PURPOSE:
There is limited evidence on the intensity of end-of-life (EOL) care for women < 65 years old, who account for about 40% of breast cancer deaths in the United States. Using established indicators, we estimated the intensity of EOL care among these women.
METHODS:
We used 2000-2014 claims data from a large US insurer to identify women with metastatic breast cancer who, in the last month of their lives, had more than one hospital admission, emergency department visit, or an intensive care unit (ICU) admission and/or used antineoplastic therapy in the last 14 days of life. Using multivariate logistic regression, we assessed whether intensity of EOL care differed by demographic characteristics, socioeconomic factors, or regions.
RESULTS:
Adjusted estimates show an increase in EOL ICU admissions between 2000-2003 and 2010-2014 from 14% (95% CI, 10% to 17%) to 23% (95% CI, 20% to 26%) and a small increase in emergency department visits from 10% (95% CI, 7% to 13%) to 12% (95% CI, 9% to 15%), both statistically significant. There was no statistically significant change in the proportions of women experiencing more than one EOL hospitalization (14% in 2010-2014; 95% CI, 11% to 17%) and of those receiving EOL antineoplastic treatment (24% in 2010-2014; 95% CI, 21% to 27%). Living in predominantly mixed, Hispanic, Black, or Asian neighborhoods correlated with more intense care (odds ratio, 1.39; 95% CI, 1.10 to 1.77 for ICU).
CONCLUSION:
Consistent with findings in the Medicare population, our results suggest an overall increase in the number of ICU admissions at the EOL over time. They also suggest that patients from non-White neighborhoods receive more intense acute care.
INTRODUCTION
Female breast cancer is the fourth leading cause (by number) of cancer death in the United States, and 40% of women dying from breast cancer are < 65 years old.1 However, most end-of-life (EOL) cancer care research has examined ≥ 65-year-old Medicare-insured patients. Far less is known about the intensity and quality of care of < 65-year-old commercially insured patients. Differences in insurance coverage, life expectancy, life circumstances, and overall health could lead to different intensity of treatment at the EOL for younger compared with older patients.
Over the past two decades, on the basis of mounting evidence of benefits and risks, multiple stakeholders have advocated less-intense EOL care and greater access to palliative care and hospice.2-7 Yet, it is unclear if care intensity has changed over time. Most studies are cross-sectional,8-12 and few recent studies analyzed the intensity of EOL cancer care over time.13,14
EOL care research has measured intensity using indicators such as hospitalization, potentially life-prolonging interventions, and potentially life-supporting interventions.2,15 US studies about EOL cancer care have focused on Medicare patients ≥ 65 years old.10,16-25 Some studies have used commercial insurance claims to investigate end-of-life care among US cancer patients younger than age 65 years,14,26 but only few with national coverage.8 Some nationally representative claims data studies have focused on costs.27,28 Other EOL care studies among patients < 65 years old have used data from the Cancer Care Outcomes Research and Surveillance study,29 the Coping with Cancer study,30 Medicaid data,31,32 or medical records at individual hospitals.33,34 There is therefore limited evidence on whether EOL care patterns among cancer patients ≥ 65 years old generalize to younger patients.
In this study, we used data from a large cohort of commercially insured women with metastatic breast cancer age 25-64 years who died between 2000 and 2014 to analyze intensity of EOL care over time using established measures.
METHODS
Data Source
This study used enrollment information and administrative claims data from Optum’s de-identified Clinformatics Data Mart Database (Eden Prairie, MN) between May 1, 2000 and December 31, 2014. These data are sourced from a large national health insurer with 48 million commercially insured members across all 50 US states. The claims data include de-identified enrollment information and all medical, pharmacy, and hospitalization claims. We linked sociodemographic variables derived from the annual American Community survey 2008-201235 using census tract. The data vendor linked month and year of death in the Death Master File to members in our cohort; we used the date of the last observed claim as the day of death within a given death month and year (Death Master File; 87%). If there was no claim in the month of death, we used the last day of enrollment (13%). Because of changes in state-level regulations in 2011, about 4.2 million historical death records were removed from the Death Master File, and since 2011 about 40% new death records are missing from the Death Master File.36,37
Study Population
We used a claims-based algorithm38-40 and relevant International Classification of Diseases, 9th Revision (ICD-9) codes to identify women with metastatic breast cancer who died between May 2000 and December 2014.
We first identified women age 25-64 years at the time of diagnosis of metastatic cancer. The date of diagnosis of metastatic cancer was determined based on the time of the first of two secondary malignant neoplasm diagnoses (ICD-9 codes 197.x, 198.xx, and 199.0) on separate days up to 90 days apart. We defined the first diagnosis date as the disease index date.
To identify women with metastatic breast cancer, we then limited this cohort of women with secondary metastatic diagnosis to women with at least two claims for breast cancer (ICD-9 codes 174-174.9, 233.0) during the 365 days before the disease index date (first inclusion rule) or at least one breast cancer diagnosis within 365 days before and one breast cancer diagnosis up to 90 days after the disease index date (second inclusion rule).
We excluded patients with a diagnosis of cancer other than breast cancer before the index date (ICD-9 codes 140.xx-165.xx, 170.xx-172.xx, 175.xx, 176.xx, 179.xx-195.xx, 199.1, 199.2, and 200.xx-209.xx) to avoid including patients whose primary cancer diagnosis was not breast cancer. Finally, we limited the cohort to women with a death recorded in the Death Master File and who were enrolled in the commercial insurance plan in the month of and in the month before death.
Outcomes
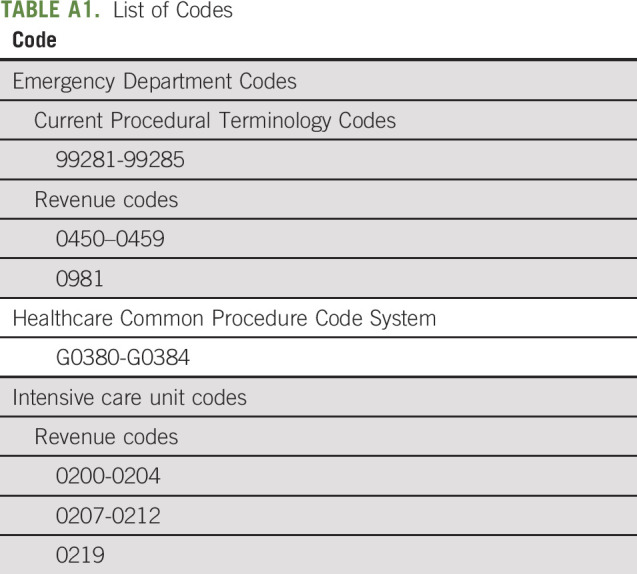
We constructed some of the measures of EOL care intensity developed by Earle et al15 that are part of the National Voluntary Consensus Standards for Quality of Cancer Care (National Quality Forum).7 Specifically, we defined the proportions of patients, who in the last 30 days of life: (1) had more than one hospital admission, (2) had more than one emergency department (ED) visit, or (3) were admitted to the intensive care unit (ICU); and (4) in the last 14 days of life received anticancer therapy. In the following sections, we refer to these measures as EOL hospitalizations, ED visits, ICU admissions, and antineoplastic treatment. Appendix Table A1 (online only) includes billing codes used to define these outcomes that we identified based on published literature.8,10,14,41-43 We define antineoplastic treatment in last 14 days of life on the basis of receipt of traditional chemotherapy and/or targeted therapies. For oral drugs, we used the date of last supply (ie, dispensing date + number of days supplied) and for injectable products the date of administration. For antineoplastic treatments, Healthcare Common Procedure Code System (HCPCS) and National Drug Code (NDC) code lists as well as National Comprehensive Cancer Network guidelines were used. Our list of antineoplastic billing codes includes drug administration codes (diagnosis [ICD-9], diagnostic-related groups, procedure [Current Procedural Terminology (CPT), revenue], and HCPCS codes) and substance codes (HCPCS and NDC codes) for traditional chemotherapy (eg, cytotoxic chemotherapy) and targeted therapy (including small molecule targeted and immunotherapies). It does not include endocrine therapy (eg, aromatase inhibitors).
Covariates
We used version 10 of The Johns Hopkins Adjusted Clinical Groups (ACG) System version 11.1 to calculate participants’ morbidity scores over the 6-month period before the month of death (ie, excluding the month of death).44 We used 2015 Area Deprivation Index deciles calculated by the University of Wisconsin based on the 2011-2015 American Community Survey 5-year estimates at the Census Block level.45 We used geocoding, at census tract level, to classify women residing in predominantly non-Hispanic White neighborhoods versus predominately mixed, Hispanic, Black, or Asian neighborhoods using the 2008-2012 American Community Survey (for more details on how race/ethnicity was defined, see Wharam et al46). We then applied a superseding ethnicity assignment (at the individual level) using flags created by the E-Tech system (Ethnic Technologies), which analyzes full names and geographic locations of individuals.47,48 In the following sections, we call the different neighborhoods predominantly White versus non-White. Other covariates include age at death, months between first observed secondary metastatic diagnosis and death (categories were selected based on tercile), US region, and time period of death (2000-2003, 2004-2006, 2007-2009, 2010-2014). Similar to previous cross-sectional8 and longitudinal trend studies,14 we grouped patients into the following categories on the basis of their year of death (2000-2003, 2004-2006, 2007-2009, 2010-2014) to achieve similar numbers of deaths and larger samples to estimate proportions in a given period.
Statistical Analysis
We used multivariate logistic regression to analyze the correlation between covariates and outcomes and estimated adjusted rates of outcome variables using the Stata margins postestimation command. We used separate models for each outcome measure. We tested for changes over time (by year) using the first-order ARIMA autoregressive model.49 This study was approved by the Harvard Pilgrim Health Care Institute Institutional Review Board.
RESULTS
Descriptive Analysis
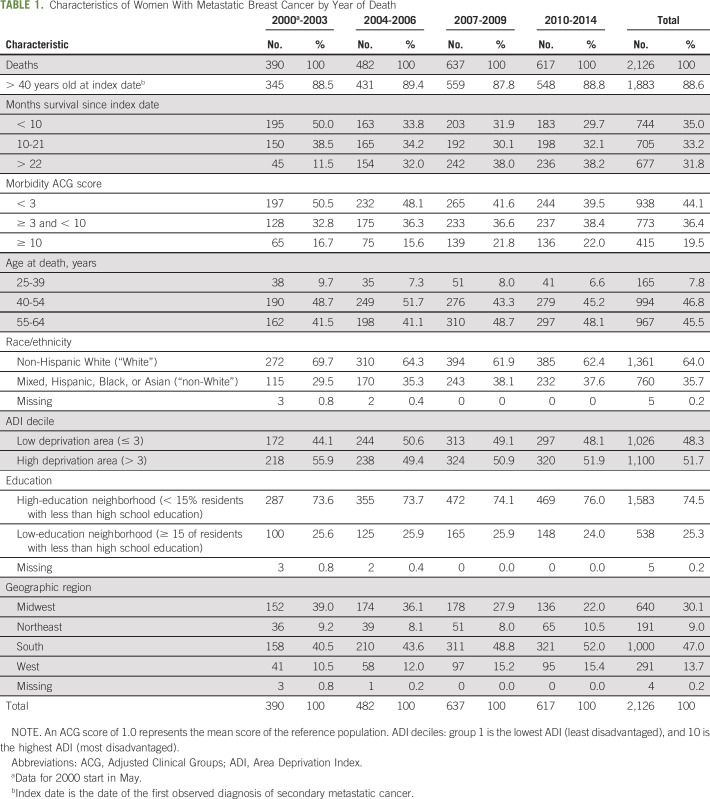
Between November 2000 and December 2014, 2,126 women were identified as having died with metastatic breast cancer. Most women (89%) were > 40 years of age when they were diagnosed (Table 1) and resided in predominantly non-Hispanic White neighborhoods (64%). Almost half (47%) lived in the South and 30% in the Midwest.
TABLE 1.
Characteristics of Women With Metastatic Breast Cancer by Year of Death
Statistical Analysis
Multivariable analysis.
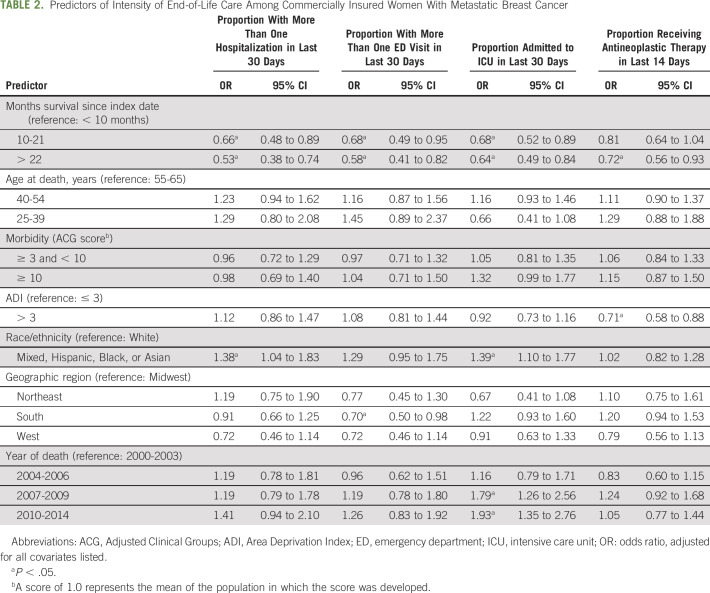
Longer time intervals (10-21 and > 22 months compared with < 10 months) between the first observed secondary metastatic diagnosis and death correlated with between 53% and 72% lower likelihood of intense EOL care (Table 2).
TABLE 2.
Predictors of Intensity of End-of-Life Care Among Commercially Insured Women With Metastatic Breast Cancer
Women living in predominantly mixed, Hispanic, Black, or Asian neighborhoods were more likely to be hospitalized (odds ratio [OR], 1.38; P = .03) and be admitted to the ICU (OR, 1.39; P = .01) at the EOL. Women living in more deprived neighborhoods were less likely to receive EOL antineoplastic treatment (OR, 0.71; P < .01). Patients living in the South were less likely than patients living in the Midwest to experience EOL ED visits (OR, 0.70; P = .04).
A higher percentage of women experienced EOL ICU admissions during 2007-2009 (OR, 1.79; P < .001) and 2010-2014 (OR, 1.93; P < .001) compared with 2000-2003.
Intensity of EOL care by time period.
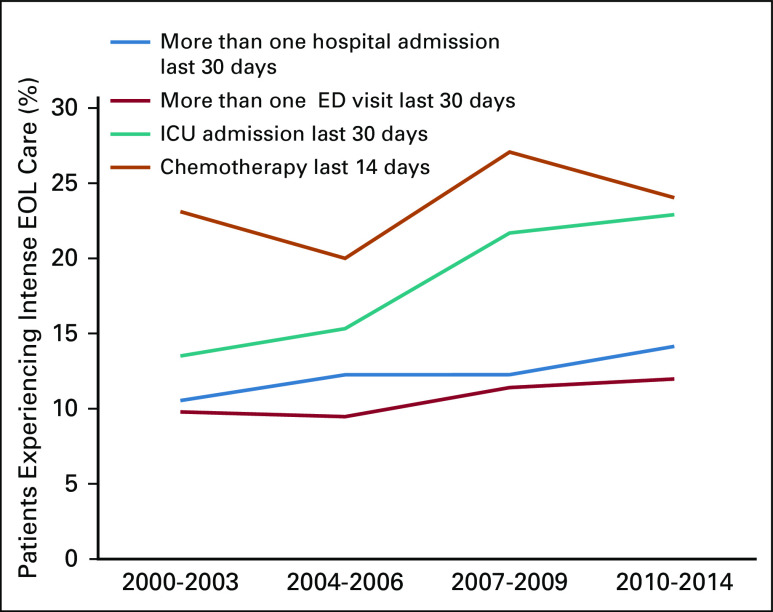
The proportion of women experiencing EOL hospitalizations was 11% (95% CI, 8% to 14%) in 2000-2003 and 14% (95% CI, 11% to 17%) in 2010-2014; the annual increase was not statistically significant (ARIMA model: P = .086). EOL ED visits increased from 10% (95% CI, 7% to 13%) to 12% (95% CI, 9% to 15%); EOL ICU admissions increased from 14% (95% CI, 10% to 17%) to 23% (95% CI, 20% to 26%). Changes in EOL ED visits and ICU admissions over time were statistically significant (ARIMA model: ED, P = .018; ICU, P < .01). The proportion of women on EOL antineoplastic therapy was 23% (95% CI, 19% to 27%) in 2000-2003, 20% (95% CI, 16% to 24%) in 2004-2006, 27% (95% CI, 24% to 31%) in 2007-2009, and 24% (95% CI, 21% to 27%) in 2010-2014; there was no statistically significant change over time (ARIMA: P = .56; Fig 1).
FIG 1.
Intensity of end-of-life (EOL) care over different time periods (adjusted rates). ED, emergency department; ICU, intensive care unit.
DISCUSSION
This study contributes longitudinal evidence on EOL care intensity in a population of younger commercially insured patients. We find that commercially insured women who died with metastatic breast cancer between 2000 and 2014 received more intense EOL care when they were living in predominantly mixed, Hispanic, Black, or Asian neighborhoods or (with respect to antineoplastic treatment) when living in less-deprived neighborhoods; when time between diagnosis of secondary metastatic cancer and death was shorter; and when they died in more recent years (with respect to ICU and, to a smaller extent, ED visits).
Previous studies showed an effect of race/ethnicity on EOL in the Medicare population. We provide evidence for this in younger commercially insured patients. A study of patients with breast cancer using SEER-Medicare (2007-2012) data found that Black patients were more likely to experience EOL hospitalizations, ED visits, or ICU admission than White patients (risk ratio, 1.30; 95% CI, 1.02 to 1.65).11 Additional studies in SEER-Medicare have found more intense care for Black patients with cancer.10,21,22,50
There may be several reasons that patients living in predominantly mixed and/or non-White neighborhoods experience more hospital and ICU admissions. In addition to availability of palliative and hospice services, trust in the health care system, prognostic understanding, knowledge of risk and benefits of treatment, religious beliefs, and provider communication skills influence patient and family preferences toward EOL care.51
Few studies assessed the association between time since diagnosis and intensity of EOL. A study of women with stage IV breast cancer on Medicare found that patients surviving > 6 months after diagnosis were less likely to receive aggressive EOL care in comparison with patients surviving ≤ 6 months (OR, 0.47; 95% CI, 0.39 to 0.58 among patients who lived 6-12 months, and OR, 0.44; 95% CI, 0.38 to 0.52 among patients who lived > 12 months).50
In our study, about 11% of women were hospitalized at the EOL in 2000-2003 and 14% in 2010-2014, but this increase was not statistically significant. In an analysis of MarketScan MEDSTAT data, 9.7% of commercially insured patients with cancer had more than one hospital admission in the last month of life during 1991-2003.16 Among patients with cancer (colorectal, breast, lung, prostate, and hematologic) on Medicare, < 10% experienced more than one EOL hospitalization during 1993-2000.16 Among 482 patients with breast cancer age ≥ 18 years from west Washington state (53% of whom were ≥ 65 years old), 4.4% were hospitalized more than once at the EOL during 2007-2015.14 Differences between our results and those of previous studies are likely due to different types of cancers included in the earlier studies (not only breast cancer), patient ages, the time period of analysis, and the geographical coverage of the studies.
We found a small but statistically significant increase in the percentage of patients with metastatic breast cancer who visited the ED at the EOL: 10% in 2000-2003 and 12% in 2010-2014. These results are consistent with earlier findings (1993-1999) from Medicare patients (7.8%-10.4%)16 and more recent findings (2007-2013) from SEER-Medicare (11.1%).13 Results from a study in western Washington State found that only 1.5% patients with breast cancer visited the ED more than once in the last month of life.14 This is similar to results from another study of commercially insured patients < 65 years old with breast cancer from across the four US regions (1.7%).8 Differences in how ED visits were identified may explain some of these differences. We used HCPCs, revenue codes, and CPT codes, whereas the national study on commercially insured patients used place of service revenue codes on facility claims.8
In line with findings from studies using Medicare data, we find an increasing percentage of patients admitted to the ICU in the last month of life, from 14% during 2000-2003 to 23% during 2010-2014. This increase was statistically significant. Goodman et al52 found that the percentage of Medicare patients admitted to the ICU in the last month of life increased from 23.7% in 2003-2007 to 28.8% in 2010. Similar increases were found in other studies of patients with cancer and patients with other conditions like COPD and dementia on Medicare.53,54 Findings for women with breast cancer > 18 years of age in western Washington State found that 34% of the women were admitted to the ICU in the last month of life.14 The percentage was lower for Medicare patients, 18.5% among patients with various cancers during 2007-2013,13 and, in another study of commercially insured patients, 17.5% for patients with breast cancer during 2007-2014.8 Both these studies used CPT codes for critical care (99291, 99292) to identify ICU admissions. We used revenue codes validated by Weissman et al,55 which may explain why the results of the former studies differ from ours.
EOL antineoplastic therapy in the last 14 days of life remained stable at 23%-24% between 2000-2003 and 2010-2014, with some variation in years 2004-2006 (20%) and 2007-2009 (27%) but no statistically significant change over time. Overall, the percentage of patients receiving EOL antineoplastic therapy was similar to results from western Washington State in patients > 18 years old (21.8%).14 Antineoplastic therapy use in our study was higher than reported by Falchook et al8 for a national sample of commercially insured patients with breast cancer (14.1%). This study did not include NDC codes to identify antineoplastic use, which may explain the lower value.
Our data included month but not day of death. It is possible that by assigning last claims or last enrolled date as death date, our EOL intensity measures covered slightly more or fewer than 30 or 14 days before death. This is likely to affect more the receipt of antineoplastic treatment during the last 14 days, given the shorter time span it covers. We therefore estimated the proportion of patients on antineoplastic treatment if the date of death was 5 days after our estimated one. The proportion of patients receiving antineoplastic treatment then would be reduced (2000-2003: 12%; 2004-2006: 12%; 2007-2009: 16%; 2010-2014: 15%). These results still indicate that antineoplastic treatment is used very close to death. Due to changes in state legislation affecting reporting of deaths to the Death Master File, death information after 2011 is partially complete,36 leading to undercounting of deaths. Because outcome measures are based on proportions of deaths, declining numbers of deaths would only confound the results if unreported deaths were systematically related to intensity of EOL care, which is unlikely given the dropout is at the state level and not at individual level. To affect the results, states that dropped would need to include patients with characteristics that both differed from patients in states that did not drop and were associated with intensity of EOL care.
This is a retrospective study of care received at the EOL by women who died with metastatic breast cancer. The study design does not allow us to assess the care received by severely ill women who survived. Because of lack of information on cause of death, this study reports the experience of patients who died with metastatic breast cancer. We did not have information from cancer registry or electronic health records to confirm stage at diagnosis or metastatic status. Instead, we used a claims-based algorithm, based on the literature,38-40 to identify patients with metastatic breast cancer. These limitations affect most national studies using claims data of commercially insured patients.
In this study, we find that, despite broadening consensus on less intense EOL care for patients with cancer, frequency of ICU admissions and, to a smaller extent, EOL ED visits in a cohort of commercially insured women who died with metastatic breast cancer has increased over time. Patients living in predominantly mixed, Hispanic, Black, or Asian neighborhoods experienced more intense EOL care, and shorter time intervals between first observed diagnosis of secondary metastatic cancer and death were correlated with more intense EOL care. These findings provide new evidence on EOL care in an understudied group, < 65-year-old commercially insured patients, and longitudinal data on the intensity of EOL care over time.
ACKNOWLEDGMENT
We thank Jamie Wallace, MPH, for project management and for analytic development and data processing of the socioeconomic variables in the study. We thank Matthew Callahan, MS, MPH, for initial project management during his tenure at the Institute.
Appendix
TABLE A1.
List of Codes
DISCLAIMER
Xin Xu conducted the analyses prior to changing employer. At the time the analyses were conducted, he was employed at the Harvard Pilgrim Health Care Institute.
SUPPORT
Supported by a postdoctoral fellowship from the Swiss National Science Foundation (A.F.), the Department of Population Medicine’s Ebert Award (A.K.W.), and a grant from the National Cancer Institute (D.R.D., F.Z., J.F.W., and A.K.W.; NCI Grant Nos. 5R01CA172639-05 and 4R01CA172639-04).
AUTHOR CONTRIBUTIONS
Conception and design: Alessandra Ferrario, Dennis Ross-Degnan, J. Frank Wharam, Anita K. Wagner
Administrative support: J. Frank Wharam
Provision of study material or patients: J. Frank Wharam
Collection and assembly of data: Alessandra Ferrario, Xin Xu, J. Frank Wharam
Data analysis and interpretation: Alessandra Ferrario, Xin Xu, Fang Zhang, Dennis Ross-Degnan, J. Frank Wharam, Anita K. Wagner
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Intensity of End-of-Life Care in a Cohort of Commercially Insured Women With Metastatic Breast Cancer in the United States
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Xin Xu
Employment: Takeda
Stock and Other Ownership Interests: Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1. National Cancer Institute: Cancer Stat Facts: Female breast cancer. https://seer.cancer.gov/statfacts/html/breast.html.
- 2.Luta X, Maessen M, Egger M, et al. : Measuring intensity of end of life care: A systematic review. PLoS One 10:e0123764, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choosing Wisely: American Society of Clinical Oncology: Ten things physicians and patients should question. http://www.choosingwisely.org/societies/american-society-of-clinical-oncology/
- 4.Institute of Medicine : Approaching Death: Improving Care at the End of Life. Washington, DC, The National Academies Press, 1997 [PubMed] [Google Scholar]
- 5.National Research Council : Describing Death in America: What We Need to Know. Washington, DC, The National Academies Press, 2003 [PubMed] [Google Scholar]
- 6.Institute of Medicine : Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC, The National Academies Press, 2015 [PubMed] [Google Scholar]
- 7. National Quality Forum: National Voluntary Consensus Standards for Quality of Cancer Care. A consensus report. Washington, DC, National Quality Forum. [Google Scholar]
- 8. doi: 10.1093/jnci/djx028. Falchook AD, Dusetzina SB, Tian F, et al: Aggressive end-of-life care for metastatic cancer patients younger than age 65 years. J Natl Cancer Inst 109:djx028, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerillo JA, Stuver SO, Fraile B, et al. : Understanding oral chemotherapy prescribing patterns at the end of life at a comprehensive cancer center: Analysis of a Massachusetts payer claims database. J Oncol Pract 11:372-377, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Abdollah F, Sammon JD, Majumder K, et al. : Racial disparities in end-of-life care among patients with prostate cancer: A population-based study. J Natl Compr Canc Netw 13:1131-1138, 2015 [DOI] [PubMed] [Google Scholar]
- 11. Check DK, Samuel CA, Rosenstein DL, et al: Investigation of racial disparities in early supportive medication use and end-of-life care among medicare beneficiaries with stage IV breast cancer. J Clin Oncol 34:2265-2270, 2016. [DOI] [PMC free article] [PubMed]
- 12.Clough JD, Strawbridge LM, LeBlanc TW, et al. : Association of practice-level hospital use with end-of-life outcomes, readmission, and weekend hospitalization among medicare beneficiaries with cancer. J Oncol Pract 12:e933-e943, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Fang P, Jagsi R, He W, et al. : Rising and falling trends in the use of chemotherapy and targeted therapy near the end of life in older patients with cancer. J Clin Oncol 37:1721-1731, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott CL, Fedorenko C, Kreizenbeck K, et al. : End-of-life services among patients with cancer: Evidence from cancer registry records linked with commercial health insurance claims. J Oncol Pract 13:e889-e899, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earle CC, Park ER, Lai B, et al. : Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 21:1133-1138, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Earle CC, Landrum MB, Souza JM, et al. : Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 26:3860-3866, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earle CC, Neville BA, Landrum MB, et al. : Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22:315-321, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Guadagnolo BA, Huo J, Buchholz TA, et al. : Disparities in hospice utilization among American Indian Medicare beneficiaries dying of cancer. Ethn Dis 24:393-398, 2014 [PubMed] [Google Scholar]
- 19.Morden NE, Chang C-H, Jacobson JO, et al. : End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood) 31:786-796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obermeyer Z, Makar M, Abujaber S, et al. : Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA 312:1888-1896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith AK, Earle CC, McCarthy EP: Racial and ethnic differences in end-of-life care in fee-for-service Medicare beneficiaries with advanced cancer. J Am Geriatr Soc 57:153-158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miesfeldt S, Murray K, Lucas L, et al. : Association of age, gender, and race with intensity of end-of-life care for Medicare beneficiaries with cancer. J Palliat Med 15:548-554, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tschirhart EC, Du Q, Kelley AS: Factors influencing the use of intensive procedures at the end of life. J Am Geriatr Soc 62:2088-2094, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. doi: 10.1136/bmj.328.7440.607. Wennberg J, Fisher E, Stukel T, et al: Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States. BMJ 328:607, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Setoguchi S, Earle C, Glynn R, et al: Testing cancer quality measures for end-of-life care. Effective Health Care Research Report No. 21. Rockville, MD, Agency for Healthcare Research and Quality, 2010.
- 26.Stuver SO, McNiff K, Fraile B, et al. : Novel data sharing between a comprehensive cancer center and a private payer to better understand care at the end of life. J Pain Symptom Manage 52:161-169, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Kolodziej M, Hoverman JR, Garey JS, et al. : Benchmarks for value in cancer care: An analysis of a large commercial population. J Oncol Pract 7:301-306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chastek B, Harley C, Kallich J, et al. : Health care costs for patients with cancer at the end of life. J Oncol Pract 8:75s-80s, 2012. (suppl 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks GA, Cronin AM, Uno H, et al. : Intensity of medical interventions between diagnosis and death in patients with advanced lung and colorectal cancer: A CanCORS analysis. J Palliat Med 19:42-50, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker-Seeley RD, Abel GA, Uno H, et al. : Financial hardship and the intensity of medical care received near death. Psychooncology 24:572-578, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mack JW, Chen K, Boscoe FP, et al. : Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol 31:2569-2579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang A, Goldin D, Nova J, et al. : Racial disparities in health care utilization at the end of life among New Jersey medicaid beneficiaries with advanced cancer. JCO Oncol Pract 16:e538-e548, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinall MC, Jr, Ehrenfeld JM, Guillamondegui OD: Religiously affiliated intensive care unit patients receive more aggressive end-of-life care. J Surg Res 190:623-627, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Taylor JS, Brown AJ, Prescott LS, et al. : Dying well: How equal is end of life care among gynecologic oncology patients? Gynecol Oncol 140:295-300, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. US Census Bureau: American Community Survey (ACS). https://www.census.gov/programs-surveys/acs.
- 36.da Graca B, Filardo G, Nicewander D: Consequences for healthcare quality and research of the exclusion of records from the Death Master File. Circ Cardiovasc Qual Outcomes 6:124-128, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Levin MA, Lin H-M, Prabhakar G, et al. : Alive or dead: Validity of the Social Security Administration Death Master File after 2011. Health Serv Res 54:24-33, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. doi: 10.1007/s10549-016-3875-z. Leopold C, Wagner AK, Zhang F, et al: Racial disparities in all-cause mortality among younger commercially insured women with incident metastatic breast cancer. Breast Cancer Res Treat 158:333-340, 2016 [Erratum: Breast Cancer Res Treat 160:385, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurvitz S, Guerin A, Brammer M, et al. : Investigation of adverse-event-related costs for patients with metastatic breast cancer in a real-world setting. Oncologist 19:901-908, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whyte JL, Engel-Nitz NM, Teitelbaum A, et al. : An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med Care 53:e49-e57, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Du X, Goodwin JS: Increase of chemotherapy use in older women with breast carcinoma from 1991 to 1996. Cancer 92:730-737, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du X, Goodwin JS: Patterns of use of chemotherapy for breast cancer in older women: Findings from Medicare claims data. J Clin Oncol 19:1455-1461, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Sinaiko AD, Chien AT, Hassett MJ, et al. : What drives variation in spending for breast cancer patients within geographic regions? Health Serv Res 54:97-105, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johns Hopkins HealthCare Solutions: ACG System: Population health analysis tool. https://www.johnshopkinssolutions.com/solution/acgsystem/
- 45. University of Wisconsin School of Public Health: Neighborhood atlas. https://www.neighborhoodatlas.medicine.wisc.edu/
- 46.Wharam JF, Zhang F, Wallace J, et al. : Vulnerable and less vulnerable women in high-deductible health plans experienced delayed breast cancer care. Health Aff (Millwood) 38:408-415, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiscella K, Fremont AM: Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res 41:1482-1500, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ethnic Technologies: Frequently asked questions. https://www.ethnictechnologies.com/faq.
- 49. stata.com: ARIMA. https://www.stata.com/manuals13/tsarima.pdf.
- 50.Accordino MK, Wright JD, Vasan S, et al. : Association between survival time with metastatic breast cancer and aggressive end-of-life care. Breast Cancer Res Treat 166:549-558, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prigerson HG, Maciejewski PK: Dartmouth Atlas: Putting end-of-life care on the map but missing psychosocial detail. J Support Oncol 10:25-28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goodman D, Morden NE, Chang C-H, et al: Trends in cancer care near the end of life. A Dartmouth Atlas of Health Care Brief. https://www.dartmouthatlas.org/downloads/reports/Cancer_brief_090413.pdf. [PubMed]
- 53.Teno JM, Gozalo PL, Bynum JPW, et al. : Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 309:470-477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Teno JM, Gozalo P, Trivedi AN, et al: Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA 320:264-271, 2018. [DOI] [PMC free article] [PubMed]
- 55.Weissman GE, Hubbard RA, Kohn R, et al. : Validation of an administrative definition of ICU admission using revenue center codes. Crit Care Med 45:e758-e762, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]