Abstract
Low participation of Black Americans in cancer clinical trials is a well-established predicament. Many factors resulted in this current dilemma with racism being the fundamental unit. Here, we discuss some current challenges and proposed solutions to help in increasing the enrollment of Black Americans in cancer clinical trials. We suggest implementing the least acceptable race-specific percentage as a new bar that registrational clinical trials need to pass before cancer drugs approval. Clinical trials will continue to draw the future of cancer therapeutics in which we believe that a prompt improvement of Black Americans participation is warranted.
INTRODUCTION
Clinical trials provide the highest level of evidence in evaluating the safety and efficacy of new cancer therapies. Lack of participation in clinical trials was associated with worse survival outcomes in some malignancies. Moreover, clinical trial participants were reported to live longer than patients receiving Medicare who were treated with US Food and Drug Administration (FDA)–approved cancer drugs.1,2 For some patients with cancer, a clinical trial may be the only remaining treatment option.3
Black Americans constitute at least 13% of the general population in the United States, and the percentage will continue to grow further in the coming years.4 The death rate from cancer is more common in Black Americans, especially males. The largest difference is for cancers of prostate, stomach, uterine corpus, and multiple myeloma (MM) where Black American death rates are about twice as high when compared with Whites.4
Black Americans are under-represented in cancer clinical trials. Taking MM as an example, a cancer in which Black Americans account for about 22% of yearly cases, 32,270 new cases are estimated to occur per year.5 The age-adjusted incidence rate difference in the period 2011-2015 for development of MM was 8.4 and 7.2 per 100,000 population higher for male and female Black Americans, respectively, when compared with Whites.5
In trials submitted in support of MM indications, the median enrollment percentage of Black Americans was 4.5%.6 Across all studies that resulted in a US FDA cancer drug approval in a 5-year period, Black Americans were under-represented.7 Participation to prevalence ratio was low in all cancer subtypes with an average Black Americans enrollment percentage of 7%.7 Reporting race in cancer clinical trials is suboptimal and needs further improvement.8
Improving Black American enrollment in cancer clinical trials is important not only to ensure generalizability of the results but also to provide an opportunity to study cancer biology and response to therapies across different patients' subgroups. This will provide an opportunity to study therapeutic response across different sociodemographic groups. Differences in biologic responses may result in increased resistance to some medications.9,10 Improving Black Americans' enrollment in cancer clinical trials is also important to provide high-quality and potentially beneficial care to the participants.
Sampling bias or ascertainment bias is a consequence of poor enrollment of Black Americans in clinical trials. It results from a lower sampling probability of Black Americans as part of the intended study population. In other words, Black Americans are not equally likely to be selected as participants in clinical trials, and this will result in selection bias. This bias will result in a nonrandom sample (predominately White participants), and the results will not be generalizable to the population with the studied cancer. This is especially significant when the studied cancer is more common in Black Americans.
Here, we discuss some current challenges and proposed solutions to help in increasing the enrollment of Black Americans in cancer clinical trials. We propose the least acceptable race-specific percentage as a new bar that registrational clinical trials need to pass before cancer drugs approval.
BARRIERS FOR BLACK AMERICANS PARTICIPATION IN CANCER CLINICAL TRIALS
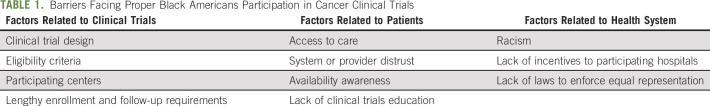
Multiple studies provided potential reasons for lack of appropriate representation of Black Americans in cancer clinical trials. Barriers can be divided into three major categories, which interact and overlap with each other (Table 1).
TABLE 1.
Barriers Facing Proper Black Americans Participation in Cancer Clinical Trials
Factors Related to Clinical Trials
The design of clinical trials, which includes strict inclusion or exclusion criteria, can render many Black Americans ineligible for participation.11 A single-institution study of 235 Black Americans cancer patients found that only 20 patients (8.5%) were eligible based on inclusion criteria with most patients excluded for co-existing comorbidities.12 Even among eligible patients, the enrollment rate was 60%. It is also important to take biologic factors into consideration when designing a clinical trial, for example, one prostate cancer trial required serum testosterone levels to be < 50 ng/dL and did not take into consideration that Black Americans have a 15% higher testosterone levels compared with their White counterparts.13 Other biologic differences should be considered such as glomerular filtration rate and hemoglobin level although the use of race-based cutoffs for hemoglobin and glomerular filtration rate (and other lab tests) is highly controversial. Trials that do not consider racial difference in laboratory values would exclude potentially appropriate participants solely because of normal laboratory differences related to their race.14
To conduct a cancer clinical trial, a specific infrastructure that includes trained research staff is needed. Most of the cancer clinical trials are conducted at major academic cancer centers. Many Black Americans may not have access to those centers, which will result in lack of opportunity to participate.15 Black Americans are disproportionately more likely to receive care in under-resourced hospital systems and to be uninsured.16-18 Many clinical trials will require patients to use their insurance benefits for follow-up testing and specific treatments, which may not be available for Black Americans. On the other hand, lengthy enrollment and follow-up requirements may result in dissatisfaction and low participation in cancer clinical trials. As a result of many of the above factors, retention rate in Black Americans may be different. One study showed that Black Americans' disengagement from clinical trials is twice that of Whites.19
Factors Related to Patients
Access to care is a complex process and could limit utilization of clinical trials. For example, the rate of screening colonoscopy is 8% lower in Black Americans.20 This leads to late diagnosis, which is often associated with poor performance status and worse clinical outcomes. This may result in exclusion of those patients from participation in clinical trials.
Lack of comfort with the process of clinical trial, which includes fear of being treated unequally and of the outcome of experimental drug, has been identified as a barrier to enrollment in clinical trials.21-23 A systematic review of the factors that influenced participation of Black Americans in cancer clinical trials reported religious beliefs, low clinical trial awareness level, and structural barriers, such as access to health care and transportation, as potential causes for lack of appropriate participation.24
Financial constraints are another major barrier. Days off work and childcare cost are usually not covered by clinical trials. Patients in households making less than $50,000 per year were 27% less likely to participate in clinical trials.25 The median household income for Black Americans is $20,000 lower when compared with that for non-Hispanic White Americans.26 Other financial constraints include the lack of transparency in coverage policy and rising cost of cancer care. This led organizations like ASCO and Lazarex Cancer Foundation to consolidate efforts implementing legislation at the federal and state level.
With sponsorship of Lazarex Cancer Foundation, California was the first state in the United States to legally recognize the financial burdens afflicting patients with cancer seeking treatment in clinical trials. The California law differentiates between inducement and reimbursement with recognition of ancillary costs as a barrier to clinical trial participation.27 It identifies the allowable expenses that can be reimbursed to patients and encourages industry support of these costs. Lazarex helps patients with advanced-stage cancer and the medically underserved with costs for FDA trial participation, identification of clinical trial options, community outreach, and education. Improving Patient Access to Cancer Clinical Trials, one of the initiatives launched by Lazarex, showed preliminary promising results with approximately 60% participation from minority patients.28
Lower level of health literacy may contribute to low participation in cancer clinical trials. Health literacy includes a wide range of skills that people need to make informed choices.29 Black Americans were reported to have lower health literacy levels when compared with non-Hispanic Whites.23
A study looking at attitudes of Black Americans toward medical research found that many participants assumed that the trial consent forms were protecting hospitals and doctors from any legal responsibility.30 The same study showed that most participants were in favor of medical research if they were not treated as guinea pigs, which reflects a lack of proper clinical trials education.
Factors Related to Health Systems
Racism, research misconduct, and low-quality health care have led many Black Americans to be skeptical regarding health care institutions in general and clinical research enrollment specifically.31-33
Health care providers play a crucial role in the enrollment of Black Americans patients into clinical trials.34,35 Patient accrual was affected by provider attitude related to patient adherence to the study protocol.35 A study of Black women to explore factors associated with the decision to participate in cancer screening trial showed that almost all women reported that their doctor had never talked to them about participating in a clinical trial.36
Other health system–related factors include lack of incentives to participating hospitals and lack of laws to enforce equal representation. Implicit bias, which includes conscious and unconscious portions, among physicians may lead physicians to believe that Black Americans are less likely to be complaint with the study.37,38
PROPOSED SOLUTIONS
Since the problem is complex, the solution will not be straightforward. Previous efforts to minimize lack of minority representation in clinical trials include the revitalization act of 1993, which requires that clinical trials funded by the National Institutes of Health to include women and participants from minority groups. However, in the field of oncologic clinical trials, many trials are industry-sponsored, which may not adhere to similar regulations. This may result in even lower participation in industry-sponsored clinical trials.39 In 2003, ASCO established a formal group of cancer health disparities and health equity experts to focus on understanding and eliminating disparities in cancer care. This was followed by the first ASCO policy statement on cancer care disparities, which highlighted the society's commitment to enhancing awareness, support of research on heath disparities, and improving cancer care access for minority groups.40 In 2013, ASCO established a Health Disparities Committee, which later became the Health Equity Committee. This committee is responsible for collaboration within ASCO and with other cancer care stakeholders to improve health equity for patients with cancer. Recently, an updated statement issued by ASCO stressed on the need to move beyond descriptions of differences in cancer outcomes toward cancer health equity.41,42
Efforts from National Institutes of Health, FDA, ASCO, and other groups draw a path for a change; however, such efforts will need the appropriate support to succeed. Health equity–promoting agencies should be empowered to set and enforce guidelines for the recruitment of minorities in clinical trials and should require scientific justification for limited or selected study population enrollment.
Cancer Clinical Trial Design Modification
Biased statistical design can help in diluting the sampling bias. It means to enroll more Black Americans in cancer clinical trials to try to eliminate the sampling bias. The ideal way of doing that is to aim for a proportion of Black American participation that is similar to their proportion in the incident cases.
For example, if we want to match the enrollment proportion of Black Americans with Whites in MM clinical trials, we will need to enroll 22% of the calculated sample size as Black Americans in a study that will look into patients with newly diagnosed MM in the United States
The lowest acceptable race-specific percentage should be a new bar that clinical trials need to overcome before drug approval. This should be applied initially for registrational trials to ensure that an approved cancer drug will be a suitable option for all patients under the studied cancer. A 10-year implementation goal can be entertained (Fig 1). If such a model is enforced by institutions like the FDA, it will direct the sponsor of the trial to the hospitals or clinics that serve more Black Americans to open sites of enrollment.
FIG 1.
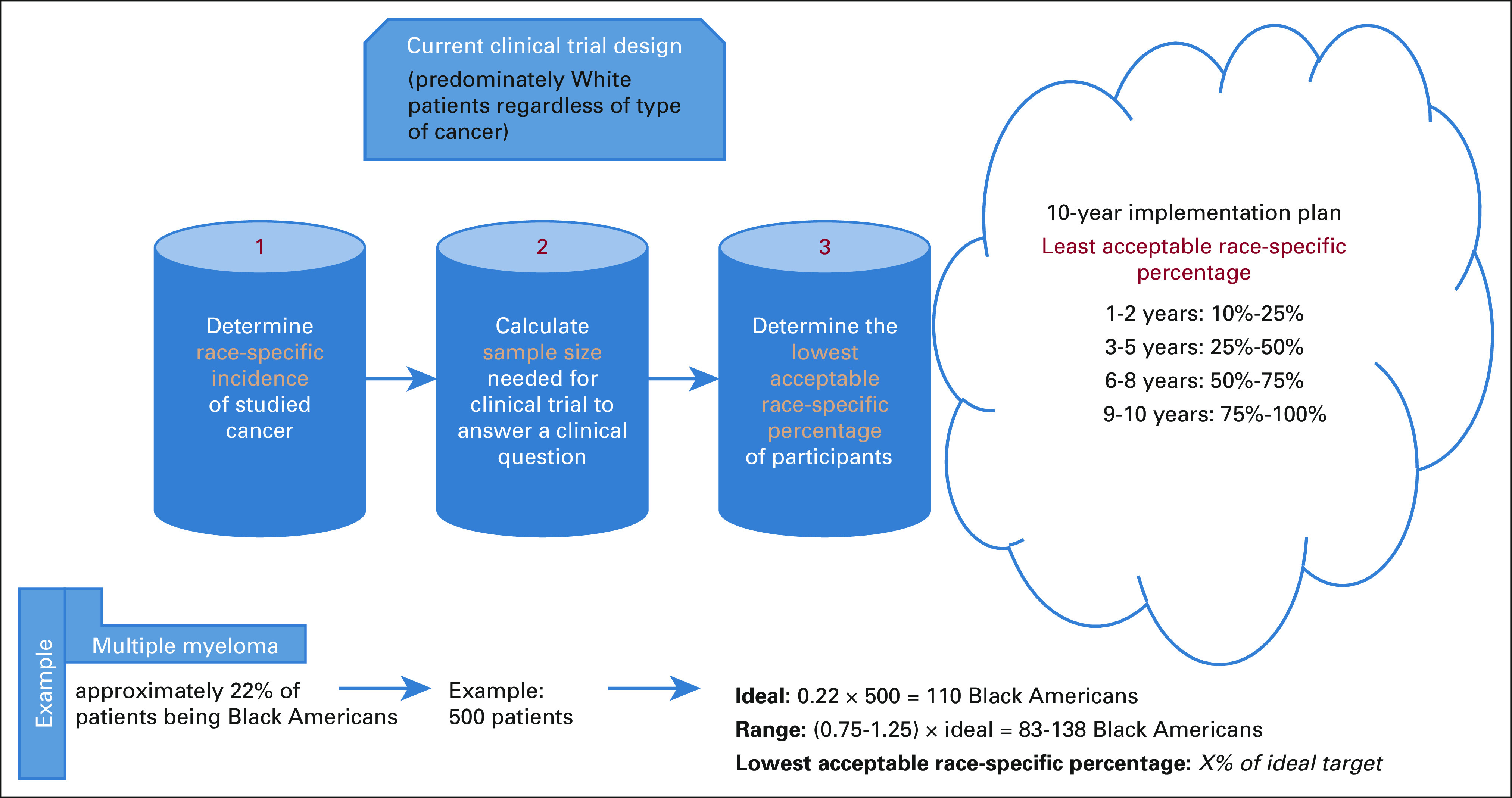
A proposed implementation plan of the least acceptable race-specific percentage.
Finding the right balance between attempts to recruit a diverse patient population and trial logistics is an inherently difficult and costly endeavor. Attempts to increase diversity may result in trial delays, and this should be balanced with the need to get drugs and therapies approved. It is also known that increasing trial time maybe more expensive, and this could possibly lead to price increase by pharmaceutical companies to offset rising costs.
Financial Support
Although the impact of incentives on recruitment and retention in practice is still not well-described, attempts to lessen the financial burden associated with participating in clinical trials by covering not only the cost of the drug but also copayments and coinsurance will be helpful. Covering indirect expenses like time off from work, childcare, and transportation would theoretically improve access and participation in clinical trials especially for lower-income patients. The ASCO Health Disparities Committee issued a policy statement with recommendations to overcome financial barriers. Some of the recommendations included the use of a transparent process to estimated out-of-pocket cost of participation in clinical trials and provide financial support to cover those costs.43 Medicaid, which covers nearly 20% of Americans, is not federally required to cover routine costs of participation in clinical trials, and many states do not explicitly require such coverage. Reduction in co-pays and deductibles for clinical trial participants can be a potential option.
Funding agencies should include ethnicity and race as criteria for assigning priority scores. These scores are currently used to evaluate the expected impact of a particular research and include assessment of significance, innovation, and approach. The final score usually determines the grant funding. By incorporating ethnicity and race, researchers applying for funding will at least be required to actively try to recruit Black Americans. Similar approaches have been taken when evaluating sex or gender inclusion.44
Improving Accessibility and Community Reach Programs
Allowing study centers with easy access to Black Americans to be part of cancer clinical trials will help in recruiting patients. Black American researchers, research coordinators, patient advocates, and previous trial participants will help in recruiting more Black American patients.35 The benefits from enrolling in cancer clinical trials can be more attractive for Black Americans given possible higher responses to studied therapies.45,46 Increasing community involvement, incentivizing collaboration among different groups, and involving community hospitals in the recruitment to clinical trials will not only increase access for minority groups but would likely also result in a quicker enrollment process. National Cancer Institute (NCI)–designated centers have launched an NCI community oncology research program to bring cancer clinical trials and care delivery studies to people in their communities. This is particularly important in the current era of genomics and molecular targeted therapy as the majority of cancer care takes place in the community setting.47 The increasingly complex requirements of genomic testing, such as next-generation sequencing and tissue biopsy testing, may serve as an extra obstacle for improving community oncology research participation. Some potential solutions include improving coordination between community oncology centers or oncology practices and a nearby NCI-designated cancer center(s) along with backup support system to provide community sites with several linings of support according to their need.
Patient-Centered Approaches
Targeting enrollment toward specific cultural backgrounds and literacy levels may improve recruitment of under-represented populations.17 Patient navigation models, which involve the process of providing educational and facilitative services to the patient, have been proposed to improve the retention of Black Americans in cancer clinical trials with one study showing an increase of participation from 9% to 16%.48
Several patient-focused interventions have been developed. In a multicenter, randomized trial of a web-based interactive, education tool in patient with cancer was conducted. Its aim was to increase knowledge, decrease attitudinal barriers, and improve preparation for making decisions about clinical trials. The control group received a standard text-based general information about clinical trials from NCI, whereas the intervention group watched a brief, individually tailored video addressing knowledge and attitudinal barriers to clinical trials participations. Although both groups showed improved knowledge, the intervention group has a significant increase in their preparedness to consider participation in clinical trials.49
Health Care–Centered Approaches
Attempts to reduce the impact of bias in clinical interactions have been made. They include training health care professionals in the use of high-quality patient-centered communication. To successfully enroll Black Americans in clinical trials, health care providers must be culturally sensitive with appropriate communication skills.50 Specific training curriculum to recognize race and ethnic cultural barriers to participation and understand the structure and function of family units and sex dynamics in different cultures emphasis should be implemented to bridge cultural gaps between participants and research professionals.51
To mitigate the lack of clinical trials in under-resourced hospitals servicing Black American patients, several associations have established programs to increase the enrollment among minority groups. The NCI established the Minority-Based Community Clinical Oncology program (MBCCOP), which is a national network of health professionals, investigators, and organizations that conducts cancer-related research across the United States.50 The network developed outreach programs (eg, minority-targeted media outlet, presentations at minority churches, and distributed fliers at minority health fairs) at institutes that serve large numbers of minorities with cancer. The network enrolled more than 5,500 minority patients with cancer in NCI-sponsored clinical trials, although overall successful it failed to show improved enrollment of premenopausal Black American women in breast cancer trials.52 Barriers to MBCCOP success include many of the factors we discussed above such as inclusion or exclusion criteria and poor national adoption. We believe that programs such as MBCCOP have the potential to succeed although it will require collaboration between health care microsystems and adopting a systematic series of improvement strategies.
Digital clinical trials, which use digital technologies as part of their design and conduct, can help in enrolling more patients from minority ethnic groups. This becomes particularly helpful for patients who do not live close to a research site or have scheduling constraints. It can also be used to recruit, obtain informed consent, and follow up enrollment milestones. It is still in its infancy stages; however, the use of technology in clinical trials will be the future and will continue to evolve.53
In conclusion, the lack of diversity in cancer clinical trials remains a major scientific challenge. The oncology community is obligated to improve participation of Black Americans in cancer clinical trials. With the pace of scientific advancement in the field, reassessment of our current approach to clinical research and recruitment is needed. We introduce the least acceptable race-specific percentage as a statistical method to help end the long-lived disparities that Black Americans are facing. We must strive to fulfill our moral and scientific duty of achieving social justice for under-represented communities when conducting cancer clinical trials.
SUPPORT
S.A. reports salary support by National Heart, Lung, and Blood Institute Grant No. 5T32HL092332-18.
M.A. and S.A. contributed equally to this work.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI https://doi.org/10.1200/OP.21.00001.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Participation of Black Americans in Cancer Clinical Trials: Current Challenges and Proposed Solutions
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Bleyer A, Montello M, Budd T, et al. : National survival trends of young adults with sarcoma: Lack of progress is associated with lack of clinical trial participation. Cancer 103:1891-1897, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Green AK, Curry M, Trivedi N, et al. : Assessment of outcomes associated with the use of newly approved oncology drugs in Medicare beneficiaries. JAMA Netw Open 4:e210030, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conforti F, Pala L, Bagnardi V, et al. : Cancer immunotherapy efficacy and patients' sex: A systematic review and meta-analysis. Lancet Oncol 19:737-746, 2018 [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Miller KD, Goding Sauer A, et al. : Cancer statistics for African Americans, 2019. CA Cancer J Clin 69:211-233, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar V, Gormley N, Kazandjian D, et al. : FDA analysis of racial demographics in multiple myeloma trials. Blood 130:4352, 2017. (suppl 1) [Google Scholar]
- 7.Al Hadidi S, Mims M, Miller-Chism CN, et al. : Participation of African American persons in clinical trials supporting U.S. Food and Drug Administration approval of cancer drugs. Ann Intern Med 173:320-322, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loree JM, Anand S, Dasari A, et al. : Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol 5:e191870, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltayeb AE, Demas DM, Clarke R, et al. : The unfolded protein response may contribute to racial disparity in endocrine responsiveness in breast cancer. Cancer Res 75, 2015. (15 suppl; abstr 1258) [Google Scholar]
- 10.Lindner R, Sullivan C, Offor O, et al. : Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One 8:e71915, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim ES, Bruinooge SS, Roberts S, et al. : Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research statement. J Clin Oncol 35:3737-3744, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. : Enrollment of African Americans onto clinical treatment trials: Study design barriers. J Clin Oncol 22:730-734, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ross R, Bernstein L, Judd H, et al. : Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst 76:45-48, 1986 [PubMed] [Google Scholar]
- 14.Vastola ME, Yang DD, Muralidhar V, et al. : Laboratory eligibility criteria as potential barriers to participation by Black men in prostate cancer clinical trials. JAMA Oncol 4:413-414, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruner DW, Jones M, Buchanan D, et al. : Reducing cancer disparities for minorities: A multidisciplinary research agenda to improve patient access to health systems, clinical trials, and effective cancer therapy. J Clin Oncol 24:2209-2215, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Diehl KM, Green EM, Weinberg A, et al. : Features associated with successful recruitment of diverse patients onto cancer clinical trials: Report from the American College of Surgeons Oncology Group. Ann Surg Oncol 18:3544-3550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford JG, Howerton MW, Lai GY, et al. : Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer 112:228-242, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Langford AT, Resnicow K, Dimond EP, et al. : Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute's Community Cancer Centers Program. Cancer 120:877-884, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold KB, Hermos JA, Anderson KB, et al. : Retention of black and white participants in the selenium and vitamin E cancer prevention trial (SWOG-coordinated intergroup study S0000). Cancer Epidemiol Biomarkers Prev 23:2895-2905, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren Andersen S, Blot WJ, Lipworth L, et al. : Association of race and socioeconomic status with colorectal cancer screening, colorectal cancer risk, and mortality in Southern US adults. JAMA Netw Open 2:e1917995, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Advani AS, Atkeson B, Brown CL, et al. : Barriers to the participation of African-American patients with cancer in clinical trials: A pilot study. Cancer 97:1499-1506, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Avis-Williams A, Khoury A, Lisovicz N, et al. : Knowledge, attitudes, and practices of underserved women in the rural South toward breast cancer prevention and detection. Fam Community Health 32:238-246, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Evans KR, Lewis MJ, Hudson SV: The role of health literacy on African American and Hispanic/Latino perspectives on cancer clinical trials. J Cancer Educ 27:299-305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivers D, August EM, Sehovic I, et al. : A systematic review of the factors influencing African Americans' participation in cancer clinical trials. Contemp Clin Trials 35:13-32, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Unger JM, Hershman DL, Albain KS, et al. : Patient income level and cancer clinical trial participation. J Clin Oncol 31:536-542, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffin E: Median Household Income by Race or Ethnic Group 2019. Statista, 2020. https://www.statista.com/statistics/233324/median-household-income-in-the-united-states-by-race-or-ethnic-group/ [Google Scholar]
- 27.California Legislative Information : AB-1823 California Cancer Clinical Trials Program. (2015-2016). Assembly Bill No. 1823. Chapter 661. 2016. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201520160AB1823 [Google Scholar]
- 28.Dornsife D: Why improving enrollment and diversity in cancer clinical trials requires the attention of state lawmakers. CONQUER: The Patient Voice, Volume 7, 2021 [Google Scholar]
- 29.Zarcadoolas C, Pleasant A, Greer DS: Understanding health literacy: An expanded model. Health Promot Int 20:195-203, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Corbie-Smith G, Thomas SB, Williams MV, et al. : Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med 14:537-546, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banda DR, Germain DS, McCaskill-Stevens W, et al. : A critical review of the enrollment of Black patients in cancer clinical trials. Am Soc Clin Oncol Ed Book 32:153-157, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Nelson A: Unequal treatment: Confronting racial and ethnic disparities in health care. J Natl Med Assoc 94:666, 2002 [PMC free article] [PubMed] [Google Scholar]
- 33.Katz RV, Russell SL, Kegeles SS, et al. : The Tuskegee Legacy Project: Willingness of minorities to participate in biomedical research. J Health Care Poor Underserved 17:698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaskill-Stevens W, McKinney MM, Whitman CG, et al. : Increasing minority participation in cancer clinical trials: The minority-based community clinical oncology program experience. J Clin Oncol 23:5247-5254, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Howerton MW, Gibbons MC, Baffi CR, et al. : Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer 109:465-476, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Trauth JM, Jernigan JC, Siminoff LA, et al. : Factors affecting older African American women's decisions to join the PLCO Cancer Screening Trial. J Clin Oncol 23:8730-8738, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Moskowitz GB, Stone J, Childs A: Implicit stereotyping and medical decisions: Unconscious stereotype activation in practitioners' thoughts about African Americans. Am J Public Health 102:996-1001, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabin JA, Rivara FP, Greenwald AG: Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care 46:678-685, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Unger JM, Hershman DL, Osarogiagbon RU, et al. : Representativeness of Black patients in cancer clinical trials sponsored by the National Cancer Institute compared with pharmaceutical companies. JNCI Cancer Spectr 4:pkaa034, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goss E, Lopez AM, Brown CL, et al. : American Society of Clinical Oncology policy statement: Disparities in cancer care. J Clin Oncol 27:2881-2885, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Patel MI, Lopez AM, Blackstock W, et al. : Cancer disparities and health equity: A policy statement from the American Society of Clinical Oncology. J Clin Oncol 38:3439-3448, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierce LJ: A time to dig deeper and take meaningful action. J Clin Oncol 38:3361-3362, 2020 [DOI] [PubMed] [Google Scholar]
- 43.Winkfield KM, Phillips JK, Joffe S, et al. : Addressing financial barriers to patient participation in clinical trials: ASCO policy statement. J Clin Oncol 36:3331-3339, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Clayton JA, Collins FS: Policy: NIH to balance sex in cell and animal studies. Nature 509:282-283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fillmore NR, Yellapragada SV, Ifeorah C, et al. : With equal access, African American patients have superior survival compared to white patients with multiple myeloma: A VA study. Blood 133:2615-2618, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George DJ, Heath EI, Sartor AO, et al. : Abi race: A prospective, multicenter study of black (B) and white (W) patients (pts) with metastatic castrate resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP). J Clin Oncol 36, 2018. (18 suppl; abstr LBA5009) [Google Scholar]
- 47.McCaskill-Stevens W, Lyss AP, Good M, et al. : The NCI Community Oncology Research Program: What every clinician needs to know. Am Soc Clin Oncol Ed Book 33: e84-e89, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Brooks SE, Muller CY, Robinson W, et al. : Increasing minority enrollment onto clinical trials: Practical strategies and challenges emerge from the NRG oncology accrual workshop. J Oncol Pract 11:486-490, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meropol NJ, Wong Y-N, Albrecht T, et al. : Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol 34:469, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCaskill-Stevens W, Lyss AP, Good M, et al. : The NCI Community Oncology Research Program: What every clinician needs to know. Am Soc Clin Oncol Ed Book 33:e84-e89, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Niranjan SJ, Durant RW, Wenzel JA, et al. : Training needs of clinical and research professionals to optimize minority recruitment and retention in cancer clinical trials. J Cancer Educ 34:26-34, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newman LA: Breast cancer in African-American women. Oncologist 10:1-14, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Inan OT, Tenaerts P, Prindiville SA, et al. : Digitizing clinical trials. NPJ Digit Med 3:101, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]