PURPOSE:
The recent sorafenib versus radioembolization in advanced hepatocellular carcinoma (SARAH) and selective internal radiation therapy versus sorafenib in locally advanced hepatocellular carcinoma (SIRveNIB) trials showed no statistically significant difference in overall survival for randomization to selective internal radiotherapy (SIRT) versus sorafenib for locally advanced hepatocellular carcinoma, although SIRT was better tolerated. Given the high cost of both treatments, we investigated their comparative cost-effectiveness from a US healthcare sector perspective.
PATIENTS AND METHODS:
We constructed a state-transition microsimulation model to simulate patients allocated to SIRT versus sorafenib according to an intention-to-treat principle. Hazard rates of disease progression and death were based on pooled individual patient data generated from the SARAH and SIRveNIB trials’ Kaplan-Meier curves. Inputs for adverse events, treatment adherence, and quality of life utility weights were derived from trial data as well. Costs were based on Medicare reimbursement rates and literature. We performed probabilistic sensitivity analysis and estimated costs and quality-adjusted life years (QALYs) over a 5-year time horizon. We evaluated sensitivity to uncertainty of key model parameters.
RESULTS:
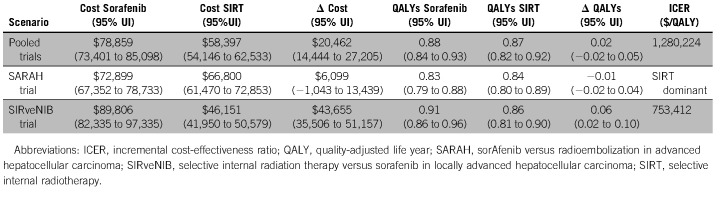
Costs were $78,859 v $58,397 (difference $20,462; 95% uncertainty interval $14,444 to 27,205) and QALYs were 0.88 v 0.87 (difference 0.02, −0.02 to 0.05) for sorafenib versus SIRT, respectively. The incremental cost-effectiveness ratio (ICER) of sorafenib was $1,280,224/QALY. The likelihood that sorafenib would be cost effective did not exceed 1%, assuming cost-effectiveness thresholds up to $200k/QALY. If the monthly price of sorafenib decreased from $16,390 to below $7,000, the ICER of sorafenib fell below $200k/QALY, and an ICER < $100k/QALY was reached if the monthly price fell below $6,600.
CONCLUSION:
Sorafenib is unlikely to provide a gain in quality-adjusted survival compared with SIRT at an acceptable cost for the US healthcare sector. Only if the current price decreased by more than 50% would sorafenib be considered economically attractive.
BACKGROUND
Primary liver cancer is the second leading cause of cancer-related death worldwide, accounting for an estimated 745,000 deaths in 2012 alone.1 Hepatocellular carcinoma (HCC) accounts for 70%-90% of these cases,2 with an increase in HCC incidence observed over the past several decades in the United States that has largely been attributed to the high prevalence of chronic hepatitis C infection.3 While patients diagnosed with early-stage HCC can benefit from potentially curative treatments, most cases are diagnosed with intermediate or advanced stage disease, for which limited treatment options are available and prognosis is poor.4
The oral multikinase inhibitor sorafenib has been the reference treatment for locally advanced and metastatic HCC, given that it was shown to significantly extend overall survival (OS) compared with placebo (10.7 v 7.9 months [hazard ratio 0.69, 95% CI, 0.55 to 0.87] in the SHARP trial).5,6 Despite its efficacy, sorafenib is associated with significant toxicities that often lead to dose discontinuation or reduction.5,6 Selective internal radiotherapy (SIRT) is an alternative therapy that delivers yttrium-90 (Y-90) microspheres via the hepatic artery, allowing for targeted delivery of high-dose radiation directly to the tumor. Retrospective studies have associated SIRT with promising potential as a safe and efficacious treatment option for locally advanced HCC.7–10
The recently published SorAfenib versus Radioembolization in Advanced HCC (SARAH) and Selective Internal Radiation Therapy Versus Sorafenib in Locally Advanced HCC (SIRveNIB) randomized trials showed no statistically significant difference in OS, the primary end point, for randomization to SIRT versus sorafenib.11,12
While both SIRT and sorafenib continue to be a mainstay of treatment for patients with locally advanced HCC, there remains uncertainty in guidance on the optimal first-line treatment for this patient population, with clinical practice guidelines continuing to recommend systemic treatment in the absence of definitive data suggesting superiority of alternate therapies.13 Given that both the SARAH and SIRveNIB trials failed to meet their superiority end point, the potential differences in quality of life and costs between sorafenib and SIRT may come to the forefront of treatment decision making. Therefore, we sought to investigate the cost-effectiveness of these two modalities of treatment for locally advanced, inoperable HCC.
METHOD
Study Population
Our analysis was based on pooled individual patient time-to-event data derived from the SARAH (N = 237 in SIRT arm, N = 222 in sorafenib arm) and SIRveNIB (N = 182 in SIRT arm, N = 178 in sorafenib arm) trials of patients with advanced HCC, who were randomly assigned to receive either SIRT or sorafenib. Eligible patients had an Eastern Cooperative Oncology Group performance status score of 0 or 1, Child-Pugh liver function class A or B (score of 7 or lower), and locally advanced disease (Barcelona Clinic Liver Cancer stage B or C with limited or no distant metastases).14 In the pooled SIRT arm (N = 419), the majority of suitable patients received a single SIRT administration, with a minority receiving multiple treatments based on tumor location or incomplete or unsuccessful initial administration (N = 58 received 2 treatments; N = 11 received 3 treatments). In the pooled sorafenib arm (N = 400), oral sorafenib treatment was started at 400 mg twice daily and was continued until intolerable adverse events (AEs), radiological progression, or death. Institutional review board approval was not relevant to this project since no human subjects were involved.
State-Transition Microsimulation Model
We developed a state-transition microsimulation model to analyze the cost-effectiveness of SIRT versus sorafenib in advanced HCC from a US healthcare sector perspective. The health states included stable disease, progression, and death, with all patients beginning in the stable disease state and with death being a terminal state (Fig 1). The model simulated transitions from stable disease to progression and progression to death with a cycle length of 1 month for 5 years. The cycle length was selected given that key events in the model occurred within months of randomization, while the 5-year time horizon was based on the projected survival at this time point on the published trial curves. Following disease progression, patients were treated with a second-line regimen or supportive care until death. Postprogression second-line regimens were modeled after trial-reported data and included transarterial chemoembolization (TACE), SIRT, continuation of sorafenib, or stereotactic body radiotherapy (SBRT) for the sorafenib strategy, and TACE, sorafenib, repeat SIRT, or SBRT for the SIRT arm. In addition to disease progression, the model tracked serious (grade ≥ 3) AEs as reported in the trials. The most commonly reported AEs in prior literature were included in the model.15–17
Fig 1.
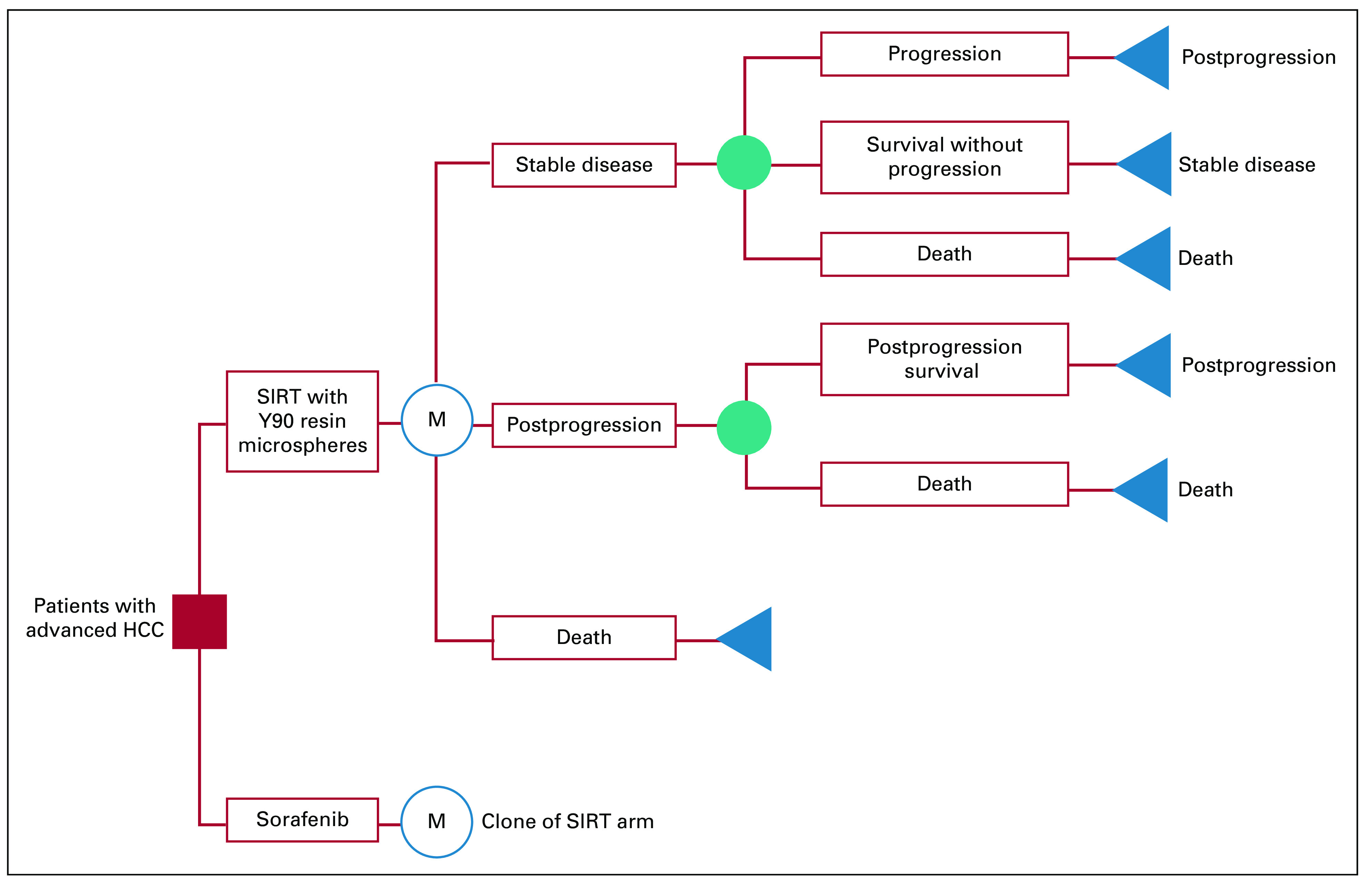
Schematic state-transition diagram. HCC, hepatocellular carcinoma; SIRT, selective internal radiotherapy.
Transition Probabilities
Hazard rates of disease progression and death were based on a pooled analysis of individual patient time-to-event data generated from Kaplan-Meier curves as reported for the intention-to-treat analysis of the SARAH and SIRveNIB trials. Using the software DigitizeIt, we traced coordinates of the published Kaplan-Meier curves for OS and progression-free survival. We used these coordinates with information on the total number of events and numbers at risk at several time points as inputs into a previously published algorithm18 to generate the individual patient survival and follow-up times from both trials separately. Nelson-Aalen estimates of the cumulative hazard functions were fitted for OS and progression-free survival. We used cubic spline functions of the log cumulative hazard and log time to model and extrapolate 1-month hazards beyond trial duration, and progression rates were adjusted to competing death risk. The microsimulation model’s predictions of progression-free and OS were compared against those observed in the trial demonstrating good validity (Appendix Fig A1, online only).
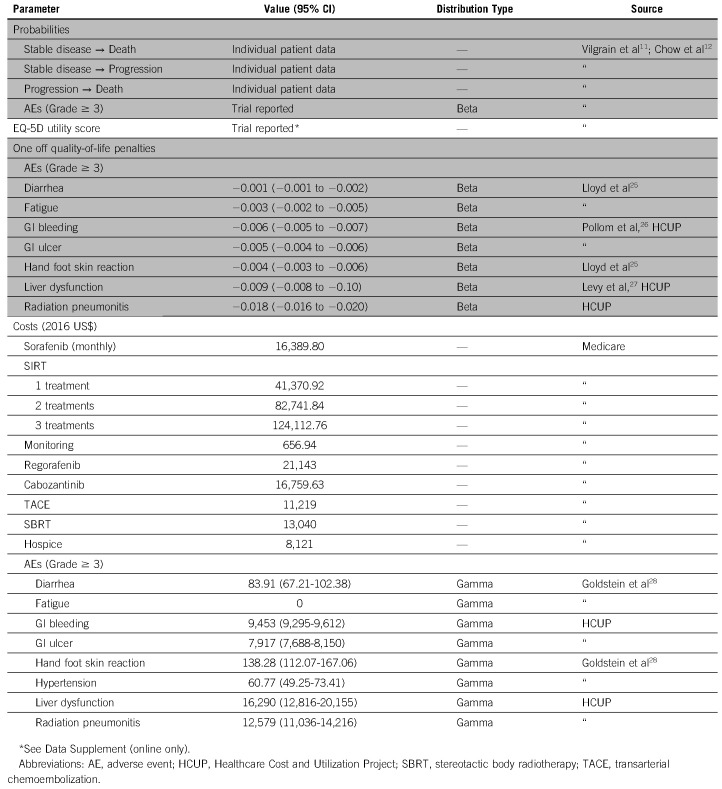
Costs and Utilities
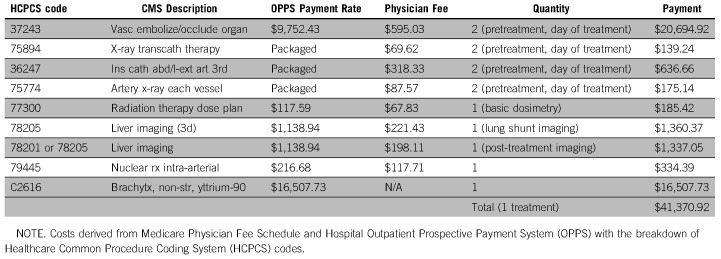
The monthly cost of sorafenib was derived from the Medicare Part D drug pricing schedule.19 The cost of SIRT was derived from the 2017 Medicare Physician Fee Schedule (MPFS)20 and Hospital Outpatient Prospective Payment System (OPPS)21 using a detailed breakdown of Current Procedural Terminology codes (Appendix Table A1). The cost of subsequent SIRT treatments was calculated in the same manner as the initial administration. For all patients, monitoring and follow-up involved an outpatient oncology visit and computerized tomography scan of the abdomen every 3 months. The associated costs were derived from MPFS and OPPS payment rates. For second-line regimens, costs were based on Medicare data for Part D drug pricing and physician fee schedule. AE-associated costs were derived from Healthcare Cost and Utilization Project (HCUP)22 data and the literature. Treatment adherence and crossover were modeled based on the trials. We assumed these payment estimates to be fixed based on set Medicare payment schedules. Costs were adjusted to 2016/2017 US dollars using Personal Consumption Expenditure Health indices.
Health-related quality of life was based on EuroQol 5-D (EQ-5D) data published in the SIRveNIB trial and EQ-5D utility values with utilities estimated from European Organization for Research and Treatment of Cancer Quality of Life Questionnaire data23 reported in the SARAH trial (see Appendix Table A2). We modeled major AEs (grade ≥ 3) with one-time quality-of-life penalties based on length-of-stay values or duration of symptoms derived from the literature or HCUP data. We discounted all costs and utility weights accounting for time preference at a 3% discount rate.24 See Table 1 for model parameters.
TABLE 1.
Model Parameters
Cost-Effectiveness Analyses
For the base-case, we used pooled individual patient time-to-event data from the two trials and ran a probabilistic sensitivity analysis by drawing 1,000 random samples from prespecified parametric distributions. We performed 1,000 microsimulations with each set of sampled parameters. We tracked cumulative costs and quality-adjusted life years (QALYs) over time for each simulation and calculated averages and 95% uncertainty intervals (95% UIs) for the 1,000 samples.
We calculated the incremental cost-effectiveness ratio (ICER) as when one of the treatment strategies was both more expensive and effective than the other treatment, ie absence of absolute dominance. We assessed probabilities of cost-effectiveness (acceptability) by evaluating the number of samples including dominance or a favorable ICER assuming cost-effectiveness or willingness-to-pay (WTP) thresholds of $100,000 and $200,000 per QALY gained.29
In sensitivity analyses, we modeled the SARAH and SIRveNIB trials separately, using trial-specific time-to-event and quality of life data. We also modeled the pooled trials assuming equal OS between both arms in each simulation. Additionally, we considered two scenarios in which regorafenib or cabozantinib was administered to patients as second-line treatments, based on recent trials showing a survival benefit associated with these agents compared with placebo following progression on sorafenib.30,31 Finally, we varied the cost of sorafenib and SIRT in one-way sensitivity analyses to determine the cost at which the sorafenib strategy could be considered cost effective at each of the WTP thresholds.
Analyses were performed using R and TreeAge Pro 2018 (TreeAge Software, Williamstown, MA).
RESULTS
For the base-case, the average costs and effectiveness of sorafenib were $78,859 (95% UI, 73,041-85,098) and 0.88 (0.84-0.93) QALYs over the 5-year time horizon. For SIRT, the average costs and QALYs were $58,397 (54,146-62,533) and 0.87 (0.82-0.92), respectively, and the resulting ICER for sorafenib was $1,280,224/QALY (Table 2).
TABLE 2.
Incremental Costs and Quality-Adjusted Life Years Over 5-Year Time Horizon
When the SARAH trial was modeled separately, sorafenib was dominated by SIRT due to sorafenib’s less favorable costs and effectiveness of $72,899 (67,352-78,733) and 0.83 (0.79-0.88): $6,099 (−1,042-13,439) more expensive and 0.01 (−0.04-0.02) QALYs less effective than SIRT. For the SIRveNIB trial, costs and QALYs for the sorafenib arm were $89,806 (82,335-97,335) and 0.91 (0.86-0.96), respectively, which was $43,655 (35,506-51,157) more expensive and 0.06 (0.02-0.10) QALYs more effective than SIRT. The resulting ICER for sorafenib was $753,412/QALY for the SIRveNIB trial (Table 2).
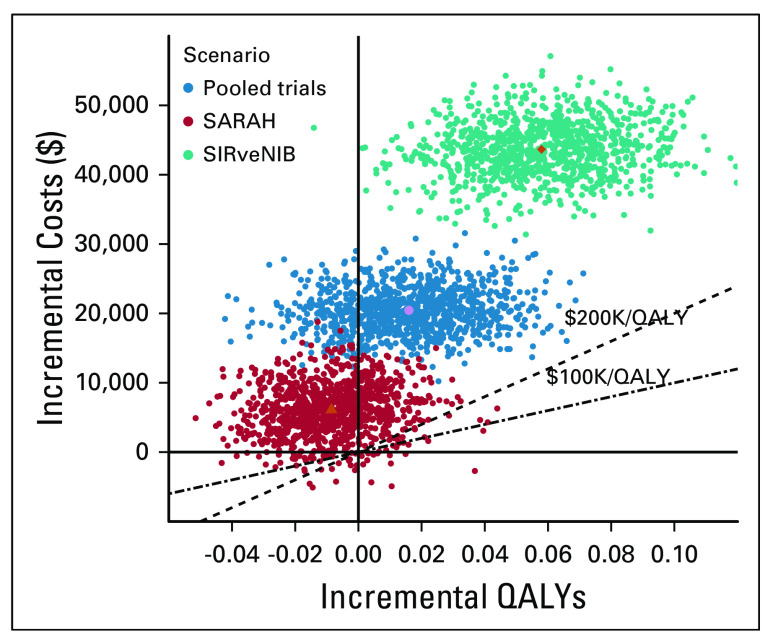
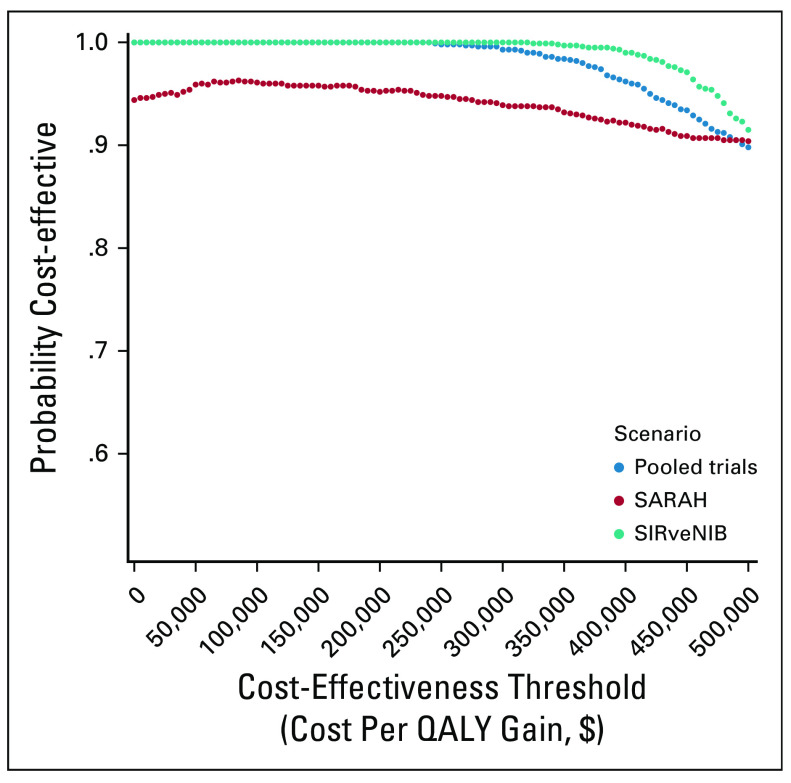
Figure 2 summarizes the results of the base-case probabilistic sensitivity analysis as a scatter plot. In this figure, none of the simulations (0/1,000) fell below either the $200,000/QALY or the $100,000/QALY cost-effectiveness threshold. For a wide range of cost-effectiveness thresholds ($0-500,000/QALY), there was a high likelihood that SIRT would be considered more cost-effective, with likelihoods of approximately 99%-100% in both the base-case and analysis of the SIRveNIB trial, and 90%-94% in the SARAH trial analysis (Fig 3).
Fig 2.
Scatter plot showing incremental costs and QALYs comparing sorafenib versus SIRT from the probabilistic sensitivity analysis. QALY, quality-adjusted life year; SIRT, selective internal radiotherapy.
Fig 3.
Probability of SIRT being cost effective at different cost-effectiveness thresholds. The probability of sorafenib being cost effective equals 100% minus the depicted probability of SIRT being cost effective. QALY, quality-adjusted life year; SIRT, selective internal radiotherapy.
When equal OS was assumed between the two treatment arms, SIRT dominated with average costs and effectiveness of $59,918 (55,604-64,202) and 0.95 (0.89-1.00), compared with $78,859 (73,041-85,098) and 0.88 (0.84-0.93) for sorafenib. For the scenario where regorafenib was administered to patients receiving second-line treatment, the ICER of sorafenib decreased to $696,324/QALY. Similarly, modeling the scenario where cabozantinib was administered as the second-line treatment resulted in an ICER for sorafenib of $1,028,532/QALY. Finally, when the cost of sorafenib was varied in a one-way sensitivity analysis, the ICER for sorafenib would only fall below $200,000/QALY when the monthly price of sorafenib was changed from $16,390 to below $7,000, a decrease of more than 50%. The ICER of sorafenib would fall below $100,000/QALY assuming a monthly price below $6,600. The ICER of the sorafenib strategy would also fall below $200,000/QALY or $100,000/QALY when the cost of SIRT was changed from $41,371 to higher than $75,000 or $77,000, respectively.
DISCUSSION
The recently published SARAH and SIRveNIB trials were large randomized, open-label phase III trials that were designed to assess the efficacy and safety of SIRT versus sorafenib in patients with locally advanced HCC.11,12 Both trials showed no significant difference in OS, while the SARAH trial reported that SIRT significantly improved tumor response. Given this context established by the two trials, we sought to investigate the cost-effectiveness of SIRT and sorafenib for locally advanced, inoperable HCC from the perspective of the US healthcare sector. Our study based on pooled SARAH and SIRveNIB trial data demonstrated that, at its current price, a strategy of sorafenib would cost $1,280,224 per QALY gained as compared with SIRT, an ICER well above the commonly accepted US cost-effectiveness thresholds.29 Only if the monthly price of sorafenib decreased by more than 50% did the ICER of sorafenib fall below $200,000/QALY. When the SARAH trial was modeled separately, sorafenib became less effective than SIRT and remained more expensive, making SIRT the dominant treatment strategy.
In modeling the SARAH and SIRveNIB trials separately, incremental QALYs varied between the two analyses. For the SARAH trial, the marginally lower quality-adjusted survival with sorafenib arose from the significantly lower quality of life and less favorable toxicity profile associated with sorafenib, whereas OS was similar among the two arms. The SIRveNIB trial, however, did not find a difference in quality of life scores between the treatment arms, whereas the survival curve point estimates were more separated, favoring sorafenib. Therefore, this resulted in higher QALYs for sorafenib, despite the greater number of AEs occurring in this arm. Furthermore, incremental costs also differed between the two analyses. A relatively higher cost of SIRT in the SARAH trial resulted from the multiple SIRT treatments received by some patients in the SARAH trial, decreasing the difference in costs across treatment arms, although sorafenib remained more expensive. A relatively higher cost of sorafenib seen in the SIRveNIB trial arose from its higher point estimates of disease progression rates, further increasing the difference in costs in favor of SIRT.
Of note, although the trials were designed to demonstrate superiority, both failed to reach this end point, with point estimate HRs favoring sorafenib. To establish equivalent efficacy between SIRT and sorafenib, a larger trial would be required to prove noninferiority. In the absence of such a trial, however, clinical decisions regarding initiation of first-line treatment must still be made in the face of this uncertainty. Thus, in designing the current study, we used the best available evidence and modeled the survival data based on trial-reported survival curves, without assuming equality of the treatment arms.
Consistent with our findings, previously published economic analyses looking at first-line treatments in the setting of advanced HCC have demonstrated that sorafenib is an expensive treatment option from various international healthcare perspectives. One study from the Chinese payer’s perspective evaluated the cost-effectiveness of sorafenib versus best supportive care using individual patient data.32 Their model estimated an ICER of $101,399/QALY that was well above the commonly accepted WTP threshold in China, leading to the conclusion that sorafenib is not a cost-effective first-line treatment for these patients. Another study by Rognoni et al33 used observational data from Italian oncology centers to compare the cost-effectiveness of SIRT and sorafenib in patients with intermediate and advanced HCC. The authors found that SIRT dominated for patients with advanced disease, while for intermediate stage, the model estimated an incremental cost-utility ratio of €3,302/QALY for SIRT. These findings supported their conclusion that SIRT is a cost-effective treatment strategy compared with sorafenib from the Italian healthcare service perspective. Furthermore, the National Institute for Health and Care Excellence in the United Kingdom has reported that commercially presented ICER estimates of approximately £52,000/QALY for sorafenib versus placebo based on the SHARP trial substantially surpassed the threshold delineating acceptable use of the United Kingdom’s National Health Service resources.34 This has led to the recommendation that sorafenib would only be a reasonable option to treat advanced HCC under the circumstance that the company provide the drug within terms of a specific access agreement. In other words, sorafenib was only deemed an attractive treatment recommendation when offered at a significant discount.
Our study has several important strengths. First, our model was based on detailed individual patient survival data generated from two large, randomized trials, in which sorafenib and SIRT were directly compared and analyzed using the intention-to-treat principle. Quality of life data were collected as a secondary outcome for both trials, allowing for the incorporation of data reflective of the modeled trial population. Our model also mirrored the rates of treatment adherence and crossover and the incidence of AEs in the trial population. Our results were robust against various uncertainty analyses including additionally modeling each trial separately to account for differences in design and patient case-mix.
Despite these strengths, our study is not without limitations. First, although there are significant advantages to modeling our analysis based on randomized trials, our model can be limited by the generalizability of these trials. The SARAH and SIRveNIB trial populations, for instance, may not be representative of the advanced HCC patient population in the United States since the trials were conducted in France and the Asia-Pacific region, thus potentially limiting the interpretation of our results given that our analysis was performed from a US healthcare sector perspective. For example, the distribution of HCC etiologies and treatment decisions may differ geographically and result in differences in outcomes. Additionally, the trials used resin Y-90 microspheres for the SIRT administration. Some studies have reported potential favorable survival with glass versus resin microspheres,35 and currently both Y-90 microsphere types are used for unresectable HCC in the United States. Our study could therefore potentially underestimate the effectiveness of SIRT for the contemporary US practice. Second, our study lacked individual-level data for quality-of-life utilities since both trials reported only mean values for each arm at specific time points, thus limiting our ability to correlate model parameters such as time to disease progression with utility weights and to use available mapping algorithms to estimate EQ-5D scores from European Organization for Research and Treatment of Cancer Quality of Life Questionnaire values. Therefore, we used published parameters to estimate scores from the reported mean values assuming linearity, potentially underestimating the uncertainty given that currently published algorithms show a large variation among mapped values and limited accuracy in comparison with observed EQ-5D scores.36 Third, we did not include all reported AEs in our model and only included the most relevant AEs (grade ≥ 3) with a relatively frequent occurrence. Minor AEs of grade < 3 were expected to resolve without substantial utility penalty and healthcare costs, and other less frequent AEs were assumed not to have a significant difference in incidence between the two treatment modalities. A further limitation of this study was the use of Medicare data for cost inputs, although variations may exist in reimbursement rates between the private and public sectors. However, using cost inputs based on Medicare rates is considered an acceptable practice given that Medicare payments are generally based on estimates of actual costs to health systems37 and influence reimbursement decisions made by private payers.38 Thus, Medicare is a reasonable proxy for actual healthcare costs, particularly given that cost information directly obtained from all payers is generally not available. Finally, while the results of this analysis are most applicable at the population level and may help to inform policy decisions, the findings are difficult to extend to individual patient conversations given differences in preferences of patients and providers.
The landscape of first-line treatments for locally advanced and metastatic HCC is rapidly evolving. Lenvatinib, another multi-kinase inhibitor, recently gained FDA approval as a first-line agent for unresectable HCC following the results of the REFLECT trial,39 while SBRT has been associated with high rates of local control.40 Future research evaluating the cost-effectiveness of lenvatinib versus sorafenib based on the REFLECT trial given the demonstration of noninferiority in OS and in comparison to SIRT may help further inform treatment decisions among this patient population. Nivolumab has shown clinically meaningful results, although it failed to reach its primary end point of OS versus sorafenib in the CHECKMATE-459 trial.41 Moreover, ongoing trials are investigating the efficacy of other immunotherapy agents and combinations of established HCC treatments, including sorafenib and SIRT,42,43 and SBRT followed by sorafenib.44 As these trials move toward completion, future studies will be required to evaluate the cost-effectiveness of newly approved and combined treatment regimens. Additionally, further studies are required to consider the effects of second-line agents on the cost-effectiveness of HCC treatment strategies with several recent trials reporting a survival benefit associated with certain systemic therapies compared with placebo in the second-line setting.30,31 Tyrosine kinase inhibitors regorafenib and cabozantinib are two such therapies, which we modeled separately in sensitivity analyses. Alternative options include nivolumab and pembrolizumab, which have recently been approved as second-line treatments based on recently published trials,45,46 but the lack of comparative data in this setting necessitates further studies to ultimately evaluate the cost-effectiveness of these therapies.
In conclusion, our analysis suggests that sorafenib is unlikely to provide a gain in quality-adjusted survival compared with SIRT in locally advanced HCC at a cost considered to be acceptable according to current US cost-effectiveness thresholds. Under these thresholds, our study suggests that sorafenib may be considered economically attractive only if the current price decreased by more than 50%. Yet, it should be noted that health systems’ willingness to pay may be greater at the end of life since QALYs gained at the end of life may be assigned a higher value.47 Given that the treatments for advanced HCC are typically noncurative, quality of life and value of care are important considerations, and our findings may serve as a further evidence base for the ongoing conversation on the challenges of the care for patients with advanced HCC.
APPENDIX
Fig A1.
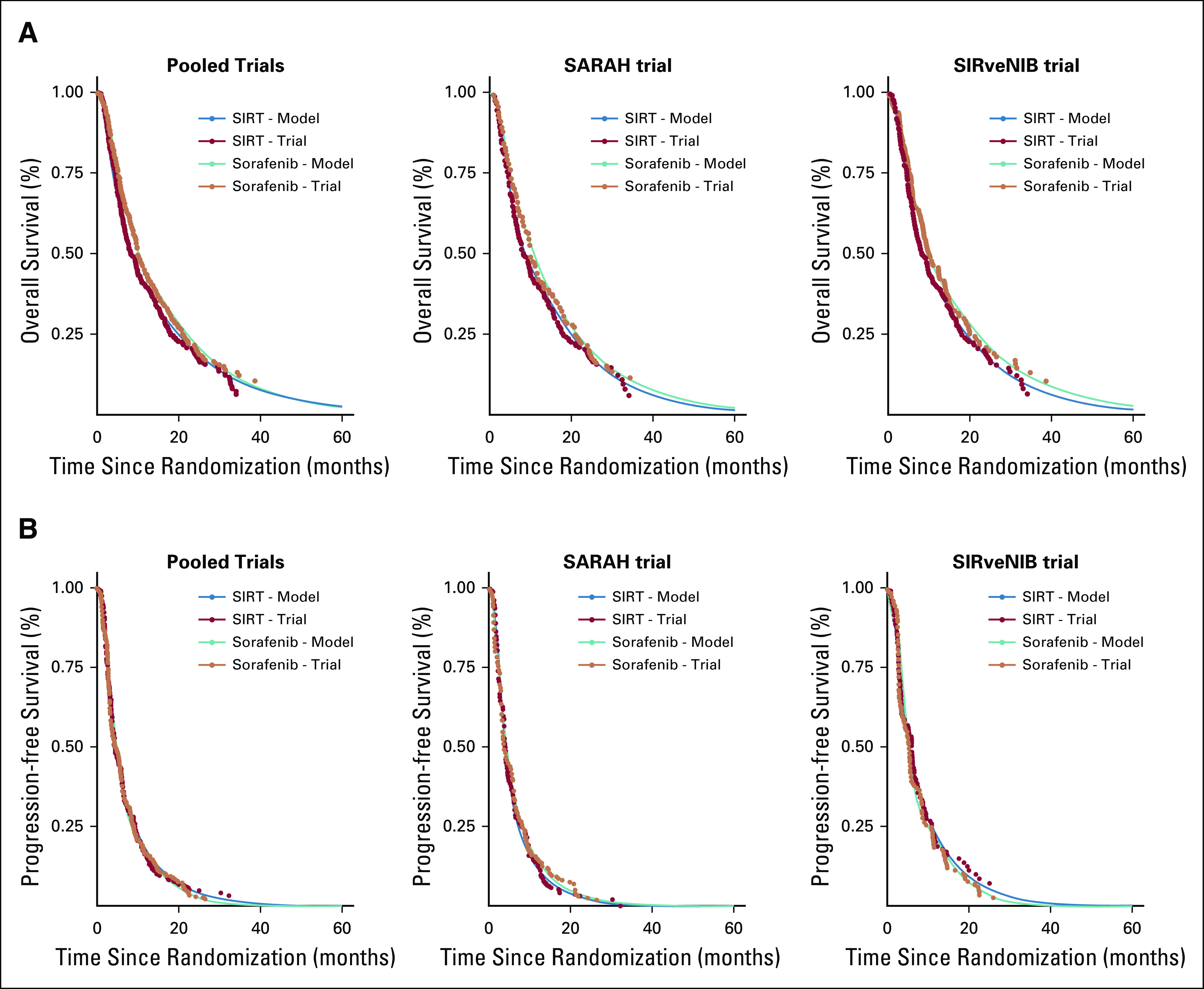
Internal validation of model predictions: (A) overall survival and (B) progression-free survival. SIRT, selective internal radiotherapy.
TABLE A1.
Cost of Selective Internal Radiotherapy (SIRT)
TABLE A2.
EuroQol 5-D (EQ-5D) Utility Scores
PRIOR PRESENTATION
Presented as a poster at the American Society for Radiation Oncology 60th Annual Meeting, San Antonio, TX, October 23, 2018.
SUPPORT
NCI Cancer Center Support Grant P30 CA196521-01 (Ferket, Mazumdar); Biostatistics Shared Resource Facility, Icahn School of Medicine at Mount Sinai (Mazumdar).
SENIOR AUTHORS
M.B. and B.S.F. are joint senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Kathryn E. Marqueen, Michael Buckstein, Bart S. Ferket
Financial support: Madhu Mazumdar
Administrative support: Madhu Mazumdar
Collection and assembly of data: Kathryn E. Marqueen, Edward Kim, Bart S. Ferket
Data analysis and interpretation: Kathryn E. Marqueen, Celina Ang, Madhu Mazumdar, Michael Buckstein, Bart S. Ferket
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Edward Kim
Consulting or Advisory Role: Ethicon, a subsidiary of Johnson and Johnson, Boston Scientific, Bristol-Myers Squibb
Speakers' Bureau: Boston Scientific
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. : Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. : Global cancer statistics, 2012. CA Cancer J Clin 65:87-108, 2015 [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Davila JA, Petersen NJ, et al. : The continuing increase in the incidence of hepatocellular carcinoma in the United States: An update. Ann Intern Med 139:817-823, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J: Hepatocellular carcinoma. Lancet 379:1245-1255, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. : Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378-390, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, et al. : Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25-34, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Sangro B, Carpanese L, Cianni R, et al. : Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology 54:868-878, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Inarrairaegui M, Thurston KG, Bilbao JI, et al. : Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 21:1205-1212, 2010 [DOI] [PubMed] [Google Scholar]
- 9.D'Avola D, Lnarrairaegui M, Bilbao JI, et al. : A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology 56:1683-1688, 2009 [PubMed] [Google Scholar]
- 10.Kulik LM, Carr BI, Mulcahy MF, et al. : Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 47:71-81, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Vilgrain V, Pereira H, Assenat E, et al. : Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol 18:1624-1636, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Chow PKH, Gandhi M, Tan SB, et al. : SIRveNIB: Selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 36:1913-1921, 2018 [DOI] [PubMed] [Google Scholar]
- 13.EASL Clinical Practice Guidelines : Management of hepatocellular carcinoma. J Hepatol 69:182-236, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Forner A, Reig ME, de Lope CR, et al. : Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis 30:61-74, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Marrero JA, Kudo M, Venook AP, et al. : Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol 65:1140-1147, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Golfieri R, Bilbao JI, Carpanese L, et al. : Comparison of the survival and tolerability of radioembolization in elderly vs. younger patients with unresectable hepatocellular carcinoma. J Hepatol 59:753-761, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Mantry PS, Mehta A, Madani B, et al. : Selective internal radiation therapy using yttrium-90 resin microspheres in patients with unresectable hepatocellular carcinoma: A retrospective study. J Gastrointest Oncol 8:799-807, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyot P, Ades AE, Ouwens MJ, et al. : Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 12:9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bach PB: Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med 360:626-633, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Overview of the Medicare Physician Fee Schedule, Centers for Medicare & Medicaid Services. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx
- 21.Hospital Outpatient PPS, Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS
- 22.Healthcare Cost and Utilization Project. https://hcupnet.ahrq.gov/#setup
- 23.Kontodimopoulos N, Aletras VH, Paliouras D, et al. : Mapping the cancer-specific EORTC QLQ-C30 to the preference-based EQ-5D, SF-6D, and 15D instruments. Value Health 12:1151-1157, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Sanders GD, Neumann PJ, Basu A, et al. : Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA 316:1093-1103, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Lloyd A, Nafees B, Narewska J, et al. : Health state utilities for metastatic breast cancer. Br J Cancer 95:683-690, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollom EL, Lee K, Durkee BY, et al. : Cost-effectiveness of stereotactic body radiation therapy versus radiofrequency ablation for hepatocellular carcinoma: A Markov Modeling Study. Radiology 283:460-468, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy AR, Kowdley KV, Iloeje U, et al. : The impact of chronic hepatitis B on quality of life: A multinational study of utilities from infected and uninfected persons. Value Health 11:527-538, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DA, Ahmad BB, Chen Q, et al. : Cost-effectiveness analysis of regorafenib for metastatic colorectal cancer. J Clin Oncol 33:3727-3732, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumann PJ, Cohen JT, Weinstein MC: Updating cost-effectiveness—The curious resilience of the $50,000-per-QALY threshold. N Engl J Med 371:796-797, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Bruix J, Qin S, Merle P, et al. : Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56-66, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Abou-Alfa GK, Meyer T, Cheng AL, et al. : Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379:54-63, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P, Yang Y, Wen F, et al. : Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol 27:853-859, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Rognoni C, Ciani O, Sommariva S, et al. : Real-world data for the evaluation of transarterial radioembolization versus sorafenib in hepatocellular carcinoma: A cost-effectiveness analysis. Value Health 20:336-344, 2017 [DOI] [PubMed] [Google Scholar]
- 34.National Institute for Health and Clinical Excellence : Sorafenib for treating advanced hepatocellular carcinoma: NICE technology appraisal guidance TA474, 2017 [Google Scholar]
- 35.Biederman DM, Titano JJ, Tabori NE, et al. : Outcomes of radioembolization in the treatment of hepatocellular carcinoma with portal vein invasion: Resin versus glass microspheres. J Vasc Interv Radiol 27:812-821.e2, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Crott R: Mapping algorithms from QLQ-C30 to EQ-5D utilities: No firm ground to stand on yet. Expert Rev Pharmacoecon Outcomes Res 14:569-576, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Tumeh JW, Moore SG, Shapiro R, et al. : Practical approach for using Medicare data to estimate costs for cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res 5:153-162, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Clemens J, Gottlieb JD: In the shadow of a giant: Medicare's influence on private physician payments. J Polit Econ 125:1-39, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudo M, Finn RS, Qin S, et al. : Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391:1163-1173, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Bujold A, Massey CA, Kim JJ, et al. : Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 31:1631-1639, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Yau T, Park JW, Finn RS, et al. : LBA38_PR - CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol 30:v874-v875, 2019 [Google Scholar]
- 42.Chauhan N, Bukovcan J, Boucher E, et al. : Intra-arterial therasphere yttrium-90 glass microspheres in the treatment of patients with unresectable hepatocellular carcinoma: Protocol for the STOP-HCC phase 3 randomized controlled trial. JMIR Res Protoc 7:e11234, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricke J, Sangro B, Amthauer H, et al. : The impact of combining selective internal radiation therapy (SIRT) with sorafenib on overall survival in patients with advanced hepatocellular carcinoma: The soramic trial palliative cohort. J Hepatol 68:S102, 2018 [Google Scholar]
- 44.Sorafenib tosylate with or without stereotactic body radiation therapy in treating patients with liver cancer.
- 45.El-Khoueiry AB, Sangro B, Yau T, et al. : Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492-2502, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu AX, Finn RS, Edeline J, et al. : Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol 19:940-952, 2018 [DOI] [PubMed] [Google Scholar]
- 47.Lakdawalla DN, Doshi JA, Garrison LP, Jr, et al. : Defining elements of value in health care-A health economics approach: An ISPOR Special Task Force Report [3]. Value Health 21:131-139, 2018 [DOI] [PubMed] [Google Scholar]