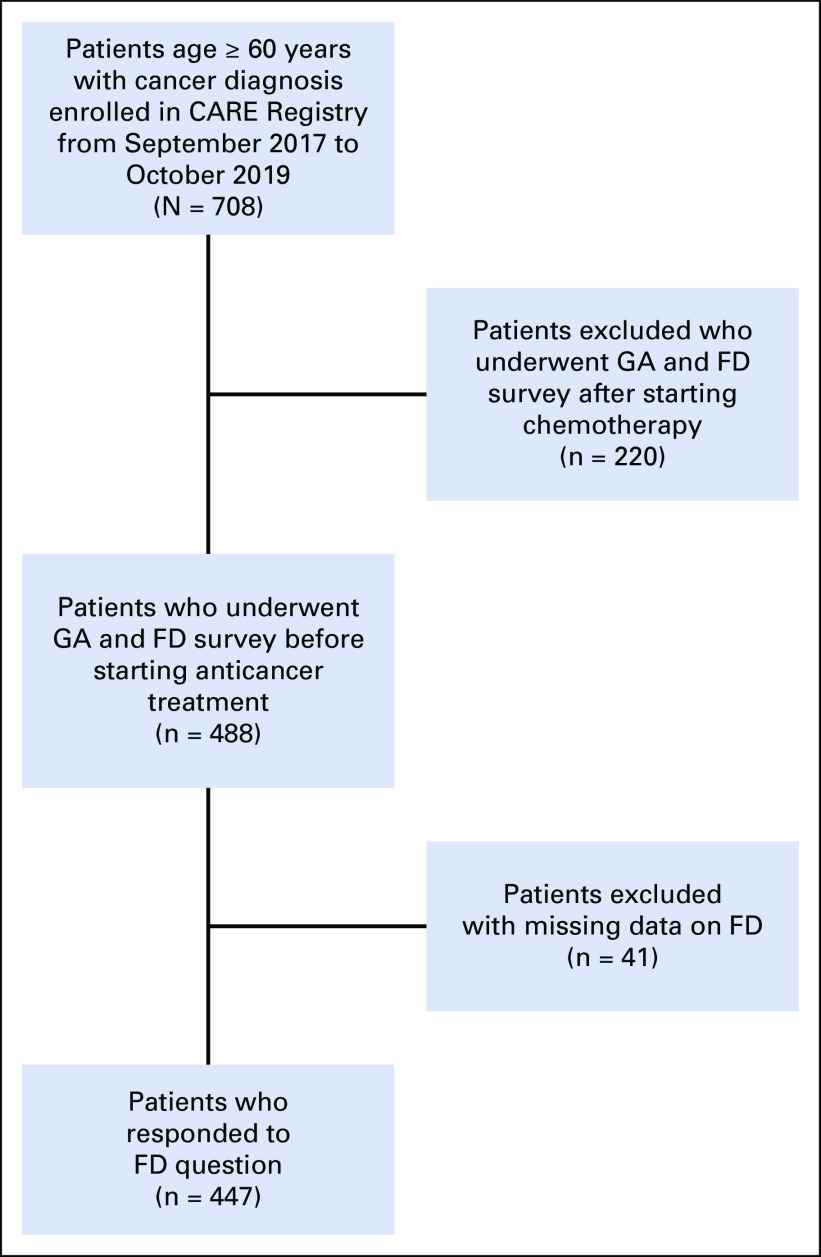
Of 708 adults age ≥ 60 years enrolled in the CARE registry between September 2017 and October 2019, we identified 447 (63%) eligible participants who underwent GA during their initial visit at the medical oncology clinic and had available information on FD (Appendix Fig A1, online only).
Table 1 lists the demographic characteristics of the study participants. Overall, the median age was 69 years; 59.5% of participants were men, and 75.2% were non-Hispanic Whites. A majority of patients (59.1%) were retired, whereas 19% were employed; 51.5% had Medicare, 42.3% reported private insurance, and 2.7% had Medicaid. Only 3.6% of the study population had uninsured/self-pay status. The most common cancer types included colorectal (25.5%), pancreatic (19.2%), and hepatobiliary (13.0%) cancers, with most patients having advanced stage III/IV disease (71.8%).
TABLE 1.
Patient Characteristics and Comparison of Those With FD Versus Those Without (N = 447)
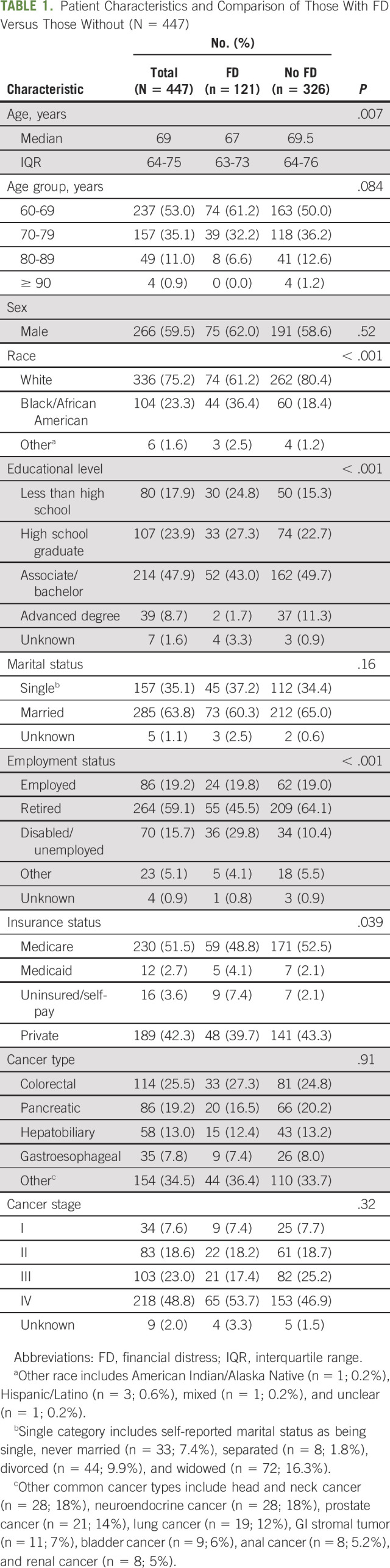
FD was defined using a single-item screening question from the PSQ-18, which was agreement with the following phrase: “Do you have to pay for more medical care than you can afford?” Overall, 27.1% (n = 121) had evidence of FD (ie, reported that they had to pay more for medical care than they could afford). Patients with FD were more likely to be non-White (40% v 20%; P < .001), have an educational level of high school graduate or lower (52% v 38%; P < .001), be disabled or unemployed (30% v 10%; P < .001), and be uninsured/self-paying (7% v 2%; P = .039). There was no significant difference in cancer type or stage between the two groups (Table 1).
As summarized in Table 2, according to our study, race (Black v White; OR, 2.26; 95% CI, 1.35 to 3.81; P = .002) and employment status (disabled/unemployed v other; OR, 2.60; 95% CI, 1.17 to 5.76; P = .019) were more likely to be associated with FD. Meanwhile, patients who possessed an advanced degree (OR, 0.13; 95% CI, 0.03 to 0.65; P = .012) were less likely to have FD, as compared with those with less than a high school education.
TABLE 2.
Multivariable Logistic Regression Model to Identify Characteristics Associated With FD in Study Population
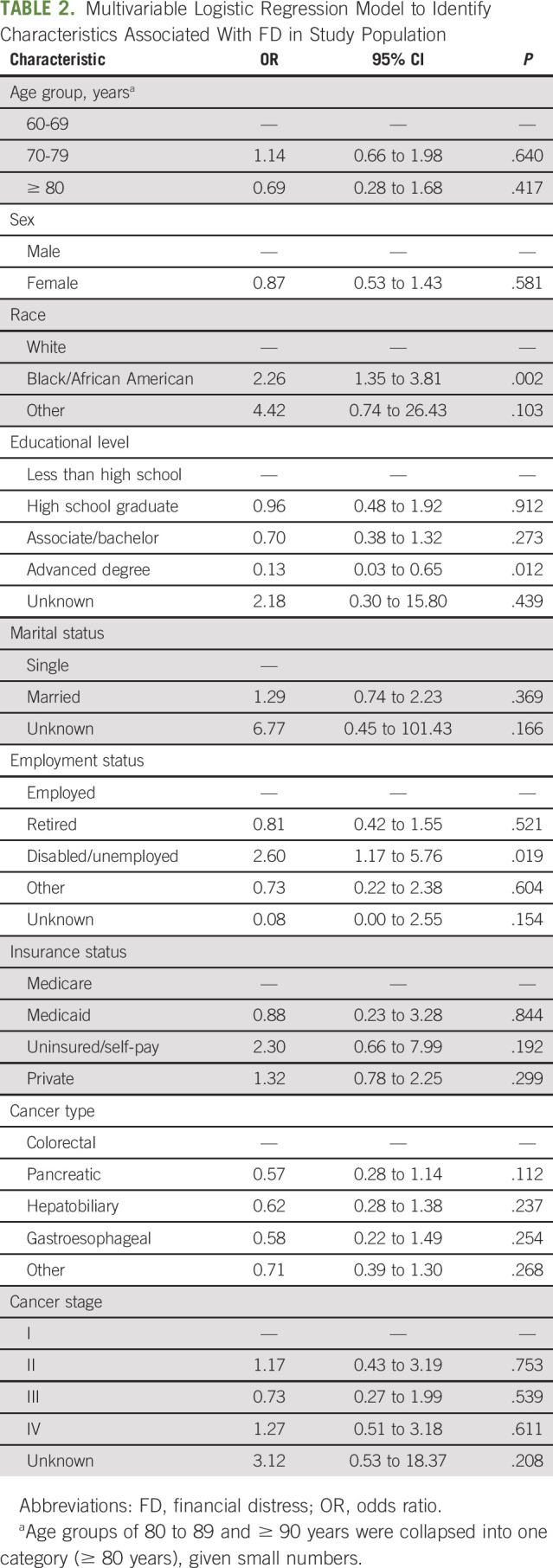
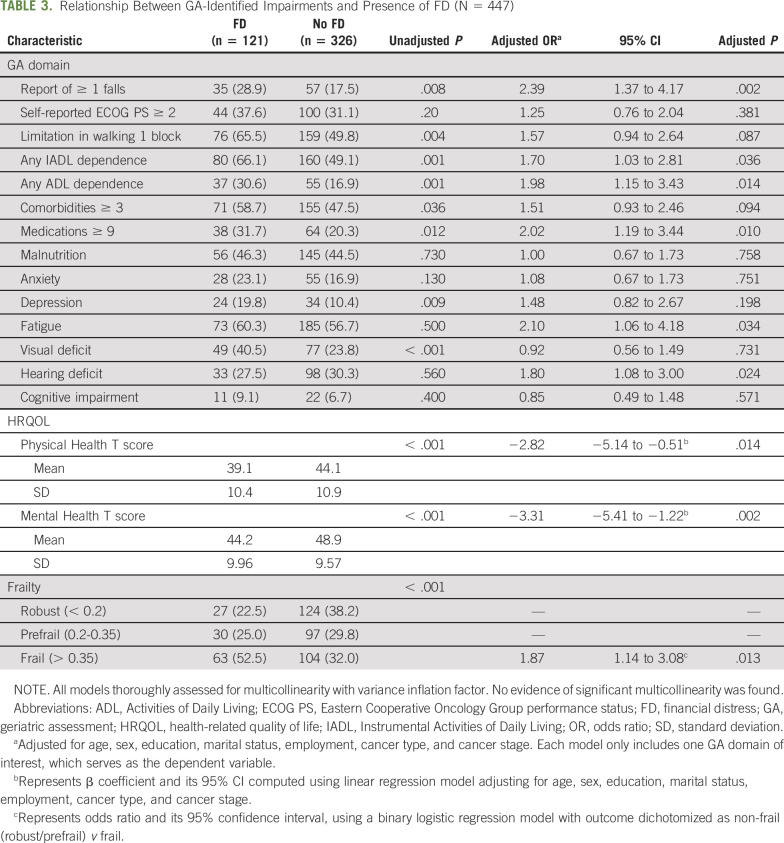
Table 3 summarizes the relationship between GA-identified impairments and presence of FD. Patients reporting FD were more likely to report having ≥ one falls within the past 6 months (OR, 2.39; 95% CI, 1.37 to 4.17; P = .002), report IADL (OR, 1.68; 95% CI, 1.02 to 2.76; P = .042) or ADL dependence (OR, 2.0; 95% CI, 1.16 to 3.46; P = .042), or report taking ≥ nine medications on a daily basis (OR, 2; 95% CI, 1.18 to 3.40; P = .010). These patients were also more likely to experience depression (OR, 2.10; 95% CI, 1.06 to 4.18; P = .034), report visual deficit (OR, 1.80; 95% CI, 1.0), and have reduced PROMIS Global Physical Health (β = −2.82; P = .014) and Mental Health scores (β = −3.31; P = .002). These associations remained significant even after adjusting for age, sex, education, marital status, employment, cancer type, and cancer stage. There was no evidence of multicollinearity in any of these models, as supported by a VIF < 2 for all variables.
TABLE 3.
Relationship Between GA-Identified Impairments and Presence of FD (N = 447)
DISCUSSION
The results from our study indicate that more than a quarter of adults age ≥ 60 years with cancer reported FD at the time of initial presentation to a medical oncologist. Potential factors associated with FD in this population included race, educational level, employment status, and insurance status. Additionally, we noted that those experiencing FD had an increased prevalence of depression and lower physical and mental HRQOL, in addition to other domain-specific GA impairments, including falls, limitations in walking one block, IADL and ADL impairments, visual impairment, multimorbidity, and polypharmacy. The risk factors identified in this study may allow early recognition and intervention among older adults who are at the greatest risk of experiencing FD.
In general, patients diagnosed with cancer have high rates of FD.33 However, FD for older adults with cancer can be quite different from that for younger adults. For example, patients age ≥ 65 years with cancer are more likely to have basic health insurance through Medicare and have a lower fixed income from pension, social security, and retirement payouts. However, there is no out-of-pocket (OOP) maximum with Medicare, and > 4 million Medicare beneficiaries do not have supplemental insurance to help minimize OOP costs for a cancer diagnosis.8 Meanwhile, younger patients with cancer tend to be more dependent on employment for health insurance and income, which is not the case in most older patients.9 Few studies have shown that as compared with older patients with cancer, younger patients may have a higher rate of FD, hypothesizing that older patients with cancer are afforded protective economic benefits through social security and Medicare, which is not reliant on current employment.9,10 However, the true prevalence of FD among older adults with cancer remains unclear, given that prior studies often included a convenience sample of a heterogeneous group of patients interviewed at different phases of their cancer treatment.34 To that end, we have used an older adult prospective cancer registry and screened patients at the time of initial presentation to the medical oncology clinic, thus providing reliable estimates of prevalence and predictors of FD in this population.
Several tools and questionnaires exist for measuring FD among patients with cancer. The Comprehensive Score for Financial Toxicity tool, developed by de Souza et al,35 is an 11-item patient-reported outcome measure that is widely used and validated among older adults with cancer. However, some studies have used more abbreviated questionnaires to screen for financial toxicity, as in our study. In 1988, Knight et al16 studied the presence of financial toxicity using a single-item questionnaire similar to ours among adults age ≥ 18 years with cancer seen at a single cancer center. Interestingly, the reported prevalence of financial toxicity (26%) was remarkably similar to that seen in our study, even though the Knight et al study involved a much younger patient population (median age, 59 years; age > 65 years, 33%). Importantly, those patients found to have FD were more likely to report noncompliance with medication as a result of the inability to afford prescription drugs and to report forgoing mental health care, physician visits, and medical tests because of cost. In a study of older adults age ≥ 70 years with advanced cancer, Arastu et al36 used a three-item questionnaire and found financial toxicity reported by 18% of patients, a percentage substantially lower than that found in our study. Differences in financial toxicity measurement tools as well as study populations (clinical trial cohort in Arastu et al v patients seen in routine care in our study) may potentially explain these differences.
The higher prevalence of financial toxicity among Black, disabled/unemployed, and uninsured/self-paying patients and those with lower educational status is consistent with prior published literature.16,34,35 However, FD has been reported even among well-educated White patients and those with Medicare insurance, indicating that race and socioeconomic or insurance status do not completely determine the level of FD among older adults with cancer.5,34 Similarly, we found a significantly higher prevalence of depression and a trend toward increased anxiety and inferior HRQOL among older adults with FD, consistent with published evidence.35,36 In addition, we also found a greater prevalence of frailty and several domain-specific GA impairments, although the directionality of this association cannot be inferred given the cross-sectional nature of this study.
By asking patients the single-item PSQ-18 question (ie, “Do you have to pay more for medical care than you can afford?”), providers will be able to quickly identify patients who may have a higher risk of experiencing financial difficulties and future noncompliance with physician appointments and treatments.16 Identifying vulnerable populations will allow tailored interventions to address specific issues to help ensure they continue to receive their cancer treatments. Some potential barriers to cancer treatment that can lead to delays in care include issues with transportation, paid sick leave from work, and insurance coverage, among others. Social work referrals may be helpful in educating patients about lower-cost sources of medical care and finding them financial assistance programs to help lessen their cancer-related financial burden.37
We need to acknowledge some noteworthy limitations. Use of a single-item survey question to screen for FD may have resulted in a broad definition of FD. However, Knight et al16 demonstrated that FD, captured using the same question, is associated with relevant clinical outcomes including significant delays in seeking medical care for conditions, abandoning treatment, and forgoing other health care services. Additional limitations include the absence of a healthy cohort or a younger population of patients with cancer (age < 60 years) for comparison. Furthermore, given the cross-sectional nature of this study, cause-and-effect relationships could not be determined between demographic and GA domains in relation to FD. The study participants completed the CARE survey at the time of their initial visit to the clinic; ongoing follow-up assessments may allow us to understand the trajectory of FD. Our study sample consisted of patients with cancer from a single clinic in the southeastern United States, which may not be representative of all older adults with cancer. Our registry initially started enrolling patients from the GI oncology clinic and was later expanded to other malignancies. As a result, a majority of patients in our registry have GI cancers, whereas many other common cancers, such as breast, prostate, and lung cancers, are under-represented in our registry. Our findings need to be confirmed across other cancer types as well. By limiting our sample to patients completing GA at their initial visit, we may have excluded patients with severe illness and those requiring hospitalization for urgent treatment or hospice, potentially leading to an underestimation of the prevalence of FD. Similarly, exclusion of patients who are already receiving systemic therapy or those with missing data may have resulted in an underestimation of the prevalence of FD, because the prevalence of FD is expected to increase along the continuum of cancer treatment, and those with significant FD may be reluctant to report their problem for fear of undertreatment. Finally, there are also several other variables that were missing from this study, such as income and treatment drug information, which limited our analysis.
To our knowledge, this is one of the first studies assessing baseline patient-reported FD in older adults with cancer. Previous studies have typically identified younger adults with cancer as more vulnerable to FD.7,16 However, there are limited data on identifying risk factors for FD in older patients, and given the aging population, the burden of FD in this population will need further assessment. Use of a patient-reported GA in addition to routine clinical assessment may help clinicians better predict patients’ likelihood of benefit or risk of toxicity from cancer treatment. Routinely assessing access to care during normal clinic workflow is vital to improving the care of older adults with cancer, especially for those who belong to more vulnerable populations.
APPENDIX
FIG A1.
Study flow diagram illustrating the cohort selection process. After excluding patients who completed the Cancer and Aging Resilience Evaluation (CARE) survey after starting chemotherapy (n = 220) and patients with missing data on financial distress (FD; n = 41), 447 patients were included for final analysis. GA, geriatric assessment.
Smith Giri
Honoraria: CareVive, OncLive
Research Funding: Carevive Systems, Pack Health
Ravi Paluri
Honoraria: Ipsen, Amgen
Speakers’ Bureau: Ipsen
Olumide Gbolahan
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis
Grant R. Williams
Honoraria: Carevive Systems, Cardinal Health
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as a poster at the ASCO Gastrointestinal Cancers Symposium, San Francisco, CA, January 17-19, 2019.
SUPPORT
Supported in part by the Walter B. Frommeyer Fellowship in Investigative Medicine at the University of Alabama at Birmingham and the National Cancer Institute of the National Institutes of Health (K08CA234225).
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Smith Giri, Mustafa Al-Obaidi, Will Varnado, Seema Kumar, Smita Bhatia, Grant R. Williams
Financial support: Grant R. Williams
Administrative support: Grant R. Williams
Provision of study material or patients: Mustafa Al-Obaidi, Will Varnado, Ravi Paluri, Grant R. Williams
Collection and assembly of data: Smith Giri, Deanna Clark, Mustafa Al-Obaidi, Will Varnado, Ravi Paluri, Grant R. Williams
Data analysis and interpretation: Smith Giri, Mustafa Al-Obaidi, Will Varnado, Olumide Gbolahan, Smita Bhatia, Grant R. Williams
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Financial Distress Among Older Adults With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Smith Giri
Honoraria: CareVive, OncLive
Research Funding: Carevive Systems, Pack Health
Ravi Paluri
Honoraria: Ipsen, Amgen
Speakers’ Bureau: Ipsen
Olumide Gbolahan
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis
Grant R. Williams
Honoraria: Carevive Systems, Cardinal Health
No other potential conflicts of interest were reported.
REFERENCES
- 1. National Cancer Institute: Surveillance, Epidemiology, and End Results Program: Cancer stat facts: Common cancer sites. https://seer.cancer.gov/statfacts/html/common.html.
- 2. American Cancer Society. Cancer Facts & Figures 2019. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html.
- 3.Mariotto AB, Yabroff KR, Shao Y, et al. : Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 103:117-128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver KE, Rowland JH, Bellizzi KM, et al. : Forgoing medical care because of cost: Assessing disparities in healthcare access among cancer survivors living in the United States. Cancer 116:3493-3504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zafar SY, Peppercorn JM, Schrag D, et al. : The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 18:381-390, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doshi JA, Li P, Huo H, et al. : Association of patient out-of-pocket costs with prescription abandonment and delay in fills of novel oral anticancer agents. J Clin Oncol 36:476-482, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Smith BD, Smith GL, Hurria A, et al. : Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 27:2758-2765, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Narang AK, Nicholas LH: Out-of-pocket spending and financial burden among Medicare beneficiaries with cancer. JAMA Oncol 3:757-765, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meeker CR, Wong Y-N, Egleston BL, et al. : Distress and financial distress in adults with cancer: An age-based analysis. J Natl Compr Canc Netw 15:1224-1233, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knight TG, Deal AM, Muss HB, et al: Financial distress among older versus younger adults with cancer. J Clin Oncol 34, 2016 (suppl; abstr e18268)
- 11.Rowland D, Lyons B: Medicare, Medicaid, and the elderly poor. Health Care Financ Rev 18:61-85, 1996 [PMC free article] [PubMed] [Google Scholar]
- 12.Williams GR, Kenzik KM, Parman M, et al. : Integrating geriatric assessment into routine gastrointestinal (GI) consultation: The Cancer and Aging Resilience Evaluation (CARE). J Geriatr Oncol 11:270-273, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aleixo GFP, Choi SK, Tan AJ, et al. : Is “geriatric” assessment just for older patients? Oncologist 25:355-358, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams GR, Al Obaidi M, Weaver A, et al: Association between chronological age and geriatric assessment (GA) to identify deficits in elderly adults with cancer: Findings from the Care Registry. J Clin Oncol 38, 2020 (suppl; abstr 12048)
- 15.Thayaparan AJ, Mahdi E: The Patient Satisfaction Questionnaire Short Form (PSQ-18) as an adaptable, reliable, and validated tool for use in various settings. Med Educ Online 18:21747, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight TG, Deal AM, Dusetzina SB, et al: Financial toxicity in adults with cancer: Adverse outcomes and noncompliance. JCO Oncol Pract 14:e665-e673, 2018 [Google Scholar]
- 17.Hurria A, Gupta S, Zauderer M, et al. : Developing a cancer-specific geriatric assessment: A feasibility study. Cancer 104:1998-2005, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Mohile SG, Velarde C, Hurria A, et al. : Geriatric assessment-guided care processes for older adults: A Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw 13:1120-1130, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella D, Riley W, Stone A, et al. : Initial adult health item banks and first wave testing of the Patient-Reported Outcomes Measurement Information System (PROMIS) network: 2005-2008. J Clin Epidemiol 63:1179-1194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hays RD, Bjorner JB, Revicki DA, et al. : Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 18:873-880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer J, Capra S, Ferguson M: Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 56:779-785, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Vigano AL, di Tomasso J, Kilgour RD, et al. : The abridged patient-generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J Acad Nutr Diet 114:1088-1098, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Williams GR, Deal AM, Lund JL, et al. : Patient-reported comorbidity and survival in older adults with cancer. Oncologist 23:433-439, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolly TA, Deal AM, Nyrop KA, et al. : Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist 20:379-385, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saffer BY, Lanting SC, Koehle MS, et al. : Assessing cognitive impairment using PROMIS applied cognition-abilities scales in a medical outpatient sample. Psychiatry Res 226:169-172, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Mir N, MacLennan P, Al-Obaidi M, et al. : Patient-reported cognitive complaints in older adults with gastrointestinal malignancies at diagnosis: Results from the Cancer & Aging Resilience Evaluation (CARE) study. J Geriatr Oncol 11:982-988, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerard EJ, Deal AM, Chang Y, et al. : Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw 15:894-902, 2017 [DOI] [PubMed] [Google Scholar]
- 28. Guerard EJ, Deal AM, Williams GR, et al: Construction of a frailty index for older adults with cancer using a geriatric assessment. J Clin Oncol 33, 2015 (suppl; abstr 9535)
- 29.Searle SD, Mitnitski A, Gahbauer EA, et al. : A standard procedure for creating a frailty index. BMC Geriatr 8:24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroenke K, Yu Z, Wu J, et al. : Operating characteristics of PROMIS four-item depression and anxiety scales in primary care patients with chronic pain. Pain Med 15:1892-1901, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrielson DK, Scaffidi D, Leung E, et al. : Use of an abridged scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer 65:234-239, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Williams GR, Al-Obaidi M, Dai C, et al: Association of malnutrition with geriatric assessment impairments and health-related quality of life among older adults with gastrointestinal malignancies. Cancer 126:5147-5155, 2020 [Google Scholar]
- 33.Bestvina CM, Zullig LL, Yousuf Zafar S: The implications of out-of-pocket cost of cancer treatment in the USA: A critical appraisal of the literature. Future Oncol 10:2189-2199, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Huntington SF, Weiss BM, Vogl DT, et al. : Financial toxicity in insured patients with multiple myeloma: A cross-sectional pilot study. Lancet Haematol 2:e408-e416, 2015 [DOI] [PubMed] [Google Scholar]
- 35.de Souza JA, Yap BJ, Wroblewski K, et al. : Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer 123:476-484, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arastu A, Ciminelli J, Culakova E, et al: The impact of financial toxicity on quality of life in older patients with cancer: Baseline data from the University of Rochester NCI Community Oncology Research Program (NCORP). J Clin Oncol 36, 2018 (suppl; abstr 87)
- 37.Kent EE, Forsythe LP, Yabroff KR, et al. : Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer 119:3710-3717, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]