PURPOSE:
Hospitalizations during cancer treatment are costly, can impair quality of life, and negatively affect therapy completion. Our objective was to identify risk factors for unplanned hospitalization among older adults receiving chemotherapy.
METHODS:
This is a secondary analysis of a multisite cohort study (N = 750) of patients ≥ 65 years of age evaluated with a geriatric assessment (GA) to predict chemotherapy toxicity. The primary outcome of this analysis was unplanned hospitalizations during treatment; the secondary outcome was length of stay (LOS) of the first hospitalization. Independent variables included pretreatment GA measures, laboratory values, cancer type and stage, and treatment intensity characteristics. We used logistic regression to estimate the odds of hospitalization and generalized linear models for LOS in multivariable analyses.
RESULTS:
The sample median age was 72 years (range, 65-94 years); 59% had stage IV disease. At least one unplanned hospitalization occurred in 193 patients (25.7%) during receipt of chemotherapy. In multivariable analyses controlling for cancer type, the following baseline characteristics were significantly associated with increased odds of hospitalization: needing help bathing or dressing (odds ratio [OR], 1.8; 95% CI, 1.0 to 3.1), polypharmacy (≥ 5 meds) (OR, 1.6; 95% CI, 1.1 to 2.4), more comorbid conditions (OR, 1.1; 95% CI, 1.0 to 1.3), availability of someone to take them to the doctor (OR, 2.0; 95% CI, 1.0 to 4.1), CrCl < 60 mL/min (OR, 1.7; 95% CI, 1.1 to 2.4), and albumin < 3.5 g/dL (OR, 1.8; 95% CI, 1.2 to 2.8). In multivariable analyses, older age, self-reported presence of liver or kidney disease, living alone and depressive symptoms were associated with longer LOS.
CONCLUSION:
Readily available GA variables and laboratory data, but not age, were associated with unplanned hospitalizations among older adults receiving chemotherapy. If validated, these data can inform prediction models and the design of interventions to decrease unplanned hospitalizations.
INTRODUCTION
Older patients with cancer are at risk for high healthcare utilization after a cancer diagnosis and during cancer treatment.1,2 Unplanned hospitalizations are a major concern for patients, practitioners, and the healthcare system. Hospitalizations are costly, impair quality of life, increase the risk of disability, and can negatively affect completion of therapy.3-5 Identification of factors associated with unplanned hospitalizations during chemotherapy can inform the design of preventative interventions and avoid their subsequent negative effects on treatment and survivorship.
Compared to younger patients, older adults are more likely to have vulnerabilities such as comorbidity, impaired physical function, social support constraints, and cognitive dysfunction. These geriatric impairments may increase risk of hospitalization independent of tumor or treatment-related factors.6-8 However, few studies have addressed risk factors for hospitalization among older adults during chemotherapy. Several large cohort studies that investigated geriatric assessment (GA) as a predictor of chemotherapy toxicity have captured hospitalization as a secondary outcome.9,10 These studies have shown that a GA-derived toxicity score or a frailty assessment can identify patients at higher risk for both chemotherapy toxicity and hospitalizations. Understanding which vulnerabilities increase the odds of hospitalization is a next step that could inform interventions to minimize risk.
The primary goal of this analysis was to identify factors associated with unplanned hospitalizations among older patients receiving chemotherapy for cancer. We hypothesized that pretreatment GA impairments inclusive of physical function and comorbidity would be associated with unplanned hospitalization. A secondary objective was to evaluate factors associated with length of hospitalization.
METHODS
Analysis Cohort
This is a secondary analysis of data from an institutional review board–approved, prospective cohort study, which aimed to develop and validate a predictive model of severe chemotherapy toxicity in older adults.9,11 The City of Hope institutional review board provided approval for conduct of this analysis; the parent study was approved by all site institutional review boards. Eligible patients were 65 years of age or older starting a new course of chemotherapy for a solid tumor diagnosis. Between 2006 and 2009, 500 patients enrolled in the development cohort, and between 2008 and 2012, 250 enrolled in the validation cohort. Eligibility criteria were identical for both cohorts. The development cohort included seven accruing academic institutions across the United States.9 Participants included in the validation cohort were recruited from eight institutions in the United States, six had participated in the development cohort and two new sites were added.11 The two cohorts were combined to maximize the number of events observed.
Predictor Variables
Clinical data, abstracted from the medical record, included routinely collected laboratory values (albumin, white cell count, blood urea nitrogen, creatinine, and hepatic function tests), cancer type, stage, treatment (single-agent v multi-agent; standard dose versus dose reduction at first cycle), use of growth factor, and receipt of prior chemotherapy. Creatinine clearance was calculated using the Jelliffe formula with ideal body weight. Participants completed a GA before starting chemotherapy. The GA tool includes a healthcare provider-administered assessment and a self-administered patient questionnaire.9,11 The healthcare provider-administered questionnaire included the following assessments: (1) Karnofsky Performance Scale (KPS); (2) timed up and go (TUG) (a performance-based measure of physical function; time assessed in seconds for those who could complete the test or recorded as unable to perform)12; (3) Blessed Orientation Memory Concentration test (score ≥ 11 indicating impairment)13; (4) recording of height and weight (current and 6 months prior) to evaluate nutritional status including calculation of body mass index.
The self-administered patient questionnaire included the following surveys: self-reported measures of physical function and activities (activities of daily living [ADL] subscale of Medical Outcomes Study physical health survey and the instrumental activities of daily living subscale of the Older Americans Resources and Services survey),14,15 a patient-rated KPS,16 self-reported falls in the past 6 months, self-reported comorbid conditions and a rating of the degree to which each causes interference in activities (Physical Health Section Subscale of the Older Americans Resources and Services survey),14 number and type of medications, assessment of psychologic state (symptoms of anxiety and depression using the Mental Health Inventory-17),17 social activity, and social support (Medical Outcomes Study social activity and social support surveys).15
Outcomes
The primary outcome of this secondary analysis was incident unplanned hospitalization(s) during chemotherapy treatment, excluding any planned or scheduled admissions. The secondary outcome was the length of stay (LOS) of the first hospitalization. Data on hospitalization were abstracted from the medical records at each site and underwent review by two physicians. This process included review of notes, discharge summaries, phone notes, and scanned media to capture hospitalizations that happened within or outside of the institutions' electronic medical records system. The treating physician was queried to resolve any uncertainty. Reasons for hospitalization were recorded and adjudicated by two-physician review. If more than one reason was listed, the highest-grade toxicity resulting in hospitalization was reported.
Statistical Approach
We calculated descriptive statistics summarizing patient demographic, clinical characteristics, and GA domains. Univariate logistic regression models were used to obtain odds ratios (ORs), corresponding 95% CIs, and P values for each variable associated with hospitalization. These variables included demographic or disease characteristics, GA measures of function, comorbidity, cognition, nutrition, and psychosocial status, and laboratory values including albumin, hemoglobin, creatinine, white cell count, and liver function tests.
Variables with univariate P value < .1 were included in the multivariable logistic regression for stepwise selection. Forward selection and backward elimination was used to select the final set of variables independently associated with hospitalization. Collinearity among predictors was evaluated and they were not highly correlated (data not shown). Of note, the Cancer and Aging Research Group toxicity score, previously developed to predict grade 3-5 toxicity,9 was not included as a variable in this analysis because (1) the objective was to identify individual characteristics associated with hospitalization, and (2) the Cancer and Aging Research Group toxicity score includes some of the variables identified in the regression analysis (ie, creatinine clearance and cancer type). We evaluated the discrimination ability of the final set of variables by calculating the model's area under the curve. We also conducted an exploratory analysis among those with advanced-stage disease only (N = 436).
Next, we created an index indicating the number of risk factors for each patient from those identified in multivariate analyses (number of comorbid conditions, albumin, creatinine clearance, GI cancer, polypharmacy, requiring assistance with dressing or bathing, and having someone available to take them to the doctor most or all of the time). For this purpose, we dichotomized the continuous variables using Youden's index to determine the best cutoff point for each. The cutoff points for number of comorbidities, albumin, and creatinine clearance were three conditions, 3.5 g/dL, and 60 mL/min, respectively.
Similarly, a generalized linear model was used to examine risk factors associated with LOS among those who were hospitalized. LOS was unavailable for a small number of patients (N = 12). Baseline characteristics were similar between those with missing LOS (N = 12) and those with available LOS (N = 181) (Appendix Table A1, online only). We also conducted sensitivity analyses by assigning patients with missing LOS of 1 day or 4 (median) days of stay as well as multiple imputations using a fully conditional specification method to assess the impact of missing values on the final findings; the results were similar (Appendix Tables A2 and A3 [online only]) and complete case analysis results were presented.
All statistical tests were two‐sided, and P values < .05 were considered statistically significant. Data were analyzed using SAS 9.4 analytic software (SAS Institute, Cary, NC).
RESULTS
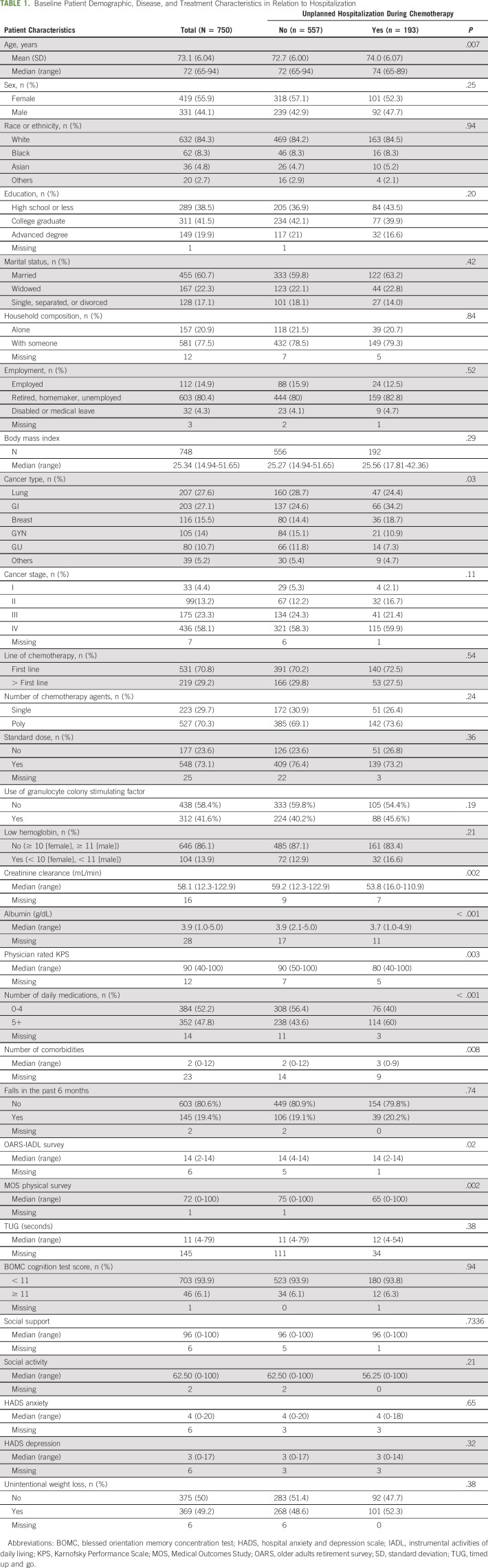
Baseline characteristics of the study population are described in Table 1. Among the 750 study participants, 193 (25.7%) experienced at least one unplanned hospitalization. Overall, their median age was 72 years (range, 65-94 years); more than half (55.9%) were female; the majority were White (84.3%) with college or higher education (61.5%), married (60.7%), and living with someone (77.5%). The most common four types of cancer were lung (27.6%), GI (27.1%), breast (15.5%), and gynecologic (14.0%), and the majority were stage IV at enrollment (58.1%). Most were receiving first-line chemotherapy (70%), were on a combination treatment (70.3%), and started with a standard dose regimen (73.1%). Their median KPS was 90 (range, 40-100), they had a median of two comorbidities (range, 0-12), and about half were taking five or more medications. About 19% of the patients reported a fall in the prior 6 months and about half had unintentional weight loss (49.2%). Few screened positive for cognitive impairment (6.1%).
TABLE 1.
Baseline Patient Demographic, Disease, and Treatment Characteristics in Relation to Hospitalization
Of the hospitalized patients, the majority (N = 162, 83.9%) were hospitalized once, and 31 (16.1%) experienced at least two hospitalizations during chemotherapy. Median LOS for the first incident hospitalization was 4 days (range, 1-66 days) with data available on 181 (93.8%) of the hospitalized patients. Reasons for hospitalization were available for 191 of the hospitalized patients and are reported in Appendix Table A4 (online only). The most common reasons for hospitalization were infection (51%) followed by GI symptoms (14.5%).
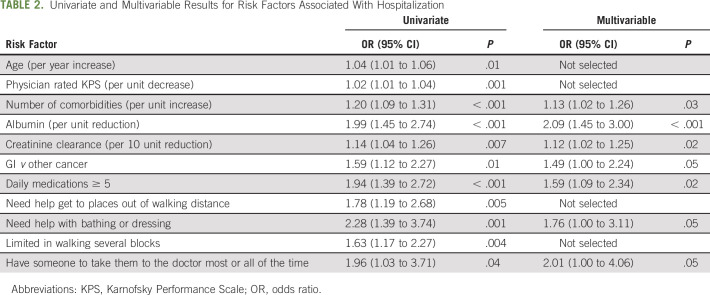
Compared to nonhospitalized patients, in univariate analyses, those who were hospitalized were older and more likely to have GI cancer, self-reported limitation in physical function (specific items were self-reported mobility limitation, difficulty walking several blocks, and requiring assistance with bathing or dressing), polypharmacy (≥ 5 daily medications), higher number of comorbid conditions, available social support (having someone to take them to the doctor most or all of the time), lower MD-rated KPS, lower creatinine clearance, and lower albumin (Table 2).
TABLE 2.
Univariate and Multivariable Results for Risk Factors Associated With Hospitalization
In multivariable analyses, the following characteristics remained independently associated with hospitalization: GI cancer type (OR, 1.5; 95% CI, 1.0 to 2.2), requiring assistance with bathing or dressing (OR, 2.1; 95% CI, 1.0 to 4.1), higher number of daily medications (OR, 1.8; 95% CI, 1.0 to 3.1 for ≥ 5 v < 5), greater number of comorbid conditions (OR, 1.1; CI, 1.0 to 1.3 per additional comorbid condition), availability of someone to take them to the doctor most or all of the time (OR, 2.0; 95% CI, 1.0 to 4.1), lower creatinine clearance per 10 mL/min reduction (OR, 1.1; 95% CI, 1.0 to 1.3), and lower albumin per 1 g/dL reduction (OR, 2.1; 95% CI, 1.5 to 3.0) (Table 2). Chronologic age was no longer significant and did not change results when forced into the model (both area under the curve = 0.68). When restricting the analysis to only those with advanced-stage disease, the effect sizes were similar (Appendix Table A5 [online only]).
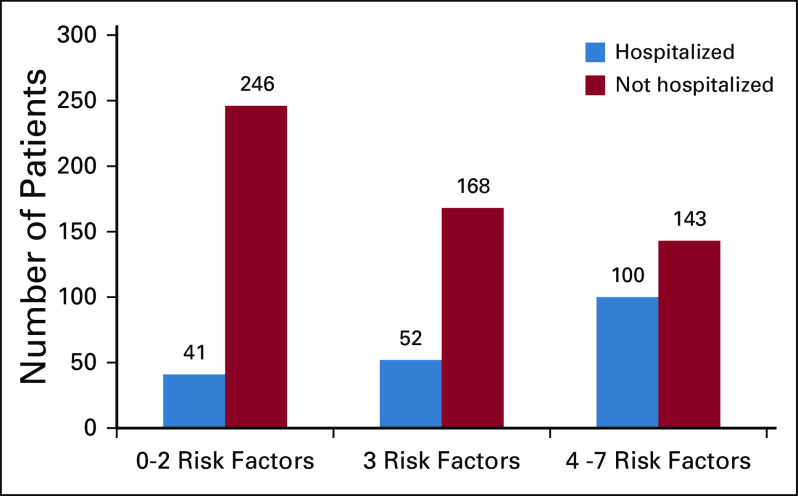
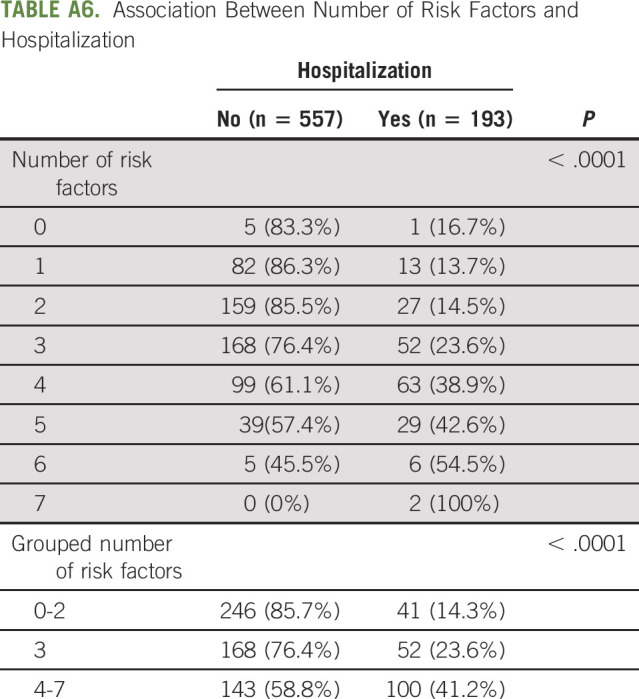
To ease interpretation, we dichotomized albumin, creatinine clearance, and number of comorbidities and created an index of number of risk factors that each patient had (0-7). The proportions of hospitalized patients among those with 0-2, 3, or 4-7 risk factors were 14.3%, 23.6%, and 41.2% (P < .001) respectively (Fig 1). The association between number of risk factors and hospitalization is detailed in Appendix Table A6 (online only).
FIG 1.
Number of older adults hospitalized during chemotherapy by presence of number of identified risk factors. Risk factors included ≥ 3 self-reported comorbid conditions, albumin < 3.5 g/dL, creatinine clearance ≤ 60 mL/min, GI cancer, polypharmacy (≥ 5 medications), requiring assistance with dressing or bathing, and having someone available to take them to the doctor most or all of the time.
In addition, we also examined risk factors associated with LOS among hospitalized patients. In univariate analyses, LOS was associated with older age, living alone, less social support, use of single-agent chemotherapy versus polychemotherapy, worse self-reported physical function, fall in the past 6 months, self-reported comorbidity (specifically, presence of liver or kidney disease), anxiety or depression, lower KPS, lower creatinine clearance, and lower albumin. In multivariable analyses, baseline characteristics independently associated with longer hospitalization were older age, self-reported presence of liver or kidney disease, living alone, and depressive symptoms (Hospital Anxiety Depression Scale depression subscale score ≥ 6). After adjusting for age, patients with liver or kidney disease had a least square mean hospitalization duration of 11.6 (95% CI, 7.3 to 15.7) days compared with 5.9 (95% CI, 4.7 to 7.0) days for those without this comorbidity (P = .01); living alone versus living with someone resulted in a nearly 3-day longer hospitalization (8.6 [95% CI, 6.1 to 11.1] v 5.5 days [95% CI, 4.2 to 6.7], P = .03); patients with depressive symptoms (Hospital Anxiety Depression Scale ≥ 6) had a nearly double LOS compared with those without depressive symptoms (9.5 [95% CI, 7.2 to 11.8] v 5.1 [95% CI, 3.8 to 6.4] days, P = .001).
DISCUSSION
Among older adults starting a new chemotherapy regimen, characteristics derived from a GA are associated with risk of hospitalization and length of hospitalization. Risk of incident hospitalization was not associated with age or performance status but rather with the type of cancer, dependence in ADL, polypharmacy, below normal creatinine clearance and albumin levels, and availability of social support. Once hospitalized, risk factors for longer LOS differed and included older age, comorbid liver or kidney disease, depressive symptoms, and living alone. These results have implications for using readily available information to identify older adults at higher risk for hospitalization and for interventions designed to decrease hospitalization risk and LOS.
Older age is a known risk factor for hospitalization among patients diagnosed with cancer.8 Receipt of chemotherapy further increases the risk of hospitalization.1 In an analysis of older adults with advanced cancer using Surveillance Epidemiology End Results or Medicare data, the majority were hospitalized during follow-up and more than half were hospitalized for likely chemotherapy toxicity.1 However, it remains challenging to identify those at highest risk for hospitalization among older adults who are planned to receive chemotherapy treatment. There is an unmet clinical need to facilitate proactive management strategies designed to avoid costly and often complicated hospitalizations. Previously published studies have shown an association between GA-derived chemotherapy toxicity prediction tools and hospitalization risk.9,18 Similarly, frailty measures derived from an accumulation of deficits are also predictive of risk of hospitalization.10,19 Our study adds to this literature by identifying specific vulnerabilities at the time of chemotherapy initiation that increase the risk of hospitalization.
Our findings highlight specific risk factors independently associated with risk of hospitalization in this cohort of older adults initiating chemotherapy. Importantly, these risk factors are readily available (ie, routine labs and medication list) and/or require minimal effort to ascertain (ie, self-reported function). First, dependence in ADLs highlights the importance of functional disability that is not captured by routine oncology performance status assessment.20 ADL impairment has previously been associated with hospital readmission risk among older adults with cancer.21 Other measures of impaired physical function (dependence on instrumental activities of daily livings and slow gait speed) have been associated with hospitalization risk among older adults diagnosed with solid tumors and those with hematologic malignancies.7,22-24 Our study suggests that asking brief questions about need for assistance with two basic ADLs (bathing and dressing) may be high yield screening questions to help identify those older adults at higher risk for hospitalization.
Second, consistent with other reports, our study highlights the importance of recognizing polypharmacy as a potentially modifiable risk factor for hospitalization.25 Polypharmacy may increase risk of hospitalization during chemotherapy because of increased risk of drug-drug interaction or as a surrogate measure for the burden of multiple chronic conditions. For example, Beinse et al26 evaluated the association between potential drug-drug interactions and risk of unplanned hospitalization among 442 older adults with cancer. In this study, the median number of daily medications was six (consistent with other reports in the literature)27 and almost 77% had a potential drug interaction recorded. Recognition of the relevance of polypharmacy can prompt intervention including medication review by a pharmacist and active consideration of deprescribing.28
Two lab-based risk factors for hospitalization were identified, lower than normal creatinine clearance and albumin. Impaired creatinine clearance has been previously identified as a risk factor for chemotherapy toxicity29-31 and was associated with hospitalization risk in a large cohort of adults receiving chemotherapy in the community setting.8 Increased risk of toxicity related to impaired clearance of chemotherapeutic agents may be contributing to the association observed and could potentially be mitigated in some patients by appropriate dosing adjustments.32 Low albumin can be a marker of poor nutritional status and is a biomarker correlated with physical frailty in older adults.33 Poor nutritional status is a risk factor for treatment toxicity among older adults with cancer and is associated with shorter survival.29,34,35 Both of these measures are routinely available to oncology providers and provide rapid opportunity to screen for those at risk for toxicity and hospitalization.
Interestingly, we had not hypothesized that availability of social support, specifically someone available to take you to the doctor, would be a risk factor for hospitalization. However, a prior publication from this cohort has described a relationship between less social support and lower nonhematologic chemotherapy toxicity.36 Our results build on that observation and may reflect an effect of engaged or proactive caregivers on healthcare utilization. Another possibility is that patients who had more vulnerabilities at start of treatment had already engaged with caregivers for assistance. This observed association between available support and hospitalization suggests a potential value to engage caregivers in interventions designed to decrease hospitalization risk.
Finally, comorbidity burden was associated with risk of hospitalization when considered as a continuous variable. Some prior studies have shown a relationship between increased comorbidity and hospitalization risk8 during receipt of chemotherapy, although not all.37,38 Comorbidity in this analysis was self-reported, and although this correlates with other comorbidity scales,39,40 it may be a less sensitive measure of compensated versus uncompensated conditions. Availability of functional measures and polypharmacy, which are correlated with comorbidity, may better reflect vulnerability in this setting. Importantly, our exploratory analysis confirms the importance of multiple concurrent vulnerabilities on risk of hospitalization with the risk increasing from 14% for those with two or fewer risk factors to 40% for those with > 3 concurrent risk factors. Identifying those at highest risk with simple screening measures can inform clinical management and provides an opportunity to develop care delivery interventions for those most likely to benefit.
It should be noted that the effect sizes for most predictors identified were modest, which supports the premise that risk of hospitalization will be better predicted by a comprehensive evaluation typical in a GA rather than focusing on one or two characteristics alone. The more risk factors patients have, the higher odds they will be hospitalized.
Our study also identified patient-specific characteristics associated with LOS among those who were hospitalized. Importantly, risk factors for longer LOS differed from those that predisposed to hospitalization. There is limited information in the literature examining factors associated with LOS among older adults with cancer receiving chemotherapy. One study investigating the relationship of functional impairment and symptom burden with clinical outcomes among hospitalized patients with advanced cancer found an association between ADL impairment and LOS.41 Our study did not show this association, although our cohort had a lower prevalence of functional impairment with all patients receiving active chemotherapy. Regarding our observed risk factors, depressive symptoms are highlighted as a potentially modifiable risk factor and warrant screening in the inpatient setting. Patients with depressive symptom may require more posthospitalization services or may be less proactively engaged in discharge planning resulting in longer LOS. Not surprisingly, once hospitalized, patients with limited social support are likely to experience a longer hospitalization potentially because of challenges related to discharge planning. Understanding both risk factors for hospitalization and those associated with longer LOS can inform cancer care delivery and supportive care interventions designed to mitigate risk and attenuate the negative consequences of unplanned hospitalization. Conduct of GA for hospitalized older patients with cancer may identify these deficits and facilitate personalized care plans.42
This study has limitations. The analysis cohort represents a heterogeneous US-based population with varied solid tumor cancer types and stages included. We do not have data on the impact of hospitalization on subsequent treatments nor on quality of life or functional independence. Findings may not reflect risk factors for hospitalization with noncytotoxic therapies such as immunotherapy or biologics. We did not evaluate timing of hospitalization during the treatment course in this analysis. We also note a high proportion of missing data for the TUG physical performance test. We observed that in many cases, the GA was performed on the date of chemotherapy initiation and participants were no longer able to perform the TUG once chemotherapy pretreatment was started. Future studies should sequence performance testing before surveys to minimize this limitation. Strengths of this study include the large sample size of older adults derived from a multisite study and detailed characterization of patients and hospitalizations. All patients were starting a new chemotherapy regimen.
In conclusion, we identify risk factors for hospitalization among older adults starting chemotherapy, which are largely readily available in routine care. A next step is to validate these risk factors in an external data set. These risk factors, if validated, can help to screen older adults and guide interventions. Interventions to decrease hospitalization could target specific GA-identified vulnerabilities such as addressing physical limitations and polypharmacy proactively as well as care delivery interventions to navigate high-risk patients and their caregivers through their treatment. Interventions designed to minimize the negative impact of hospitalizations on patients should address the unique factors associated with increased LOS.
Appendix
TABLE A1.
Comparison of Baseline Characteristics Among Hospitalized Patients With and Without Available LOS
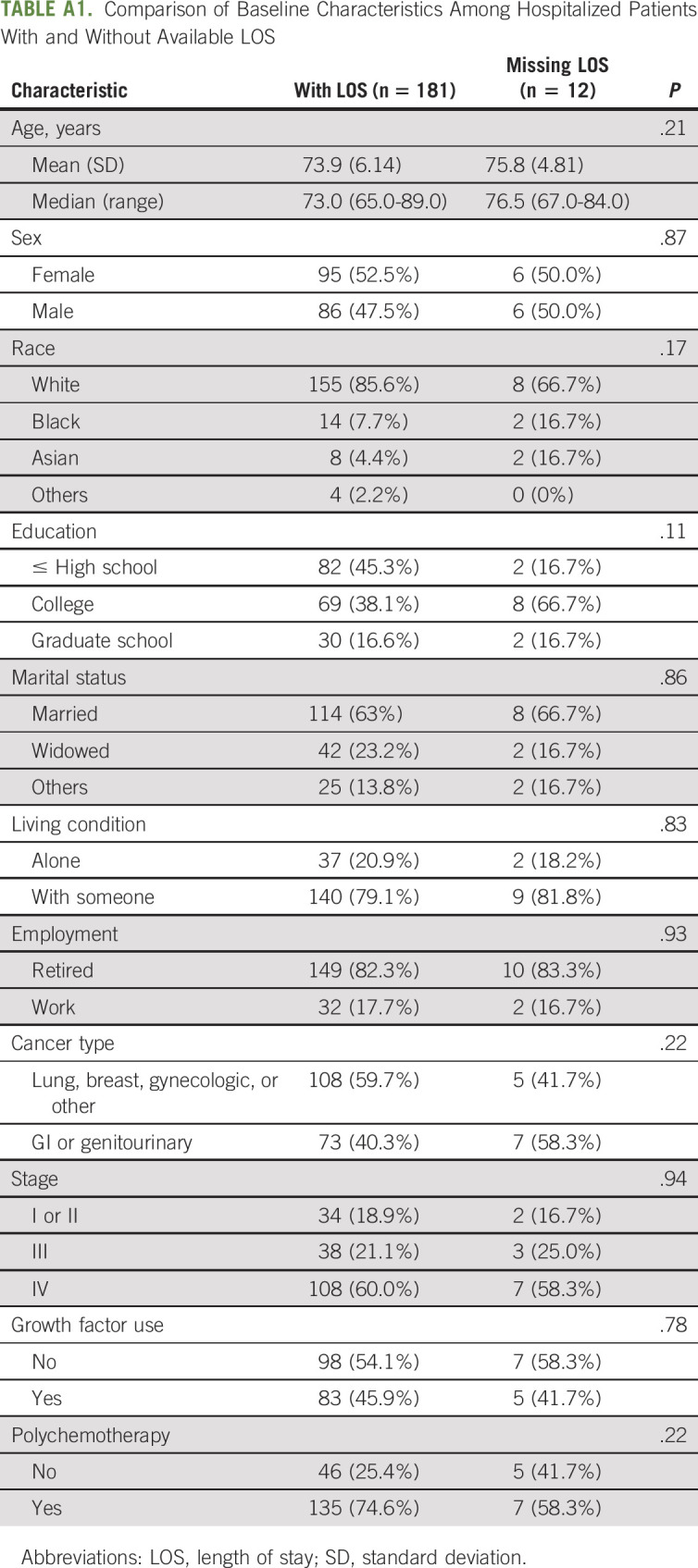
TABLE A2.
Sensitivity Analyses Results for Factors Associated With LOS
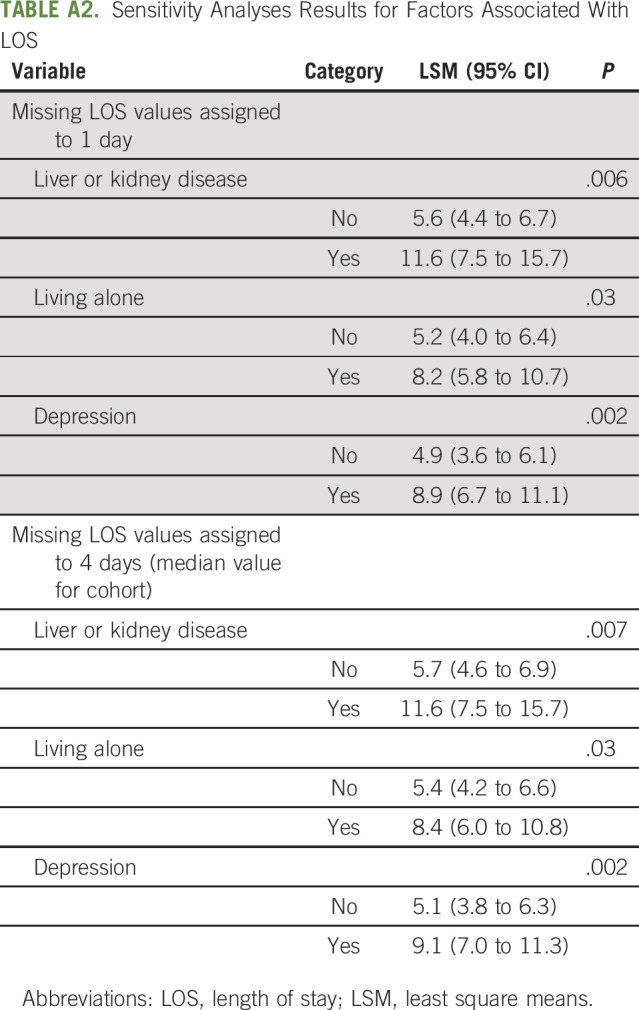
TABLE A3.
Comparison of Parameter Estimates for Factors Associated With Length of Stay Using Complete Case Versus Multiple Imputations Analysis
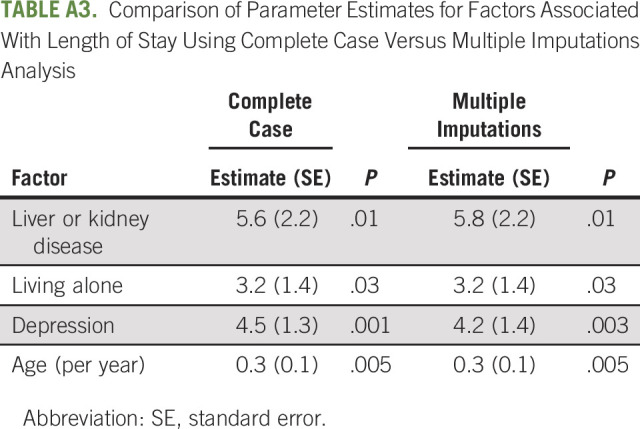
TABLE A4.
Primary Reason for Incident Hospitalization (N = 191)
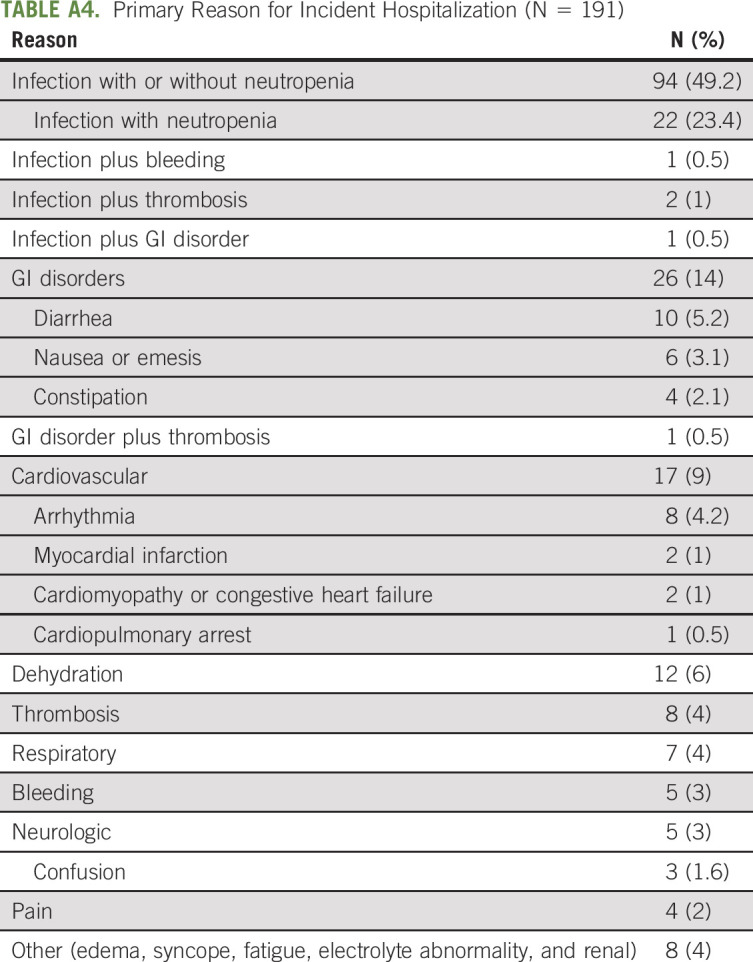
TABLE A5.
Factors Associated With Hospitalization in Multivariable Analysis Among Advanced-Stage Patients (N = 436)
TABLE A6.
Association Between Number of Risk Factors and Hospitalization
Amanda Nickles Fader
Honoraria: Ethicon, Mersana, Intuitive Surgical
Consulting or Advisory Role: Merck
Christian Meyer
Consulting or Advisory Role: Bayer
Speakers' Bureau: Novartis
Travel, Accommodations, Expenses: Plexxikon, Lilly
Other Relationship: UpToDate
Stephanie Gaillard
Consulting or Advisory Role: Immunogen, AstraZeneca, Sermonix Pharmaceuticals, Rigel
Research Funding: PharmaMar, Genentech/Roche, Iovance Biotherapeutics, Abbvie, AstraZeneca, Pfizer, Tesaro
Patents, Royalties, Other Intellectual Property: Sermonix Pharmaceuticals
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPORT
Supported by National Institute on Aging Grant No. K23AG026749-01 (Paul Beeson Career Development Award in Aging Research; A. Hurria, PI), the American Society of Clinical Oncology and Association of Specialty Professors through the Junior Development Award in Geriatric Oncology (A. Hurria, PI), and City of Hope's Center for Cancer and Aging. H.D.K. was funded by a Paul Beeson Career Development Award in Aging Research (K23AG038361; supported by National Institute on Aging, American Federation for Aging Research, John A. Hartford Foundation, and Atlantic Philanthropies), and National Institute on Aging under Award No. R33AG059206. W.D. was supported by the National Institute on Aging grant K24AG055693 (W. Dale, PI). S.M.L. was supported by National Cancer Institute Cancer Center Support Grant No. P30CA008748.
Deceased
AUTHOR CONTRIBUTIONS
Conception and design: Heidi D. Klepin, William P. Tew, Ajeet Gajra, Cary Gross, Hyman B. Muss, Harvey Jay Cohen
Financial support: William Dale
Administrative support: Vani V. Katheria, William Dale
Provision of study materials or patients: Heidi D. Klepin, William P. Tew, Supriya G. Mohile, Ajeet Gajra, Cynthia Owusu, Stuart M. Lichtman, Cary Gross, Hyman B. Muss, Andrew E. Chapman, William Dale
Collection and assembly of data: Heidi D. Klepin, Rawad Elias, William P. Tew, Supriya G. Mohile, Ajeet Gajra, Cynthia Owusu, Vani V. Katheria, Hyman B. Muss, William Dale
Data analysis and interpretation: Can-Lan Sun, David D. Smith, Rawad Elias, Kelly M. Trevino, Ashley Leak Bryant, Daneng Li, Christian Nelson, William P. Tew, Supriya G. Mohile, Ajeet Gajra, Cynthia Owusu, Stuart M. Lichtman, Hyman B. Muss, Andrew E. Chapman, Harvey Jay Cohen, William Dale
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Amanda Nickles Fader
Honoraria: Ethicon, Mersana, Intuitive Surgical
Consulting or Advisory Role: Merck
Christian Meyer
Consulting or Advisory Role: Bayer
Speakers' Bureau: Novartis
Travel, Accommodations, Expenses: Plexxikon, Lilly
Other Relationship: UpToDate
Stephanie Gaillard
Consulting or Advisory Role: Immunogen, AstraZeneca, Sermonix Pharmaceuticals, Rigel
Research Funding: PharmaMar, Genentech/Roche, Iovance Biotherapeutics, Abbvie, AstraZeneca, Pfizer, Tesaro
Patents, Royalties, Other Intellectual Property: Sermonix Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.O'Neill CB, Atoria CL, O'Reilly EM, et al. : ReCAP: Hospitalizations in older adults with advanced cancer: The role of chemotherapy. J Oncol Pract 12:151-152, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks GA, Kansagra AJ, Rao SR, et al. : A clinical prediction model to assess risk for chemotherapy-related hospitalization in patients initiating palliative chemotherapy. JAMA Oncol 1:441-447, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill TM, Allore HG, Gahbauer EA, et al. : Change in disability after hospitalization or restricted activity in older persons. JAMA 304:1919-1928, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill TM, Gahbauer EA, Han L, et al. : The role of intervening hospital admissions on trajectories of disability in the last year of life: Prospective cohort study of older people. BMJ 350:h2361, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai E, Guy GP, Dunbar A, et al. : Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract 13:e552-e561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohile SG, Dale W, Somerfield MR, et al. : Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 36:2326-2347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams GR, Dunham L, Chang Y, et al. : Geriatric assessment predicts hospitalization frequency and long-term care use in older adult cancer survivors. J Oncol Pract 15:e399-e409, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassett MJ, Rao SR, Brozovic S, et al. : Chemotherapy-related hospitalization among community cancer center patients. Oncologist 16:378-387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurria A, Togawa K, Mohile SG, et al. : Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 29:3457-3465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen HJ, Smith D, Sun CL, et al. : Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer 122:3865-3872, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurria A, Mohile S, Gajra A, et al. : Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol 34:2366-2371, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podsiadlo D, Richardson S: The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142-148, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Katzman R, Brown T, Fuld P, et al. : Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry 140:734-739, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Fillenbaum GG, Smyer MA: The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol 36:428-434, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Stewart AL, Hays RD, Ware JE, Jr: The MOS short-form general health survey. Reliability and validity in a patient population. Med Care 26:724-735, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Sherbourne CD, Stewart AL: The MOS social support survey. Soc Sci Med 32(6):705-714, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Veit C, Ware JJ: The structure of psychological distress and well-being in general populations. J Consult Clin Psychol 51:730-742, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Nishijima TF, Deal AM, Williams GR, et al. : Chemotherapy toxicity risk score for treatment decisions in older adults with advanced solid tumors. Oncologist 23:573-579, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrat E, Audureau E, Paillaud E, et al. : Four distinct health profiles in older patients with cancer: Latent class Analysis of the prospective ELCAPA cohort. J Gerontol A Biol Sci Med Sci 71:1653-1660, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolly TA, Deal AM, Nyrop KA, et al. : Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist 20:379-385, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang LY, Liu J, Flood KL, et al. : Geriatric assessment as predictors of hospital readmission in older adults with cancer. J Geriatr Oncol 6:254-261, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DuMontier C, Liu MA, Murillo A, et al. : Function, survival, and care utilization among older adults with hematologic malignancies. J Am Geriatr Soc 67:889-897, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Liu MA, DuMontier C, Murillo A, et al. : Gait speed, grip strength and clinical outcomes in older patients with hematologic malignancies. Blood 134:374-382, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aparicio T, Jouve JL, Teillet L, et al. : Geriatric factors predict chemotherapy feasibility: Ancillary results of FFCD 2001-02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J Clin Oncol 31:1464-1470, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Lu-Yao G, Nightingale G, Nikita N, et al. : Relationship between polypharmacy and inpatient hospitalization among older adults with cancer treated with intravenous chemotherapy. J Geriatr Oncol 11:579-585, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beinse G, Reitter D, Segaux L, et al. : Potential drug-drug interactions and risk of unplanned hospitalization in older patients with cancer: A survey of the prospective ELCAPA (ELderly CAncer PAtients) cohort. J Geriatr Oncol 11:586-592, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Mohamed MR, Ramsdale E, Loh KP, et al. : Associations of polypharmacy and inappropriate medications with adverse outcomes in older adults with cancer: A systematic review and meta-analysis. Oncologist 25:e94-e108, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitman A, DeGregory K, Morris A, et al. : Pharmacist-led medication assessment and deprescribing intervention for older adults with cancer and polypharmacy: A pilot study. Support Care Cancer 26:4105-4113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed M, Patrick C, Quevillon T, et al. : Prediction of hospital admissions and grade 3-4 toxicities in cancer patients 70 years old and older receiving chemotherapy. Eur J Cancer Care 28:e13144, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Peterson LL, Hurria A, Feng T, et al. : Association between renal function and chemotherapy-related toxicity in older adults with cancer. J Geriatr Oncol 8:96-101, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Extermann M, Boler I, Reich RR, et al. : Predicting the risk of chemotherapy toxicity in older patients: The chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer 118:3377-3386, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Lichtman SM, Cirrincione CT, Hurria A, et al. : Effect of pretreatment renal function on treatment and clinical outcomes in the adjuvant treatment of older women with breast cancer: Alliance A171201, an ancillary study of CALGB/CTSU 49907. J Clin Oncol 34:699-705, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mailliez A, Guilbaud A, Puisieux F, et al. : Circulating biomarkers characterizing physical frailty: CRP, hemoglobin, albumin, 25OHD and free testosterone as best biomarkers. Results of a meta-analysis. Exp Gerontol 139:111014, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Dotan E, Tew WP, Mohile SG, et al. : Associations between nutritional factors and chemotherapy toxicity in older adults with solid tumors. Cancer 126:1708-1716, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stauder R, Augschoell J, Hamaker ME, et al. : Malnutrition in older patients with hematological malignancies at initial diagnosis—association with impairments in health status, systemic inflammation and adverse outcome. Hema Sphere 4:e332, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahrokni A, Sun CL, Tew WP, et al. : The association between social support and chemotherapy-related toxicity in older patients with cancer. J Geriatr Oncol 11:274-279, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du XL, Osborne C, Goodwin JS: Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol 20:4636-4642, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KH, Lee JJ, Kim J, et al. : Association of multidimensional comorbidities with survival, toxicity, and unplanned hospitalizations in older adults with metastatic colorectal cancer treated with chemotherapy. J Geriatr Oncol 10:733-741, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bush TL, Miller SR, Golden AL, et al. : Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health 79:1554-1556, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kriegsman DM, Penninx BW, van Eijk JT, et al. : Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients' self-reports and on determinants of inaccuracy. J Clin Epidemiol 49:1407-1417, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Lage DE, El-Jawahri A, Fuh CX, et al. : Functional impairment, symptom burden, and clinical outcomes among hospitalized patients with advanced cancer. J Natl Compr Canc Netw 18:747-754, 2020 [DOI] [PubMed] [Google Scholar]
- 42.Mariano C, Williams G, Deal A, et al. : Geriatric assessment of older adults with cancer during unplanned hospitalizations: An opportunity in disguise. Oncologist 20:767-772, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]