PURPOSE:
Omic-informed therapy is being used more frequently for patients with non–small-cell lung cancer (NSCLC) being treated on the basis of evidence-based decision-making. However, there is a lack of a standardized framework to evaluate those decisions and understand the association between omics-based management strategies and survival among patients. Therefore, we compared outcomes between patients with lung adenocarcinoma who received omics-driven targeted therapy versus patients who received standard therapeutic options.
PATIENTS AND METHODS:
This was a retrospective study of patients with advanced NSCLC adenocarcinoma (N = 798) at City of Hope who received genomic sequencing at the behest of their treating oncologists. A thoracic oncology registry was used as a clinicogenomic database to track patient outcomes.
RESULTS:
Of 798 individuals with advanced NSCLC (median age, 65 years [range, 22-99 years]; 60% white; 50% with a history of smoking), 662 patients (83%) had molecular testing and 439 (55%) received targeted therapy on the basis of the omic-data. A fast-and-frugal decision tree (FFT) model was developed to evaluate the impact of omics-based strategy on decision-making, progression-free survival (PFS), and overall survival (OS). We calculated that the overall positive predictive value of the entire FFT strategy for predicting decisions regarding the use of tyrosine kinase inhibitor–based targeted therapy was 88% and the negative predictive value was 96%. In an adjusted Cox regression analysis, there was a significant correlation with survival benefit with the FFT omics-driven therapeutic strategy for both PFS (hazard ratio [HR], 0.56; 95% CI, 0.42 to 0.74; P < .001) and OS (HR, 0.51; 95% CI, 0.36 to 0.71; P < .001) as compared with standard therapeutic options.
CONCLUSION:
Among patients with advanced NSCLC who received care in the academic oncology setting, omics-driven therapy decisions directly informed treatment in patients and was correlated with better OS and PFS.
INTRODUCTION
Targeted therapy is a promising treatment that has revolutionized the management of lung cancer, a leading cause of cancer mortality in the United States. However, although randomized controlled trials (RCTs) have shown improvement in progression-free survival (PFS) with tyrosine kinase inhibitor (TKI)-based targeted therapy for genes such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) rearrangements, b-raf proto-oncogene (BRAF) V600E alterations, ros proto-oncogene 1 (ROS-1) rearrangements, and neurotrophic receptor tyrosine kinase (NTRK) fusions, the same has not been proven for overall survival (OS) as compared with standard therapy.1-5 These RCTs evaluated single-agent TKIs in the front-line setting, but real-world oncology practice is complicated further with several lines of therapy. Many patients typically receive serial TKIs during the course of their treatment, which is impossible to evaluate for PFS, and OS evaluation of these patients is not commonly performed. Testing for alterations with therapies approved by the US Food and Drug Administration (FDA) is also now routinely done in practice as part of a molecular testing panel, and this practice is endorsed by the National Comprehensive Cancer Network (NCCN), which has developed clinical pathways and guidelines to direct oncologists to proper genomic treatment management.1,6,7 Widespread use of next-generation sequencing (NGS) and the availability of targeted therapies in the clinics have complicated lung cancer treatment decision-making, with various national guidelines8 and commercial pathways9-11 coming to fruition to guide patient care. However, these guidelines and pathways are developed in “theory-free” environments,12 precluding evaluation of the accuracy of the use of NGS results and proper assignment of patients to appropriate targeted therapies informed by these guidelines and pathways. We recently proposed the use of fast-and-frugal decision trees (FFTs) as a theoretical framework for constructing clinical pathways to enable us to better assess the accuracy and the impact of the recommended management strategies on important health outcomes.12 Therefore, to address the clinical utility of omics-driven pathways and guidelines in the management of lung cancer, we constructed FFTs to provide a theoretical framework that calculates the accuracy of appropriate TKI selection based on a given mutation, as well as the impact this has on long-term outcomes.
METHODS
Patients
Patients (N = 798) at City of Hope (COH) who had pathology-confirmed metastatic lung adenocarcinoma were enrolled in this analysis and evaluated from 2008 to 2016. The data were collected between 2016 and 2018 and a retrospective chart review was performed. All patients in the study had stage IV disease and their date of diagnosis was recorded as the time of metastasis diagnosis. Patients had molecular testing performed at the discretion of their primary clinical provider, using clinically available molecular testing platforms. However, not all patients were tested on the same panel because of the variability of the treating oncologist and the type of testing available when the testing was ordered. The various NGS platforms of testing included (1) FoundationOne (Foundation Medicine, Cambridge, MA), (2) Onco48 (COH, Duarte, CA), (3) Response DX: Lung (Cancer Genetics, Los Angeles, CA), (4) LabCorp (LabCorp, Burlington, NC), (5) OncoComplete (COH), (6) Caris (Caris Life Sciences, Dallas, TX), (7) MD Anderson (MD Anderson, Houston, TX), (8) Mayo Clinic (Mayo Clinic, Rochester, MN), (9) Hopeseq Lung (COH), (10) Guardant 360 (Guardant Health, Redwood City, CA), and (11) bioT3 (bioTheranostics, San Diego, CA). Single-gene testing results were also available and performed with fluorescent in situ hybridization, immunohistochemistry, Sanger sequencing, or NGS by various pathology laboratories for alternations in the following genes: EGFR, ALK, ROS1, KRAS, BRAF, MET, and RET.
Data Source
A thoracic oncology registry (THOR) included de-identified patient data obtained under an institutional review board–approved protocol (No. 18008) with a waiver of informed consent. The study was approved by the ethics review boards and in accord with an assurance filed with and approved by the Department of Health and Human Services at the COH and was conducted according to the Declaration of Helsinki. THOR encompasses the demographically diverse population of patients at COH. The patients were all treated at the COH academic site by 4 physicians with a focus on thoracic oncology. Data collected included patient demographics, stage, age at diagnosis, race and ethnicity, smoking history, date of diagnosis of metastatic disease, metastatic sites, treatment dates, dates of progression, date of death or of last contact, histology, molecular testing results, vital status at last contact, and overall survival (OS). Molecular testing results were abstracted from sequencing reports in patients’ charts. Only patients with stage IV disease who had a confirmed diagnosis of metastatic disease were included in this study.
Decision-Making Analysis: Clinical Practice Guidelines, Pathways, and FFTs
Clinical practice guidelines, such as those from the NCCN, are commonly used to aid decision-making. They are often converted into easy-to-follow algorithms, flow-charts, or clinical pathways. Pathways are typically ad hoc developed constructs by experts in an unsystematic, “theory-free” environment, which, in turn, precludes the quantitative evaluation of the outcomes based on the management strategies recommended by guidelines and pathways. The quantitative analysis of the accuracy of clinical management strategies (eg, whether the recommendation was true positive or negative) and assessment of its impact on health outcome is possible by converting pathways into FFT heuristics.12 FFTs are highly effective, simple decision trees composed of sequentially ordered cues (tests) and binary (yes or no) decisions formulated via a series of if-then statements.13 The binary (yes or no) responses determine the ratio between false-negative and false-positive recommendations, which, in turn, allow the application of Bayesian methods to calculate the accuracy of the entire FFT (ie, the entire clinical management strategy).13
Statistical Analysis
We first determined positive and negative predictive values related to the choice of appropriate targeted therapy (ie, whether management was based solely on available mutations, in which case targeted therapy was chosen or was affected by other factors prompting the use of chemotherapy [ie, nontarget therapy]). We then calculated survival and PFS as a function of the management driven by targeted versus nontargeted therapy. PFS was determined on the basis of physician notes from the medical records. Survival and PFS estimates for the study’s patients were generated using the Kaplan-Meier method supplemented by a multivariable Cox regression model to adjust the analysis for other relevant clinical factors. The distribution of cohort characteristics and the type of treatment assignment between targeted therapy and nontargeted therapy groups were compared using χ2 tests.
RESULTS
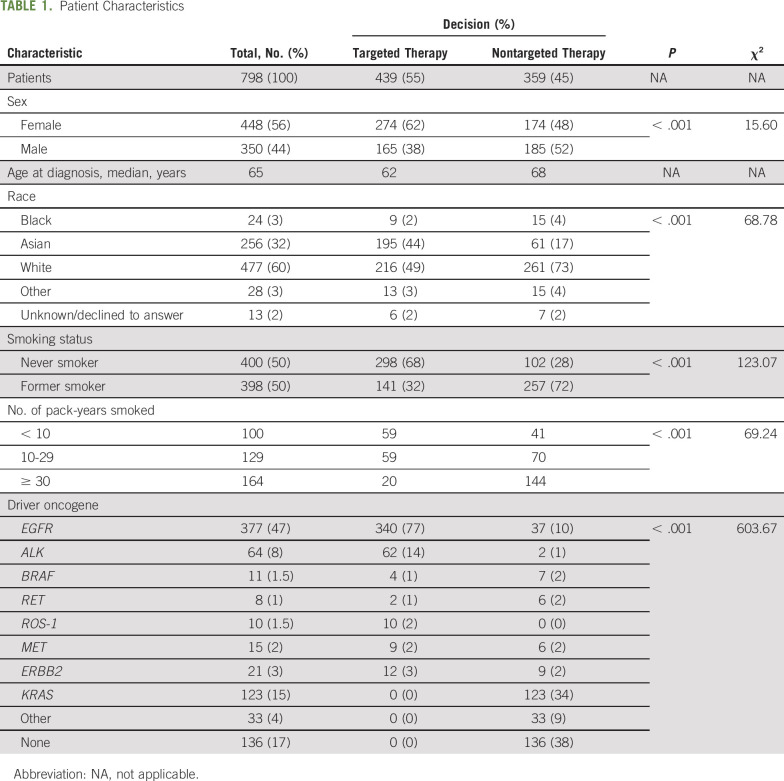
The clinical and demographic features of all patients included in this analysis are described in Table 1. In this study, 798 individuals with lung adenocarcinoma were identified in THOR who were treated or were intended to be treated (before their death or hospice care) at COH. The median age at metastatic diagnosis was 65 years (range, 22-99 years) for the entire cohort. The majority of patients were female (56%), the major race groups were White (60%), Asian (32%), and Black (3%); and 398 patients (50%) had a history of smoking, among whom 164 (21%) had a history of > 30 pack-years. For the targeted-therapy group, the majority of patients were female (62%), never smokers (68% v 28% in nontargeted-therapy group), and there was a distinctly high percentage of Asians (44%) as compared with 17% Asian patients in the nontargeted-therapy decision group.
TABLE 1.
Patient Characteristics
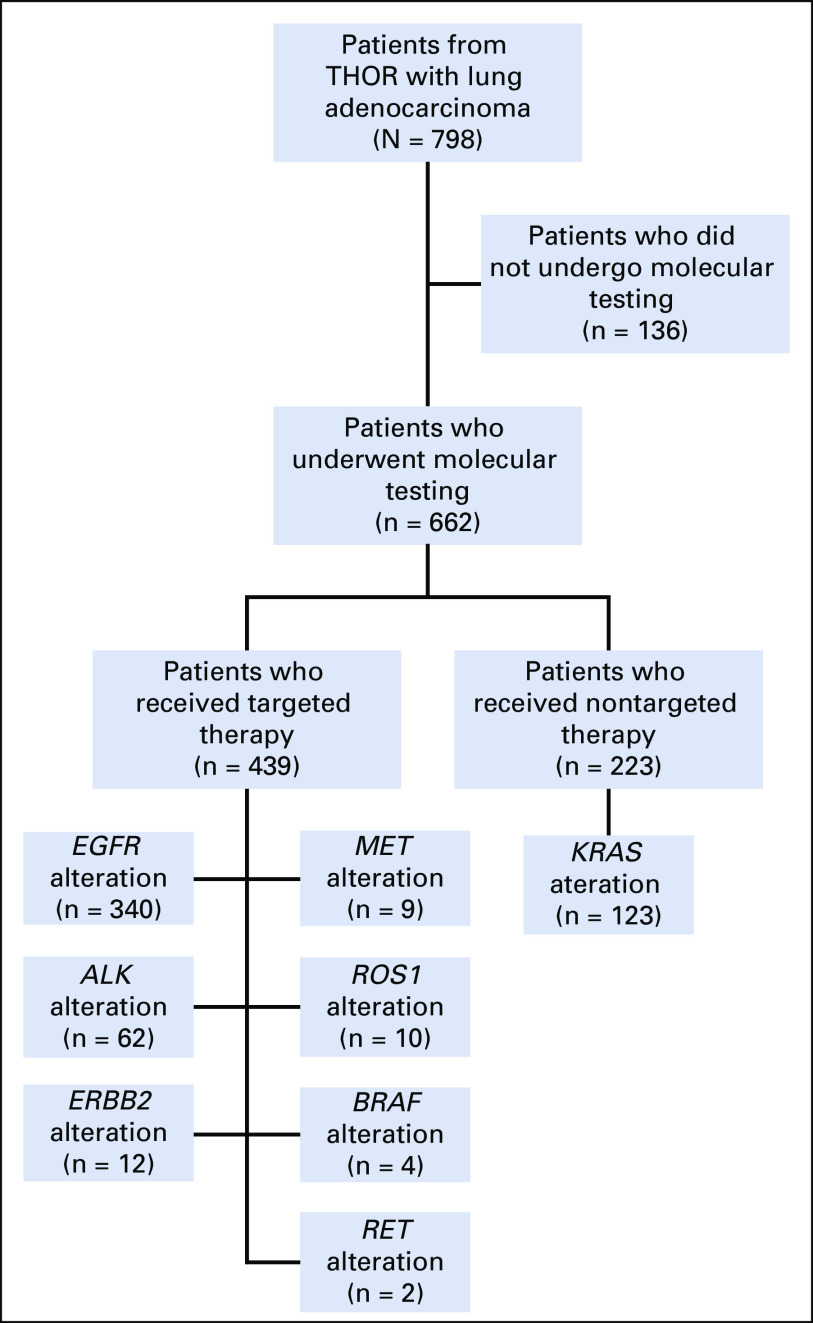
The breakdown of NGS testing and the distribution of targeted therapy and nontargeted therapy across the different genes is shown in Figure 1. The most common alterations were in EGFR (47%), and these patients mostly were treated with erlotinib (68%; Data Supplement, online only). Of the 662 patients who underwent molecular testing, 485 (73%) had an alteration detected with an available FDA-approved or clinically significant therapy and 88% of the patients (n = 427 of 485) were appropriately matched to a targeted therapy based on the oncologist’s decision. Overall, 90.18% of patients (n = 340 of 377) with an EGFR mutation, 96.87% of patients (n = 62 of 64) with an ALK rearrangement, and 100% of patients (n = 10 of 10) with a ROS1 fusion were appropriately treated with targeted therapy based on their mutational status. Similar rates were not observed in BRAF V600E (36.6%; n = 4 of 11), MET exon 14 (59.99%; n = 9 of 15), or RET fusion (24.98%; n = 2 of 8); Fig 1).
FIG 1.
Flow diagram for patient participation. THOR, thoracic oncology registry.
Although stage IV lung adenocarcinoma with MET exon 14 alterations, ERBB2 mutations, and RET fusions did not have an FDA-approved therapy at the time of our analysis, which is common in the real-world, off-label use in oncology practice, information related to these mutations informed treatment decisions in our cohort. Alternative therapeutic options for patients who had actionable alterations, such as in EGFR, ALK, and BRAF, included chemotherapy, immunotherapy, palliative care, hospice, among others (Data Supplement). The majority of patients with a nontargeted-therapy decision had a KRAS alteration (n = 123 of 359; 34%) or no molecular testing performed (n = 136 of 359; 38%) and were treated with chemotherapy (54%) or immunotherapy (13% single; 5% combination).
FFT for lung Cancer Management
Figure 2 shows FFT representing a comprehensive strategy for the management of metastatic lung cancer based on molecular testing and administration of targeted therapy. The overall positive predictive value of our FFT for predicting decisions regarding the use of TKI targeted therapy was 88% and the negative predictive value was 96%, suggesting that lung cancer management strategies are almost entirely driven by the availability of targeted therapy and other clinical factors played a relatively minor role.
FIG 2.
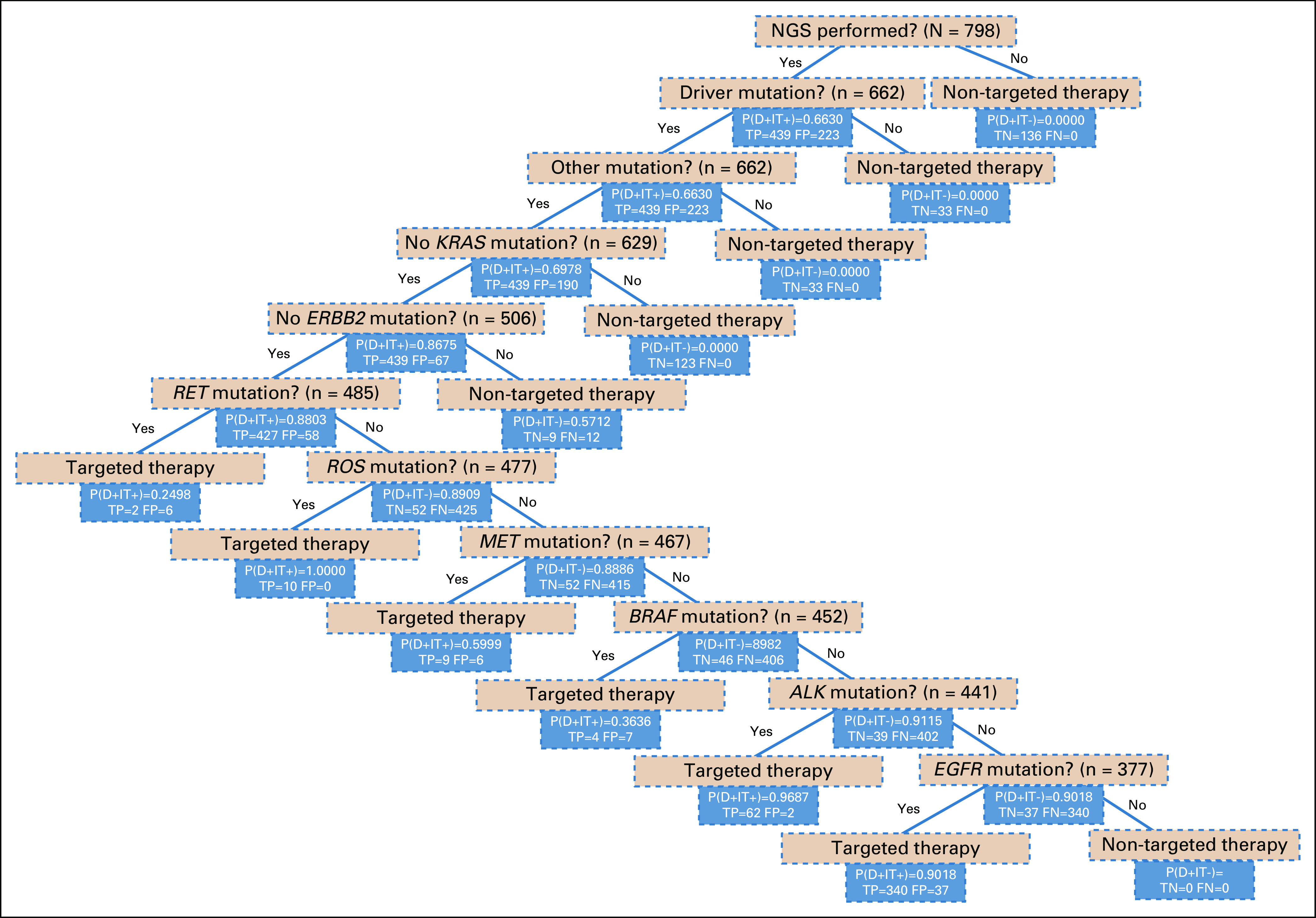
A model fast-and frugal decision tree (FFT) showing cues that represent a comprehensive management strategy for lung cancer decision-making based on molecular testing and administration of targeted therapy. An FFT comprises sequentially ordered cues where cues (eg, was next-generation sequencing [NGS] performed?) and accompanying decisions (eg, targeted therapy v nontargeted therapy) are binary (yes or no), thus we can frame their relationship with “if-then” statements. In this example, if a patient had an EGFR mutation, they would be given targeted therapy. Furthermore, the FFT also evaluates whether the decision made was a true positive (TP) or a false positive (FP), based on the detected alteration. P(D+|T+), probability of selecting targeted therapy (ie, probability of selection of a tyrosine kinase inhibitor [TKI] given a positive mutation [positive predictive value]); P(D+|T−), probability of selecting nontargeted therapy (ie, probability of selecting a non-TKI therapy given the absence of mutation). The figure shows the predictive value after using each cue (mutation). Using Bayes formula for taking into consideration conditional dependency of cues, we calculated that the overall positive predictive value of the entire FFT strategy for predicting decisions regarding the use of TKI targeted therapy was 88%, whereas the negative predictive value (NPV) was 96%.12,13 FN, false negative; FP, false positive.
Impact of FFT-Driven Targeted Therapy on Survival and PFS
In an intention-to-treat analysis, the targeted-therapy treatment decision was correlated with survival benefit as compared with nontargeted-therapy decisions. FFT-based targeted-therapy decision-making showed a significant benefit, with a median survival of 38 months as compared with 26 months in the nontargeted-therapy decision-making group (P < .001; Fig 3A). This was also evident in the PFS analysis, where patients in the targeted-therapy decision-making group had a median survival of 9 months, as compared with 5 months in the other group (P < .001; Fig 3B). In the unadjusted Cox regression analysis, the hazard ratio (HR) was 0.53 (95% CI, 0.43 to 0.65; P < .001) for OS and 0.54 (95% CI, 0.45 to 0.64; P < .001) for PFS, both favoring better outcomes with the FFT-driven therapy decision (Data Supplement). An adjusted Cox regression analysis demonstrated that, as expected, OS benefits were associated with age (HR, 1.03; 95% CI, 1.01 to 1.05; P < .001), with younger patients faring better. More importantly, OS was improved with the FFT-driven therapeutic strategy (HR, 0.51; 95% CI, 0.36 to 0.71; P < .001; Data Supplement). However, in the PFS adjusted Cox analysis, the FFT-driven therapy decision was the only significant variable (HR, 0.56; 95% CI, 0.42 to 0.74; P < .001; Data Supplement).
FIG 3.
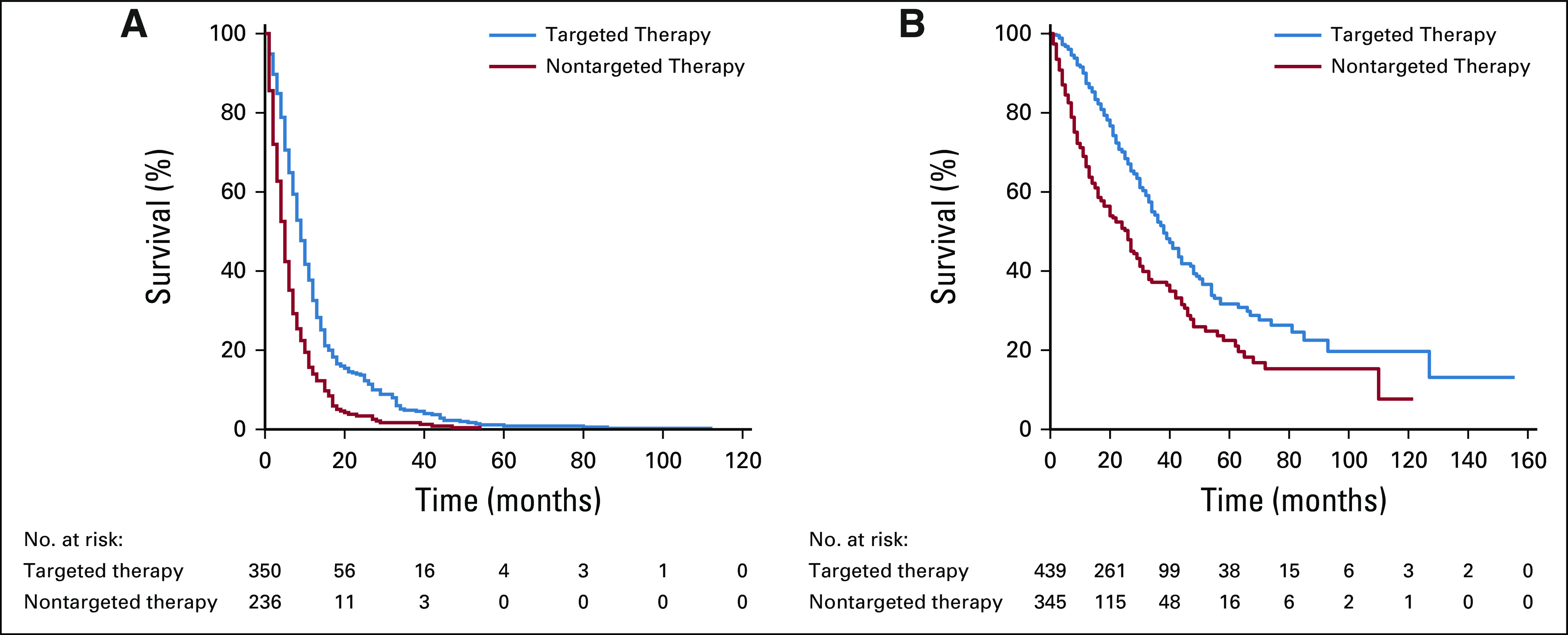
Association of survival with the decision made based on targeted versus nontargeted therapy. (A) Kaplan-Meier estimates of progression-free survival between a targeted therapy and nontargeted therapy decision. (B) Kaplan-Meier estimates of overall survival between a targeted therapy and nontargeted therapy decision.
DISCUSSION
Overall, patients who received targeted therapy had improved short-term PFS long-term OS when compared with patients who received a nontargeted standard-of-care therapy. This survival advantage was correctly predicted by our FFT analysis, especially when taking into account the entire treatment management plan, which used a number of cues. Although targeted therapy has been shown in several RCTs to have superior PFS,14-18 the same has not been proven for OS. This is partly because the advantage of treatment effects related to PFS is diluted by crossover or subsequent therapies.19 However, statistically proven incremental gains in OS are difficult to achieve without negative effects on quality of life.19 Although first-generation TKIs such as geftinib and erlotinib showed minimal median OS improvements, more mature data from gefitinib and erlotinib trials showed that patients who received sequential combination of EGFR-TKI and chemotherapy had significantly improved OS, suggesting that TKI-related improvement in OS lies in sequential therapy.2,20 Furthermore, recent trials, including the FLAURA trial, have shown incremental improvements in OS alongside improvements in quality of life, as compared with first-generation TKIs.21 However, the ARCHER 1050 trial showed that although OS was improved with dacomitinib compared with gefitinib, the improvements in quality of life were only seen in patients treated with geftinib.22,23 We had previously shown in a retrospective meta-analysis of > 1,000 clinical trials that enrolled > 80,000 patients with oncologic malignancies that the long-term outcomes in patients with personalized treatment strategies were superior to those in patients in nonpersonalized therapy arms.24-28 However, to our knowledge, this is the first formal study that applied a standardized theoretical framework to evaluate lung cancer decision-making demonstrating that an omic-based management strategy leads to superior outcomes compared with standard chemotherapy or immunotherapy (ie, non–omics-based treatment). Therefore, we believe the widespread availability of NGS testing would improve outcomes beyond our single-institution experience. Indeed, we had previously shown that adherence to clinical guidelines improves biomarker testing and appropriate first-line therapy in academic and community settings.11,29 In our current study, overall testing rates for ALK, EGFR, and other actionable mutations was 83% (n = 662 of 798). The rates were similarly high for appropriate assignment to treatment upon detection of targeted alterations in EGFR (n = 340 of 377; 90%), ALK (n = 62 of 64; 97%), and ROS1 (n = 10 of 10; 100%), but were much lower with BRAF (n = 4 of 11; 36%), RET (n = 4 of 8; 25%), and MET (n = 9 of 15; 60%). This may be because the guidelines have only recently incorporated the other actionable alterations, and other alterations, such as MET exon 14, ERBB2 mutations, and RET fusions, at the time these patients were treated (2008-2016), did not yet have FDA-approved therapies.30,31
With these biomarker testing and adherence rates in mind, our FFT approach was able to identify a distinct pattern of improved OS of 38 months in the FFT-driven targeted-therapy decision-making group as compared with 26 months in the nontargeted-therapy decision-making group. Therefore, we have shown that the confluence between omics-driven therapeutics and lung cancer decision-making yields significant benefits to the patient and that applying the FFT approach may simplify the decision-making process for oncologists beyond what could be offered by the available clinical pathways and guidelines. In our Cox regression analyses, 2 factors were significantly associated with better survival: younger age and FFT-driven therapeutic decisions. In regard to PFS, the FFT-driven therapeutic decision was the sole significant factor associated with better outcomes signifying the superiority of omic-driven management strategies as compared with standard therapeutic options. Although previous studies have attempted to evaluate specific cases using limited-panel molecular testing for specific mutations,25,32-34 this study shows a statistically significant increase in both PFS and OS based on a molecular-informed therapy strategy in patients with lung cancer at a single academic site.
A limitation of this study is that this is a single-institution study that focused on a retrospective analysis, with relatively few but key variables of prognostic or predictive significance. With a relatively small sample size in our study, it would be important to perform a large multi-institutional study to better understand the utility of the FFT as a theoretical framework for lung cancer decision-making. This would also alleviate the concern that our study only included patients of 4 oncologists at a single academic institution who undoubtedly confer with each other for their academic expertise when evaluating patients. Inclusion of community sites or oncologists from the community setting in future studies would offer a more robust conclusion. It would also be important in the future to delineate the immunotherapy subgroups into prognostic factors such as PD-L1 to better understand the effect it may have on targeted therapy.35 Nevertheless, it remains a remarkable finding that the availability of mutation- or targeted-therapy dominated decision-making in almost 90% of cases with improved durable survival.
In conclusion, among patients who received care for advanced non–small-cell lung cancer in the academic setting, omics-driven therapy decision directly informed treatment in patients and was closely correlated with better survival.
ACKNOWLEDGMENT
We thank the City of Hope nurses and support staff for their dedication to their patients.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health (Grants No. P30CA033572, U54CA209978, and R01CA218545 [R.S.]).
R.S. and I.M. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Ravi Salgia, Isa Mambetsariev, Marianna Koczywas, Karen Reckamp, Benjamin Djulbegovic
Financial support: Ravi Salgia
Administrative support: Ravi Salgia
Provision of study material or patients: Ravi Salgia, Karen Reckamp, Benjamin Djulbegovic
Collection and assembly of data: Ravi Salgia, Isa Mambetsariev, Rebecca Pharaon, Jeremy Fricke, Angel Ray Baroz, Marianna Koczywas, Karen Reckamp
Data analysis and interpretation: Ravi Salgia, Isa Mambetsariev, Rebecca Pharaon, Jeremy Fricke, Iztok Hozo, Chen, Marianna Koczywas, Erminia Massarelli, Karen Reckamp, Benjamin Djulbegovic
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evaluation of Omics-Based Strategies for the Management of Advanced Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ravi Salgia
Consulting or Advisory Role: Iovance Biotherapeutics, AbbVie, Octimet, Novartis, ARIAD
Speakers' Bureau: AstraZeneca, Merck
Marianna Koczywas
Speakers' Bureau: AstraZeneca, Celgene
Erminia Massarelli
Honoraria: AstraZeneca, Merck
Consulting or Advisory Role: Roche, Nektar
Speakers' Bureau: Merck, AstraZeneca
Research Funding: Merck (Inst), AstraZeneca (Inst), Pfizer (Inst), Tessa Therapeutics (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck, Roche, Pfizer, AstraZeneca
Karen Reckamp
Consulting or Advisory Role: Amgen, ARIAD, Astellas Pharma, Euclises, Tesaro, Boehringer Ingelheim, Takeda, AstraZeneca, Exelixis, Guardant Health, Loxo, Seattle Genetics, Precision Health Economics, Calithera Biosciences, Genentech
Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), ARIAD (Inst), Xcovery (Inst), Adaptimmune (Inst), Roche (Inst), Boehringer Ingelheim (Inst), AbbVie (Inst), ACEA Biosciences (Inst), Loxo (Inst), GlaxoSmithKline (Inst), Guardant Health (Inst), Janssen Oncology (Inst), Seattle Genetics (Inst), Zeno Pharmaceuticals (Inst), Molecular Partners (Inst)
Benjamin Djulbegovic
Research Funding: GlaxoSmithKline (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lindeman NI, Cagle PT, Aisner DL, et al. : Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 13:323-358, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Mok TS, Wu YL, Thongprasert S, et al. : Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947-957, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Planchard D, Kim TM, Mazieres J, et al. : Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: A single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 17:642-650, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Ou SH, Bang YJ, et al. : Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371:1963-1971, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drilon A, Laetsch TW, Kummar S, et al. : Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 378:731-739, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reckamp KL: Targeted therapy for patients with metastatic non-small cell lung cancer. J Natl Compr Canc Netw 16(5S, 5s)601-604, 2018 [DOI] [PubMed] [Google Scholar]
- 7. https://www.businesswire.com/news/home/20171130006320/en/ Foundation Medicine: FDA approves Foundation Medicine’s FoundationOne CDx, the first and only comprehensive genomic profiling test for all solid tumors incorporating multiple companion diagnostics. 2017.
- 8.Ettinger DS, Wood DE, Aggarwal C, et al. : NCCN guidelines insights: Non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw 17:1464-1472, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Daly B, Zon RT, Page RD, et al. : Oncology clinical pathways: Charting the landscape of pathway providers. J Oncol Pract 14:e194-e200, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Zon RT, Edge SB, Page RD, et al. : American Society of Clinical Oncology criteria for high-quality clinical pathways in oncology. J Oncol Pract 13:207-210, 2017 [DOI] [PubMed] [Google Scholar]
- 11. Mason C, Ellis PG, Lokay K, et al: Patterns of biomarker testing rates and appropriate use of targeted therapy in the first-line, metastatic non-small cell lung cancer treatment setting. J Clin Pathw 4:49-54 (2018) [DOI] [PMC free article] [PubMed]
- 12.Djulbegovic B, Hozo I, Dale W: Transforming clinical practice guidelines and clinical pathways into fast-and-frugal decision trees to improve clinical care strategies. J Eval Clin Pract 24:1247-1254, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Hozo I, Djulbegovic B, Luan S, et al. : Towards theory integration: Threshold model as a link between signal detection theory, fast-and-frugal trees and evidence accumulation theory. J Eval Clin Pract 23:49-65, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Soria J-C, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal GM, Karuri SW, Zhang H, et al. : Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol 33:1008-1014, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simeone JC, Nordstrom BL, Patel K, et al. : Treatment patterns and overall survival in metastatic non-small-cell lung cancer in a real-world, US setting. Future Oncol 15:3491-3502, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Arbour KC, Riely GJ: Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. JAMA 322:764-774, 2019 [DOI] [PubMed] [Google Scholar]
- 18. Buyse ME, Squifflet P, Laporte S, et al: Prediction of survival benefits from progression-free survival in patients with advanced non small cell lung cancer: Evidence from a pooled analysis of 2,838 patients randomized in 7 trials. J Clin Oncol 26:8019, 2008 (15 suppl)
- 19.Villaruz LC, Socinski MA: The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement. Clin Cancer Res 19:2629-2636, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou C, Wu YL, Chen G, et al. : Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 26:1877-1883, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Leighl NB, Karaseva N, Nakagawa K, et al. : Patient-reported outcomes from FLAURA: Osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer 125:49-57, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Wu YL, Cheng Y, Zhou X, et al. : Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol 18:1454-1466, 2017 [DOI] [PubMed] [Google Scholar]
- 23. Mok T, Cheng Y, Zhou X, et al. Dacomitinib (daco) versus gefitinib (gef) for first-line treatment of advanced NSCLC (ARCHER 1050): Final overall survival (OS) analysis. J Clin Oncol 36:9004 2018 (15 suppl)
- 24.Gong J, Pan K, Fakih M, et al. : Value-based genomics. Oncotarget 9:15792-15815, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janku F, Berry DA, Gong J, et al. : Outcomes of phase II clinical trials with single-agent therapies in advanced/metastatic non-small cell lung cancer published between 2000 and 2009. Clin Cancer Res 18:6356-6363, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Jardim DL, Schwaederle M, Wei C, et al. : Impact of a biomarker-based strategy on oncology drug development: A meta-analysis of clinical trials leading to FDA approval. J Natl Cancer Inst 107:djv253, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwaederle M, Zhao M, Lee JJ, et al. : Impact of precision medicine in diverse cancers: A meta-analysis of phase II clinical trials. J Clin Oncol 33:3817-3825, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwaederle M, Zhao M, Lee JJ, et al. : Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: A meta-analysis. JAMA Oncol 2:1452-1459, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Mambetsariev I, Pharaon R, Nam A, et al. : Heuristic value-based framework for lung cancer decision-making. Oncotarget 9:29877-29891, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. doi: 10.1097/JTO.0000000000000405. Morgensztern D, Campo MJ, Dahlberg SE, et al: Molecularly targeted therapies in non-small-cell lung cancer annual update 2014. J Thorac Oncol 10:S1-S63, 2015 (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennell NA, Arcila ME, Gandara DR, et al. : Biomarker testing for patients with advanced non-small cell lung cancer: Real-world issues and tough choices. Am Soc Clin Oncol Educ Book 39:531-542, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Le Tourneau C, Delord JP, Gonçalves A, et al. : Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol 16:1324-1334, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Schram AM, Hyman DM: Quantifying the benefits of genome-driven oncology. Cancer Discov 7:552-554, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singal G, Miller PG, Agarwala V, et al. : Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA 321:1391-1399, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, Zeng D, Ou Q, et al. : Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: A meta-analysis and individual patient-level analysis. JAMA Netw Open 2:e196879, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]