Abstract
The pandemic of coronavirus disease 2019 (COVID-19) still remains on an upsurge trend. The second wave of this disease has led to panic in many countries, including India and some parts of the world suffering from the third wave. As there are no proper treatment options or remedies available for this deadly infection, supportive care equipment's such as oxygen cylinders, ventilators and heavy use of steroids play a vital role in the management of COVID-19. In the midst of this pandemic, the COVID-19 patients are acquiring secondary infections such as mucormycosis also known as black fungus disease. Mucormycosis is a serious, but rare opportunistic fungal infection that spreads rapidly, and hence prompt diagnosis and treatment are necessary to avoid high rate of mortality and morbidity rates. Mucormycosis is caused by the inhalation of its filamentous (hyphal form) fungi especially in the patients who are immunosuppressed. Recent studies have documented alarming number of COVID-19 patients with mucormycosis infection. Most of these patients had diabetes and were administered steroids for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection and were consequently more prone to mucormycosis. Hence, the present review emphasizes mucormycosis and its related conditions, its mechanism in normal and COVID-19 affected individuals, influencing factors and challenges to overcome this black mold infection. Early identification and further investigation of this fungus will significantly reduce the severity of the disease and mortality rate in COVID-19 affected patients.
Keywords: COVID-19, Mucormycosis, Organ damage, Diabetes, Immunosuppression, Steroids, Environmental pollution
Abbreviations: AML, Acute Myeloid Leukemia; CD4+ T, cluster of differentiation 4 T-helper cells; CNS, Central Nervous System; CotH, Spore coat protein; COVID19, Coronavirus Disease 2019; CT, Computed Tomography; ICU, Intensive Care Unit; IFN-γ, Interferon-gamma; IL-10, Interleukin −10; IL-17, Interleukin −17; IL-4, Interleukin-4; LIFE, Leading International Fungal Education; NK cells, Natural Killer cells Normal T-cell Expressed and Secreted; PDGFRB, Platelet-Derived Growth Factor Receptor B; RANTES, Regulated upon Activation Normal T-cell Expressed and Secreted; ROCM, Rhino-Orbital Cerebral Mucormycosis; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SSTI, Skin and Soft Tissue Infection; WHO, World Health Organization
1. Introduction
Mucormycosis is a rare fungal infection caused by exposure to mucor mold commonly found in soil, manure, plants, decaying fruits and vegetables, air and even in the mucus of healthy people. It affects the sinus, brain and lungs and can be life-threatening in diabetic or severely immunocompromised individuals. The year 2020 was devastating for global health as an uncommon virus raced worldwide, emerging rapidly as one of the top killers laying bare the inadequacies of the health systems. Today, health services in all regions are struggling to tackle COVID-19 and provide people with vital care. As the global COVID-19 pandemic enters the second year, countries around the world are racing to vaccinate their populations as novel variants emerge. The general population is relatively more complacent towards physical distancing, mask-wearing, and other public health interventions. India continued to maintain a downward trend in daily COVID-19 cases until the number of cases hovered above 3,00,000 in the deadly second wave of the infection. As of June 7, 2021, the country has recorded 28,252 cases of mucormycosis from 28 states. There are 24,370 cases with a history of COVID-19 and 17,601 cases with a history of diabetes. India had recorded its highest number (6329) of mucormycosis cases (Adil, 2021). The virus is spreading faster than ever before, in India, despite previous high infection rates in megacities, which should have conferred some protection. The pandemic is sweeping through India at a pace that has staggered scientists. Daily cases have exploded since early March 2021. Among the world, the European countries such as France and Germany, the Brazil and the United States are also currently experiencing large outbreaks, reporting high infection rates at around 70,000 a day. Hospitals are scrabbling for beds and oxygen in response to a deadly second surge in infections. India has reported for nearly half the COVID-19 cases worldwide recently and a quarter of the deaths (World Health Organization, 2021a). The number of COVID-19 cases globally remains at the highest levels since the beginning of the pandemic with over 175 million new weekly cases (World Health Organization, 2021b). At present, there are four variants of concern that WHO is tracking around the world. The B.1.1.7 was first identified in the United Kingdom, B.1.351, was first identified in South Africa, and the P.1 variant was first identified in Japan (World Health Organization, 2021c). Genomic surveillance data shows that the variant B.1.1.7, from the United Kingdom has become the dominant form of the virus in the Indian states. And a new and potential variant of concern, first identified in India late last year, known as B.1.617, has become dominant in the state of Maharashtra. B.1.617 has drawn attention because it contains two mutations linked to increased transmissibility and an ability to evade immune protection. It has now been detected in 20 other countries as well (Mallapaty, 2021). Physicians are documenting an alarming number of cases of mucormycosis among COVID-19 patients. Most of these patients had diabetes and are treated with steroids for SARS-CoV-2 infection and this combination might have made them more prone to fungal attack. People with a compromised immune system or bone marrow transplant with fewer neutrophils are more vulnerable to mucormycosis (Dantas et al., 2021; Sarvestani et al., 2013; Shariati et al., 2020; Suganya et al., 2019). COVID-19 patients are prescribed with heavy doses of steroids resulting in weakened immune system and are susceptible to mucormycosis. In addition, steroids can cause blood sugar levels to spike, which is challenging for patients with uncontrolled diabetes and the acidic environment due to this condition favors the fungal (Mucorales) growth. Inhalation of filamentous fungi by patients weakens the immune defense pathways. Mucormycosis has also been associated with various underlying conditions that predispose an individual to the infection. Hospitals around the country continue to report a growing trend of mucormycosis cases in COVID-19 patients and this disease has been declared as an epidemic. Hence, the aim of the present review emphasizes the history of mucormycosis, its related diseases, its process in normal individuals, immune-compromised and COVID-19 affected subjects, the various risk factors and its effect on multiple organs and challenges to overcome this infection. With increasing pressure on healthcare infrastructure during the COVID-19 pandemic, this review will provide a general evidence base for optimal treatment outcomes and prevention from this fungal infection.
2. Origin of mucormycosis
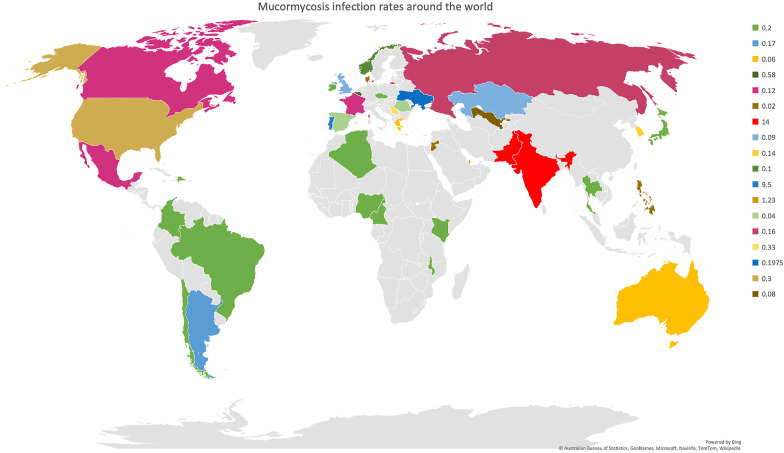
Mucormycosis (also called zygomycosis) is a serious but rare fungal infection caused by a group of molds called mucormycetes. Mucormycosis, or the deadly black fungus, is a life-threatening fungal infection caused by fungi that belongs to the subphylum Mucoromycotina and order Mucorales (Chegini et al., 2020; Chibucos et al., 2016). Mucorales fungi are the most common fungi found in hematological malignancies, hematopoietic stem cell transplantation and solid organ transplantation after Aspergillus (Jeong et al., 2019). Eleven genus and ~27 species under the order Mucorales cause mucormycosis (Gomes et al., 2011). Among the predominant genera that cause mucormycosis, Rhizopus is the most common followed by Mucor and Lichtheimia. Mucorales are generally found in soil, decaying food, manure and dust (Asghar et al., 2019; Chakrabarti et al, 2006, 2009bib_Chakrabarti_et_al_2009 bib_Chakrabarti_et_al_2006; Chow et al., 2015; Reid et al., 2020). Mucormycosis was initially described in 1855, as this was the first authentic human case of this condition (Küchenmeister, 1855). In 1876, pulmonary mucormycosis was discovered by Furbringer in Germany in a cancer patient who presented with a hemorrhagic infarct in the right lung that consisted of fungal hyphae and spores (Furbringer, 1876). Mucormycosis was first seen in an autopsy in the year 1956 (Baker, 1956). The main mode of infection of mucormycosis is through the inhalation of spores, consumption of contaminated food and inoculation of the fungi into abrasions or cuts on the skin (Chibucos et al., 2016; Gomes et al., 2011; Jeong et al., 2019; Prakash and Chakrabarti, 2019; Reid et al., 2020). In addition, outbreaks of mucormycosis have also been linked to contamination of medical devices, ventilation systems and hospital disposables like bandages, hospital linen etc. (Rammaert et al., 2012). Mucormycosis mostly infects immunocompromised individuals whose immune system lacks the ability to mitigate the fungi. It is mainly diagnosed by laboratory analysis of the biopsy isolated from the site of infection. In addition, other imaging tests like CT are also beneficial for diagnosis (Prakash and Chakrabarti, 2019). This condition can be classified into six forms namely rhino-orbital cerebral mucormycosis (ROCM), pulmonary, cutaneous, gastrointestinal, disseminated and uncommon sites based on the location of their occurrence. Among them, ROCM is the most commonly occurring one. Among the species that cause mucormycosis, the Rhizopus species was linked with ROCM. At the same time Cunninghamella was found in the pulmonary or disseminated form, while Apophysomyces and Saksenaea were seen in the cutaneous type (Jeong et al., 2019). The most common sites of infection are sinuses (39%), lungs (24%), disseminated (23%); and skin and soft tissue infection (19%) (Reid et al., 2020). The fungi begin by invading the blood vessels, which results in thrombosis and infarction of the tissue. When the spores of the fungus, comes in contact with the endothelial cells, angioinvasion occurs. More interaction with the receptors of these cells results in cell damage and fungal spread (Spellberg et al., 2005). In healthy people, the fungi often get eradicated by the polymorphonuclear phagocytes. Hence fungal growth is usually present in individuals with defects in this mechanism. In addition, Mucorales are sometimes resistant to these mechanisms making them more virulent (Chamilos et al., 2008; Ibrahim and Kontoyiannis, 2013; Kontoyiannis and Lewis, 2006). Despite the increase in mucormycosis, the prevalence data is not completely compiled due to the lack of population-based studies (Skiada et al., 2020). In a study comprising of 600 articles, it was found that majority of mucormycosis occurred in Europe (34%), followed by Asia (31%), North or South America (28%), Africa (3%), Australia and New Zealand (3%) (Jeong et al., 2019). The Leading International Fungal Education (LIFE) estimates 10,000 cases globally, excluding India, and this number increased to 9,10,000 after including the data from India (Prakash and Chakrabarti, 2019). In India, the prevalence was 0.14 cases per 1000 population, astoundingly higher than in developed countries (Skiada et al., 2020). The estimation of mucormycosis in countries around the world is illustrated in Fig. 1 . In most cases, unless surgery and antifungal therapy are administered promptly, the condition deteriorates rapidly leading to death. The mortality rate was 54% in this condition and vastly depends on the site of infection, underlying comorbidities and type of fungus. The mortality rate was the highest for individuals with disseminated mucormycosis (96%), followed by pulmonary (76%) and sinus (46%) infections (Roden et al., 2005). The extremely high mortality rate, negative effects of surgery and lack of therapeutic options makes it vital to develop early diagnostic and prevention strategies (Chibucos et al., 2016).
Fig. 1.
Mucormycosis infections around the world: The estimated rates of mucormycosis infection per 100 K individuals around the world is illustrated in this figure. Among the countries, the mucormycosis burden is the highest in countries like India and Pakistan and followed by Portugal. This estimation was compiled by The Leading International Fungal Education (LIFE) portal.
3. Underlying conditions associated with mucormycosis
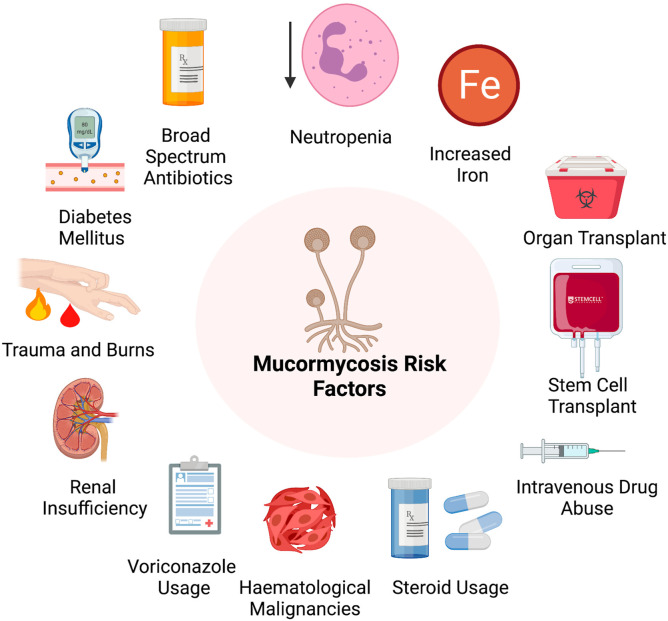
Mucormycosis has been associated with various underlying conditions that predispose an individual to the infection. Some of these factors include diabetes, neutropenia, organ or stem cell transplantation, trauma and burns, hematological disorders, steroidal use, metabolic acidosis, intravenous drug usage, renal insufficiency, broad-spectrum antibiotics, increase in iron in the system, malnutrition, usage of voriconazole (Fig. 2 ) (Dantas et al., 2021; Sarvestani et al., 2013; Shariati et al., 2020; Suganya et al., 2019). A previous study from Europe (Skiada et al., 2011) showed that, the most significant underlying causes were hematological malignancies, while it was diabetes mellitus in India (Chakrabarti et al., 2009), Iran (Dolatabadi et al., 2018), Middle East, North Africa (Stemler et al., 2020) and Mexico (Corzo-León et al., 2018). Among the different forms of mucormycosis, ROCM has been concomitant with the presence of diabetes. The cutaneous form was more prominent in individuals with trauma, and organ transplant was related to the pulmonary, gastrointestinal and disseminated type. In addition, underlying hematological malignancies were present in disseminated type and neutropenia in the pulmonary form (Jeong et al., 2019). Mucormycosis occurs mainly in individuals with uncontrolled diabetes, and this is because the innate immunity in these individuals, impacts the polymorphonuclear phagocytes to destroy the fungi. In patients with diabetes, the sinus was the most affected area followed by the pulmonary areas (Rammaert et al., 2012). Individuals with hematological malignancies were also predisposed to mucormycosis during the neutropenia phase of the ailment. The presence of mucormycosis in hematological malignancies can be attributed to chemotherapy and the usage of voriconazole used in the treatment of aspergillosis (Shadrivova et al., 2019). Mucormycosis was also more common in patients with acute leukemia than other types of malignancies. The main modes of prevention of mucormycosis in patients with hematological malignancies include avoidance of environmental exposures and the treatment strategies were surgery, antifungal treatment and reversal of neutropenia (Pagano et al., 1997). Transplantation therapies have also been diagnosed as risk factors for mucormycosis. However, the incidence of the condition varies based on the type of organs that are being transplanted. Since recipients of transplantation therapies are administered immunosuppressants and high doses of steroids, it makes them more vulnerable to mucormycosis (Almyroudis et al., 2006). In addition, corticosteroids also suppress macrophages and neutrophils inhibiting the ability of the body to fight the infection (Mcnulty, 1982). Steroid administered individuals also fall under the high-risk category. Stem cell therapy patients are also treated with voriconazole which influences the occurrence of mucormycosis when used prophylactically (Lionakis et al., 2018). Another factor contributing to mucormycosis is iron overload and deferoxamine therapy, which is being used to treat patients with diabetic ketoacidosis, haemodialysis and renal failure. However, this deferoxamine therapy makes the patients more likely to develop mucormycosis. The iron that is removed by the drug is used by the fungi to grow making a favorable condition for its development (Boelaert et al., 1991). Interestingly, mucormycosis is not just observed in patients with comorbid conditions, and it can also be seen in individuals after surgery, probably after using contaminated products (Jeong et al., 2019). A detailed explanation regarding various studies related to mucormycosis and its clinical studies is provided in Table 1 . Since invasive mucormycosis is also predominant in clinical settings, it is imperative to maintain a sterile environment that is safe for patients. In addition, care should be taken while assisting patients with chronic conditions in clinical settings to prevent the onset of mucormycosis.
Fig. 2.
Risk factors associated with the development of mucormycosis: Various factors that contribute to mucormycosis development; this includes the presence of underlying conditions like renal insufficiency, diabetes mellitus and hematological malignancies. Medications like voriconazole, broad-spectrum antibiotics and steroids are also known to predispose an individual to mucormycosis. In addition, an increase in iron levels in the circulation, neutropenia and immunosuppressant treatments like stem cell therapy and organ transplant make individuals more vulnerable to the condition. Exposure to spores through cuts and burns on the skin and intravenous drug usage are also risk factors for mucormycosis.
Table 1.
Predisposing conditions for mucormycosis.
| Underlying Disease | Organ/region infected with fungus | Fungus/Disease 3. Disease entities in mucormycosis |
Objective | No. Of patients | Country | Methodology | Results & Conclusion |
Reference |
|---|---|---|---|---|---|---|---|---|
| ROCM and Stroke | Paranasal region and Brain | Mucormycosis | Administration of amphotericin B and its complications to stroke and vasculopathy | 6 cases | Taiwan | Questionnaire survey |
|
Thajeb et al. (2004) |
| Gerstmann syndrome | Brain | Rhizomucor | Clinical examination and discussion of a case with aggressive mucormycosis | A 60-year-old women | USA |
|
|
Stretz et al. (2017) |
| Cerebral mucormycosis | Brain – basal ganglia | Rhizopus | Analysis of a case with mucormycosis along with multiple risk factors | A 28-year-old man | USA |
|
|
Malik et al. (2014) |
| Cerebral Lymphoma, vision loss, cirrhosis, diabetes | Brain | Mucormycosis | Analysis of fungal infection in orbital and CNS inflammation | A 61-year-old man | USA |
|
|
Beketova et al. (2018) |
| Rhinocerebral mucormycosis | Paranasal sinus, orbital and intra-cranial | Mucormycosis | To analyse the infection in CT scan to determine paranasal sinus, orbital and intra-cranial involvement | 17 cases | India |
|
|
Kulkarni et al. (2005) |
| Diabetes | Lungs | Pulmonary mucormycosis and tuberculosis | A diabetic case with fungal co-infection | A 56-year old female | Netherlands |
|
|
Jiménez-Zarazúa et al. (2019) |
| Chronic lymphocytic leukemia | Blood and bone marrow | Aspergillosis and Mucormycosis | Assessment of fungal infection in leukemia patient | A 79-year-old man | USA |
|
|
Tsikala-Vafea et al. (2020) |
| Septic shock | Intestine | Mucormycosis | Examination of fungal infection in immunocompetent individual | A 40-year-old male | Ethiopia |
|
|
Wotiye et al. (2020) |
| Hypothyroidism | Throat | Aspergillosis and Mucormycosis | A case study treated with corticosteroids developed fungal infections | A 55-year-old female | Italy |
|
|
Mantero et al. (2019) |
| Diabetes mellitus | Pansinusitis | Mucormycosis | Analysis of infection in a diabetic patient | A 56-year-old male | India |
|
|
Kumar, 2021 |
| Diabetes mellitus | Eye | Mucormycosis | Optic nerve infarction due to mucormycosis in a diabetes case | A 51-year-old male | Texas |
|
|
Chaulk et al. (2021) |
| Optic neuropathy | Eye | Mucormycosis | A case with retrobulbar optic neuropathy linked with mucormycosis. | A 94-year-old women | Japan |
|
|
Sano et al. (2018) |
| Renal failure and diabetes mellitus | Eye | Mucormycosis | ROCM observed in a case with ophthalmic nerve infection. | A 34-year-old man | Taiwan |
|
|
Lau et al. (2011) |
| Seizure | Brain | Rhizopus | Examination of the fungal infection in a case | A 49-year-old male | USA |
|
|
Verma et al. (2006) |
| Diabetic ketoacidosis with ophthalmoplegia | Nostril region | mucormycosis | Recovery from mucormycosis infection in a case | A 22-year-old women | USA |
|
|
Zafar and Prabhu (2017) |
| Diabetes mellitus | Orbital region | Rhizopusorzae | Two cases treated with posaconazole and amphotericin B with sinus surgical debridement. | 2 cases | China |
|
|
Zhang et al. (2013) |
| Diplopia, otalgia and right side numbness. Autoimmune hepatitis | Cerebral region | Mucormycosis | Assessment of fungal infection in a 12 year old girl | A 12-year-old girl | USA |
|
|
Ibrahim et al. (2009) |
| Diabetes, kidney failure, myelodysplastic syndrome, acute leukemia, | Cerebral region | Mucormycosis | Retrospective study of 36 cases with mucormycosis. | 36 cases | Mexico |
|
|
Rangel-Guerra et al. (1996) |
| HIV infection and diabetes | Cerebral region | Mucormycosis | Mucormycosis with vasculitis in a diabetic case | A 54-year-old woman | Brazil |
|
|
de Moura Feitoza et al. (2019) |
| Diabetes mellitus | Cerebral region | Mucormycosis | Progressive ophthalmoplegia and blindness in infection | 18-year-old woman | USA |
|
|
Hu et al. (2006) |
| Chronic lymphocytic leukemia | Cerebral region | Rhizomucorpusillus | A case study with mucormycosis in an immunocompromised host | 61-year-old man | USA |
|
|
Farid et al. (2017) |
| Eye movement syndrome | Sphenoid sinus | Mucormycosis | Patient with the infection suffered simultaneous carotid artery occlusion with infarction and a contralateral horizontal gaze palsy. | 54-year-old man | San Antonio |
|
|
Carter and Rauch (1994) |
| Diabetes mellitus (three patients) and Chronic leukemia (one patient) | Cerebral region | Mucormycosis | Examination of fungal infection in 4 cases with underlying diseases | 4 cases | Turkey (Abstract) |
|
|
Karakurum et al. (2005) |
| Diabetes mellitus with Cushing's syndrome | Cerebral region | Mucormycosis | Infection is associated with Cushing's syndrome and solid tumors | 42-year-old women | Mexico |
|
|
Salinas-Lara et al. (2008) |
| Acute lymphoblastic leukemia | Cerebral region | Mucormycosis | A case of fatal invasive ROCM with thrombotic occlusion of the internal carotid arteries following hematopoietic stem cell transplantation for acute lymphoblastic leukemia. | A 5-year-old boy | Switzerland |
|
|
Abela et al. (2013) |
| Stroke | Cerebral region | Mucormycosis | Outcome of stroke occurring in pregnancy and puerperium | 36 patients | USA |
|
|
Skidmore et al. (2001) |
| Hodgkin's lymphoma | Cerebral region | Mucormycosis | Assessment of mucormycosis in lymphoma patient which ended in multiple stroke | A 56-year-old man | Spain |
|
|
Jiménez Caballero et al. (2012) |
| Diabetes mellitus and immunosuppression conditions | Cerebral region | Mucormycosis | Regional differences in the infection and its causes | – | Middle East and North Africa |
|
|
Stemler et al. (2020) |
| Chronic lymphocytic leukemia | Cerebral region | Mucormycosis | A case of mucormycosis with cerebral involvement which ended in ischemic stroke | A 68-year-old man | Pennsylvania |
|
|
Ermak et al. (2014) |
| Diabetes | Cerebral region | Mucormycosis | A case with diabetes infected with mucormycosis | Elder man | Victoria |
|
|
Macdonell et al. (1987) |
| Diabetes mellitus with Garcin syndrome | Cerebral region | Mucormycosis | Analysis of infection and tuberculosis meningitis in a case with underlying disease. | – | China |
|
|
Yang and Wang (2016) |
| Diabetes mellitus | Cerebral region | Mucormycosis | To identify the prevalence and predisposing factors of mucormycosis in diabetes mellitus patients | 162 patients | Iran |
|
|
Sarvestani et al. (2013) |
| Acute lymphoblastic leukemia | Cerebral region | Mucormycosis | Treatment for leukemia resulted with infection and neuropathy | 17-year-old-female | USA |
|
|
Dworsky et al. (2017) |
| Leukemia | Cerebral region | Mucormycosis | Isavuconazole treatment risk assessment in leukemia patients | 100 patients | Houston |
|
|
Rausch et al. (2018) |
| Acute leukemias | Cerebral region | Rizopusspp | Assess the risk factors of infection in children with leukemia | 1136 subjects | Israel |
|
|
Elitzur et al. (2020) |
| Diabetes mellitus | Cerebral region | Mucormycosis | Identification of infection in diabetic patient with complications to acute infarction. | 57-year-old man | Iran |
|
|
Sasannejad et al. (2015) |
| Diabetes mellitus | Sinus region | Rhizopusarrhizus | To estimate the distribution of infection and its associated factors | 208 cases | Iran |
|
|
Dolatabadi et al. (2018) |
| Multiple diseases | Cerebral region | Rhizopusoryzae and Apophysomyceselegans | Clinical course of mucormycosis | 75 cases | India |
|
|
Chakrabarti et al. (2009) |
| CSS | Cerebral region | Mucormycosis | To assess the clinical and etiological profile of patients with CSS | 73 patients | India |
|
|
Bhatkar et al. (2017) |
| Diabetes mellitus and Hypothyroidism | Cerebral region | Rhizopussps | A progressive bilateral visual loss from mucormycosis due to bilateral optic nerve and retinal infarction in a patient with diabetes | 62-year-old woman | New York |
|
|
Merkler et al. (2016) |
| Parkinsonism | Cerebral region | Mucormycosis | Parkinsonism disease with mucormycosis infection | A 24-year-old man | USA |
|
|
Adler et al. (1989) |
| Hematologic malignancies or HCT recipients | – | Mucorales | Effect of isavuconazole in hematologic malignancies or HCT recipients | 145 patients | Portland |
|
|
Fontana et al. (2020) |
| Hematologic malignancies or HCT recipients | rhino-orbital-cerebral, pulmonary, disseminated, gastrointestinal and cutaneous | Mucormycosis | Antifungal treatment for hematologic malignancies or HCT recipients who were affected with mucormycosis. | 64 patients | USA |
|
|
Miller et al. (2021) |
| Lymphoid cancers | Cerebral region | Mucorales | Assessment of children with lymphoid cancers who developed fungal abscesses. | 8 children | India |
|
|
Ramanathan et al. (2020) |
| Hematological diseases | Cerebral region | Mucorales | Patients with hematological diseases assessed for fungal infections | 689 patients | South Korea |
|
|
Lee et al. (2020) |
| Diabetes and non-diabetic patients | rhino-orbito-cerebral | Mucorales | Compare the fungal infection in diabetic and non-diabetic patients | 63 patients | Iran |
|
|
Abdolalizadeh et al. (2020) |
| Diabetes mellitus, Malignancy, transplant | rhino-orbital | Rhizopus | Prospective observational study with mucormycosis across 12 centres in India | 465 patients | India |
|
|
Patel et al. (2020) |
| Lymphoid cancers | Lungs, CNS, sinus, liver and orbital regions | Mucorales | Evaluation of mycotic infection in hematological malignancies | 37 patients | Italy |
|
|
Pagano et al. (1997) |
| Renal transplant | Rhino-cerebral and pulmonary regions | Mucorales | Investigation of infection in renal transplant patients | 25 patients | Iran |
|
|
Einollahi et al. (2011) |
| Acute lymphocytic leukemia | cerebral | Mucormycosis | A case with leukemia reported with mucormycosis | 3-year-old girl | France |
|
|
Cornu et al. (2018) |
| Congenital neutropenia | – | Mucormycosis | Neutropenia patient with recurrent infections. | – | Iran |
|
|
Fahimzad et al. (2008) |
ROCM: rhino-orbito-cerebral mucormycosis; MRI: Magnetic resonance imaging; CT: computed tomography; CTA: computed tomography angiography; GMS: Grocott–Gomorimethenamine silver stain; PAS: periodic acid–Schiff; HR-VWI: high-resolution vessel wall imaging; ACTH: adrenal corticotropic hormone; CSS: cavernous sinus syndrome; HIV: human immunodeficiency virus; CSF: cerebrospinal fluid; RT-PCR: reverse transcription polymerase chain reaction; NGS: next-generation sequencing; HCT: hematopoietic cell transplant; PCR: polymerase chain reaction; qPCR: quantitative polymerase chain reaction; HAX1: HCLS1 Associated Protein X-1.
4. Mucormycosis mode of action in normal individuals
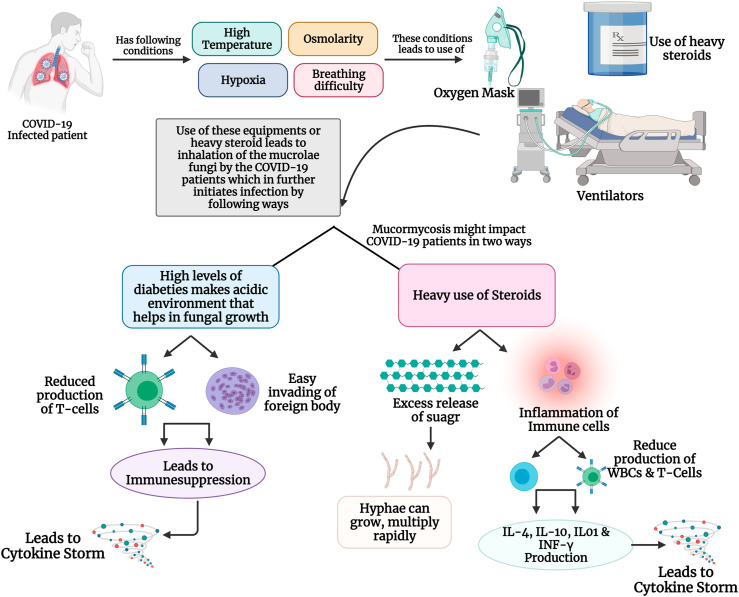
Mucormycosis is caused by the inhalation of its filamentous (hyphal form) fungi, especially in patients who have undergone weakness in the arsenal of immune defense. The characteristic phenotype of this disease is the growth of hyphae in and around the blood vessels, leading to life-threatening scenarios in severely immunocompromised patients. Once the fungal spores invade the human system, the hyphae intrude on the blood vessels, resulting in tissue infarction, necrosis and thrombosis. Mucormycosis occurs in the host by two steps: 1) by evading the immune system and surviving inside the host cell, 2) perturbation of the immune system further damaging the host cell (Brunke et al., 2016). In immunocompromised patients, iron is abundantly released via sequestering proteins which creates a favorable environment for the growth of fungi inside the human body. Also, Mucorales fungi consume iron using high-affinity iron permease and transport iron to development inside the host cell (Artis et al., 1982). The virulence factors of the pathogen play a key role to accomplish the damage. Spore coat (CotH) protein which is present on the spore surface of the Mucorales is responsible for penetrating, disrupting and damaging the immune cells (Gebremariam et al., 2014). The epithelial cells are the first line of contact to fungal pathogens, especially the mucoralean fungi damaging the epithelial cell via increased signaling of platelet-derived growth factor receptor B (PDGFRB) provides the proper growth factors to the fungi. The neutrophils are the first line of defense against these fungi, as these are an important part of the innate immune system and regulate the adaptive immune system (Jaillon et al., 2013). In diabetic or steroid use patients due to ketoacidosis or hyperglycemic conditions, the chemotactic factors released by neutrophils decrease thereby increasing the fungal hyphae in human hosts (Roilides et al., 2012). The Mucorales, after entering the host cell produces Mucorales-specific T-cells which generates interleukins (IL-4, IL-10 and IL-17) and IFN-γ. These pro-inflammatory cytokines further stimulate CD4+ T cells and damage the host cell (Castillo et al., 2018; Potenza et al., 2011). Also, the fungal hyphae reduce the release of various immunomodulatory molecules such as RANTES (regulated upon activation, normal T-cell expressed and secreted) and IFN-γ, which are secreted by NK cells which appears in the early stage of infection (Schmidt et al, 2013, 2016bib_Schmidt_et_al_2016 bib_Schmidt_et_al_2013). These key findings explain the susceptibility of immunocompromised patients to mucormycosis and its possible mechanism of action inside the host cell (Fig. 3 A).
Fig. 3.
(A): Mechanism of mucormycosis in healthy individuals: When the Mucorales enters an immune-compromised patient through inhalation, or through wounds, it is initially gets attached to the epithelial cells receptor using its CotH receptors. Further, the PDGFRB signaling pathways provides essentials for the proper development and growth of the fungal hyphae. Also, if the patients have diabetes, ketoacidosis and hyperglycaemia damage the neutrophils, making it easy for the fungi spread. Once the fungi are developed it starts to produce Mucorales-specific T cells which has various pro-inflammatory cells such as IL-4, IL-10, IL-17, IFN-γ, which triggers the cytokine storm resulting into cellular damages. (B): Possible mechanism of mucormycosis in COVID-19 infected patients: Mucormycosis is becoming common among COVID-19 patients, especially due to physiological stressors such as high body temperature osmolarity, hypoxia which are common conditions when affected with SARS-CoV-2. Also, these patients undergo heavy intake of steroids, use oxygen masks and ventilators to combat SARS-CoV-2 infection, which turns as an entry pass to the body for the Mucorales fungus. Further, this fungal infection could impact the COVID-19 in two-way scenario: 1) when the COVID-19 patients who have diabetes as co-morbidity, create an acidic environment that enables a unique environment for these fungi to grow. Also, due to hyperglycaemia, there is a decrease production of T-cells and immunosuppression, resulting in a cytokine storms. 2) heavy intake of steroids also release a huge amount of sugar which helps in the rapid multiplication and growth of fungal hyphae. Also, steroids tend to inflammation the immune cells leading to cytokine storm and damage to cellular organs.
5. COVID-19 and mucormycosis: a tangled relation
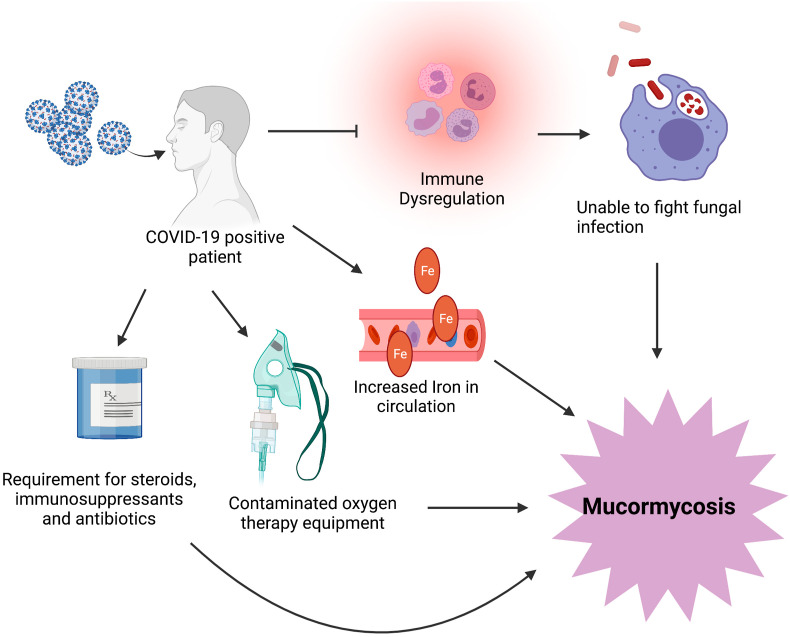
The advent of COVID-19 has sprung upon the world myriad of conditions and complications (Balachandar et al., 2020a; Iyer et al., 2020; Mahalaxmi et al., 2021). Mucormycosis is an another milestone added in COVID-19, that has emerged as a deadly complication associated with COVID-19. In March 2021, 41 cases of COVID-19 associated mucormycosis has been reported worldwide and 70% were from India. There is a surge in these cases amidst the second wave in India (Monica Slavin and Karin Thursky, 2021). There have been 2245 cases and 120 deaths from the infection in Maharashtra state (Barnagarwala, 2021). As of June 5, 2021, Rajasthan reported 2651 cases and 85 deaths (Mukherjee, 2021) and Telangana reports around 50 cases daily (Ali, 2021). In Tamil Nadu, till June 9, 2021, total mucormycosis reported being to be 1196 (Josephine, 2021). The mucormycosis stemming from COVID-19 patients has been more commonly observed in patients with a history of diabetes mellitus and 95% of individuals with severe or critical COVID-19 (Alekseyev et al., 2021; John et al., 2021). In addition to this, a two month old heart transplant patient developed mucormycosis three months after the COVID-19 diagnosis. This was of the cutaneous type as the old intravascular device location and despite aggressive treatment, the patient had died (Khatri et al., 2021). This evidence indicates that transplant patients need more vigilant care in the clinical setting while managing COVID-19 as these patients are already predisposed to mucormycosis (Fig. 4 ). Similarly, a patient with acute myeloid leukemia (AML) also suffered mucormycosis after the COVID-19 infection (Zurl et al., 2021). Although these factors such as diabetes, organ transplantation and hematological factors are commonly linked to mucormycosis, it is evident that COVID-19 infection also acts as a trigger in these situations. In addition, it has also been observed that people with no history of any underlying condition can also be diagnosed with mucormycosis post-COVID-19 infection (Maini et al., 2021). There are many plausible explanations for the occurrence of mucormycosis post-COVID-19 infection. COVID-19 patients exhibit a wide array of pulmonary changes (Subramaniam et al., 2020; Vishnupriya et al., 2021) which may be a focal point for fungal initiation. Moreover, COVID-19 is also associated with immune dysfunction (Renu et al., 2020) preventing the polymorphonuclear phagocytes from attacking the fungal spores upon entry (Jayaramayya et al., 2020). Patients with severe COVID-19 also require a prolonged hospital stay and mechanical ventilation (Balachandar et al., 2020b); the occurrence of fungal spores in this equipment could also contribute to mucormycosis in these individuals (Khatri et al., 2021). The immunosuppressants and corticosteroid medications that are warranted in COVID-19 can contribute significantly to the occurrence of mucormycosis (Khatri et al., 2021). Further, in addition to hyperglycemia, COVID-19 also contributes to changes in iron metabolism. High ferritin levels have been observed in COVID-19, the high iron concentrations release reactive oxygen species while damaging the nearby tissue. The cytokines released during COVID-19 further increases intracellular iron and leakage of iron into the circulation, posing as a risk factor for the development of mucormycosis (John et al., 2021). Although the diagnosis of mucormycosis is mainly made by observing fungus in biopsies and culturing the tissue (Johnson et al., 2021), waiting for the cultures in the dire COVID-19 situation may be impractical as the progression of mucormycosis is speedy (Dallalzadeh et al., 2021). Also, other therapeutic options besides reversal of underlying cause must be implemented as the reversal of these conditions may not be possible while treatment is ongoing for COVID-19. This is especially true due to the need to use high doses of steroids for the treatment of COVID-19 (Moorthy et al., 2021; Sharma et al., 2021). In this condition, it is evident that the use of antibiotics and steroids may be dangerous for some patients as they may trigger the onset of these life-threatening fungal infections. It is extremely important for the doctors treating patients with COVID-19 to be mindful of patients with underlying illnesses and prescribed steroids or immunosuppressants (Sarkar et al., 2021). Despite the tremendous burden on the healthcare system due to the overwhelming increase in cases, more vigilance is required in utilizing preventive measures for this condition (Kanwar et al., 2021). Also, it is important to periodically check the air in the hospital wards and the oxygen therapy machinery for spores (Suryanarayanan and Shaanker, 2021). In addition, recovered patients should also be advised to stay indoors for few weeks to build up their immunity and follow-up studies to prevent any adverse complications. Since it's important that care should be taken in disposal of solid waste of COVID-19 patients, similar measures must be undertaken for individuals with the infection (Iyer et al., 2021). Moreover, there is an urgent need to develop prompt diagnostic measures to manage mucormycosis in time (Veisi et al., 2021). The proper management of mucormycosis must be prioritized to prevent more COVID-19 related deaths.
Fig. 4.
COVID-19 and mucormycosis: A COVID-19 infected patient may be more susceptible to mucormycosis because, of a dysregulated immune system and may receive immune suppressant drugs that prevent the phagocytic cells in the body from attacking the fungus at an optimum level. COVID-19 also increases iron in the circulation and the fungus uses this iron to grow and proliferate and making the individual more vulnerable to infection. COVID-19 infected individuals are often given oxygen therapy. Contamination in these devices can serve as points of mucormycosis infection. The steroid therapy offered to COVID-19 patients places them at further risk for this condition.
6. Possible role of action of mucormycosis among COVID-19 patients
The symptoms of COVID-19 includes a rise in body temperature, osmolarity, hypoxia and breathlessness (Balachandar et al., 2020a). The COVID-19 recovered patients of late have been distressed with a new infection called mucormycosis disease. This fungal disease could easily invade the sinus and lungs, making its way to intra-orbital and intracranial regions of the body (Sundaram et al., 2014). The main symptoms of COVID-19 create a perfect environment for the growth and development of Mucorales inside the human body. Hosts susceptible to mucormycosis include diabetics, those on systemic corticosteroid use, patients with neutropenia and hematologic malignancies, stem cell transplant patients and immunocompromised individuals (Binder et al., 2014). Reports suggest that diabetic patients are more prone to acquire COVID-19 accompanied by mucormycosis infection (Mehta and Pandey, 2020; Mekonnen et al., 2021; Ahmadikia et al., 2021; Alekseyev et al., 2021; Garg et al., 2021, Ravani et al., 2021). The potential mechanism by which diabetes increases COVID-19 morbidity, and mortality is a) reduced viral clearance, b) decrease in T-cell function, c) high cytokine storm, d) immune-suppression (Balachandar et al., 2020b). Hyperglycemia worsens the cytokine storm by disrupting endothelial cells leading to multi-organ damage in COVID-19 patients. During diabetic ketoacidosis, the acidic environment and increase in the levels of free ferric ions support the growth of Mucorales. These circumstances support the invasion and successful attachment of the hyphae from the Mucorales inside the body. Individuals with chronic diabetes accompanied with foot ulcers are prone to this infection as any injured skin tissue is an easy entry route for this fungus. Further, treatment for COVID-19 is still preliminary (Kar et al., 2020; Kinoshita et al., 2021), and to combat the effect of SARS-CoV-2 infection, patients are given heavy doses of steroids (corticosteroids), as it reduces the inflammation in the lungs and might also curb the damages that had happened in the body due to the cytokine storm. Meanwhile patients affected with this new strain of COVID-19 are mostly treated with heavy steroids, extreme use of oxygen masks and ventilators which makes these patients more susceptible to mucormycosis. Steroids reduce both inflammation and the activity of the immune system, where the production of white blood cells (WBCs) and T-helper cells are decreased, making it easy for any foreign substances to invade and completely corrode the immune system in the host cell. Also, these steroids could trigger the uncontrolled release of sugar, which also enables the Mucorales to grow, multiply and invade at a rapid rate (“What patients with diabetes, cancer and kidney disorders need to know about black fungus,” 2021). Only a few case reports have been published regarding the impact of mucormycosis on COVID-19 affected patients (Table 2 ). Hence, these possible mechanisms have been listed based on the points and suggestions provided by various doctors and researchers (Fig. 3B), where the above-explained reasons may be potential factors for the occurrence of mucormycosis among COVID-19 recovered or infected patients.
Table 2.
Recent studies on COVID-19 cases with Mucormycosis.
| Age of COVID-19 case | Symptoms | Clinical History | Clinical examination | Treatment | Study Findings and Conclusion | Reference |
|---|---|---|---|---|---|---|
| 60-year-old male | severe breathlessness, pyrexia, tachypnea, and generalized malaise | diabetic (>10 years) pulse rate was 80/minute, blood pressure was 150/90 mmHg, Patient was afebrile on admission, respiratory rate was 26/minute, with a specific oxygen saturation of 86% on oxygen supplementation (10 L/min) bilateral crepts at the lung non-healing ulcer with the diabetic peripheral vascular disease was observed on right foot |
|
oral anti hypoglycemic tablets intravenous meropenem, oral oseltamivir with intravenous methylprednisolone and dexamethasone subcutaneous enoxaparin (40mg/0.4 ml) twice daily |
|
Mehta and Pandey, (2020) |
| 33-year-old Somali female | hypertension and asthma with altered mental status | patient began with symptoms of vomiting, cough, and shortness of breath 2 days prior to presentation. Signs of mild tachycardia, hypertension, and tachypnea. Afebrile and normal oxygen saturation. Left eye ptosis with 1 cm proptosis |
|
Vancomycin and piperacillin-tazobactam, Amphotericin B |
|
Werthman-Ehrenreich (2021) |
| 60-year-old man | diabetes, asthma, hypertension, hyperlipidemia and recent travel with dyspnea and hypoxia | Reported COVID-19 negative, and was discharged and later noticed with elevated level of glucose with oxygen demand. Was tested COVID-19 with ARDS |
|
Intravenous vancomycin and cefepime, antifungal coverage with liposomal amphotericin B, and strict glucose management. Dexamethasone 6 mg daily and a single dose of convalescent plasma as a treatment for COVID-19 |
|
Mekonnen et al, (2021) |
| 9 patients | COVID-19 infection | Post-mortem examination conducted. |
|
– |
|
Hanley et al. (2020) |
| 86-year-old male | arterial hypertension with acute diarrhea, cough, dyspnea, and fever | Throat swab confirmed COVID-19 |
|
ceftriaxone, azithromycin, oseltamivir, and hydrocortisone was provided. |
|
do Monte Junior et al. (2020) |
| 44-year-old women | Diabetes mellitus observed. Fever, malaise, myalgia, dry cough and partial dyspnoea was noticed. | Positive for influenze and negative for COVID-19 |
|
amphotericin B and posaconazole was administered. |
|
Ahmadikia et al. (2021) |
| 41-year-old man | Diabetes mellitus with loss of taste and cough. | Deep pain in the nose which radiated to throat. Oral cavity noted with black eschar |
|
Cefepime and IV abelcet, which is amphotericin B complexed with two phospholipids |
|
Alekseyev et al. (2021) |
| 31 patients | Diabetes, COVID-19 | Vision diminution and ophthalmoplegia |
|
amphotericin B |
|
Ravani et al. (2021) |
| 6 patients | COVID-19 | ptosis and ophthalmoplegia, edema, ptosis and proptosis, conjunctival congestion, and severe chemosis |
|
Corticosteroids, posaconazole was initiated |
|
Sen et al. (2021) |
| 55-year-old man | diabetes mellitus, hypertension, and ischemic cardiomyopathy presented with fever, dry cough, and progressive breathlessness | Respiratory rate was 26 breaths/minute, blood pressure 110/80 mmHg, and heart rate of 90 beats/minute. The oxygen saturation was 84% |
|
examethasone and remdesivir |
|
Garg et al. (2021) |
| 66-year-old male | COVID-19 positive | Deterioration of oxygen. |
|
hydroxychloroquine and lopinavir-ritonavir |
|
Pasero et al. (2020) |
| 38-year-old male | COVID-19 positive. | high grade fever, body ache, cough and shortness of breath |
|
Methylprednisolone, Dexamethasone. Intravenous Fluconazole and Amphotericin B |
|
Maini et al. (2021) |
| 32 year old women | Diabetes with ptosis and left facial pain |
|
– |
|
Saldanha et al. (2021) | |
| Middle aged women | Diabetes mellitus with ptosis | Sinuses on the left side |
|
Amphotericin B and aspirin. Antifungal treatment |
|
Revannavar et al. (2021) |
| 40-year old woman and a 54-year old man | COVID-19 | – |
|
corticosteroid therapy and amphotericin B |
|
Veisi et al. (2021) |
| 79-year old male | diabetes mellitus and hypertension | fevers, rigors, dry cough, and worsening shortness of breath |
|
Ceftriaxone, azithromycin, remdesivir, dexamethasone, voriconazole |
|
Johnson et al. (2021) |
| 68-year old male | Hyperglycemia and acute renal failure | non-productive cough with non-bloody diarrhea, fever |
|
Prednisone, mycophenolate mofetil, tacrolimus, atovaquone, nystatin, valganciclovir, hydroxychloroquine |
|
Khatri et al. (2021) |
| 2 cases (Abstract) | diabetes mellitus and ketoacidosis | – | – | corticosteroids |
|
Dallalzadeh et al. (2021) |
| 24-year-old female | Obesity | COVID-19 with respiratory failure and oxygen saturation |
|
– |
|
Waizel-Haiat et al. (2021) |
| 49-year-old male | – | fever, cough, and shortness of breath |
|
ceftriaxone and azithromycin, enoxaparin, remdesivir |
|
Placik et al. (2020) |
| 53-year old male | acute myeloid leukemia, myelodysplastic syndrome, obesity and depression | sore throat, parageusia, dysosmia and fever |
|
Corticosteroids and antibacterial therapy |
|
Zurl et al. (2021) |
| 66-year-old male | Diabetes mellitus | – |
|
– |
|
Rao et al. (2021) |
| 56-year-old man | Renal disease | fatigue and shortness of breath |
|
methylprednisolone and tocilizumab |
|
Kanwar et al. (2021) |
| 31 patients | Different diseases | COVID-19 symptoms |
|
– |
|
Rashid et al. (2021) |
| 18 patients | 16 patients were diabetic | facial cellulitis, maxillary sinusitis, headache, necrosis of palatal bone/mucosa or acute loss of vision |
|
voriconazole, posaconazole |
|
Moorthy et al. (2021) |
| 55-year-old man | follicular lymphoma | Inflammatory response |
|
amphotericin B |
|
Bellanger et al. (2021) |
| 23 patients | Diabetes mellitus, renal failure and hypertension | COVID-19 positive cases |
|
Steroids |
|
Sharma et al. (2021) |
ARDS: acute respiratory distress syndrome; EGD: Esophagogastroduodenoscopy; GMS: Grocott's methenamine staining; H&E: haematoxylin and eosin; LCB: Lactophenol cotton blue; BAL: bronchoalveolar lavage; PAS: periodic acid–Schiff; KOH: potassium hydroxide; MALDI-TOF: Matrix-assisted laser desorption ionization time-of-flight; MRI: magnetic resonance imaging, CT: computed tomography; COVID-19: coronavirus disease 2019; RT-PCR: reverse transcription polymerase chain reaction.
7. Unravelling the factors supporting mucormycosis in COVID-19
Mucormycosis is an invasive infection caused by naturally occurring fungus in the soil and human beings get infected by inhaling the spores floating in the air. These spores get lodged in the nasal passages and sinuses and cause the disease. Black Fungus is a rare fungal infection and is primarily contracted in the Intensive Care Unit (ICU) of a hospital and can be fatal, causing loss of vision and even death. Earlier symptoms include greyish-black pigmentation in the nose or oral cavity and blockage of the nasal cavity. Spores of fungus near the eyes leads to ocular swelling, and a few patients may get lesions over the cheeks. At a later stage, this fungus can make its way to the brain. It is quite severe, and the fatality rate can go up to 50%. Treatments include antifungal drugs like amphotericin-B that are given intravenously and supportive therapy. Healthcare workers and ICU technicians should be educated to change flow meters frequently and to sterilize oxygen tubing. Besides alveolar damage with severe inflammatory exudation, COVID-19 patients always have immunosuppression with decreased CD4 + T and CD8 + T cells (Yang et al., 2020a, 2020b). Critically ill patients, especially the patients admitted to the ICU with mechanical ventilation or who had a longer duration of hospital stay, are more likely to develop fungal co-infections (Yang et al., 2020a, 2020b). The symptoms of mucormycosis includes infection that can vary from person to person, these symptoms include headache, fever, facial, nasal pain, blackish nasal discharge, loss of vision, toothache (Loss of teeth, swelling in the upper jaw) and paralysis. Unless treated, this infection can cross the central nervous system (CNS) and become a life-threatening disease. Early diagnosis can be lifesaving, but the infections can be extremely challenging to treat, even at an early stage. These patients are treated with amphotericin-B injections, but these drugs can induce substantial side effects, including kidney damage. In less severe cases, endoscopy has been inserted into the nasal cavity to remove the fungus. In the severe spread of infections, the surgeon will remove the infected part. Earlier identifications of fungal co-infections can significantly reduce the mortality rate.
8. Depletion of organs due to mucormycosis
Mucormycosis can frequently infect the sinuses, brain or lungs but it has been said that it can also the impact on oral cavity, gastrointestinal tract, skin and other organs. The infection of mucormycosis could result in the following outcomes in different organs 1) when infected in the sinusitis, it blocks the nasal cavity leading to blackish or bloody discharge; 2) Face-local pain on cheekbone, one-sided facial pain or numbness; 3) Oral cavity-loosening of teeth or jaw; 4) Eye-blurred/double vision, vision loss; 5) Skin-thrombosis or necrotic skin lesion; 6) Lungs-chest pain, worsening of respiratory symptoms. However, in COVID-19 affected patients, mostly it affects the eye, oral region and brain.
8.1. Eye
The mucormycosis infection classically starts its journey inside the human body in the nasal or maxillary sinus and spreads to sphenoid or ethmoid sinus. After which it intrudes the orbit via ethmoid foramina or nasolacrimal duct or by splitting lamina papyracea (Sundaram et al., 2014; Teixeira et al., 2013). The lesion in the eye occurs due to the angioinvasion of the germinated hyphae resulting in dry gangrene. Whereas when the angioinvasion occurs via cavernous sinus thrombosis/internal carotid artery results in cerebral infarction, mycotic abscesses or aneurysms and hematogenous dissemination (Ochiai et al., 1993; Sundaram et al., 2014). When the blood vessel necrosis occurs in the ophthalmic artery it might lead to blindness, cranial nerve palsies and other motor and sensory deficits.
8.2. Mouth
Mucormycosis occurs in the oral cavity mainly due to spread of spores through inhalation, open oral wounds, ulceration or an extraction socket in the mouth, particularly in the patients who are immune-compromised (Rajashri et al., 2020). Diagnosis can be made based on the appearance of necrotic lesions in the form of pressure sores in the orbital-nasal region, the palate or the floor of the mouth. The infection of mucormycosis especially in the maxillofacial regions spreads to regions such as the oral cavity, maxilla, palate, nose, paranasal sinuses and finally into CNS (Bakathir, 2006). Angioinvasion of Mucorales and its spores into the blood vessels leads to the thrombus formation, which causes progressive necrosis of associated hard and soft tissues.
8.3. Brain
The manifestation of mucormycosis in the CNS mainly questions the survival rate and proper functioning of the organs in the infected individual. After the entry of the fungus, the invasion occurs either via hematogenous spread or by direct cranial dispersion from the paranasal sinuses. Patients who have diabetes mellitus as a major co-morbidity for mucormycosis, the CNS becomes the third most common site of infection (Bannykh et al., 2018; Higo et al., 2015). The fungal hypha develops in the internal elastic lamina and spreads to the arterial lumen, eradicating intravascular thrombosis. Vascular occlusion leading to cerebral infarction and hemorrhagic necrosis, even before hyphal invasion in brain tissue (Economides et al., 2017). Hyphal invasion of the necrotic brain parenchyma occurs in advanced CNS mucormycosis and might lead to death (Malik et al., 2014). Hence, the growing piece of evidence shows that it is important to have options open to diagnose this disease in different dimensions. It has several sites of infection mainly attacking the immune system and causing infection in individuals.
9. Challenges faced due to mucormycosis
Mucormycosis is a fungal infection with a high mortality rate of 50 percent. An increasing number of COVID-19 patients have been developing this infection while still at the hospital or after discharge. Patients hospitalized for COVID-19 and particularly those who require oxygen therapy during COVID-19 illness are at a much higher risk of mucormycosis. Inhalation of Mucor spores by patients with a compromised immune system will lead to colonization of the fungus, invasion of the host and development of mucormycosis. Individuals with uncontrolled diabetes are at a higher risk of mucormycosis because the high blood sugar levels make it easier for the fungi to grow and survive. Their weaker immune systems offers less protection against the infection. Hot and humid conditions, oxygen, humidifiers and oxygen delivery masks may contribute to the spread of infection. Delay in diagnosis and treatment can make the impact of this fungal infection deadlier.
10. Conclusion
COVID-19 has put the entire world in turmoil situation, and an exact cure for this deadly infection has not been found yet. Its an infection, the consequential immunosuppression, former co-morbidities and its medications have made the patients susceptible to secondary fungal infections such as Mucormycosis. This is an opportunistic fungal infection that is caused due to mucor hyphae that are commonly available in soil, plants, dungs, rotting fruits and vegetables. The COVID-19 affected patients who are more susceptible to these infections are immunocompromised, have diabetes, and are prescribed heavy steroids. As mucormycosis is angioinvasive, once inhaled, its spores begin to grow, and the fungal hyphae invade the blood vessels, further contributing to tissue infarction, necrosis and thrombosis. This fungal infection is life-threatening as it occurs among those who have immunosuppression accompanied with diabetic ketoacidosis, neutropenia, increased serum levels of iron, excess release of sugar due to overtake of steroids which finally results in a decrease in levels of WBCs, T-cells and other immunomodulatory cells and triggers the cytokine storm that damages the cellular organs. Therefore, researchers and healthcare professionals should promptly control this mucormycosis infection by understanding its influence and range of severity, especially on COVID-19 patients. A multidisciplinary approach should include prompt diagnosis, treatment with antifungals, any appropriate surgical consultation and treatment, which may reverse the underlying condition. Additional research in this area is recommended to investigate the mucormycosis in COVID-19 infected and recovered patients. Hence vigorous investigations to emphasize the root cause of mucormycosis, specifically in COVID-19, should be under the scope of research. A diagnostic study for this opportunistic pathogen should not be ignored in case the patient is COVID-19 positive and immunosuppressed.
Funding
This work was supported by the Project funded by MHRD-RUSA 2.0 – BEICH (Dr.VB).
Credit author statement
Conceptualization: BV; IM; KJ; MDS. Data curation: IM; KJ; MDS; DV; KR; PV. Project administration: BV; AN; AVG; PS; MDS; NSK; KRSSR. Supervision: BV; MDS; NSK; KRSSR. Validation: IM; KJ; MDS; DV; KR. Roles/Writing - original draft: IM; KJ; MDS; DV; BV. Writing - review & editing: IM; KJ; MDS; DV; AN; AVG; PS; MDS; NSK; KRSSR; BV.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author Dr. VB would like to thank Bharathiar University and Mizoram University (DBT- Advanced State Biotech Hub)for providing the necessary infrastructure facility and the RUSA 2.0 BEICH Project (Bharathiar University) for providing necessary help in carrying out this review process of the manuscript.
References
- Abdolalizadeh P., Kashkouli M.B., Khademi B., Karimi N., Hamami P., Es’ haghi A. Diabetic versus non‐diabetic rhino‐orbito‐cerebral mucormycosis. Mycoses. 2020;63:573–578. doi: 10.1111/myc.13078. [DOI] [PubMed] [Google Scholar]
- Abela L., Toelle S.P., Hackenberg A., Scheer I., Güngör T., Plecko B. Fatal outcome of rhino-orbital-cerebral mucormycosis due to bilateral internal carotid occlusion in a child after hematopoietic stem cell transplantation. Pediatr. Infect. Dis. J. 2013;32:1149–1150. doi: 10.1097/INF.0b013e31829e69e7. [DOI] [PubMed] [Google Scholar]
- Adil A. Anadolu Agency; 2021. Over 28,200 'black Fungus' Cases Recorded in India.https://www.aa.com.tr/en/asia-pacific/over-28-200-black-fungus-cases-recorded-in-india/2266396 2021. accessed 6.21.21. [Google Scholar]
- Adler C.H., Stem M.B., Brooks T.L. Parkinsonism secondary to bilateral striatal fungal abscesses. Mov. Disord.: Off. J. Movement Disord. Soc. 1989;4:333–337. doi: 10.1002/mds.870040407. [DOI] [PubMed] [Google Scholar]
- Ahmadikia K., Hashemi S.J., Khodavaisy S., Getso M.I., Alijani N., Badali H., Mirhendi H., Salehi M., Tabari A., MohammadiArdehali M. The double‐edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza‐associated mucormycosis versus COVID‐19 associated mucormycosis. Mycoses. 2021 doi: 10.1111/myc.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyev K., Didenko L., Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J. Med. Cases. 2021;12:85. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. The Hans India; 2021. Telangana: Black Fungus Patients Losing Sight.https://www.thehansindia.com/news/cities/hyderabad/telangana-black-fungus-patients-losing-sight-689329 accessed 6.21.21. [Google Scholar]
- Almyroudis N., Sutton D., Linden P., Rinaldi M., Fung J., Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. Am. J. Transplant. 2006;6:2365–2374. doi: 10.1111/j.1600-6143.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Artis W.M., Fountain J.A., Delcher H.K., Jones H.E. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31:1109–1114. doi: 10.2337/diacare.31.12.1109. [DOI] [PubMed] [Google Scholar]
- Asghar S.A., Majid Z., Tahir F., Qadar L.T., Mir S. Rhino-oculo cerebral mucormycosis resistant to amphotericin B in a Young patient with diabetic ketoacidosis. Cureus. 2019;11 doi: 10.7759/cureus.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakathir A.A. Mucormycosis of the jaw after dental extractions: two case reports. Sultan Qaboos Univ. Med. J. 2006;6:77–82. [PMC free article] [PubMed] [Google Scholar]
- Baker R.D. Pulmonary mucormycosis. Am. J. Pathol. 1956;32:287. [PMC free article] [PubMed] [Google Scholar]
- Balachandar V., Kaavya J., Mahalaxmi I., Arul N., Vivekanandhan G., Bupesh G., Singaravelu G., Anila V., Dhivya V., Harsha G. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar V., Mahalaxmi I., Devi S.M., Kaavya J., Kumar N.S., Laldinmawii G., Arul N., Reddy S.J.K., Sivaprakash P., Kanchana S. Follow-up studies in COVID-19 recovered patients-is it mandatory? Sci. Total Environ. 2020:139021. doi: 10.1016/j.scitotenv.2020.139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh S.I., Hunt B., Moser F. Intra-arterial spread of Mucormycetes mediates early ischemic necrosis of brain and suggests new venues for prophylactic therapy: arterial occlusion in mucormycosis. Neuropathology. 2018;38:539–541. doi: 10.1111/neup.12501. [DOI] [PubMed] [Google Scholar]
- Barnagarwala T. Indian Express; 2021. Mucormycosis: 2,245 Cases So Far in Maharashtra, 30 Dead in Last Six Days.https://indianexpress.com/article/cities/mumbai/mucormycosis-2245-cases-so-far-in-maharashtra-30-dead-in-last-six-days-7330313/ accessed 6.21.21. [Google Scholar]
- Beketova T.R., Bailey L., Crowell E.L., Supsupin E.P., Adesina O.O. Orbitocerebral mucormycosis in a patient with central Nervous system lymphoma. Ophthalmic Plast. Reconstr. Surg. 2018;34:e197–e201. doi: 10.1097/IOP.0000000000001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatkar S., Goyal M., Takkar A., Mukherjee K., Singh P., Singh R., Lal V. Cavernous sinus syndrome: a prospective study of 73 cases at a tertiary care centre in Northern India. Clin. Neurol. Neurosurg. 2017;155:63–69. doi: 10.1016/j.clineuro.2017.02.017. [DOI] [PubMed] [Google Scholar]
- Binder U., Maurer E., Lass‐Flörl C. Mucormycosis–from the pathogens to the disease. Clin. Microbiol. Infect. 2014;20:60–66. doi: 10.1111/1469-0691.12566. [DOI] [PubMed] [Google Scholar]
- Boelaert J.R., Fenves A.Z., Coburn J.W. Deferoxamine therapy and mucormycosis in dialysis patients: report of an international registry. Am. J. Kidney Dis. 1991;18:660–667. doi: 10.1016/s0272-6386(12)80606-8. [DOI] [PubMed] [Google Scholar]
- Bellanger A.-P., Navellou J.-C., Lepiller Q., Brion A., Brunel A.-S., Millon L., Berceanu A. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a Severe Acute Respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect. Dis. Now. 2017 doi: 10.1016/j.idnow.2021.01.010. S2666- 9919(21)00030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke S., Mogavero S., Kasper L., Hube B. Virulence factors in fungal pathogens of man. Curr. Opin. Microbiol. 2016;32:89–95. doi: 10.1016/j.mib.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Carter J.E., Rauch R.A. One-and-a-half syndrome, type II. Arch. Neurol. 1994;51:87–89. doi: 10.1001/archneur.1994.00540130121020. [DOI] [PubMed] [Google Scholar]
- Castillo P., Wright K.E., Kontoyiannis D.P., Walsh T., Patel S., Chorvinsky E., Bose S., Hazrat Y., Omer B., Albert N., Leen A.M., Rooney C.M., Bollard C.M., Cruz C.R.Y. A new method for reactivating and expanding T cells specific for Rhizopus oryzae. Mol. Ther. - Methods Clin. Dev. 2018;9:305–312. doi: 10.1016/j.omtm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A., Das A., Mandal J., Shivaprakash M., George V.K., Tarai B., Rao P., Panda N., Verma S.C., Sakhuja V. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Sabouraudia. 2006;44:335–342. doi: 10.1080/13693780500464930. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A., Chatterjee S., Das A., Panda N., Shivaprakash M., Kaur A., Varma S., Singhi S., Bhansali A., Sakhuja V. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad. Med. 2009;85:573–581. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- Chamilos G., Lewis R., Lamaris G., Walsh T., Kontoyiannis D. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob. Agents Chemother. 2008;52:722–724. doi: 10.1128/AAC.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaulk A.L., Do T.H., Supsupin E.P., Bhattacharjee M.B., Richani K., Adesina O. A unique radiologic case of optic nerve Infarction in a patient with mucormycosis. J. Neuro Ophthalmol. Off. J. North Am. Neuro Ophthalmol. Soc. 2021 doi: 10.1097/WNO.0000000000001179. [DOI] [PubMed] [Google Scholar]
- Chegini Z., Didehdar M., Khoshbayan A., Rajaeih S., Salehi M., Shariati A. Epidemiology, clinical features, diagnosis and treatment of cerebral mucormycosis in diabetic patients: a systematic review of case reports and case series. Mycoses. 2020;63:1264–1282. doi: 10.1111/myc.13187. [DOI] [PubMed] [Google Scholar]
- Chibucos M.C., Soliman S., Gebremariam T., Lee H., Daugherty S., Orvis J., Shetty A.C., Crabtree J., Hazen T.H., Etienne K.A. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 2016;7:1–11. doi: 10.1038/ncomms12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow V., Khan S., Balogun A., Mitchell D., Mühlschlegel F.A. Invasive rhino-orbito-cerebral mucormycosis in a diabetic patient–the need for prompt treatment. Med. Mycol. Case Rep. 2015;8:5–9. doi: 10.1016/j.mmcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M., Bruno B., Loridant S., Navarin P., François N., Lanternier F., Amzallag-Bellenger E., Dubos F., Mazingue F., Sendid B. Successful outcome of disseminated mucormycosis in a 3-year-old child suffering from acute leukaemia: the role of isavuconazole? a case report. BMC Pharmacol. Toxicol. 2018;19:81. doi: 10.1186/s40360-018-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo-León D.E., Chora-Hernández L.D., Rodríguez-Zulueta A.P., Walsh T.J. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med. Mycol. 2018;56:29–43. doi: 10.1093/mmy/myx017. https://doi.org/0.1093/mmy/myx017. [DOI] [PubMed] [Google Scholar]
- Dallalzadeh L.O., Ozzello D.J., Liu C.Y., Kikkawa D.O., Korn B.S. Orbit; 2021. Secondary Infection with Rhino-Orbital Cerebral Mucormycosis Associated with COVID-19; pp. 1–4. [DOI] [PubMed] [Google Scholar]
- Dantas K.C., Mauad T., de André C.D.S., Bierrenbach A.L., Saldiva P.H.N. A single-centre, retrospective study of the incidence of invasive fungal infections during 85 years of autopsy service in Brazil. Sci. Rep. 2021;11:1–10. doi: 10.1038/s41598-021-83587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura Feitoza L., Altemani A., da Silva N.A., Reis F. Teaching neuroimages: mucormycosis-associated vasculitis: a new sequence to show an old invasive infection. Neurology. 2019;92:e1796–e1797. doi: 10.1212/WNL.0000000000007275. [DOI] [PubMed] [Google Scholar]
- do Monte Junior E.S., Dos Santos M.E.L., Ribeiro I.B., de Oliveira Luz G., Baba E.R., Hirsch B.S., Funari M.P., De Moura E.G.H. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin. Endosc. 2020;53:746–749. doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolatabadi S., Ahmadi B., Rezaei-Matehkolaei A., Zarrinfar H., Skiada A., Mirhendi H., Nashibi R., Niknejad F., Nazeri M., Rafiei A. Mucormycosis in Iran: a six-year retrospective experience. J. Mycol. Medicale. 2018;28:269–273. doi: 10.1016/j.mycmed.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Dworsky Z.D., Bennett R., Kim J.M, Kuo D.J. Severe medication-induced peripheral neuropathy treated with topical doxepin cream in a paediatric patient with leukaemia. Case Rep. 2017 doi: 10.1136/bcr-2017-219900. bcr2017219900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economides M.P., Ballester L.Y., Kumar V.A., Jiang Y., Tarrand J., Prieto V., Torres H.A., Kontoyiannis D.P. Invasive mold infections of the central nervous system in patients with hematologic cancer or stem cell transplantation (2000–2016): uncommon, with improved survival but still deadly often. J. Infect. 2017;75:572–580. doi: 10.1016/j.jinf.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Einollahi B., Lessan-Pezeshki M., Aslani J., Nemati E., Rostami Z., Hosseini M.J., Ghadiani M.H., Ahmadpour P., Shahbazian H., Pour-Reza-Gholi F. Two decades of experience in mucormycosis after kidney transplantation. Ann. Transplant. 2011;16:44–48. doi: 10.12659/aot.881994. [DOI] [PubMed] [Google Scholar]
- Elitzur S., Arad‐Cohen N., Barg A., Litichever N., Bielorai B., Elhasid R., Fischer S., Fruchtman Y., Gilad G., Kapelushnik J. Mucormycosis in children with haematological malignancies is a salvageable disease: a report from the Israeli study group of childhood leukemia. Br. J. Haematol. 2020;189:339–350. doi: 10.1111/bjh.16329. [DOI] [PubMed] [Google Scholar]
- Ermak D., Kanekar S., Specht C.S., Wojnar M., Lowden M. Looks like a stroke, acts like a stroke, but it’s more than a stroke: a case of cerebral mucormycosis. J. Stroke Cerebrovasc. Dis. 2014;23:e403–e404. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Fahimzad A., Chavoshzadeh Z., Abdollahpour H., Klein C., Rezaei N. Necrosis of nasal cartilage due to mucormycosis in a patient with severe congenital neutropenia due to HAX1 deficiency. J. Investig. Allergol. Clin. Immunol. 2008;18:469–472. [PubMed] [Google Scholar]
- Farid S., AbuSaleh O., Liesman R., Sohail M.R. Isolated cerebral mucormycosis caused by Rhizomucor pusillus. Case Rep. 2017 doi: 10.1136/bcr-2017-221473. bcr-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Perlin D.S., Zhao Y., Noble B.N., Lewis J.S., Strasfeld L., Hakki M. Isavuconazole prophylaxis in patients with hematologic malignancies and hematopoietic cell transplant recipients. Clin. Infect. Dis. 2020;70:723–730. doi: 10.1093/cid/ciz282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbringer M. Zur vergleichenden anatomie der Schultermuskeln. Morphol. Jahrb. 1876;1:636–816. [Google Scholar]
- Garg D., Muthu V., Sehgal I.S., Ramachandran R., Kaur H., Bhalla A., Puri G.D., Chakrabarti A., Agarwal R. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186:289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremariam T., Liu M., Luo G., Bruno V., Phan Q.T., Waring A.J., Edwards J.E., Filler S.G., Yeaman M.R., Ibrahim A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Invest. 2014;124:237–250. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M.Z., Lewis R.E., Kontoyiannis D.P. Mucormycosis caused by unusual mucormycetes, non-Rhizopus,-Mucor, and-Lichtheimia species. Clin. Microbiol. Rev. 2011;24:411–445. doi: 10.1128/CMR.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., Thursz M., Manousou P., Corbett R., Goldin R. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo T., Kobayashi T., Yamazaki S., Ando S., Gonoi W., Ishida M., Okuma H., Nakamura F., Ushiku T., Ohtomo K., Fukayama M., Kurokawa M. Cerebral embolism through hematogenous dissemination of pulmonary mucormycosis complicating relapsed leukemia. Int. J. Clin. Exp. Pathol. 2015;8:13639–13642. [PMC free article] [PubMed] [Google Scholar]
- Hu W.T., Leavitt J.A., Moore E.J., Noseworthy J.H. MRI findings of rapidly progressive ophthalmoplegia and blindness in mucormycosis. Neurology. 2006;66:E40. doi: 10.1212/01.wnl.0000204231.85308.7e. [DOI] [PubMed] [Google Scholar]
- Ibrahim M., Chitnis S., Fallon K., Roberts T. Rhinocerebral mucormycosis in a 12-year-old girl. Arch. Neurol. 2009;66:272–273. doi: 10.1001/archneurol.2008.546. [DOI] [PubMed] [Google Scholar]
- Ibrahim A.S., Kontoyiannis D.P. Update on mucormycosis pathogenesis. Curr. Opin. Infect. Dis. 2013;26:508. doi: 10.1097/QCO.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer M., Jayaramayya K., Subramaniam M.D., Lee S.B., Dayem A.A., Cho S.-G., Vellingiri B. COVID-19: an update on diagnostic and therapeutic approaches. BMB Rep. 2020;53:191. doi: 10.5483/BMBRep.2020.53.4.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer M., Tiwari S., Renu K., Pasha M.Y., Pandit S., Singh B., Raj N., Saikrishna K., Kwak H.J., Balasubramanian V. Environmental Survival of SARS-CoV-2–A solid waste perspective. Environ. Res. 2021;111015 doi: 10.1016/j.envres.2021.111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon S., Galdiero M.R., Del Prete D., Cassatella M.A., Garlanda C., Mantovani A. Neutrophils in innate and adaptive immunity. Semin. Immunopathol. 2013;35:377–394. doi: 10.1007/s00281-013-0374-8. [DOI] [PubMed] [Google Scholar]
- Jayaramayya K., Mahalaxmi I., Subramaniam M.D., Raj N., Dayem A.A., Lim K.M., Kim S.J., An J.Y., Lee Y., Choi Y. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB Rep. 2020;53:400. doi: 10.5483/BMBRep.2020.53.8.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W., Keighley C., Wolfe R., Lee W., Slavin M., Kong D., Chen S.-A. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Jiménez Caballero P.E., Falcón García A.M., Portilla Cuenca J.C., Casado Naranjo I. Multiple subcortical strokes caused by mucormycosis in a patient with lymphoma. Arquivos de neuro-psiquiatria. 2012;70:69–70. doi: 10.1590/s0004-282x2012000100014. [DOI] [PubMed] [Google Scholar]
- Jiménez-Zarazúa O., Vélez-Ramírez L., Alcocer-León M., Utrilla-Álvarez J., Martínez-Rivera M., Flores-Saldaña G., Mondragón J. A case of concomitant pulmonary tuberculosis and mucormycosis in an insulin-dependent diabetic patient. J. Clin. Tubercul. Mycobact. Dis. 2019;16:100105. doi: 10.1016/j.jctube.2019.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J. Fungi. 2021;7:298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.K., Ghazarian Z., Cendrowski K.D., Persichino J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med. Mycol. Case Rep. 2021;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephine S.M. The Hindu; 2021. 1,196 Cases of Mucormycosis in T.N., More Drugs Required.https://www.thehindu.com/news/national/tamil-nadu/1196-cases-of-mucormycosis-in-tn-more-drugs-required/article34784997.ece accessed 6.21.21. [Google Scholar]
- Kanwar A., Jordan A., Olewiler S., Wehberg K., Cortes M., Jackson B.R. A fatal case of Rhizopus azygosporus pneumonia following COVID-19. J. Fungi. 2021;7:174. doi: 10.3390/jof7030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P., Kumar V., Vellingiri B., Sen A., Jaishee N., Anandraj A., Malhotra H., Bhattacharyya S., Mukhopadhyay S., Kinoshita M. Anisotine and amarogentin as promising inhibitory candidates against SARS-CoV-2 proteins: a computational investigation. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1860133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakurum B., Karatas M., Cagici A.C., Uncu H., Yildirim T., Hurcan C., Karaca S., Kizilkilic E., Tan M. Mucormycosis presenting with painful ophthalmoplegia. Acta Neurol. Belg. 2005;105:201–205. [PubMed] [Google Scholar]
- Khatri A., Chang K.-M., Berlinrut I., Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient–case report and review of literature. J. Med. Mycol. 2021;101125 doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., Sato K., Vellingiri B., Green S.J., Tanaka M. Inverse association between hypertension treatment and COVID-19 prevalence in Japan. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis D.P., Lewis R.E. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect. Dis. Clin. 2006;20:581–607. doi: 10.1016/j.idc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Küchenmeister F. GB Teubner; 1855. Die in und an dem körper des lebenden menschen vorkommenden parasiten: Ein lehr-und handbuch der diagnose und behandlung der thierischen und pflanzlichen parasiten des menschen. [Google Scholar]
- Kulkarni NS., Bhide A.R., Wadia R.S. Rhinocerebral mucormycosis: an analysis of probable mode of spread and its implication in an early diagnosis and treatment. Indian J. Otolaryngol. Head Neck Surg. 2005;57:121–124. doi: 10.1007/BF02907665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. NDTV; 2021. Centre Asks States to Notify “Black Fungus” under Epidemic Diseases Act. [Google Scholar]
- Lau C.-I., Wang H.-C., Yeh H.-L., Li C.-H. Isolated orbito-cerebral mucormycosis. Neurologist. 2011;17:151–153. doi: 10.1097/NRL.0b013e3182173395. [DOI] [PubMed] [Google Scholar]
- Lee H., Cho S., Lee D., Park C., Chun H., Park Y. Characteristics and risk factors for mortality of invasive non‐Aspergillus mould infections in patients with haematologic diseases: a single‐centre 7‐year cohort study. Mycoses. 2020;63:257–264. doi: 10.1111/myc.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis M.S., Lewis R.E., Kontoyiannis D.P. Breakthrough invasive mold infections in the hematology patient: current concepts and future directions. Clin. Infect. Dis. 2018;67:1621–1630. doi: 10.1093/cid/ciy473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonell R.A., Donnan G.A., Kalnins R.M., Richards M.J., Bladin P.F. Otocerebral mucormycosis--a case report. Clin. Exp. Neurol. 1987;23:225–232. [PubMed] [Google Scholar]
- Mahalaxmi I., Kaavya J., Mohana Devi S., Balachandar V. COVID‐19 and olfactory dysfunction: a possible associative approach towards neurodegenerative diseases. J. Cell. Physiol. 2021;236:763–770. doi: 10.1002/jcp.29937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini A., Tomar G., Khanna D., Kini Y., Mehta H., Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int. J. Surg. Case Rep. 2021:105957. doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A.N., Bi W.L., McCray B., Abedalthagafi M., Vaitkevicius H., Dunn I.F. Isolated cerebral mucormycosis of the basal ganglia. Clin. Neurol. Neurosurg. 2014;124:102. doi: 10.1016/j.clineuro.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. India's massive COVID surge puzzles scientists. Nature. 2021;592:667–668. doi: 10.1038/d41586-021-01059-y. [DOI] [PubMed] [Google Scholar]
- Mantero V., Basilico P., Pozzetti U., Tonolo S., Rossi G., Spena G., Rigamonti A., Salmaggi A. Concomitant cerebral aspergillosis and mucormycosis in an immunocompetent woman treated with corticosteroids. J. Neurovirol. 2019;26:277–280. doi: 10.1007/s13365-019-00804-4. [DOI] [PubMed] [Google Scholar]
- Mcnulty J.S. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope. 1982;92:1140–1143. [PubMed] [Google Scholar]
- Mekonnen Z.K., Ashraf D.C., Jankowski T., Grob S.R., Vagefi M.R., Kersten R.C., Simko J.P., Winn B.J. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast. Reconstr. Surg. 2021;37:e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler A.E., Duggal I., Kaunzner U., Maciel C.B., Miller A.M., Scognamiglio T., Dinkin M.J. Rapidly progressive bilateral optic nerve and retinal infarctions due to rhinocerebral mucormycosis and pseudoephedrine use. Neurol. Clin. Pract. 2016;6:549–552. doi: 10.1212/CPJ.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.10726. S2666- 9919(21)00030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy A., Gaikwad R., Krishna S., Hegde R., Tripathi K., Kale P.G., Rao P.S., Haldipur D., Bonanthaya K. SARS-CoV-2, uncontrolled diabetes and corticosteroids—an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J. Maxillofac. Oral Surg. 2021;1–8 doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M. Indian Express; 2021. Inside Black Fungus Wards in Rajasthan: amid Injection Shortage, Increasing Patients, Many End up Losing Vision.https://indianexpress.com/article/india/in-black-fungus-wards-in-rajasthan-many-end-up-losing-vision-amid-injection-shortage-rising-patient-numbers-7345983/ accessed 6.21.21. [Google Scholar]
- Miller M.A., Molina K.C., Gutman J.A., Scherger S., Lum J.M, Mossad S.B, Burgess M, Cheng M.P, Chuang S.T., Jacobs S.E. Presented at the Open Forum Infectious Diseases. Oxford University Press; US: 2017. Mucormycosis in hematopoietic cell transplant recipients and in patients with hematological malignancies in the era of new antifungal agents. 8, ofaa646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H., Iseda T., Miyahara S., Goya T., Wakisaka S. Rhinocerebral mucormycosis —case report—. Neurol. Med.-Chir. 1993;33:373–376. doi: 10.2176/nmc.33.373. [DOI] [PubMed] [Google Scholar]
- Pagano L., Ricci P., Tonso A., Nosari A., Cudillo L., Montillo M., Cenacchi A., Pacilli L., Fabbiano F., Del Favero A. Mucormycosis in patients with haematological malignancies: a retrospective clinical study of 37 cases. Br. J. Haematol. 1997;99:331–336. doi: 10.1046/j.1365-2141.1997.3983214.x. [DOI] [PubMed] [Google Scholar]
- Pasero D., Sanna S., Liperi C., Piredda D., Branca G.P., Casadio L., Simeo R., Buselli A., Rizzo D., Bussu F. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020:1–6. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Kaur H., Xess I., Michael J., Savio J., Rudramurthy S., Singh R., Shastri P., Umabala P., Sardana R. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020;26:944.e9–944.e15. doi: 10.1016/j.cmi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 2020;15:2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza L., Vallerini D., Barozzi P., Riva G., Forghieri F., Zanetti E., Quadrelli C., Candoni A., Maertens J., Rossi G., Morselli M., Codeluppi M., Paolini A., Maccaferri M., Del Giovane C., D'Amico R., Rumpianesi F., Pecorari M., Cavalleri F., Marasca R., Narni F., Luppi M. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood. 2011;118:5416–5419. doi: 10.1182/blood-2011-07-366526. [DOI] [PubMed] [Google Scholar]
- Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. J. Fungi. 2019;5:26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashri R., Muthusekhar M.R., Kumar S.P. Mucormycosis following tooth extraction in a diabetic patient: a case report. Cureus. 2020 doi: 10.7759/cureus.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S., Kate S., Kembhavi S., Cheriyalinkal Parambil B., KC A., Bhat V., Prasad M., Moiyadi A., Biswas S., Narula G. A retrospective analysis of Invasive Fungal Diseases (IFD) of the central nervous system in children with lymphoid malignancies. J. Pediatr. Hematol. Oncol. 2020;42:e202–e206. doi: 10.1097/MPH.0000000000001690. [DOI] [PubMed] [Google Scholar]
- Rammaert B., Lanternier F., Zahar J.-R., Dannaoui E., Bougnoux M.-E., Lecuit M., Lortholary O. Healthcare-associated mucormycosis. Clin. Infect. Dis. 2012;54:S44–S54. doi: 10.1093/cid/cir867. [DOI] [PubMed] [Google Scholar]
- Rangel-Guerra R.A., Martínez H.R., Sáenz C., Bosques-Padilla F., Estrada-Bellmann I. Rhinocerebral and systemic mucormycosis. Clinical experience with 36 cases. J. Neurol. Sci. 1996;143:19–30. doi: 10.1016/s0022-510x(96)00148-7. [DOI] [PubMed] [Google Scholar]
- Rao R., Shetty A.P., Nagesh C.P. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: clinico-radiological features. Indian J. Ophthalmol. 2021;69:1627–1630. doi: 10.4103/ijo.IJO_1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid H.U., Rashid M., Khan N., Ansari S.S., Bibi N. Taking a step down on the reconstruction ladder for head and neck reconstruction during the COVID-19 pandemic. BMC Surg. 2021;21:120. doi: 10.1186/s12893-021-01134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch C.R., DiPippo A.J., Bose P., Kontoyiannis D.P. Breakthrough fungal infections in patients with leukemia receiving isavuconazole. Clin. Infect. Dis. 2018;67:1610–1613. doi: 10.1093/cid/ciy406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravani S.A., Agrawal G.A., Leuva P.A., Modi P.H., Amin K.D. Rise of the phoenix: mucormycosis in COVID-19 times. Indian J. Ophthalmol. 2021;69:1563–1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Lynch J.P., III, Fishbein M.C., Clark N.M. Thieme Medical Publishers; 2020. Mucormycosis. Presented at the Seminars in Respiratory and Critical Care Medicine; pp. 99–114. [DOI] [PubMed] [Google Scholar]
- Renu K., Subramaniam M.D., Chakraborty R., Haritha M., Iyer M., Bharathi G., Kamalakannan S., Balachandar V., Abilash V. The role of Interleukin-4 in COVID-19 associated male infertility–A hypothesis: running title: IL-4 and its role in COVID-19 associated male infertility. J. Reprod. Immunol. 2020;103213 doi: 10.1016/j.jri.2020.103213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revannavar S.M., Supriya P., Samaga L., Vineeth V. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L., Sein M., Sein T., Chiou C.C., Chu J.H. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- Roilides E., Kontoyiannis D.P., Walsh T.J. Host defenses against zygomycetes. Clin. Infect. Dis. 2012;54:S61–S66. doi: 10.1093/cid/cir869. [DOI] [PubMed] [Google Scholar]
- Saldanha M., Reddy R., Vincent M.J. of the Article: Paranasal Mucormycosis in COVID-19 Patient. Indian J. Otolaryngol. Head Neck Surg. 2021:1–4. doi: 10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Lara C., Rembao-Bojórquez D., de la Cruz E., Márquez C., Portocarrero L., Tena-Suck M.L. Pituitary apoplexy due to mucormycosis infection in a patient with an ACTH producing pulmonary tumor. J. Clin. Neurosci. 2008;15:67–70. doi: 10.1016/j.jocn.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Sano T., Kobayashi Z., Takaoka K., Ota K., Onishi I., Iizuka M., Tomimitsu H., Shintani S. Retrobulbar optic neuropathy associated with sphenoid sinus mucormycosis. Neurol. Clin. Neurosci. 2018;6:146–147. doi: 10.1111/ncn3.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Gokhale T., Choudhury S.S., Deb A.K. COVID-19 and orbital mucormycosis. Indian J. Ophthalmol. 2021;69:1002. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvestani A.S., Pishdad G., Bolandparvaz S. Predisposing factors for mucormycosis in patients with diabetes mellitus; an experience of 21 years in southern Iran. Bull. Emerg. Trauma. 2013;1:164. [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Tramsen L., Perkhofer S., Lass-Flörl C., Hanisch M., Röger F., Klingebiel T., Koehl U., Lehrnbecher T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology. 2013;218:939–944. doi: 10.1016/j.imbio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Sasannejad P., Ghabeli-Juibary A., Aminzadeh S., Olfati N. Cerebellar infarction and aneurysmal subarachnoid hemorrhage: an unusual presentation and rare complications of rhinocerebral mucormycosis. Iran. J. Child Neurol. 2015;14:222. [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Schneider A., Demir A., Lass-Flörl C., Lehrnbecher T. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses. 2016;59:34–38. doi: 10.1111/myc.12431. [DOI] [PubMed] [Google Scholar]
- Sen M., Lahane S., Lahane T.P., Parekh R., Honavar S.G. Mucor in a Viral Land: a tale of two pathogens. Indian J. Ophthalmol. 2021;69:244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadrivova O.V., Burygina E.V., Klimko N.N. Molecular diagnostics of mucormycosis in hematological patients: a literature review. J. Fungi. 2019;5:112. doi: 10.3390/jof5040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariati A., Moradabadi A., Chegini Z., Khoshbayan A., Didehdar M. An overview of the management of the most important invasive fungal infections in patients with blood malignancies. Infect. Drug Resist. 2020;13:2329. doi: 10.2147/IDR.S254478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021:1–6. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiada A., Pagano L., Groll A., Zimmerli S., Dupont B., Lagrou K., Lass-Florl C., Bouza E., Klimko N., Gaustad P. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European confederation of medical mycology (ECMM) working group on zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011;17:1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- Skiada A., Pavleas I., Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J. Fungi. 2020;6:265. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore F.M., Williams L.S., Fradkin K.D., Alonso R.J., Biller J. Presentation, etiology, and outcome of stroke in pregnancy and puerperium. J. Stroke Cerebrovasc. Dis. 2001;10:1–10. doi: 10.1053/jscd.2001.20977. [DOI] [PubMed] [Google Scholar]
- Slavin Monica, Thursky Karin. BBC; 2021. Mucormycosis: the Black Fungus Hitting Covid-19 Patients. [Google Scholar]
- Spellberg B., Edwards J., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemler J., Hamed K., Salmanton‐García J., Rezaei‐Matehkolaei A., Gräfe S.K., Sal E., Zarrouk M., Seidel D., Abdelaziz Khedr R., Ben‐Ami R. Mucormycosis in the Middle East and North Africa: analysis of the FungiScope® registry and cases from the literature. Mycoses. 2020;63:1060–1068. doi: 10.1111/myc.13123. [DOI] [PubMed] [Google Scholar]
- Stretz C., Mook A., Modak J.M., Rodriguez J.M., Nouh A.M. Gerstmann syndrome in a patient with aggressive mucormycosis. Neurohospitalist. 2017;7:102–103. doi: 10.1177/1941874416663282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M.D., Venkatesan D., Iyer M., Subbarayan S., Govindasami V., Roy A., Narayanasamy A., Kamalakannan S., Gopalakrishnan A.V., Thangarasu R. Biosurfactants and anti-inflammatory activity: a potential new approach towards COVID-19. Curr. Opin. Environ. Sci. Health. 2020 doi: 10.1016/j.coesh.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganya R., Malathi N., Karthikeyan V., Janagaraj V.D. Mucormycosis: a brief review. J. Pure Appl. Microbiol. 2019;13:161–165. doi: 10.22207/JPAM.13.1.16. [DOI] [Google Scholar]
- Sundaram C., Sravani T., Uppin S., Uppin M. Rhinocerebral mucormycosis: pathology revisited with emphasis on perineural spread. Neurol. India. 2014;62:383. doi: 10.4103/0028-3886.141252. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan T.S., Shaanker U. COVID-19 and black fungus: what is mucormycosis? Health Sci. 2021 [Google Scholar]
- Teixeira C.A., Medeiros P.B., Leushner P., Almeida F. Rhinocerebral mucormycosis: literature review apropos of a rare entity. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-008552. bcr2012008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thajeb P., Thajeb T., Dai D. Fatal strokes in patients with rhino-orbito-cerebral mucormycosis and associated vasculopathy. Scand. J. Infect. Dis. 2004;36:643–648. doi: 10.1080/00365540410020794. [DOI] [PubMed] [Google Scholar]
- Tsikala-Vafea M., Cao W., Olszewski A.J., Donahue J.E., Farmakiotis D. Fatal mucormycosis and aspergillosis in an atypical host: what do we know about mixed invasive mold infections? Case Rep. Infect. Dis. 2020;2020:8812528. doi: 10.1155/2020/8812528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veisi A., Bagheri A., Eshaghi M., Rikhtehgar M.H., Rezaei Kanavi M., Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur. J. Ophthalmol. 2021;11206721211009450 doi: 10.1177/11206721211009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Brozman B., Petito C.K. Isolated cerebral mucormycosis: report of a case and review of the literature. J. Neurol. Sci. 2006;240:65–69. doi: 10.1016/j.jns.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Vishnupriya M., Naveenkumar M., Manjima K., Sooryasree N., Saranya T., Ramya S., Winster S.H., Paulpandi M., Balachandar V., Arul N. Post-COVID pulmonary fibrosis: therapeutic efficacy using with mesenchymal stem cells–How the lung heals. Eur. Rev. Med. Pharmacol. Sci. 2021;25:2748–2751. doi: 10.26355/eurrev_202103_25438. [DOI] [PubMed] [Google Scholar]
- What Patients with Diabetes, Cancer and Kidney Disorders Need to Know about Black Fungus. Indian Express; 2021. [Google Scholar]
- Waizel-Haiat S., Guerrero-Paz J.A., Sanchez-Hurtado L., Calleja-Alarcon S., Romero-Gutierrez L. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021;13 doi: 10.7759/cureus.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2021. Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports 2021. accessed 6.21.21. [Google Scholar]
- World Health Organization . World Health Organization; 2021. Weekly Epidemiological Update on COVID-19 - 15 June 2021.https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---15-june-2021 2021. accessed 6.21.21. [Google Scholar]
- World Health Organization . World Health Organization; 2021. Episode #39 - Update on Virus Variants.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/media-resources/science-in-5/episode-39---update-on-virus-variants 2021. accessed 6.21.21. [Google Scholar]
- Wotiye A.B., Poornachandra K., Ayele B.A. Invasive intestinal mucormycosis in a 40-year old immunocompetent patient-a rarely reported clinical phenomenon: a case report. BMC Gastroenterol. 2020;20:61. doi: 10.1186/s12876-020-01202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A., Dai J., Sun Q., Zhao F., Qu J., Yan F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang C. Looks like tuberculous meningitis, but not: a case of rhinocerebral mucormycosis with Garcin syndrome. Front. Neurol. 2016;7:181. doi: 10.3389/fneur.2016.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar S., Prabhu A. Rhino-orbito-cerebral mucormycosis: recovery against the odds. Practical. Neurol. 2017;17:485–488. doi: 10.1136/practneurol-2017-001671. [DOI] [PubMed] [Google Scholar]
- Zhang J., Kim J.D., Beaver H.A., Takashima M., Lee A.G. Rhino-orbital mucormycosis treated successfully with posaconazole without exenteration. Neuro Ophthalmol. 2013;37:198–203. doi: 10.3109/01658107.2013.809463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurl C., Hoenigl M., Schulz E., Hatzl S., Gorkiewicz G., Krause R., Eller P., Prattes J. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. J. Fungi. 2021;7:88. doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]