Abstract
There is a growing need to provide effective adventitious agent mitigation for high risk upstream cell culture raw materials used for the production of biologics. It is also highly important in the growing fields of cell and gene therapies. Glucose is a critical raw material necessary for effective cell growth and productivity; however, glucose is the highest risk animal‐origin‐free raw material for viral contamination, and often the highest risk raw material in the upstream process as more companies move to chemically defined media. This study examines the efficacy of utilizing High Temperature Short Time (HTST) pasteurization for inactivation of physiochemically resistant, worst‐case parvovirus using a bench‐scale HTST system. We demonstrated approximately six log inactivation of Minute Virus of Mice (MVM) in concentrated glucose feeds without impacting the subsequent performance of the glucose in a Chinese Hamster Ovary (CHO) expression system.
Keywords: high temperature short time, minute virus of mice, pasteurization, upstream cell culture risk mitigation
Abbreviations
- (w/v)
weight by volume
- CAACB
consortium on adventitious agent contamination in biomanufacturing
- CHO
chinese hamster ovary
- DNA
deoxyribonucleic acid
- HTST
high temperature short time
- iCIEF
imaged capillary isoelectric focusing
- LRV
log reduction value
- mAb
monoclonal antibody
- MIT
Massachusetts Institute of Technology
- MVM
murine viruse of mice
- UV‐C
ultraviolet C
1. INTRODUCTION
Mammalian cell cultures are exposed to the risk of adventitious agent contamination from numerous sources. Viral contamination requires increased focus as it is much harder to detect compared to other microbial agents [1]. The smaller physical size of most viruses does not allow removal by standard sterilizing upstream filters [2] and the risk of human pathogen replication in mammalian cell culture can affect a biopharmaceutical manufacturing process [1, 3, 4].
Although the number of incidents of known viral contamination is low; with 26 reported cases in the last 36 years, the impact of a viral bioreactor contamination can be tremendous [5]. The costs for corrective and preventive actions, manufacturing downtime, lost revenue, fines from the regulatory authorities and a decrease in the company's value caused by public relation problems can reach toward $1 Billion [6]. In severe cases, contamination events can create drug shortages that affect patient quality of life [5, 7, 8] additionally, competitors could profit from accelerated drug approval processes [5, 9, 10].
A recent publication of the Consortium on Adventitious Agent Contamination in Biomanufacturing (CAACB), a biotech industry consortium, together with the Massachusetts Institute of Technology (MIT), gives an excellent overview of viral bioreactor contamination reported by the 20 member companies of that consortium [5]. Noteworthy is that 45% of those companies have already faced at least one viral contamination event in the past.
Approximately 70% of all biotech products are produced by Chinese Hamster Ovary (CHO) cell lines [11] which are typically more resilient than other alternative expression systems [12]. However, they can still be subject to infection by adventitious agents. Viral infection can negatively impact the performance of an expression system and lead to a failed harvest.
PRACTICAL APPLICATION
To facilitate targeted upstream viral mitigation strategies, companies need to understand the sources of risk and how they can be mitigated. This manuscript illustrates that concentrated glucose feeds are the highest risk (non‐animal derived) raw material required for therapeutic production. No other published works have assessed the suitability of pasteurizing a concentrated glucose feed. Our data shows that a high level of viral clearance is achieved, and the treated glucose can be used effectively in a CHO system. Other papers have examined this topic using whole cell culture media, but never specifically targeting glucose which is the worst case for contamination and due to its high viscosity, is the most challenging solution for virus mitigation. Our work will help companies justify the targeting and treatment of the highest risk feed without having to pasteurize complete media which can be more expensive and technically challenging due to heat labile components.
The use of chemically defined animal origin‐free media greatly reduces the risk for upstream viral contamination. However, this risk is not fully eliminated, since evidence for Minute Virus of Mice (MVM) contamination in animal origin‐free media has been previously reported [5].
MVM can be carried by small rodents and is a robust physiochemically‐resistant parvovirus. It has an icosahedral capsid structure that is approximately 20–25 nm [13] in diameter, therefore not removable by sterilizing grade filters. The virus has a non‐enveloped capsid containing single‐stranded DNA. MVM's small size and lack of a lipid envelope make it more challenging to inactivate and filter compared to other viruses [2, 13, 14]. Therefore, MVM is widely regarded as a worst‐case model virus and in fact is a very relevant model in that it has been responsible for several contamination events in the biopharmaceutical industry [5, 12, 15].
Systems designed to mitigate MVM risk are considered to reduce risk from other less robust, larger viruses even more effectively [15].
There are several potential viral contamination sources, including operators, facility and utilities that could result in a contamination event; however according to the CAACB study, the main source of the viral contamination originates from raw materials [5, 16, 17]. Importantly, for some of the reported contamination events, raw material testing alone was not always sufficient to detect virus [1]. The reasons for this can be heterogeneity of the viral load distribution and the sensitivity of viral detection methods [5]. These contamination events highlight the need for overarching approaches for prevention, removal and inactivation of virus in complete cell culture media and media components [5].
Glucose is a critical component of most cell culture media formulations [18, 19], and is typically added periodically as a feed during a bioreactor run, and is generally regarded as one of the highest risk raw materials for viral contamination. Even though it is animal origin‐free, it has a similar viral risk profile as animal‐derived material. This high‐risk designation arises from its innate attractiveness to virus‐carrying rodents [20], and the fact that it is often stored in unregulated warehouse conditions before purchase by biopharmaceutical consumers or their intermediate suppliers.
This combination of factors can easily lead to a situation where virus could be transmitted from rodent urine or faeces to stored sugar. Typical processing or repackaging operations do not include virus removal steps; therefore, virus contamination is very much a risk that is difficult to address.
Different technologies for upstream viral risk mitigation exist, like pasteurization, nanofiltration, and UV‐C or gamma‐irradiation, but treatment efficacy is dependent on the volumetric and physio‐chemical properties of the raw material, equipment design and control as well as the target virus susceptibilities [21, 22, 23].
There is a rising trend in industry to separate the glucose from cell culture media and charge into the bioreactor as a separate feed. This can yield a number of benefits from mitigating media glycation, reducing cold storage requirements for media and facilitating a targeted risk based viral mitigation approach [24].
In fed‐batch processes, where large volumes of glucose are often used as a highly concentrated 40% or 50% (w/v) solutions, solution viscosity presents a challenge for virus filtration. The high viscosity requires a larger filter area, and therefore a higher cost for this process step. It can also be significantly time consuming to setup, integrity test, filter and re‐integrity test these filters for the typical glucose volumes required. An alternative approach for virus mitigation often needs to be utilized.
HTST pasteurization is commonly used in the biopharmaceutical industry for mitigation of upstream viral contamination [5, 13, 15] HTST technology involves the heating of a feed solution to a pathogen‐inactivating temperature; holding the solution at this temperature for a specified residence time, and then rapidly cooling the solution. This thermal spike denatures the protein capsid around the virus, rendering it non‐infectious [16, 25].
Previous studies have shown that HTST treatment of cell culture media works effectively in viral risk mitigation [15, 25, 26, 27, 28, 29]. However, there is a gap in understanding if HTST treatment can effectively reduce the viral load in a viscous solution such as concentrated glucose, a solution that is up to 40 times more viscous [30, 31] than common cell culture media. In this study we present evidence for effective treatment of 40% and 50% (w/v) glucose solution achieving LRV of more than six. Moreover, we show that HTST treatment of glucose does not impact the cell culture media performance in a standard CHO expression system.
2. MATERIALS
2.1. Virus inactivation
Unless otherwise stated, solutions, media and cell line were provided by MilliporeSigmaTM.
Sterile 40% (w/v) glucose solution.
Sterile 50% (w/v) glucose solution.
MVM virus: High titer MVM stock: 5E + 09TCID50/mL Cell line for virus quantitation: Adherent human, 324 K SV40‐transformed newborn kidney cell line ‐ P. Tattersall, Yale University.
Heated circulating bath, VWR, Model 116 OS.
Heated circulating bath, Cole‐Palmer, Model 12108–10.
Isotemp circulating bath, Fisher Scientific, Model 5150‐R28.
Pure silicone fluid 5 CST 1 Gal, Fisher Cat # NC0569236.
Stainless steel 1.5 mL Micro VLS Tube, Fisher Cat# NC0348280.
Silicon rubber caps, Fisher Cat# NC0465419.
Temperature probe thermocouple.
Thin wire thermocouple probes 0.003″ diameter bead, Omega Cat# 5SC‐TT‐K‐40‐72.
Stainless steel tubing (0.042″ OD, 0.005″ wall) McMaster‐Carr Cat#8987K51.
Fluke 5627A probe calibration reference instruments.
Custom Labview VI (Data Acquisition Software).
NI USB Chassis, National Instruments Cat# cDAQ‐9171.
Thermocouple I/O module, National Instruments Cat# NI‐9213.
MATLAB software (The MathWorks, Inc.).
Cell Viability Testing.
Reagents and media provided by MilliporeSigma.
D(+)‐Glucose anhydrous (powder, untreated)
Hydrated to 50% (w/v) with Water For Injection.
Glucose solution 50% (w/v), HTST treated).
Ex‐CellTM Advanced CHO Fed‐batch Medium.
Ex‐Cell Advanced CHO Feed.
CHOZN GS‐/‐ ZFN‐modified CHO cell line (Clone#14‐2‐13), passage 9.
TPP Polypropylene tubes.
Shaking Kuhner incubator.
Countstar cell counter.
ForteBio Octet Interferometer system.
3. METHODS
3.1. Virus inactivation
3.1.1. Bench‐scale system development
The heat transfer rates of the pilot and commercial HTST systems were examined, and this was used to develop the bench‐scale viral clearance testing apparatus. The bench‐scale HTST system used for viral inactivation consisted of a series of water or silicone oil baths. Each was set to a different temperature to represent different sections of the commercial HTST system. Development work was required to modify the apparatus to provide adequate heat transfer and temperature homogeneity. An additional pump was installed to facilitate faster oil recirculation in the "ramp" bath designed to mimic the heating section of the HTST system. No modification was required for the equilibrium water bath or the "hold" oil bath which was designed to represent the residence section of the commercial HTST system. A dip rack system was developed which held stainless steel 1.5 mL vessels in an array that provided the most equivalent environment within each bath. Surrogate vessels contained non‐virus‐spiked glucose and had temperature probes designed for fast response time and low mass inserted into them. This rack is moved sequentially through the different water or oil baths. Temperature probes recorded the temperature at 0.01 s intervals and generated a real‐time average which was recorded by data acquisition software. The software also provided both audible and visual prompts that the rack needed to be moved to the next position.
3.2. Test article preparation
MVM stock virus was added to the glucose solution to a target titer of 107 TCID50/mL, corresponding to a 0.2% (v/v) virus spike. This solution was then aliquoted into test vessels to be used in the clearance experiment.
Test vessels were filled with 1370 ± 20 μL of glucose solution (40% or 50% [w/v]), and volume was confirmed by weight. This was thoroughly mixed prior to equilibration in the water bath.
Temperature probes were inserted through silicone stoppers into the surrogate vessels so that the probe was immersed in the solution. The same volume was placed into the test and surrogate vessels to ensure that the same level and mass was achieved. Two stainless steel vessels and two polypropylene vessels were filled with 1370 ± 20 μL of virus‐spiked glucose solution, which were not subjected to HTST treatment, to use as the hold control to determine virus log reduction values of the HTST treatment. Polypropylene vessels were used to confirm that stainless steel vessels were not contributing to viral inactivation.
3.3. Virus inactivation
The dip rack containing the test vessels and surrogate vessel was placed in a circulating water bath at 22°C and equilibrated for 3–5 min. The rack was then shaken to remove excess water and moved to the "ramp" oil bath at 130°C. The rack was held in the "ramp" oil bath until a pre‐determined trigger temperature was reached in the surrogate vessel. The trigger temperature is the defined treatment temperature for each run (90, 102, 105, 108, 110°C). Upon reaching this trigger temperature the rack was quickly moved to the "hold" oil bath which was set to the target residence temperature for the run. The rack was held in the hold bath for the target hold time and then quickly moved to an ice bath to cool the vessels to approximately 25°C. Once cooled, sample aliquots were transferred to cryotubes. One sample was assayed immediately, the other stored at 80°C.
The 40% (w/v) glucose was tested at the following residence times (10, 20, 30, and 40 s) and temperatures (90, 102, 105, 108, 110°C) in quadruplicate. The 90°C temperature was selected to confirm industry observation that sub‐100°C treatment is not effective [13, 15]. The 50% glucose was tested at 10 and 40 s at 90, 102 and 105°C in triplicate.
3.4. Virus quantitation
Infectious virus titer was determined using the Tissue Culture Infectious Dose 50% methodology.
For all incubation temperatures except 90°C, samples were diluted 1:10 in cell culture media and were plated into 80 wells of a 96‐well microplate containing a monolayer of 324 k indicator cells in culture media (100 μL of sample into 100 μL of cell culture). Serial 10‐fold dilutions (10−1 and 10−2) of the prediluted sample (1:10) were prepared and each were plated into eight wells of a 96‐well microplate (into 100 μL cell culture per well). For the 90°C condition, samples were serially diluted (10−2, 10−3, 10−4, 10−5, and 10−6) and plated in 8 or 16 wells of a 96‐well microplate containing a monolayer of indicator cells. The assay strategy for 90°C samples was based on an estimation of incomplete inactivation under this condition.
Plates were inspected for cytopathic effect (CPE) by microscopic observation after incubation for 10 days at 37°C, 5% CO2. The titer was determined by utilizing the Spearman‐Kärber method. Dependent on frequency of observed CPE, titer was estimated using several accepted statistical methods, either the Taylor method or a derivation of the Poisson distribution based on the volume of sample assayed and the limit of detection [32].
3.5. Cell viability studies
3.5.1. HTST treatment of glucose
A solution of 50% (w/v) glucose in WFI was pasteurized at standard processing conditions of 103°C for 11 s (acceptable treatment range for this batch was 102–106°C for 10–12 s). The glucose was produced from the MilliporeSigma commercial scale HTST process in Irvine, Scotland.
3.6. Cell viability and mAb expression analysis
CHOZN GS‐/‐ ZFN‐modified CHO cells (Clone#14‐2‐13) were seeded at 0.5 × 106 viable cells/mL in in EX‐CELL Advanced CHO Fed‐batch medium and incubated with shaking in a Kuhner incubator at 37°C, 5% CO2 with humidity control for 12 days. The cultures were fed with 5% EX‐CELL Advanced CHO Feed 1 on day 3, 5, 7, 9, 11, and glucose concentration of between 2 and 4 g/L was maintained with HTST treated or non‐HTST treated glucose. The trypan blue exclusion method was used to determine growth and viability with CountstarTM cell counter. The experiment was terminated after the viability of the cultures dropped below 75%.
To determine IgG productivity, culture samples were collected on days 7, 9, 11, and 12 and analyzed with a ForteBio Octet Interferometer system.
Spent media samples from Day 12 were collected and mAb was purified by one‐step Protein A capture. Glycan analysis was undertaken using the 2‐AB(2‐aminobenzamide) UPLC method and charge variant analysis was performed using iCIEF (imaged Capillary Isoelectric Focusing).
4. RESULTS
For fed‐batch processes, glucose is often added as a concentrated solution of 40% or 50% (w/v). Due to economic and time reasons, HTST treatment is the method of choice. HTST efficacy must be assessed in high viscosity solutions, in order to select optimum heating rates and residence times to avoid harsh conditions that eventually could trigger degradation of the sugar.
These experiments must be performed with a scale‐down system as virus cannot be introduced into a GMP manufacturing plant for testing at commercial scale, and it is prohibitive to impossible to generate the quantity of virus that would be needed to demonstrate adequate inactivation in a commercial‐scale platform. Viral inactivation was conducted in a representative bench‐scale HTST system that was demonstrated to provide a similar temperature ramp profile as the commercial system. MVM was used as a “worst‐case” virus due to its physicochemical resistance to many inactivation methods.
4.1. Viral clearance efficacy assessment
Figure 1 illustrates the inactivation results obtained with 40% (w/v) glucose.
FIGURE 1.
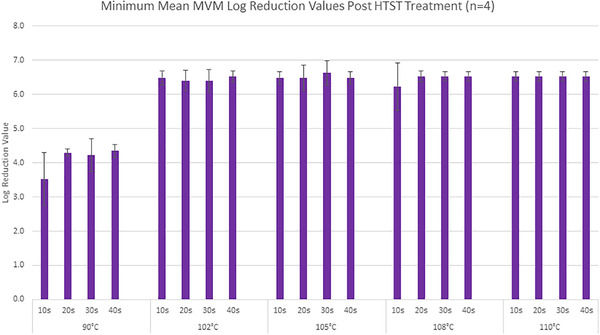
Minimum mean log reduction value (LRV) across all 40% (w/v) glucose runs
Treatment at 102°C and above achieved a log reduction of 6.2–6.6. A six log, or 99.9999%, reduction significantly reduces the risk of an infectious adventitious agent in a raw material feed. For most of the samples, there was no detectable virus following HTST treatment (Figures S1‐S4, Supporting Information) however, virus may have remained below the limit of detection.
HTST treatment of 50% (w/v) glucose also achieved clearance in excess of six log reduction in MVM titer. (Figure 2)The inactivation results in both glucose concentrations demonstrates that processing at 102°C or higher is effective at a high virus titer and high glucose concentration. (Figures 1 and 2)
FIGURE 2.
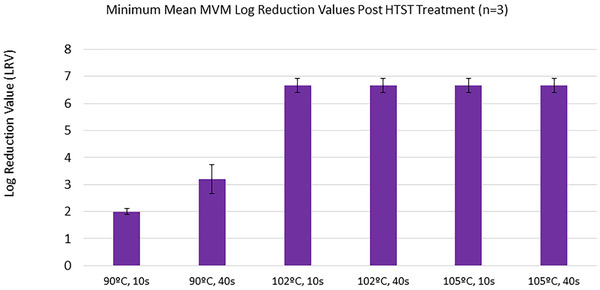
Minimum mean log reduction value (LRV) across All 50% (w/v) glucose runs
For all runs above 90°C there was no detectable virus remaining in post‐HTST treatment assay (Figure S5).
The 90°C treatment of 50% (w/v) glucose shows a reduction in viral inactivation efficacy compared to 90°C treatment of 40% (w/v) glucose. At 10 s treatment time, 40% (w/v) and 50% (w/v) glucose resulted in 3.5 and 2.0 LRVs respectively, and 40 s treatment time resulted in 4.2 and 3.1 LRVs, suggesting that at lower treatment temperatures, viscosity may be a factor in viral inactivation efficacy. However further study is required to investigate this mechanism.
4.2. Assessment of HTST‐treated glucose suitability in CHO expression system
Mammalian cells require an energy source such as glucose to proliferate and maintain viability. Heating glucose to high temperatures will eventually lead to degradation with the potential formation of several by‐products [33]. that may affect cellular performance.
To demonstrate that HTST treatment does not negatively affect cell culture viability growth or performance, a mAb‐expressing CHO cell culture was assessed for cell titer, viable cell density, viability and mAb titer from cultures supplied with HTST‐treated or untreated glucose feeds.
The viable cell density of CHOZN GS‐/‐ ZFN‐modified CHO cells fed with HTST treated and untreated glucose was determined over 12 days with no difference observed between the treated and untreated feeds (Figure 3).
FIGURE 3.
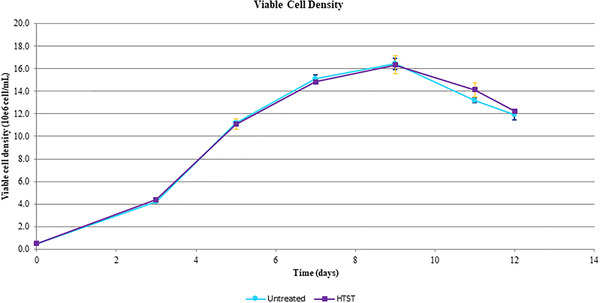
Comparison of viable cell density for treated and non‐treated glucose
There is no significant difference on cell viability and mAb titer between the CHO cultures fed with HTST‐treated or untreated glucose. (Figures 4 and 5)
FIGURE 4.
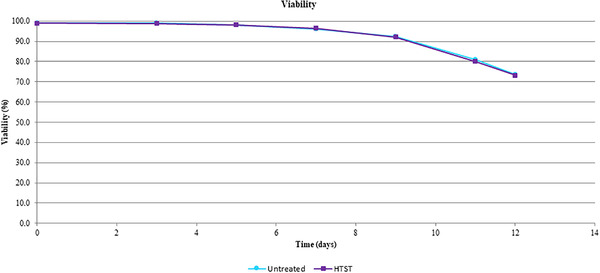
Comparison of viability of Chinese hamster ovary (CHO) expression system with treated and non‐treated glucose
FIGURE 5.
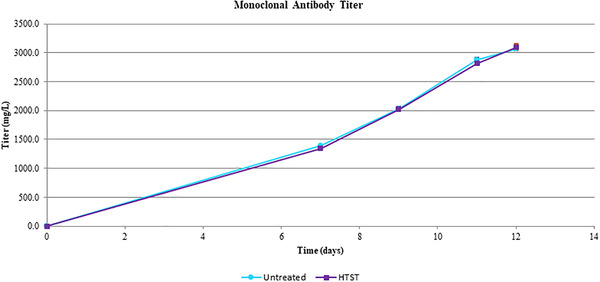
Comparison of productivity of Chinese hamster ovary (CHO) expression system with treated and non‐treated glucose
In addition to cell productivity, heat treatment of glucose should not result in any changes in critical quality attributes of the therapeutic mAb proteins produced by the cells.
Specific glycoforms are required to ensure that the antibody has effective functionality and is able to bind to the target antigen as well as ensuring sufficient stability and immunogenicity [34]. A selection of five standard glycans were compared, examining the relative abundance of each produced in a CHO expression system fed with untreated and HTST treated glucose [35].
The relative abundance of each selected glycan in the panel was comparable between treated glucose and untreated controls (Figure 6). Post translational modification assessments are necessary to ensure that HTST treatment has not altered how the cells utilize the glucose molecules [36].
FIGURE 6.
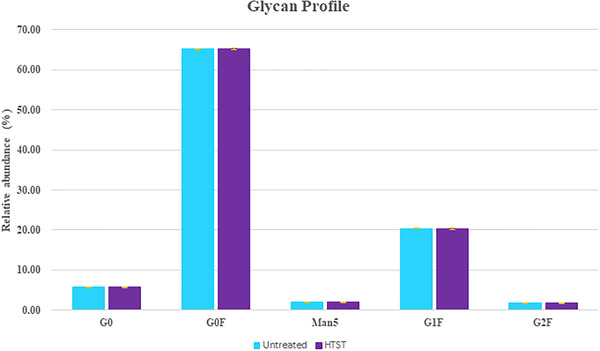
Comparison of glycan profile for treated and non‐treated glucose
Charge heterogeneity of the mAb was also assessed. There is a negligible difference between the charge variant profile indicating that the recombinant protein produced by the expression system fed with HTST‐treated glucose is comparable to the system fed with untreated glucose. (Figure 7)
FIGURE 7.
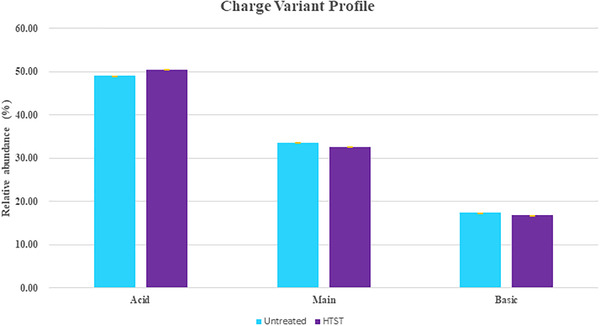
Comparison of charge variant profile for treated and non‐treated glucose
Glucose, as an important raw material, is used in large quantities during the culture process. These studies have demonstrated that compared with untreated glucose, neither the growth and expression of cells, mAb titer, charge variant nor the post‐translational modification of the expressed protein are affected by the HTST treatment of the glucose raw material feed.
5. DISCUSSION
In contrast to downstream purification of mAbs that requires several orthogonal process steps that provide viral reduction, there is no regulatory requirement for upstream viral barriers such as nanofiltration or HTST pasteurization. A contaminated batch is seen as a business risk. This viewpoint combined with the common heterogeneity of virus distribution in raw materials and limited detection sensitivity has led to several contamination events in the past with severe consequences leading to cumulative costs of up to $1 Billion and a shortage of drug supply ultimately affecting patient quality of life [6].
With the increasing industry prevalence for high‐titer processes in mAb manufacturing [37], there is a requirement for higher consumption of glucose. Novel modalities such as cell and gene therapies pose greater challenges for adventitious virus removal in the downstream purification process, with nanofiltration not even possible, so upstream viral safety is even more critically important [38].
To avoid costly contamination events, HTST‐treatment is commonly used for complete cell culture media [5, 13, 15, 25, 26, 27, 39]. Pasteurizing the whole media formulation can create problems if there are heat labile components in the bulk. Careful consideration and development works are required to mitigate this risk. These challenges can be overcome, but a more effective approach is to identify the risk components within the bulk media and treat as a separate feed, this addresses the viral risk and can lower the cost for viral mitigation as a smaller volume is being treated.
Due to its plant‐based origin and rodent attractant nature, Glucose is considered as one of the highest‐risk raw materials in animal‐free media. However, there is limited data on pasteurization efficacy for viscous glucose solutions that demonstrates effective virus inactivation without impacting on cell culture parameters and protein expression.
In this study, effective treatment conditions for 40% and 50% (w/v) glucose solutions that are commonly used for fed‐batch processes were assessed. The results of the viral clearance study show that high log reduction (LRV >6) of MVM spiked solutions can be achieved and that therefore HTST treatment for concentrated glucose feeds could be an effective barrier technology to viral contamination of the bioreactor. Since parvovirus is considered as a "worst case" scenario due to its small size and physicochemical resistance, HTST treatment will also inactivate enveloped viruses or other pathogens such as bacteria or fungi [15].
When utilizing risk mitigation strategies such as HTST treatment or viral filtration, it is necessary to remember that total elimination of viral particulates is not possible. This cannot be confirmed due to a combination of assay limits of detection, practicality of testing large raw material quantities and limits of the removal or inactivation technology. These methods should be used as part of a holistic upstream viral mitigation strategy and be viewed as a risk reduction only.
In the literature, lower LRV have been demonstrated for MVM using HTST pasteurization [13, 14, 15]. However, direct comparisons of LRV can be complicated by several factors. First, the magnitude of the LRV demonstrated will depend on the total viral load of the sample and assay sensitivity, independent of the inactivation efficiency. Secondly, the efficiency of virus inactivation has been shown to depend on the initial viral concentration [16] and may depend on the matrix in which the virus is present as well as the type of HTST equipment that is used. For virus inactivation in upstream raw materials, the ultimate goal is prevention of even a single virus particle entry into the bioreactor, so determining and comparing treatment conditions that result in virus inactivation to undetectable levels is very important.
While heat transfer systems can be designed to suitably raise concentrated solutions to viral inactivating temperatures, the virus may be "shielded" from the full effect of heat inactivation by the glucose molecules surrounding the capsid.
The data generated by the viral clearance studies has shown that a potential correlation between viscosity and resulting HTST treatment viral clearance may be valid at lower treatment temperatures. The higher the viscosity (caused by increased glucose concentration), the lower the viral clearance efficacy in the 90°C treatment runs. For 50% (w/v) glucose, there is approx. one log lower inactivation than for 40% (w/v) glucose at the same conditions, indicating either a difference in heat transfer to the virus or some protective effect of the higher concentration of glucose.
Although this finding is of interest, it does not affect the optimal clearance condition that we determined. Further investigation into the effect of viscosity impact on inactivation efficacy was outside the scope for this project and further works would be required to assess if this is a factor. Similar to reports in literature [5, 13, 14, 39] we found that temperatures above 102°C with a holding time >10 s provides effective virus inactivation.
We also demonstrated that HTST‐treated glucose is suitable for use in biopharmaceutical processing such as mAb production. HTST treatment of glucose feeds did not have an effect on cell viability, density, productivity.
The critical quality attributes of an antibody molecule may be affected by the cell clone, cell culture medium and culture process. In particular, variance in the cell culture medium raw materials often cause differences in the post‐translational modification, such as changes in the glycosylation profile of the therapeutic protein [40]. These differences may affect the safety and efficacy of antibody therapeutics.
The glycosylation profile comparison is a key factor as studies have shown that limiting glucose availability during cell culture processes can lead to an increased heterogeneity in glycosylation profile [40, 41] which may alter the pharmacokinetic properties of the mAb [42]. The performance and viability study undertaken has shown that HTST‐treatment of feeds does not impact key quality indicators. This strongly suggests that expression products will be as effective as those produced from a system without viral mitigated feeds.
The charge variant analysis also strongly suggests that recombinant proteins expressed in systems fed with viral mitigated glucose will have a similar isoelectric point and will therefore be suitable for purification in existing downstream purification trains, suggesting that legacy operations could utilize viral mitigated glucose without modification.
Our cell‐based performance results are consistent with previous studies, in which there was no negative impact on CHO cell processes using heat‐treated glucose [24]. Interestingly, even if production of the common heat‐dependent impurity 5‐Hydroxymethylfurfural (5‐HMF) was forced by holding a glucose solution for 5 weeks at 55°C, no impact on cell toxicity, protein productivity and product quality was observed [43].
HTST‐treatment provides an additional barrier technology that can be used in conjunction with orthogonal techniques such as upstream viral filtration. HTST typically achieves greater parvovirus viral clearance than viral filtration (>6 vs. >3.8 log) [44], however, they are typically used for different target feeds.
To achieve the highest levels of risk mitigation, while minimizing operating costs, it is possible for a manufacturer to utilize viral filtration on components which cannot be pasteurized and HTST treat feeds which have higher volumes and/or viscosity making them less economical to filter [39]. The risk mitigation techniques utilized should be material‐specific and tailored to provide the greatest cost/benefit to achieve a highly robust viral mitigation strategy.
Although not required from a regulatory standpoint, many leading biopharmaceutical companies have implemented HTST treatment of cell culture media [39]. Importantly, those companies that have faced a viral contamination event and have then moved to HTST treatment of cell culture media have not seen a bioreactor contamination thereafter [5].
HTST treatment has developed as the leading upstream viral safety technology for bulk media or feeds since it comes with many advantages, such as low running costs, being able to treat large volumes, easy validation and uniformity in treatment results [39].
Utilizing a cell culture media which has glucose as part of the formulation tends to create a greater challenge for HTST treatment. While certainly possible, it requires further development work to provide a commercial solution, as the high temperatures may cause degradation or precipitation of key heat‐labile components. One such degradation pathway is the Maillard reaction which occurs when amino acids are heated with a reducing sugar [45].
The biopharmaceutical company Biogen has chosen a novel approach to look at separating glucose from the bulk cell culture media formulation, and charge into the bioreactor as a separate feed. This gives many advantages other than the ability to easily pasteurize the high‐risk glucose.
The removal and separate HTST treatment of glucose in cell culture media removes the possibility for amino acids in the mixture to undergo glycation, a degradation pathway that limits bioavailability of these media components causing cultures to underperform [46]. Reducing this degradation is the reason that cell culture media is generally stored at refrigerated temperatures, but without glucose the glycation degradation path is removed and the media formulation is much more stable at room temperature storage conditions [24]. Since media formulations often comprise the bulk of raw material used in biologic pharmaceutical manufacturing processes; manufacturing sites can benefit from reduced refrigeration requirements when formulations are designed without glucose [24].
HTST skids can either be run in‐house which come with lower running costs or pre‐treated feeds can be purchased, mitigating the risk of ever bringing a potentially contaminated raw material into the biopharmaceutical facility [39].
Consideration should be taken to select the optimum time to introduce HTST treated raw materials into your process. The general industry consensus is that introducing HTST treatment into a legacy process must be considered a "major process change."[39] As such it is better to implement it early in process development to mitigate the labor cost of documenting and justifying the change further into commercial manufacture [47, 48, 49]. This approach will also mitigate the risk of moving into commercial manufacture and utilizing higher volumes of material which will increase the risk of contamination. Industry generally does not view it necessary to undertake new animal or human clinical trials for a process change such as the implementation of HTST treatment but comparability studies involving molecular weight, protein structure, affinity and physicochemical and immunochemical properties should be undertaken to ensure that these characteristics remain unchanged [39].
Vendors such as MilliporeSigma offer HTST treatment of glucose and other high‐risk raw materials. Outsourcing the pasteurization process is a viable option for debottlenecking production lines and saving high capital, validation, training and maintenance expenditures.
The availability of pre‐treated feeds is particularly useful to Contract Manufacturing Organizations (CMOs) who may want to bid to manufacture a product for a company that has a robust upstream viral clearance strategy in operation in‐house, but do not want to go to the expense of building an HTST capability at the CMO site [39].
Due to the current global pandemic there is an increased awareness of viral safety. It is clear that a thorough viral risk mitigation approach is more important than ever. It is critical for high risk raw materials, or raw materials for applications such as cell therapy, that do not always facilitate the use of robust viral filtration techniques downstream. In the future, as industry moves to ever‐higher production outputs, achieved by fed‐batch or perfusion operational modalities, increasing volumes of raw materials will be required. The associated risk of viral contamination increases proportionally with these improved outputs. The biopharmaceutical industry needs to be ready to face these known challenges as well as unknown challenges, such as potential for cross‐species transmission of virus into a production platform.
6. CONCLUDING REMARKS
HTST pasteurization can provide significant levels of worst‐case parvovirus clearance and provide viral risk mitigation for upstream cell culture feeds such as glucose. There appears to be no impact to CHO cell viability, density or productivity using HTST‐treated glucose as part of the expression system feed. Monoclonal antibodies produced by the CHO expression system tested were of a similar quality to those fed with non‐HTST treated glucose.
A holistic approach to upstream viral safety provides optimal risk mitigation. As with all process risk reduction strategies, a multi‐layered approach has been shown to be most effective to mitigate risk [5, 50].
This will involve the adoption and utilization of multiple complementary mitigation strategies designed to work synergistically, while providing effective risk reduction with minimal process impact. These operational methodologies, raw material selection strategies or technologies to allow prevention, detection and removal or inactivation of adventitious agents is key to a successful, consistent manufacturing process.
CONFLICTS OF INTEREST
The authors declare no financial or commercial conflict of interest. Authors acknowledge that MilliporeSigma offer HTST‐treated glucose feeds at commercial‐scale volumes; however, this is not viewed as a conflict of interest as there are other vendors in the marketplace offering similar services. In addition, the treatment conditions recommended in this paper can be implemented by any organization on their internal HTST systems.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to acknowledge the following people: Mark Carroll, Lori Mullin, Michael Doty and Mary Priest from the Virology and Microbiological Sciences R&D group from the MilliporeSigma facility located Bedford, MA, USA. Yue Zhao, from the Upstream R&D team at the MilliporeSigma facility located in Shanghai, China. Dave Kolwyk, Jason Dickens, Chris Grinnell, Catherine Lynes, Jessica Mondia and Lisa Bareford from the Biogen Material Science Group.
Gemmell DK, Mack A, Wegmann S, Han D, et al. Efficacy of minute virus of mice (MVM) inactivation utilizing high temperature short time (HTST) pasteurization and suitability assessment of pasteurized, concentrated glucose feeds in Chinese hamster ovary (CHO) cell expression systems. Eng Life Sci. 2021;21:502–513. 10.1002/elsc.202100044
DATA AVAILABILITY STATEMENT
MilliporeSigma can be contacted for discussion regarding datasets shared in this manuscript. Original data may be shared upon request but this will be relieved on a case by case basis.
REFERENCES
- 1. Merten, O. W. , Virus contaminations of cell cultures—A biotechnological view. Cytotechnology 2002, 39, 91–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peter Kosiol, B. H. , Ulbricht, M. , Thom, V. , Determination of pore size distributions of virus filtration membranes using gold nanoparticles and their correlation with virus retention. J Membrane Sci 2017, 533, 289–301. [Google Scholar]
- 3. Shah, K. , Nathanson, N. , Human exposure to SV40: review and comment. Am J Epidemiol 1976, 103, 1–12. [DOI] [PubMed] [Google Scholar]
- 4. Vilchez, R. A. , Butel, J. S. , Emergent human pathogen simian virus 40 and its role in cancer. Clin Microbiol Rev 2004, 17, 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barone, P. W. , Wiebe, M. E. , Leung, J. C. , Hussein, I. T. M. , et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nat Biotechnol 2020, 38, 563–572. [DOI] [PubMed] [Google Scholar]
- 6. Rockoff, J. D. , Drug manufacturing mending after questions of quality. In Wall Street Journal, Wall Street Journal New York (USA), New York: 2010; Vol. 2010. [Google Scholar]
- 7. Ailworth, E. , W, R. , Virus shuts Genzyme plant, holds up drugs for 8,000. Boston Globe 2009. [Google Scholar]
- 8. Liu, S. , Carroll, M. , Iverson, R. , Valera, C. , et al. Development and qualification of a novel virus removal filter for cell culture applications. Biotechnol Prog 2000, 916. [DOI] [PubMed] [Google Scholar]
- 9. Bethencourt, V. , Virus stalls Genzyme plant. Nat Biotechnol 2009, 27(8), 681‐681.19668157 [Google Scholar]
- 10. Allison, M. , As Genzyme flounders, competitors and activist investors swoop in. Nat Biotechnol 2010, 28, 3–4. [DOI] [PubMed] [Google Scholar]
- 11. Dumont, J. , Euwart, D. , Mei, B. , Estes, S. , et al. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit Rev Biotechnol 2016, 36, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berting, A. , Farcet, M. R. , Kreil, T. R. , Virus susceptibility of Chinese hamster ovary (CHO) cells and detection of viral contaminations by adventitious agent testing. Biotechnol Bioeng 2010, 106, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schleh, M. , Romanowski, P. , et al. Susceptibility of mouse minute virus to inactivation by heat in two cell culture media types. Biotechnol Prog 2009, 25, 854–860. [DOI] [PubMed] [Google Scholar]
- 14. Nims, R. P.M. , Modeling the efficacy of HTST for inactivating mouse minute virus. Biopharm Int 2016, 29, 42–45. [Google Scholar]
- 15. Djemal, L. , Fournier, C. , von Hagen, J. , Kolmar, H. , Deparis, V. , Review . High Temperature Short Time Treatment of Cell Culture Media and Feed Solutions to Mitigate Adventitious Viral Contamination in the Biopharmaceutical Industry: Review, e3117. [DOI] [PubMed] [Google Scholar]
- 16. Murphy, M. , Quesada, G. M. , Chen, D. , Effectiveness of mouse minute virus inactivation by high temperature short time treatment technology: a statistical assessment. Biologicals 2011, 39, 438–443. [DOI] [PubMed] [Google Scholar]
- 17. Garnick, R. L. , Experience with viral contamination in cell culture. Dev Biol Stand 1996, 88, 49–56. [PubMed] [Google Scholar]
- 18. Garnick, R. L. , Raw materials as a source of contamination in large‐scale cell culture. Dev Biol Stand 1998, 93, 21–29. [PubMed] [Google Scholar]
- 19. Sissolak, B. , Lingg, N. , Sommeregger, W. , et al. Impact of mammalian cell culture conditions on monoclonal antibody charge heterogeneity: an accessory monitoring tool for process development. J Ind Microbiol Biotechnol 2019, 46, 1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eagle, H. , Amino acid metabolism in mammalian cell cultures. Science 1959, 130, 432–437. [DOI] [PubMed] [Google Scholar]
- 21. Sclafani, A. , Starch and sugar tastes in rodents: an update. Brain Res Bull 1991, 27, 383–386. [DOI] [PubMed] [Google Scholar]
- 22. Nims, R. W. , Gauvin, G. , Plavsic, M. , Gamma irradiation of animal sera for inactivation of viruses and mollicutes–A review. Biologicals 2011, 39, 370–377. [DOI] [PubMed] [Google Scholar]
- 23. Wang, J. , Mauser, A. , Chao, S. F. , Remington, K. , et al. Virus inactivation and protein recovery in a novel ultraviolet‐C reactor. Vox Sang 2004, 86, 230–238. [DOI] [PubMed] [Google Scholar]
- 24. Meunier, S. M. , Sasges, M. R. , Aucoin, M. G. , Evaluating ultraviolet sensitivity of adventitious agents in biopharmaceutical manufacturing. J Ind Microbiol Biotechnol 2017, 44, 893–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dickens, J. E. , Chen, R. , Bareford, L. , Talreja, G. , et al. Colorimetric and physico‐chemical property relationships of chemically defined media powders used in the production of biotherapeutics. J Pharm Sci 2020;110(4):1635‐1642. [DOI] [PubMed] [Google Scholar]
- 26. Pohlscheidt, M. , Charaniya, S. , Kulenovic, F. , Corrales, M. , et al. Implementing high‐temperature short‐time media treatment in commercial‐scale cell culture manufacturing processes. Appl Microbiol Biotechnol 2014, 98, 2965–2971. [DOI] [PubMed] [Google Scholar]
- 27. Kiss, R. D. , Practicing safe cell culture: applied process designs for minimizing virus contamination risk. PDA J Pharm Sci Technol 2011, 65, 715–729. [DOI] [PubMed] [Google Scholar]
- 28. Shiratori, M. , Kiss, R. , Risk mitigation in preventing adventitious agent contamination of mammalian cell cultures. Adv Biochem Eng Biotechnol 2018, 165, 75–93. [DOI] [PubMed] [Google Scholar]
- 29. Shiratori, M. K. , Kiss, R. D. , Prashad, H. , Iverson, R. , et al. Methods for viral inactivation and other adventitious agents. 2019.
- 30. Weaver, B. , Rosenthal, S. , Viral risk mitigation for mammalian cell culture media. PDA J Pharm Sci Technol 2010, 64, 436–439. [PubMed] [Google Scholar]
- 31. Poon, C. , Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. 2020. [DOI] [PubMed]
- 32. Telis, V. R. N. , Telis‐Romero, J. , Mazzotti, H. B. , Gabas, A. L. , Viscosity of aqueous carbohydrate solutions at different temperatures and concentrations. Int J Food Properties 2007, 10, 185–195. [Google Scholar]
- 33. Rabenau, H. F. , Schwebke, I. , Blümel, J. , Eggers, M. , et al. Guideline for testing chemical disinfectants regarding their virucidal activity within the field of human medicine. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2020, 63, 645–655. [DOI] [PubMed] [Google Scholar]
- 34. Paine, J. B. , Pithawalla, Y. B. , Naworal, J. D. , Carbohydrate pyrolysis mechanisms from isotopic labeling: Part 2. The pyrolysis of d‐glucose: General disconnective analysis and the formation of C1 and C2 carbonyl compounds by electrocyclic fragmentation mechanisms. J Anal Appl Pyrol 2008, 82, 10–41. [Google Scholar]
- 35. Zhou, Q. , Qiu, H. , The mechanistic impact of N‐glycosylation on stability, pharmacokinetics, and immunogenicity of therapeutic proteins. J Pharm Sci 2019, 108, 1366–1377. [DOI] [PubMed] [Google Scholar]
- 36. Ehret, J. , Zimmermann, M. , Eichhorn, T. , Zimmer, A. , Impact of cell culture media additives on IgG glycosylation produced in Chinese hamster ovary cells. Biotechnol Bioeng 2019, 116, 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zimmer, A. , M, R. , Wehsling, M. , Schnellbaecher, A. , Improvement and simplification of fed‐batch bioprocesses with ahighly soluble phosphotyrosine sodium salt. J Biotechnol 2014, 186, 110–118. [DOI] [PubMed] [Google Scholar]
- 38. Shukla, A. A. , Wolfe, L. S. , Mostafa, S. S. , Norman, C. , Evolving trends in mAb production processes. Bioeng Transl Med 2017, 2, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morenweiser, R. , Downstream processing of viral vectors and vaccines. Gene Ther 2005, 12, S103‐S110. [DOI] [PubMed] [Google Scholar]
- 40. Delfosse, S. , Sathavipat, M. , Hsu, N. , Croughan, M. , LaFond, M. , Trends regarding viral barrier implementation in animal cell culture processes. Pharm Bioprocess 2013, 1, 351–360. [Google Scholar]
- 41. Torkashvand, F. , Vaziri, B. , Main quality attributes of monoclonal antibodies and effect of cell culture components. Iran Biomed J 2017, 21, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jayme, D. , Watanabe, T. , Shimada, T. , Basal medium development for serum‐free culture: a historical perspective. Cytotechnology 1997, 23, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Higel, F. , Seidl, A. , Sorgel, F. , Friess, W. , N‐glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur J Pharm Biopharm 2016, 100, 94–100. [DOI] [PubMed] [Google Scholar]
- 44. Xu, H. , Templeton, A. C. , Reed, R. A. , Quantification of 5‐HMF and dextrose in commercial aqueous dextrose solutions. J Pharm Biomed Anal 2003, 32, 451–459. [DOI] [PubMed] [Google Scholar]
- 45. Germany, M. K. D. , Robust Removal of Minute Virus of Mice from Cell Culture Media with Viresolve® Barrier Filters, AN5779EN00 Ver 1.0; 2017; p 2.
- 46. Zhang, Q. , Ames, J. M. , Smith, R. D. , Baynes, J. W. , et al. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J Proteome Res 2009, 8, 754–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rabbani, N. , Thornalley, P. J. , Glycation research in amino acids: a place to call home. Amino Acids 2012, 42, 1087–1096. [DOI] [PubMed] [Google Scholar]
- 48. FDA, U. J. U. F., MD, USA , Guidance for Industry: Changes to an Approved Application for Specified Biotechnology and Specified Synthetic Biological Products. MD: FDA, UJUF; 1997. [Google Scholar]
- 49. FDA, C. , Guidance for Industry, Changes to an Approved NDA or ANDA. 2004. [PubMed]
- 50. Chirino, A. J. , Mire‐Sluis, A. J. N. B. , Characterizing biological products and assessing comparability following manufacturing changes. 2004, 22, 1383–1391. [DOI] [PubMed] [Google Scholar]
- 51. Reason, J. , Managing the Risks of Organizational Accidents. Routledge: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
MilliporeSigma can be contacted for discussion regarding datasets shared in this manuscript. Original data may be shared upon request but this will be relieved on a case by case basis.


