PURPOSE:
Minority race and lower socioeconomic status are associated with lower rates of opioid prescription and undertreatment of pain in multiple noncancer healthcare settings. It is not known whether these differences in opioid prescribing exist among patients undergoing cancer treatment.
METHODS AND MATERIALS:
This observational cohort study involved 33,872 opioid-naive patients of age > 65 years undergoing definitive cancer treatment. We compared rates of new opioid prescriptions by race or ethnicity and socioeconomic status controlling for differences in baseline patient, cancer, and treatment factors. To evaluate downstream impacts of opioid prescribing and pain management, we also compared rates of persistent opioid use and pain-related emergency department (ED) visits.
RESULTS:
Compared with non-Hispanic White patients, the covariate-adjusted odds of receiving an opioid prescription were 24.9% (95% CI, 16.0 to 33.9, P < .001) lower for non-Hispanic Blacks, 115.0% (84.7 to 150.3, P < .001) higher for Asian–Pacific Islanders, and not statistically different for Hispanics (−1.0 to 14.0, P = .06). There was no significant association between race or ethnicity and persistent opioid use or pain-related ED visits. Patients living in a high-poverty area had higher odds (53.9% [25.4 to 88.8, P < .001]) of developing persistent use and having a pain-related ED visit (39.4% [16.4 to 66.9, P < .001]).
CONCLUSION:
For older patients with cancer, rates of opioid prescriptions and pain-related outcomes significantly differed by race and area-level poverty. Non-Hispanic Black patients were associated with a significantly decreased likelihood of receiving an opioid prescription. Patients from high-poverty areas were more likely to develop persistent opioid use and have a pain-related ED visit.
INTRODUCTION
Opioid prescribing patterns for patients with cancer are under increased scrutiny in the setting of the ongoing opioid crisis.1,2 As in the general population, rates of opioid-related deaths and emergency department (ED) visits have increased among patients with cancer over the past decade.3,4 There is also concern, however, that in response to the opioid epidemic, restrictive policies or fears over addiction could lead to systematic undertreatment of cancer pain.5 Prior studies suggest that up to 50% of patients with cancer have inadequate pain management and that minority patients are especially at risk for undertreatment.1,6,7
Disparities in opioid prescribing exist in the noncancer acute care setting, with non-Hispanic White (NHW) patients being more likely to receive opioid analgesics compared with non-Hispanic Black (NHB), Hispanic, or Asian–-Pacific Islander (API) patients.7-9 Patients from more affluent and educated neighborhoods are also more likely to receive an opioid prescription when presenting to the ED.10 It is not clear whether discrepancies in opioid prescribing exist among patients with cancer, who often undergo more protracted treatment regimens with multidisciplinary care. It is also not known if discrepancies in initial opioid prescribing by race, ethnicity, or socioeconomic status correlate with persistent opioid use or inadequate pain management among patients with cancer.
The purpose of this study is to determine if race and poverty are associated with the likelihood of receiving opioid analgesics during definitive cancer treatment. Understanding potential disparities in opioid prescriptions can highlight a need for additional research or a change in practice patterns to ensure equitable care. We evaluated rates of new opioid prescriptions in patients with breast, colon, lung, or prostate cancer among Medicare beneficiaries. Opioid prescription rates during cancer treatment were compared by race, ethnicity, and neighborhood poverty levels. To determine if initial discrepancies in prescribing affect long-term opioid behavior, we also compared rates of persistent opioid use one to 2 years after treatment. Rates of pain-related ED visits were also evaluated as a potential surrogate for inadequate pain management.
METHODS AND MATERIALS
Data Source and Patient Selection
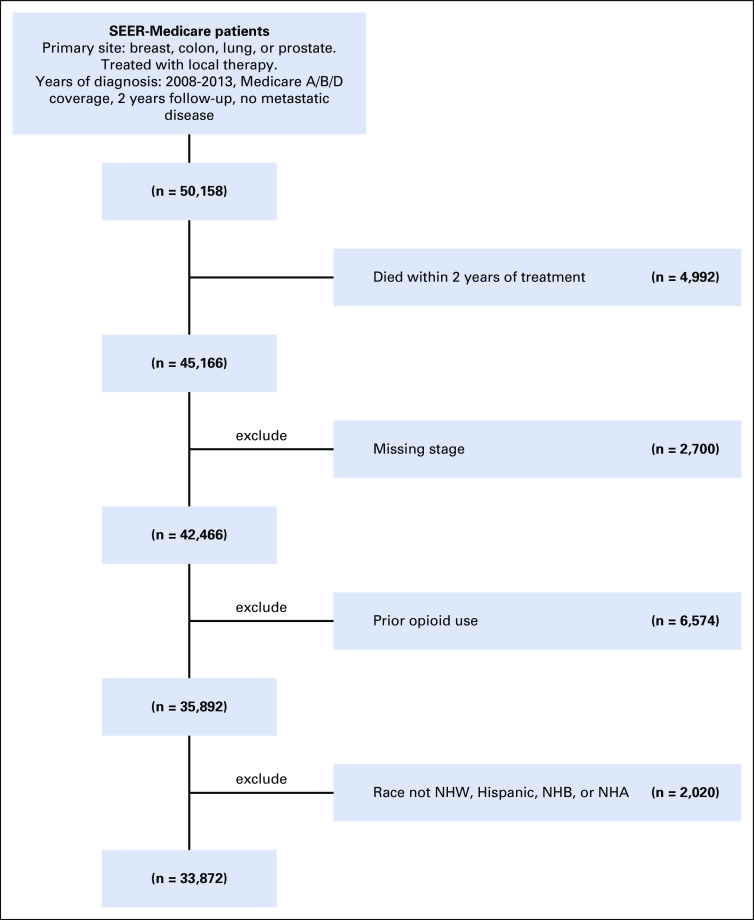
This retrospective cohort study evaluated patients in the SEER registry linked to Medicare claims data diagnosed with cancer from 2008 to 2013. SEER registry linked to Medicare claims data was chosen as a nationwide population-based data set with prescription data coupled with a cancer registry and demographic data. We included patients with one of the four most common noncutaneous solid malignancies (breast, colorectal, lung, and prostate), of age > 65 years, and with continuous Medicare Part A and B coverage, without enrollment in Medicare Part C (managed care) throughout the study period. Patients listed as other were not included because of low sample sizes. We included only patients with Medicare Part D coverage to capture opioid prescription data. We restricted the analysis to patients treated with definitive local therapy including surgery, radiation therapy, or both. Metastatic and palliative intent patients were excluded to analyze longer-term opioid prescribing patterns. This analysis only included opioid-naive patients who were defined as not having received an opioid prescription from one to twelve months prior to the cancer diagnosis.11-13 Patients were also excluded for metastatic disease, death within the first 2 years since starting treatment, or missing follow-up data. The final study cohort included 33,057 patients that met eligibility criteria. Appendix Figure A1 (online only) demonstrates the patient selection process.
Covariates, Exposures, and Outcomes
Baseline patient and cancer-related factors including age, sex, race, ethnicity, marital status, stage, and cancer type were extracted from the SEER database. Patients were categorized as NHW, API, NHB, or Hispanic. Patients were categorized as Hispanic if they were of Hispanic ethnicity irrespective of their race. Poverty rate was estimated using median household income data at the census-tract level and represents the percent of the local population living below the poverty level.14 Rural zip codes were defined as those with a population center < 20,000.15 The non–age-adjusted Charlson Comorbidity Index was calculated from Medicare claims data using the International Classification of Diseases, Ninth Edition diagnostic codes, as previously described.16 Similarly, claims and diagnostic codes were used to identify prior diagnoses of depression; alcohol abuse; and high-risk psychiatric conditions including schizophrenia, attention deficit disorder, and obsessive compulsive disease.17,18 The primary end point of a new opioid prescription during treatment was defined as receiving any opioid analgesic from one month prior to 3 months after the date of cancer diagnosis.11,12 Persistent opioid use was identified as having filled ≥ 120 days' supply or 10 or more opioid prescriptions in the window between 1 and 2 years after the start of treatment.11-13 Pain-related ED visits within 2 years of starting treatment were identified using International Classification of Diseases, Ninth Edition claims data.
Statistical Methods
The median and interquartile ranges were calculated for continuous variables by opioid prescription status and compared using a Mann-Whitney U Test. The absolute number and percentage were calculated for categorical variables and cohorts were compared using a chi-squared test.
Rates of new opioid prescriptions and persistent opioid use were calculated by race or ethnicity and area-level poverty subgroups; 95% CIs were estimated using a Pearson-Clopper interval.19 In an exploratory analysis, we also tested for differences in outcomes by poverty level when stratified by race. Multivariable logistic regression was used to estimate the odds of a new opioid prescription or persistent use when accounting for baseline patient, cancer-related, and treatment factors. Model covariates were considered significant with a two-sided P value < .05. Statistical analyses were performed using R version 3.5.3.20
RESULTS
Patient Characteristics and Outcomes
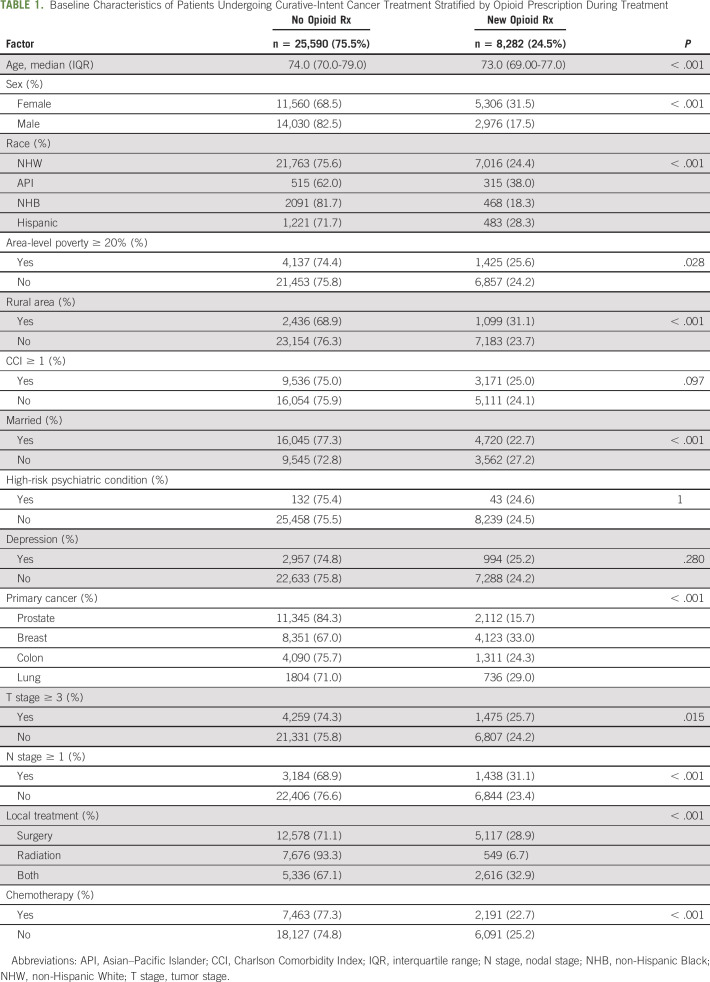
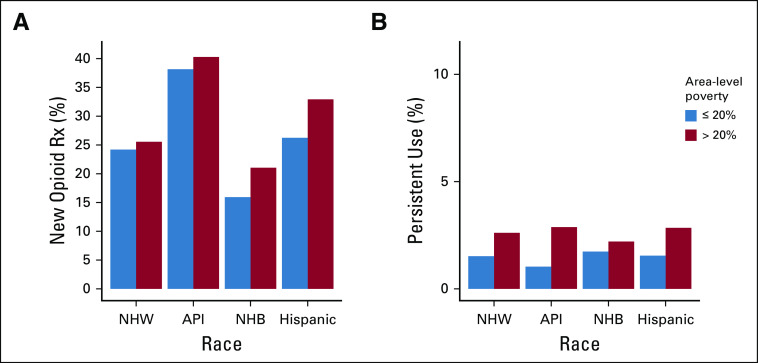
This cohort of opioid-naive patients undergoing definitive cancer treatment included 28,779 (84.9%) NHWs, 815 (2.4%) APIs, 2,559 (7.6%) NHBs, and 1,719 (5.1%) Hispanics (Table 1). There were 5,562 (16.4%) patients identified as living in areas with poverty levels ≥ 20%. When separated by race and ethnicity, rates of living in a high-poverty area were 13.0%, 17.1%, 46.0%, and 28.6% for NHW, API, NHB, and Hispanic patients, respectively. Rates of new opioid prescriptions during cancer treatment differed significantly (P < .001) among NHW (24.3%), API (38.0%), NHB (18.2%), and Hispanic (28.1%) patients (Fig 1A). Patients living in high-poverty areas had increased rates of new opioid prescriptions (25.2% v 23.9%, P = .03) (Fig 1B). On average, patients prescribed an opioid during treatment were younger than those not given a prescription (73.8 v 74.6, P < .001). Prescription rates also varied significantly by sex, marital status, cancer type, tumor stage, nodal stage, and local and systemic treatment (Table 1).
TABLE 1.
Baseline Characteristics of Patients Undergoing Curative-Intent Cancer Treatment Stratified by Opioid Prescription During Treatment
FIG 1.
Opioid prescription rates during cancer treatment by (A) race and (B) area-level poverty. API, Asian–Pacific Islander; NHB, non-Hispanic Black; NHW, non-Hispanic White; Rx, prescription.
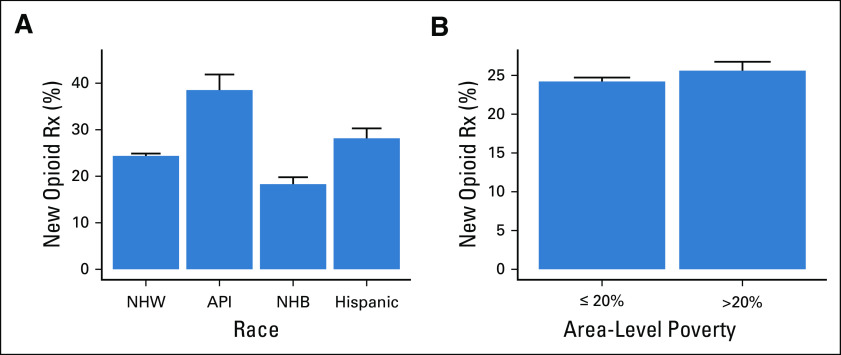
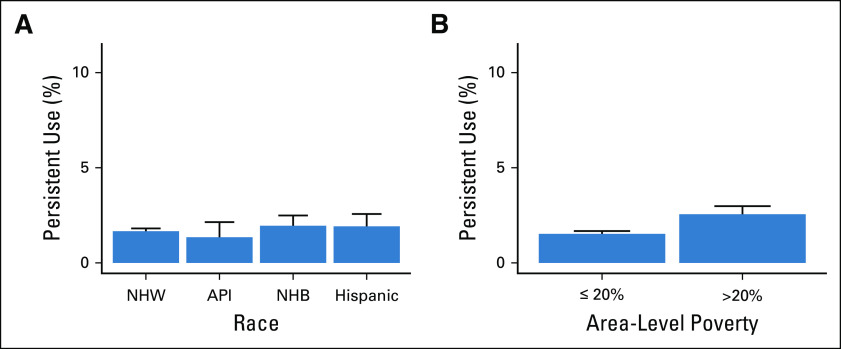
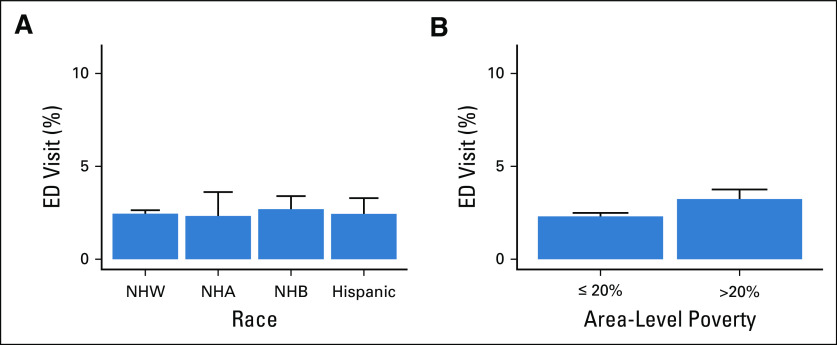
Rates of persistent opioid use did not differ significantly by race (P = .427) (Fig 2A). In contrast, patients living in communities with high poverty levels had a significantly increased rate of persistent opioid use (2.6% v 1.5%, P < .001) (Fig 2B). For the overall cohort, 2.4% of patients had a pain-related ED visit within 2 years since starting treatment. This rate did not vary significantly by race (P = .75) but did vary between patients living in high- and low-poverty areas, respectively (3.2% v 2.3%, P < .001) (Fig 3).
FIG 2.
Rates of persistent opioid use after cancer treatment by (A) race and (B) area-level poverty. API, Asian–Pacific Islander; NHB, non-Hispanic Black; NHW, non-Hispanic White; Rx, prescription.
FIG 3.
Rates of pain-related ED visits after cancer treatment by (A) race and (B) area-level poverty. API, Asian–Pacific Islander; ED, emergency department; NHB, non-Hispanic Black; NHW, non-Hispanic White.
When stratified by race and ethnicity, living in a high-poverty area was associated with significantly increased rates of new opioid prescriptions for NHB (21.1% v 15.9%, P = .001) and Hispanic (32.9% v 26.2%, P = .006) patients but not NHWs (25.6% v 24.2%, P = .075) or APIs (40.3 v 38.2, P = .71) (Appendix Fig A2, online only). Living in a high-poverty area was associated with increased rates of persistent use among NHW (2.6% v 1.5%, P < .001) patients (Appendix Fig A2). There was no significant association between poverty and increased persistent opioid use within NHB (2.2% v 1.7%, P = .48), Hispanic (2.9% v 1.6%, P = .11), or API (2.9% v 1.0%, P = .19) patients.
Multivariable Logistic Regression
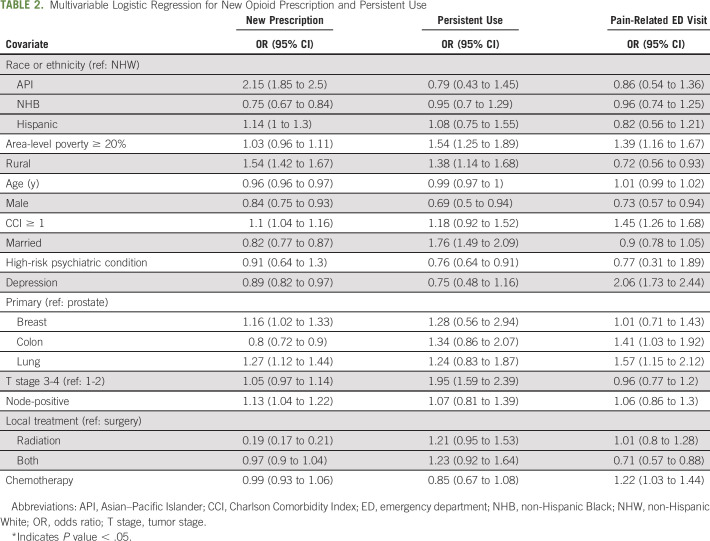
Compared with NHWs, the covariate-adjusted odds ratio for receiving a new opioid prescription was 0.75 (95% CI, 0.67 to 0.84, P < .001) for NHBs, 2.15 (1.85 to 2.50, P < .001) for APIs, and 1.14 (0.99 to 1.30, P = .06) for Hispanics (Table 2). There was no statistically significant association between race or ethnicity and persistent opioid use or pain-related ED visits. Patients living in a rural community had increased odds of receiving a new prescription (odds ratio [95% CI], 1.54 [1.42 to 1.67], P < .001) and decreased odds of having a pain-related ED visit (0.72 [0.56 to 0.93], P = .01). Living in a higher-poverty area was not associated with an increased likelihood of receiving a prescription during treatment on multivariable analysis but was associated with a higher likelihood for persistent opioid use (1.54 [1.25 to 1.89], P < .001) and having a pain-related ED visit (1.39 [1.16 to 1.67], P < .001]). Additional patient, cancer, and treatment factors correlated with receiving an opioid prescription or becoming a persistent user on multivariable analysis (Table 2).
TABLE 2.
Multivariable Logistic Regression for New Opioid Prescription and Persistent Use
DISCUSSION
In this population-based cohort study, NHBs were less likely to receive an opioid prescription during definitive cancer treatment when compared with NHWs, with no difference in rates of persistent opioid use or pain-related ED visits. APIs were more likely to receive an opioid during treatment; however, this did not translate into increased rates of persistent use. Patients in rural communities had an increased likelihood of having an opioid prescribed during treatment and a decreased likelihood of presenting to the ED for pain. We found that patients living in areas of high poverty had an increased likelihood of becoming a persistent user and having a pain-related ED visit. Within racial or ethnic groups, high area-level poverty was associated with increased rates of new opioid use for NHB and Hispanic but not NHW or API patients. High area-level poverty was associated with increased persistent use for NHW but not API, NHB, or Hispanic patients. Subgroup analysis for persistent opioid use among the NHB and Hispanic patients had limited power because of smaller sample sizes and limited events.
Associations between race and both adequate pain management and opioid prescriptions have been previously described in multiple healthcare settings.21-24 In an analysis of the National Hospital Ambulatory Medical Care Survey, NHW patients presenting to the ED for pain-related visits were more likely to receive an opioid compared with NHB, Hispanic, or API patients.8 NHB children were less likely to receive analgesia for a diagnosis of appendicitis and less likely to receive opioids for severe pain in a population-based study of ED visits.25 To what degree race is associated with opioid analgesic use among patients with cancer is less clear, especially during the era of the ongoing opioid crisis. In a cross-sectional study of older patients with cancer living in nursing homes from the 1990s, minority race and advanced age were associated with undertreatment of daily pain.6 A more recent prospective cohort study found that among ambulatory patients with cancer, the odds of undertreatment of pain was twice as high for minority patients.7
The etiology of disparities in pain management by race and ethnicity is not fully understood. Hypothesized barriers to optimal pain management include factors related to healthcare systems, providers, implicit biases, communication, and individual patient beliefs and coping strategies.22 In a survey study of minority patients with cancer and their providers, inadequate pain assessment was identified as a primary barrier for optimal pain management.26 Others have suggested that challenges in communication between NHW providers and minority patients may limit trust and result in discrepancies in the perceived levels of pain.26-28
The association between socioeconomic status, pain control, and risk for adverse opioid outcomes in patients with cancer is less studied. We found that patients from lower-income areas had increased rates of prescriptions on univariable but not multivariable analysis. Rurality was a more dominant explanatory variable with increased rates of opioid prescriptions on both univariable and multivariable analyses (results not shown). Prior studies from the noncancer acute care setting have demonstrated decreased prescription rates among low-income patients.10,29 An additional study of cancer survivors in Ontario, Canada, found a strong correlation between lower-income quantile and higher rates of opioid prescriptions among cancer survivors.30
Although the association between poverty and new opioid prescriptions differs across healthcare settings, the association between poverty and adverse opioid outcomes is more consistent. Several studies have described higher rates of adverse opioid outcomes among patients from lower-income communities, which could correspond to the higher rates of persistent use observed in our study. Our data also suggest that low-income patients are at increased risk for inadequate pain control as evidenced by the increased rate in pain-related ED visits. In a nationwide cross-sectional study of Medicare enrollees, poverty was associated with opioid-related mortality at the county level.29 Further, a population-based study of opioid-related events requiring emergency room evaluation revealed that opioid-related harms occurred nearly 2.4 times more frequently in the lowest-income quintile when compared with the highest quintile.31 High levels of opioid-related morbidity and mortality in this population could be attributed to lower healthcare utilization, especially regarding mental health and substance abuse treatment.32 There may also be decreased access to nonpharmacologic therapies in economically disadvantaged communities.33
Optimal treatment of cancer pain with opioid analgesics requires an assessment of risks and benefits for an individual patient.1 A primary limitation of this study is the inability to measure the level of pain and adequacy of pain control from the available data. Without this information, it is challenging to determine whether opioids are being prescribed appropriately. Depending on the clinical scenario, the risks associated with opioid use may outweigh benefits and lower prescription rates may be more in line with best practices.34 Given the history of undertreatment of pain in minority patients with cancer, however, our findings raise concern that NHB patients may be at risk for inadequate pain management with opioids. The higher rates of persistent use among patients from high-poverty areas has not been well-studied in the cancer population. Our findings suggest that this population may be at higher risk for adverse outcomes and could benefit from increased risk mitigation techniques such as integrating nonopioid analgesics or referrals into pain management or counseling services.35 A lack of resources for pain management is further evidenced by the increased utilization of the ED for pain among lower-income patients.
The results of this study should be interpreted in the context of its limitations. This analysis was limited to patients of age > 65 years continuously enrolled on Medicare Part D. It is not known if our findings apply to younger patients with cancer or those with varying levels of health insurance coverage. The population-based nature of the study also makes it difficult to determine an etiology for the observed discrepancies in opioid prescribing. Racial and ethnic groups beyond NHBs, Hispanics, and NHWs were underrepresented in this cohort and require future research. This data set was also limited by a lack of information on prescribers and institutions. Future research is warranted to determine if discrepancies in opioid use can be explained by institutional or prescribing practice patterns. Of note, previously observed racial disparities in cancer outcomes have been found to be reduced or absent in equal-access health systems such as the Veteran's Affairs medical system.36,37 Opioid prescribing patterns are dynamic and have been continuing to evolve over the past two decades.38 It is possible that the practice patterns, including the presence of disparities, have changed since the time frame of this cohort study from 2008 to 2013.
In conclusion, there are discrepancies in opioid prescriptions by race and area-level income for patients undergoing cancer treatment. In this cohort, NHB patients were less likely to be prescribed an opioid analgesic during cancer treatment without an increased risk for persistent use. Patients from high-poverty areas were more at increased risk for persistent opioid use and presenting to the ED for pain. Prescribing opioids for patients with cancer can involve complicated decision making requiring a personalized assessment of risks and benefits that is further confounded by the ongoing opioid epidemic.39,40 To ensure equitable, compassionate care, additional research is needed to identify the etiology of and solutions to disparities in opioid prescribing and risk for adverse outcomes.
APPENDIX
FIG A1.
Flow diagram showing patient inclusion and exclusion in observational cohort. NHW, non-Hispanic White; NHB, non-Hispanic Black; NHA, non-Hispanic Asian.
FIG A2.
Rates of (A) new opioid prescription during treatment and (B) persistent opioid use after treatment stratified by race and area-level poverty. API, Asian–Pacific Islander; NHB, non-Hispanic Black; NHW, non-Hispanic White; Rx, prescription.
Paul Riviere
Consulting or Advisory Role: Peptide Logic
Loren K. Mell
Honoraria: Nanobiotix
Consulting or Advisory Role: Bayer
Research Funding: Merck, AstraZeneca
Timothy Furnish
Travel, Accommodations, Expenses: Boston Scientific, Medtronic, Abbott Laboratories, Nevro
James D. Murphy
Consulting or Advisory Role: Boston Consulting Group
Research Funding: eContour
No other potential conflicts of interest were reported.
SUPPORT
Supported by an ASCO Conquer Cancer Foundation Young Investigator Award (L.K.V.) and supported by U54CA132384 and U54CA132379.
AUTHOR CONTRIBUTIONS
Conception and design: Lucas K. Vitzthum, Paul Riviere, Timothy Furnish, James D. Murphy
Collection and assembly of data: Lucas K. Vitzthum, Vinit Nalawade, Paul Riviere
Data analysis and interpretation: Lucas K. Vitzthum, Vinit Nalawade, Whitney Sumner, Tyler Nelson, Loren K. Mell, Timothy Furnish, Brent Rose, María Elena Martínez
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial, Ethnic, and Socioeconomic Discrepancies in Opioid Prescriptions Among Older Patients With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Paul Riviere
Consulting or Advisory Role: Peptide Logic
Loren K. Mell
Honoraria: Nanobiotix
Consulting or Advisory Role: Bayer
Research Funding: Merck, AstraZeneca
Timothy Furnish
Travel, Accommodations, Expenses: Boston Scientific, Medtronic, Abbott Laboratories, Nevro
James D. Murphy
Consulting or Advisory Role: Boston Consulting Group
Research Funding: eContour
No other potential conflicts of interest were reported.
REFERENCES
- 1.Paice JA: A delicate balance: Risks vs benefits of opioids in cancer pain. Pain 161:459-460, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Paice JA: Cancer pain management and the opioid crisis in America: How to preserve hard-earned gains in improving the quality of cancer pain management. Cancer 124:2491-2497, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Jairam V, Yang DX, Yu JB, et al. : Emergency department visits for opioid overdoses among patients with cancer. J Natl Cancer Inst 112:938-943, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chino F, Kamal A, Chino J: Opioid associated deaths in cancer survivors: A population study of the opioid epidemic. JAMA Oncol 6:1100-1102, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paice JA: Managing pain in patients and survivors: Challenges within the United States opioid crisis. J Natl Compr Canc Netw 17:595-598, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Bernabei R, Gambassi G, Lapane K, et al. : Management of pain in elderly patients with cancer. J Am Med Assoc 279:1877-1882, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Fisch MJ, Lee JW, Weiss M, et al. : Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol 30:1980-1988, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pletcher MJ, Kertesz SG, Kohn MA, et al. : Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA 299:70-78. 2008 [DOI] [PubMed] [Google Scholar]
- 9.Lawrence AE, Deans KJ, Chisolm DJ, et al. : Racial disparities in receipt of postoperative opioids after pediatric cholecystectomy. J Surg Res 245:309-314, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joynt M, Train MK, Robbins BW, et al. : The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J Gen Intern Med 28:1604-1610, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitzthum LK, Riviere P, Sheridan P, et al. : Predicting persistent opioid use, abuse and toxicity among cancer survivors. J Natl Cancer Inst 112:720-727, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JSJ, Hu HM, Edelman AL, et al. : New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol 35:4042-4049, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brummett CM, Waljee JF, Goesling J, et al. : New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 152:e170504, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schootman M, Jeffe DB, Lian M, et al. : The role of poverty rate and racial distribution in the geographic clustering of breast cancer survival among older women: A geographic and multilevel analysis. Am J Epidemiol 169:554-561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollica MA, Weaver KE, McNeel TS, et al. : Examining urban and rural differences in perceived timeliness of care among cancer patients: A SEER-CAHPS study. Cancer 124:3257-3265, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613-619, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Regier DA, Farmer ME, Rae DS, et al. : Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 264:2511-2518, 1990 [PubMed] [Google Scholar]
- 18.Webster LR, Webster RM: Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the opioid risk tool. Pain Med 6:432-442, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404, 1934 [Google Scholar]
- 20.R Core Team : A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2019. https://www.r-project.org/ [Google Scholar]
- 21.Todd KH, Samaroo N, Hoffman JR: Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA 269:1537-1539, 1993 [PubMed] [Google Scholar]
- 22.Anderson KO, Green CR, Payne R: Racial and ethnic disparities in pain: Causes and consequences of unequal care. J Pain 10:1187-1204, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Green CR, Baker TA, Sato Y, et al. : Race and chronic pain: A comparative study of young black and white Americans presenting for management. J Pain 4:176-183, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ng B, Dimsdale JE, Rollnik JD, et al. : The effect of ethnicity on prescriptions for patient-controlled analgesia for post-operative pain. Pain 66:9-12, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Goyal MK, Kuppermann N, Cleary SD, et al. : Racial disparities in pain management of children with appendicitis in emergency departments. JAMA Pediatr 169:996-1002, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson KO, Mendoza TR, Valero V, et al. : Minority cancer patients and their providers. Cancer 88:1929-1938, 2000 [PubMed] [Google Scholar]
- 27.Gordon HS, Street RL, Sharf BF, et al. : Racial differences in trust and lung cancer patients' perceptions of physician communication. J Clin Oncol 24:904-909, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Surbone A: Cultural competence in oncology: Where do we stand? Ann Oncol 21:3-5, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Grigoras CA, Karanika S, Velmahos E, et al. : Correlation of opioid mortality with prescriptions and social determinants: A cross-sectional study of Medicare enrollees. Drugs 78:111-121, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Sutradhar R, Lokku A, Barbera L: Cancer survivorship and opioid prescribing rates: A population-based matched cohort study among individuals with and without a history of cancer. Cancer 123:4286-4293, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Cairncross ZF, Herring J, van Ingen T, et al. : Relation between opioid-related harms and socioeconomic inequalities in Ontario: A population-based descriptive study. C Open 6:E478-E485, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack KA, Zhang K, Paulozzi L, et al. : Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved 26:182-198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebauer S, Salas J, Scherrer JF: Neighborhood socioeconomic status and receipt of opioid medication for new back pain diagnosis. J Am Board Fam Med 30:775-783, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Howard RA, Klueh MP, et al. : The impact of education and prescribing guidelines on opioid prescribing for breast and melanoma procedures. Ann Surg Oncol 26:17-24, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalal S, Bruera E: Pain management for patients with advanced cancer in the opioid epidemic era. Am Soc Clin Oncol Educ Book 39:24-35, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Riviere P, Luterstein E, Kumar A, et al. : Survival of African American and non‐Hispanic white men with prostate cancer in an equal‐access health care system. Cancer 126:1683-1690, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Deka R, Parsons JK, Simpson DR, et al. : African-American men with low-risk prostate cancer treated with radical prostatectomy in an equal-access health care system: Implications for active surveillance. Prostate Cancer Prostatic Dis 23:581-588, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Jones MR, Viswanath O, Peck J, et al. : A brief history of the opioid epidemic and strategies for pain medicine. Pain Ther 7:13-21, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schatz AA, Oliver TK, Swarm RA, et al. : Bridging the gap among clinical practice guidelines for pain management in cancer and sickle cell disease. JCO Oncol Pract 18:392-399, 2020 [DOI] [PubMed] [Google Scholar]
- 40.Vitzthum LK, Riviere P, Murphy JD: Managing cancer pain during the opioid epidemic—balancing caution and compassion. JAMA Oncol 6:1103-1104, 2020 [DOI] [PubMed] [Google Scholar]