Abstract
The complications of endonasal transsphenoidal surgery (ETSS) include meningitis and sinusitis, and these complications are troublesome. Some reports have investigated the type of bacteria and the susceptibility of sphenoid sinus mucosal flora to drugs. However, most specimens can be collected after perioperative antibiotic administration. In this study, 95 and 103 sphenoid sinus mucosal samples collected during ETSS from September 2013 to February 2015 and from June 2017 to January 2019, respectively, were examined for bacterial culture. Sphenoid sinus mucosal samples were collected after antibiotic administration in the first period, whereas samples were collected before antibiotic administration in the second period. Hence, the specimens in the second period were not affected by antibiotics. Moreover, drug susceptibility tests for the detected bacteria were performed. Overall, 52 and 51 bacterial isolates were collected during both periods. Gram-positive cocci (GPCs), including Staphylococcus aureus and Staphylococcus epidermidis, were more common in the non-antibiotic group than in the antibiotic group (p <0.01). However, the proportion of gram-negative rods (GNRs) did not significantly differ between the two groups (p = 0.54). The antibiotic group had a significantly higher proportion of bacteria resistant to ampicillin (p <0.01) and first-generation cephalosporin (p = 0.01) than the non-antibiotic group. In conclusion, there was a difference in bacterial flora in the sphenoid sinus mucosal samples collected before and after intraoperative antibiotic administration.
Keywords: transsphenoidal surgery, bacterial flora, sphenoid sinus
Introduction
Endonasal transsphenoidal surgery (ETSS) is the most common procedure in managing pituitary lesions, and annually, this surgery has been increasingly performed.1) Meningitis and sinusitis are the most common infectious complications associated with ETSS. The incidence of meningitis after ETSS ranges from 0.7% to 10%.2–10) There are no global guidelines on the use of prophylactic antibiotics prior to ETSS, and recent systematic reviews have not identified any effective antibiotics.11) Moreover, the American Society of Health-System Pharmacists guidelines stated that there are no appropriate antibiotics for endoscopic sinus surgery.12) Although there is no strong evidence supporting the use of perioperative antibiotics for ETSS, surgeons commonly use prophylactic drugs to safely perform the procedure.13,14)
A previous report has shown that the bacterial flora detected in the nasal cavity before ETSS differs from that in the sphenoid sinus during surgery. Moreover, the bacterial flora in the sphenoid sinus had a high rate of drug resistance; therefore, the use of perioperative prophylactic antibiotics for ETSS should not be determined based on the type of nasal bacteria alone.15) Nasal mucosal samples can be preoperatively collected, and sphenoid sinus mucosal samples can only be obtained intraoperatively. In previous reports, sphenoid sinus mucosal samples were collected after prophylactic antibiotic administration.15,16) However, no study has assessed whether samples should be collected before or after the administration of prophylactic antibiotics. Hence, samples were commonsensically assumed to be collected after the administration of prophylactic antibiotics, which might have resulted in the selective detection of resistant bacteria. That is, the examination of an appropriate perioperative antibiotic based on these results may not be accurate. We hypothesized that the culture results of specimens collected after antibiotic administration are affected by the drug and that there may be differences between specimens collected before and after antibiotic administration. Therefore, the current study aimed to identify the difference in sphenoid sinus flora before and after antibiotic administration.
Materials and Methods
Study participants
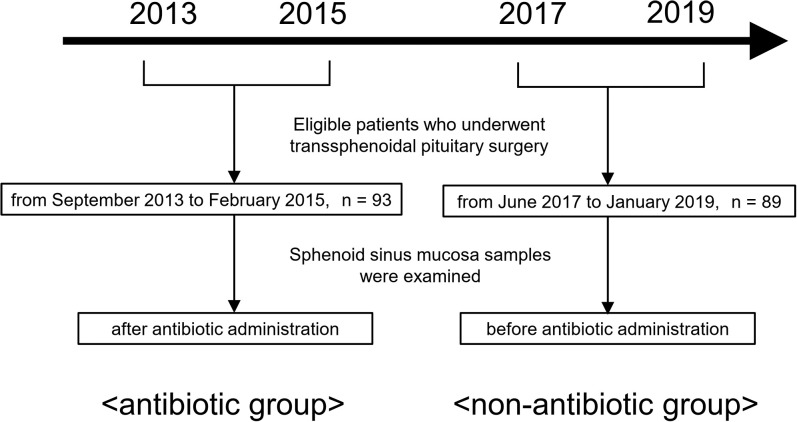
Overall, 121 and 109 consecutive ETSS were performed from September 2013 to February 2015 and from June 2017 to January 2019, respectively, at Nippon Medical School Hospital. Then, 93 and 89 patients were included in the study. Although there was a gap of several years between these two periods, the surgical procedure performed by the same surgeon who was proficient in pituitary surgery, including surgical instruments and disinfectant (0.02% benzalkonium chloride solution), did not change. In addition, we compared the bacterial flora of the entire hospital in the two groups based on bacteriological surveillance reports, and there was no apparent difference between the bacterial strains isolated during the two periods.
A previous report has shown that the sphenoid sinus mucosal samples of patients with pituitary apoplexy differed.16) Thus, patients with this condition, with the main purpose of cerebrospinal fluid (CSF) leakage repair, and those without an appropriate specimen were excluded. Patient characteristics are summarized in Table 1. Sphenoid sinus mucosal samples were collected during ETSS and were examined for bacterial culture. Samples were obtained after antibiotic administration in the first period and before antibiotic administration in the second period. The former was referred to as the antibiotic group and the latter the non-antibiotic group because the samples were not affected by antibiotic administration (Fig. 1). In the antibiotic group, sulbactam 0.5 g/ampicillin 1.0 g was immediately administered before surgery and every 3 hours thereafter. In cases in which CSF leakage was expected before surgery (e.g., patients requiring extended endoscopic ETSS), cefotaxime 1.0 g was administered. In the non-antibiotic group, antibiotics were selected using the same selection method and were immediately administered after sample collection, not before surgery and during the start of surgery. The time from the start of surgery to specimen collection was approximately 30 minutes to 1 hour in all cases. When collecting sphenoid sinus mucosal samples, caution was taken to prevent the collection device and sample from contacting the nasal corridor.
Table 1. Clinical characteristics of patients in two groups.
| Characteristics | Sample collection method | |
|---|---|---|
| Antibiotic | Non-antibiotic | |
| Total number of patients (n) | 93 | 89 |
| Sex (n) | ||
| male | 35 | 38 |
| female | 58 | 51 |
| Age, years (mean ± SD) | 51.0 ± 16.9 | 56.3 ± 13.9 |
| Disease (n) | ||
| Non-functioning pituitary adenoma | 39 | 34 |
| Functioning pituitary adenoma | 35 | 24 |
| Rathke's cleft cyst | 8 | 13 |
| Meningioma | 3 | 3 |
| Craniopharyngioma | 1 | 3 |
| Germinoma | 1 | 0 |
| Chordoma | 1 | 2 |
| Others | 5 | 10 |
Fig. 1.
Flowchart of patient selection during the two collection periods.
Bacterial culture
All specimens were subjected to culture tests with MicroScan WalkAway (Beckman Coulter, Inc., Brea, CA, USA). Then, drug susceptibility tests for the detected bacteria were performed. In this study, the drugs examined were ampicillin and first-generation (cefaclor and cefazolin), second-generation (cefmetazole, cefotiam, and flomoxef), and third-generation (cefcapene pivoxil, cefdinir, cefditoren pivoxil, cefixime, cefotaxime, ceftazidime, ceftriaxone, and sulbactam/cefoperazone) cephalosporins. The study design and protocol were approved by the Ethics Review Committee of Nippon Medical School (approval number: R1-08-1178), and a written informed consent was obtained from all patients.
Statistical analysis
Statistical differences were assessed using the chi-square test and Fisher’s exact test, and p values of <0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Table 2 shows the bacterial species detected on culture tests in the two groups. In some cases, multiple bacterial species were detected in one sample, and they were all counted. No bacteria were detected in 47 and 45 patients in the antibiotic and non-antibiotic groups, respectively. Staphylococcus aureus and Staphylococcus epidermidis were more common in the non-antibiotic group than in the antibiotic group.
Table 2. Bacterial isolates from sphenoid sinus mucosal samples in the two groups.
| Isolated bacteria | Sample collection method | |
|---|---|---|
| Antibiotic | Non-antibiotic | |
| Enterococcus faecalis | 1 | |
| Microccosus sp. | 1 | |
| MRSA | 1 | 2 |
| Parvimonas micra | 1 | |
| Peptostreptococcus | 6 | 1 |
| Staphylococcus aureus | 10 | |
| Staphyloccocus capitis | 1 | 3 |
| Staphylococcus epidermidis | 15 | 23 |
| Staphylococcus lugdunensis | 2 | 1 |
| Viridans Streptococcus | 1 | |
| Coryneform bacteria | 12 | 4 |
| Gram-positive rod | 1 | |
| Propionibacterium | 9 | 2 |
| Neisseria species | 1 | |
| Citrobacter koseri | 1 | |
| Enterobacter aerogenes | 1 | |
| Pseudomonas aeruginosa | 1 | |
| Normal bacterial flora | 2 | |
| Total number of patients (n) | 93 | 89 |
Blank indicates no detection.
MRSA: methicillin-resistant staphylococcus aureus.
In general, gram-positive coccus (GPC) and gram- negative rod (GNR) frequently cause surgical site infection.7,17,18) Hence, in the current analysis, the bacteria were classified into two groups and were then compared (Table 3). The proportion of GPCs was significantly higher in the non-antibiotic group than in the antibiotic group (p <0.01). However, there was no significant difference in proportion of GNRs between the two groups (p = 0.62).
Table 3. Gram-positive cocci and gram-negative rods isolated from sphenoid sinus mucosa samples in the two groups.
| Detected bacteria | Sample collection method | p value | |
|---|---|---|---|
| Antibiotic | Non-antibiotic | ||
| Gram-positive coccus | 27 | 42 | <0.01 |
| Gram-negative rod | 1 | 2 | 0.62 |
| Total number of bacterial isolates | 52 | 51 | |
| Total number of patients | 93 | 89 | |
Table 4 shows the detected bacteria and the number of bacteria resistant to antibacterial agents. The proportion of bacteria resistant to ampicillin (p <0.01) and first-generation cephalosporin (p = 0.01) in the antibiotic group was significantly higher than that in the non-antibiotic group.
Table 4. Distribution of bacterial species in the cultures of sphenoid sinus mucosal samples in the two groups.
| antibiotic group | |||||
|---|---|---|---|---|---|
| Total number of isolates | Ampicillin resistant | Cephalosporin resistant | |||
| First generation | Second generation | Third generation | |||
| Gram-positive coccus | 27 | 15 | 9 | 4 | 2 |
| Gram-negative rod | 1 | 1 | 1 | 1 | 0 |
| Total | 28 | 16 (57.1%) | 10 (35.7%) | 5 (17.8%) | 2 (7.1%) |
| non-antibiotic group | |||||
|---|---|---|---|---|---|
| Total number of isolates | Ampicillin resistant | Cephalosporin resistant | |||
| First generation | Second generation | Third generation | |||
| Gram-positive coccus | 42 | 10 | 5 | 0 | 0 |
| Gram-negative rod | 2 | 1 | 0 | 0 | 1 |
| Total | 44 | 11 (25.0%) | 5 (11.3%) | 0 (0%) | 1 (2.2%) |
Discussion
In the current research, the bacteria detected in the cultures of sphenoid sinus mucosal samples collected before and after antibiotic administration differed, and the results were evident. However, similar findings were not observed in previous studies. Furthermore, the rate of resistance to ampicillin and first-generation cephalosporin among bacteria detected in the sphenoid sinus mucosal samples collected before antibiotic administration was significantly lower than that in the samples collected after antibiotic administration. These results support the hypothesis that bacterial flora in the sphenoid sinus mucosal samples collected after antibiotic administration is affected by the drug.
The bacterial species in the antibiotic group in our study and those in previous studies15,16) were similar. That is, S. epidermidis and Propionibacterium, but not S. aureus, species were commonly detected. This result implied that no previous research has examined (or at least specified) the flora via sampling before antibiotic administration.
In this study, the proportion of GPCs was higher in the non-antibiotic group than in the antibiotic group. Therefore, some bacteria sensitive to the administered antibiotic were killed, and drug-resistant bacteria were selectively detected. Sulbactam/ampicillin, which was effective against most Staphylococcus species, was used in most cases. The rate of resistance to ampicillin and first-generation cephalosporin was significantly higher in the antibiotic group than in the non-antibiotic group. The detection of bacteria resistant to the antibiotics administered might have attributed to this result.
Previous reports have recommended the use of third-generation cephalosporin because of high resistance to first-generation cephalosporins.15,19) In the current study, the drug resistance rate (11.3% and 0% for the first- and second-generation cephalosporin, respectively) among unaffected bacteria in sphenoid sinus mucosal samples was not extremely high. The resistance rate was similar to that of the third-generation cephalosporin in the antibiotic group (7.1%). Hence, first-generation cephalosporins, including cefazolin, or second-generation cephalosporins, such as cefotiam, can be an effective prophylactic antibiotic. The Japanese practice guidelines on the proper use of antibacterial agents for postoperative infection recommend the use of cefazolin as a prophylactic antibacterial agent for endoscopic sinus surgery.13,19–21) This finding supports our results. However, postoperative CSF leakage is a risk factor for the development of postoperative meningitis after ETSS,2,3,22,23) and patients with diabetes or immunosuppressive drugs are at high risk, even without CSF leakage.24–29) Thus, we do not negate the use of third-generation cephalosporins in cases in which CSF leakage is expected preoperatively. After cephalosporins, penicillin-based antibiotics, including ampicillin, are the most commonly utilized antibiotics by pituitary surgeons.13) However, based on our results, ampicillin had an extremely high resistance rate; therefore, it is not effective.
The current study had several limitations. The bacterial flora might have been affected by factors such as the race of the patient and antibiotics used before admission. Although caution was taken to ensure that the collection device and sample did not come into contact with the nasal cavity upon collecting sphenoid sinus mucosal samples, the risk of intranasal contamination was undeniable.
In conclusion, there was a difference in bacterial flora in the sphenoid sinus mucosa samples collected before and after intraoperative antibiotic administration. This study proposed that first- or second- generation cephalosporins might be an effective prophylactic antibiotic for ETSS. Further, in high-risk patients or those with CSF leakage, switching to broad-spectrum antibiotics, such as third-generation cephalosporins, may be a useful option.
Conflicts of Interest Disclosure
All authors have no conflict of interest to declare.
References
- 1).Hattori Y, Tahara S, Aso S, et al. : Pituitary surgery's epidemiology using a national inpatient database in Japan. Acta Neurochir (Wien) 162: 1317–1323, 2020 [DOI] [PubMed] [Google Scholar]
- 2).Milanese L, Zoli M, Sollini G, et al. : Antibiotic prophylaxis in endoscopic endonasal pituitary and skull base surgery. World Neurosurg 106: 912–918, 2017 [DOI] [PubMed] [Google Scholar]
- 3).Ivan ME, Iorgulescu JB, El-Sayed I, et al. : Risk factors for postoperative cerebrospinal fluid leak and meningitis after expanded endoscopic endonasal surgery. J Clin Neurosci 22: 48–54, 2015 [DOI] [PubMed] [Google Scholar]
- 4).Borg A, Kirkman MA, Choi D: Endoscopic endonasal anterior skull base surgery: a systematic review of complications during the past 65 years. World Neurosurg 95: 383–391, 2016 [DOI] [PubMed] [Google Scholar]
- 5).Lai LT, Trooboff S, Morgan MK, Harvey RJ: The risk of meningitis following expanded endoscopic endonasal skull base surgery: a systematic review. J Neurol Surg B Skull Base 75: 18–26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Agam MS, Wedemeyer MA, Wrobel B, Weiss MH, Carmichael JD, Zada G: Complications associated with microscopic and endoscopic transsphenoidal pituitary surgery: experience of 1153 consecutive cases treated at a single tertiary care pituitary center. J Neurosurg 1: 1–8, 2018 [DOI] [PubMed] [Google Scholar]
- 7).Jin Y, Liu X, Gao L, et al. : Risk factors and microbiology of meningitis and/or bacteremia after transsphenoidal surgery for pituitary adenoma. World Neurosurg 110: e851–e863, 2018 [DOI] [PubMed] [Google Scholar]
- 8).Pagliano P, Caggiano C, Ascione T, et al. : Characteristics of meningitis following transsphenoidal endoscopic surgery: a case series and a systematic literature review. Infection 45: 841–848, 2017 [DOI] [PubMed] [Google Scholar]
- 9).Berker M, Hazer DB, Yücel T, et al. : Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 15: 288–300, 2012 [DOI] [PubMed] [Google Scholar]
- 10).Kassam AB, Prevedello DM, Carrau RL, et al. : Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg 114: 1544–1568, 2011 [DOI] [PubMed] [Google Scholar]
- 11).Moldovan ID, Agbi C, Kilty S, Alkherayf F: A systematic review of prophylactic antibiotic use in endoscopic endonasal transsphenoidal surgery for pituitary lesions. World Neurosurg 128: 408–414, 2019 [DOI] [PubMed] [Google Scholar]
- 12).Bratzler DW, Dellinger EP, Olsen KM, et al. : Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70: 195–283, 2013 [DOI] [PubMed] [Google Scholar]
- 13).Little AS, White WL: Prophylactic antibiotic trends in transsphenoidal surgery for pituitary lesions. Pituitary 14: 99–104, 2011 [DOI] [PubMed] [Google Scholar]
- 14).Smith EJ, Stringer S: Current perioperative practice patterns for minimizing surgical site infection during rhinologic procedures. Int Forum Allergy Rhinol 4: 1002–1007, 2014 [DOI] [PubMed] [Google Scholar]
- 15).Shibao S, Toda M, Tomita T, Ogawa K, Yoshida K: Analysis of the bacterial flora in the nasal cavity and the sphenoid sinus mucosa in patients operated on with an endoscopic endonasal transsphenoidal approach. Neurol Med Chir (Tokyo) 54: 1009–1013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Humphreys GJ, Waqar M, McBain AJ, Gnanalingham KK: Sphenoid sinus microbiota in pituitary apoplexy: a preliminary study. Pituitary 20: 619–623, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Srinivas D, Veena Kumari HB, Somanna S, Bhagavatula I, Anandappa CB: The incidence of postoperative meningitis in neurosurgery: an institutional experience. Neurol India 59: 195–198, 2011 [DOI] [PubMed] [Google Scholar]
- 18).Federico G, Tumbarello M, Spanu T, et al. : Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scand J Infect Dis 33: 533–537, 2001 [DOI] [PubMed] [Google Scholar]
- 19).Orlando R, Cappabianca P, Tosone G, Esposito F, Piazza M, de Divitiis E: Retrospective analysis of a new antibiotic chemoprophylaxis regimen in 170 patients undergoing endoscopic endonasal transsphenoidal surgery. Surg Neurol 68: 145–148; discussion 148, 2007 [DOI] [PubMed] [Google Scholar]
- 20).Little AS, White WL: Short-duration, single-agent antibiotic prophylaxis for meningitis in trans-sphenoidal surgery. Pituitary 14: 335–339, 2011 [DOI] [PubMed] [Google Scholar]
- 21).Brown SM, Anand VK, Tabaee A, Schwartz TH: Role of perioperative antibiotics in endoscopic skull base surgery. Laryngoscope 117: 1528–1532, 2007 [DOI] [PubMed] [Google Scholar]
- 22).Horowitz G, Fliss DM, Margalit N, Wasserzug O, Gil Z: Association between cerebrospinal fluid leak and meningitis after skull base surgery. Otolaryngol Head Neck Surg 145: 689–693, 2011 [DOI] [PubMed] [Google Scholar]
- 23).Kono Y, Prevedello DM, Snyderman CH, et al. : One thousand endoscopic skull base surgical procedures demystifying the infection potential: incidence and description of postoperative meningitis and brain abscesses. Infect Control Hosp Epidemiol 32: 77–83, 2011 [DOI] [PubMed] [Google Scholar]
- 24).Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D: Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg 257: 8–14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Ata A, Lee J, Bestle SL, Desemone J, Stain SC: Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg 145: 858–864, 2010 [DOI] [PubMed] [Google Scholar]
- 26).Okabayashi T, Shima Y, Sumiyoshi T, et al. : Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care 37: 1516–1524, 2014 [DOI] [PubMed] [Google Scholar]
- 27).Hendren S, Fritze D, Banerjee M, et al. : Antibiotic choice is independently associated with risk of surgical site infection after colectomy: a population-based cohort study. Ann Surg 257: 469–475, 2013 [DOI] [PubMed] [Google Scholar]
- 28).Yang ZP, Hong L, Wu Q, Wu KC, Fan DM: Preoperative infliximab use and postoperative complications in Crohn's disease: a systematic review and meta- analysis. Int J Surg 12: 224–230, 2014 [DOI] [PubMed] [Google Scholar]
- 29).Markel TA, Lou DC, Pfefferkorn M, et al. : Steroids and poor nutrition are associated with infectious wound complications in children undergoing first stage procedures for ulcerative colitis. Surgery 144: 540–545; discussion 545–547, 2008 [DOI] [PubMed] [Google Scholar]