Highlights
-
•
SAR-CoV-2 infection lead to sympathetic overactivity.
-
•
People with co morbid condition due to pre-sympathetic overactivity, there will be always possible of worse outcome.
-
•
FDA-approved drug clonidine reduces sympathetic activity during COVID-19 infection and prevent complication and death.
-
•
Clonidine should be considered early in incremental fashion to mitigate SAR-CoV-2 related complication.
-
•
This is the first case series demonstrating the effectiveness of early use of clonidine in COVID-19.
Keywords: SAR-CoV-2, Sympathetic Nervous System, Catecholamine, Clonidine
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a life-threating viral infection that is highly transmissible and be lethal. Although many patients with mild symptoms recover, an acute form of the infection is characterized by rapidly evolving respiratory failure, an acute inflammatory response, organ failure, and death. Herein, we describe the use of clonidine to modulate the acute inflammatory consequences of this infection in three cases. The patients were three men between 40–50 years from Kathmandu valley, during the peak of COVID-19 (September 2020- January 2021). All three patients presented with typical COVID-19 symptoms (daily fever, loss of smell and taste, excessive fatigue, cough) and had pneumonia with typical finding in CT Scan of chest. Patient 1was able to maintain adequate oxygenation despite having pneumonia, managed at home by regular self-monitoring of vitals and treatment with oral clonidine whereas patient 2 and 3 developed significant pneumonia and had difficult in maintaining oxygenation hence admitted in hospital and treated with clonidine and supplemental oxygen. All three patients recovered completely. In this limited report, we proposed several mechanisms by which clonidine may be useful in managing rapidly evolving SARS-CoV-2 infection based on the rationale that early clonidine administration can intervene in the catecholaminergic response that characterizes rapid clinical deterioration including presumptive cytokine storm that occurs in COVID-19 infection in vulnerable populations.
Background
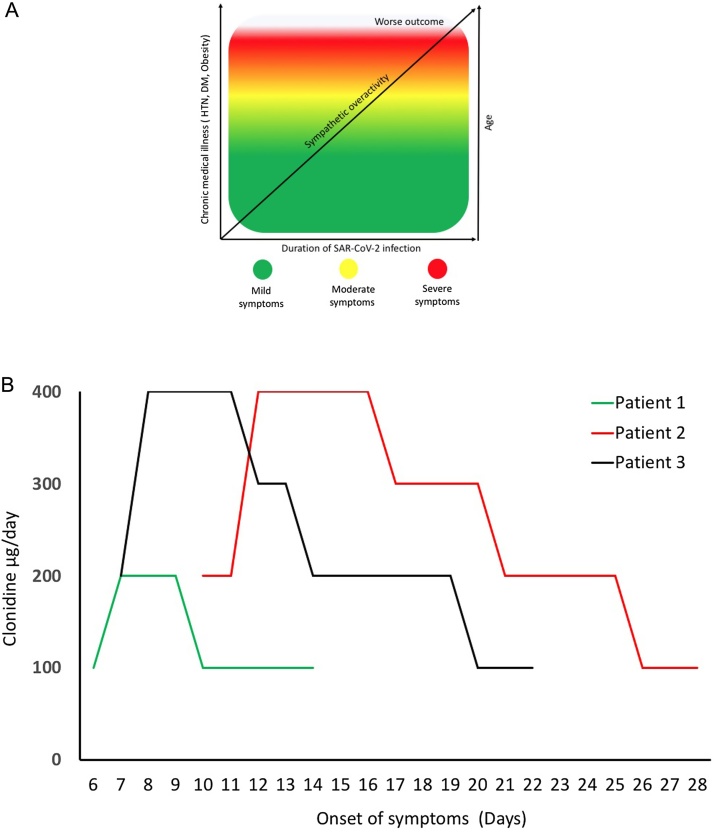
The SARS-CoV-2 pandemic has resulted in more than 4 million deaths. In healthy population, the majority of those infected with SARS-CoV-2 either have mild symptoms or remain asymptomatic. In vulnerable populations, this virus causes widespread immune dysregulation. It induces an excessive immune reaction in the host, leading to hyperinflammatory syndrome characterized by fulminant and fatal cytokines storm and viral pneumonia [1]. One of the potential mechanisms purposed for immune dysregulation is increase in proinflammatory catecholaminergic surge [2]. We [3] and other groups [4] previously hypothesized that SAR-CoV-2 infection lead to sympathetic overactivity, and in vulnerable population with comorbid conditions the pre-sympathetic overactive state [5] will likely have worse outcome. Animal models has demonstrated neurogenic pathway for SAR-CoV-2 involving the brain stem nuclei. Such findings are corelated with autopsy findings in SAR-CoV-2 infected brain specimen that show neuronal cell loss and axonal degermation brain stem nuclei such as nucleus tactus solitarii [6] Such involvement has been purposed for development autonomic dysregulation [7], leading to silent hypoxia [8] and neurological manifestation [9]. These finding have led to speculation of autonomic disturbances caused by SAR-CoV-2 [7]. Viral infection activates sympathetic activity, leading to the release of catecholamine from sympathetic nerve termini within the organs such as the lung, lymphoid tissues, bone marrow, and spleen, demonstrating the link that connects the central nervous system to immune function [10,11]. Catecholamines, such as norepinephrine can blunt innate immune responses in the lung, leading to an increase in proinflammatory cytokine that reduces viral clearance mechanisms [12]. Chronic exposure to norepinephrine can inhibit T cell proliferation thus phenotypically changing immune cells and their responses to infectious agents [13]. Therefore, in the aggregate, central sympathetic overactivity may render patients more susceptible to severe complications with increasing severity upon contacting virus (Fig. 1A).
Fig. 1.
A) Speculation: Direct correlation between sympathetic activity, age, comorbidity and disease severity once infected with SAR-CoV-2. B) Duration of clonidine treatment to all three patients.
Based on the above mechanisms, pharmacologic attenuation of the catecholaminergic surge with sympatholytic drug during acute viral infection such as with SARS-CoV-2, may have clinical benefit and prevent infection-related complication and fatal outcome. To test this, use of the FDA-approved central sympatholytic drug clonidine, an alpha2 agonist, was considered as an agent to mitigate SAR-CoV-2 related symptoms and the progression to the more severe complications of the viral infection [3]. In this report, we present three cases of moderate to severely symptomatic SAR-CoV-2 patients classified as per Ghandi et al. [14] in which there was clinical improvement with incremental dosing of clonidine (Fig. 1B).
Cases presentation
Patient 1
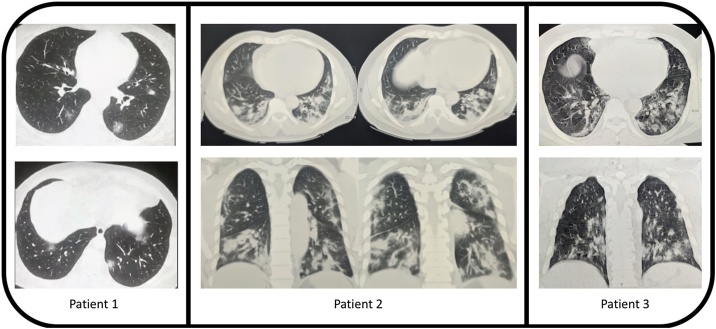
A 52-year old male with no significant past medical history tested positive for SAR-CoV-2 via nasopharyngeal swab PCR while on home isolation and presented to a virtual visit on Oct 21, 2020 with a history of 6 days of fever up to 101 °F, anorexia, anosmia and ageusia for 5 days followed by a productive cough for 3 days. Laboratory tests indicated a raised CRP, elevated liver enzymes and normal d-dimer and serum ferritin levels (Table 1). High resolution CT scan demonstrated multifocal areas of patchy ground glass opacities in all segments of bilateral lung fields with peripheral predominance suggestive of atypical viral pneumonia (COVID-19 pneumonia) with CORADS-6 score and CT severity score of 8/25 (Fig. 2). He was started on clonidine 100 microgram on day 6 of the onset of symptoms. Clonidine was increased to 200 micrograms on day 7. His fever decreased gradually and was afebrile on day 7 (24 hrs following the initiation of clonidine). His symptoms including anorexia, anosmia, ageusia resolved and his cough became non-productive on day 9 (4 days after initiation of clonidine) and gradually disappeared. Clonidine was tapered on day 10 and stopped on day 15.
Table 1.
History and lab investigation of patients.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age/ Sex | 52/M | 47/M | 49/M |
| Past Medical History | Hypertension, Hyperlipidemia | None | Overweight, Hypertension, Hypercholesterolemia |
| Duration of illness (days) | 14 days | 28 days | 22 days |
| Diagnosis date | Oct-21-2020 | Nov-21-2020 | Jan-20-2021 |
| Symptoms | High grade fever, anorexia, anosmia and ageusia, cough | High grade fever, anorexia, anosmia and ageusia, cough, shortness of breath | Fever, anosmia, ageusia, shortness of breath |
| Days of Clonidine treatment | 8 days | 18 days | 16 days |
| Lab investigation value (Normal range) | |||
| White blood cell count | 5400/mm3 (4000−11,000) | 14,460/mm3 (4000−11,000) | 3940/mm3 (4000−11,000) |
| Neutrophil count | 66 %. | 84 %. | 65 %. |
| Lymphocyte count | 32 %. | 11 %. | 32 %. |
| Platelets count | 271,000/mm3 (140,000−450,000) | 275,000/mm3 (140,000−450,000) | 160,000/mm3 (140,000−450,000) |
| Hemoglobin | 13.1gm/dL (14−18) | 10.9gm/dL (12−18) | 14.3 gm/dL (13.5−17.5) |
| C- reactive protein | positive | 73 mg/L (<5) | 35 mg/L (<5) |
| Erythrocyte Sedimentation rate | 16 mm/h. (0−20) | 45 mm/h. (0−20) | 31 mm/h. (0−20) |
| Alanine aminotransferase | 124 U/L (<45) | 27 U/L (<45) | 47 U/L (<45) |
| Aspartate aminotransferase | 101 U/L (<35) | 31 U/L (<35) | 30 U/L (<35) |
| LDH | 486 U/L (460) | 375 U/L (450) | 426 U/L (250) |
| Serum ferritin | 300 (179−464 ng/mL) | 530 ng/mL (20−400) | 525.3 ng/mL (30−400) |
| D- Dimer | 0.36 ng/dL (<0.5) | 0.3 mg/dL (0.5) | 131 ng/mL (500) |
Fig. 2.
High resolution CT scan of all three patients.
Patient 2
A 46-year old male with no medical past history who tested negative for SAR-CoV-2 via nasopharyngeal swab PCR. He was placed on home isolation when he presented on a virtual visit November 21st, 2020 with history of fever, shortness of breath with excessive fatigue for 8 days. His symptoms started with fever up to 102 °F with increasing aguesia, anosmia, dry cough during speaking and shortness of breath. High resolution CT scan of chest demonstrated patchy ground glass changes in bilateral lung parenchyma more pronounced in subpleural location in all segments with small bilateral pleural effusion consistent of COVID-19 infection, CO-RADS-5 and CT severity score of 20/25 (Fig. 2). Blood tests demonstrated an elevate leukocytosis, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and serum ferritin (Table 1) and anemia. He was hospitalized and started on oxygen via nasal cannula at 4 L/min for increasing dyspnea with hypoxia as measured by a Spo2 to < 90 %. Clonidine was started at 200 microgram/day on 10th day of onset of symptoms. As his hypoxia worsened with increasing tachypnea (respiratory rate = 38/min), clonidine was increased to 400 microgram/day on day 12. Both the hypoxia and tachypnea improved from day 16 (6 days after initiation of clonidine) and clonidine was tapered gradually eventually stopped on day 29.
Patient 3
A 49-year old male with a past medical history significant for hypertension and dyslipidemia tested positive twice for SAR-CoV-2 via nasopharyngeal swab PCR. He was tested positive at the end of October 2020 and had mild symptoms of COVID-19. He recovered on home isolation for 14 days. He then presented to a virtual visit on Jan 19, 2021 with a history of fever up to 100 °F, fatigue. Anorexia, anosmia and ageusia for 7 days followed by a cough for 3 days. Repeat nasopharyngeal swab qPCR with abTESTMCOVID-19 qPCR assay kit done on Jan 20, 2021 was positive for SAR-CoV-2 with NS1 gene 27.9, NS2 gene 27.4 and IC 27.1 ct value. Laboratory tests indicated leukopenia, raised CRP, ESR, and serum ferritin level (Table 1). High resolution chest CT scan done on same day 7 demonstrated patchy ground glass opacities in all segments of bilateral lung fields with peripheral suggestive of atypical viral pneumonia (COVID-19 pneumonia) with CORADS-6 and CT severity score of 9/25 (Fig. 2). On Day 7 his antihypertensive medication (amlodipine and losartan) was kept on hold and clonidine 200 microgram was started twice daily. Next day he was hospitalized and oxygen supplementation was given at 2 L/min flow for reduced Spo2 to <90 % and increase clonidine dose to 400 μg /per day. He became afebrile on day 11 (5 days after the initiation of clonidine). His symptoms including anorexia, anosmia, ageusia and cough gradually improved on day 9. Clonidine was tapered on day 10 and stopped on day 23.
Discussion
Since the first detection of SARS-CoV-2 in Wuhan, China, 15 months have passed and the COVID-19 pandemic has caused more than two million deaths worldwide. According to data from China, 81 % of patients with SAR-CoV-2 positive have mild to moderate symptoms whereas 14 % develop severe disease and 5% become critically ill.
In this series, we were able to successfully mitigate further complications of symptomatic moderate to severe COVID-19 with timely use of clonidine. The rationale behind using this agent is its ability to block central sympathetic outflow while titrating its effect on blood pressure and heart rate. There were no side effects of the drug itself except for dry mouth. All patients tolerated the treatment. Hypertension is one of the comorbid conditions reported for severity of SAR-CoV-2 infection [15]. As a consequence, starting clonidine may have an additive benefit given its antihypertensive properties. Besides, fever and loss of taste and smell, due to undue stress, SAR-CoV-2 positive patients have anxiety, difficulty sleeping, whole body pain, diarrhea and cough [16]. Therefore, the anxiolytic, sedative, analgesic [17] antidiarrheal [18] and central cough suppressant effects [19] of clonidine may offer a major advantage of using this drug on patients with moderate symptoms of COVID-19. All three patients developed pneumonic consolidation involving all three lobes of the lungs bilaterally, recovered completely after treatment with clonidine. It should be noted that patient 3 had re-infection. During first infection he developed mild symptoms that subsided on its own. Second time when he had COVID it was more severe and had pneumonia requiring oxygen. As a result of this experience, we propose clonidine might be considered especially in limited resources country like Nepal and started as early as possible during disease course and be titrated to achieve a maximum response while monitoring blood pressure as an adjuvant therapy. Given the low risk of this drug when properly monitored and its multiple effects that mitigate the symptoms of COVID-19, the early use of this drug is justifiable. However, we don’t know the exact mechanism yet. It will be worthwhile to explore the mechanisms such (a) blockage of central sympathetic outflow; (b) prevention of excessive catecholaminergic release; (c) immunomodulatory role of clonidine. This drug may mitigate COVID-19 symptoms and prevent complications, including hypoxemia and the need for hospital admission, especially considering emergence of new variant SAR-CoV-2 and reinfection. The other advantage of this drug if it worked in clinical trials is that it would immediately scalable globally.
Conclusion
This is the first case series demonstrating the effectiveness of early use of clonidine to treat progression of symptoms of COVID-19. We suggest that double blinded randomized placebo control trials of this agent should be considered as a stop gap measure until more effective therapy is available.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
None.
Ethical approval
Not required.
Consent
Written consent is obtained from all three patients and is available on request.
Authors contribution
Dr. Sanjiv K Hyoju: Writing, collecting data, literature review.
Dr. Bidur Baral: Manuscript revision, data verification, proof reading.
Dr. Prabin K Jha: Collecting data.
Acknowledgments
We would like to acknowledge professor Dr. Yogendra Shakya, Head of Emergency department, Institute of Medicine, Kathmandu, Nepal and assistant professor Dr. Niraj Regmi, Radiology department, BP Koirala institute of Health Science, Dharan, Nepal and Dr. Surichhya Bajracharya, MD, Advocate Children’s Hospital, Park Ridge, IL, USA for their suggestion and support.
References
- 1.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konig M.F., Powell M., Staedtke V., Bai R.Y., Thomas D.L., Fischer N. Preventing cytokine storm syndrome in COVID-19 using alpha-1 adrenergic receptor antagonists. J Clin Invest. 2020;130(7):3345–3347. doi: 10.1172/JCI139642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyoju S.K., Zaborina O., van Goor H. SARS-CoV-2 and the sympathetic immune response: dampening inflammation with antihypertensive drugs (clonidine and propranolol) Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porzionato A., Emmi A., Barbon S., Boscolo-Berto R., Stecco C., Stocco E. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J. 2020;287(17):3681–3688. doi: 10.1111/febs.15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perin P.C., Maule S., Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23(1–2):45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- 6.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein D.S. The extended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res. 2020;30(4):299–315. doi: 10.1007/s10286-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Duarte A., Norcliffe-Kaufmann L. Is’ happy hypoxia’ in COVID-19 a disorder of autonomic interoception? A hypothesis. Clin Auton Res. 2020;30(4):331–333. doi: 10.1007/s10286-020-00715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baig A.M. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26(5):499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 11.Kenney M.J., Ganta C.K. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4(3):1177–1200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grebe K.M., Takeda K., Hickman H.D., Bailey A.L., Embry A.C., Bennink J.R. Cutting edge: sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J Immunol. 2010;184(2):540–544. doi: 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slota C., Shi A., Chen G., Bevans M., Weng N.P. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun. 2015;46:168–179. doi: 10.1016/j.bbi.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 15.Huang S., Wang J., Liu F., Liu J., Cao G., Yang C. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43(8):824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant M.C., Geoghegan L., Arbyn M., Mohammed Z., McGuinness L., Clarke E.L. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamadarkhana S., Gopal S. Clonidine in adults as a sedative agent in the intensive care unit. J Anaesthesiol Clin Pharmacol. 2010;26(4):439–445. [PMC free article] [PubMed] [Google Scholar]
- 18.Fragkos K.C., Zarate-Lopez N., Frangos C.C. What about clonidine for diarrhoea? A systematic review and meta-analysis of its effect in humans. Therap Adv Gastroenterol. 2016;9(3):282–301. doi: 10.1177/1756283X15625586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cinelli E., Bongianni F., Pantaleo T., Mutolo D. Suppression of the cough reflex by alpha 2-adrenergic receptor agonists in the rabbit. Physiol Rep. 2013;1(6) doi: 10.1002/phy2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]