Abstract
Background
The COVID-19 pandemic has challenged healthcare systems, at times overwhelming intensive care units (ICUs). We aimed to describe the length and rate of ICU admission, and explore the clinical variables influencing ICU use, for COVID-19 patients with known cardiovascular diseases or their risk factors (CVDRF).
Methods and Results
A post hoc analysis was performed of 693 Japanese COVID-19 patients with CVDRF enrolled in the nationwide CLAVIS-COVID registration system between January and May 2020 (mean [±SD] age 68.3±14.9 years; 35% female); 199 patients (28.7%) required ICU management. The mean (±SD) ICU length of stay (LOS) was 19.3±18.5 days, and the rate of in-hospital death and hospital LOS were significantly higher (P<0.001) and longer (P<0.001), respectively, in the ICU than non-ICU group. Logistic regression analysis revealed that clinical variables reflecting impaired general condition (e.g., high C-reactive protein, low Glasgow Coma Scale score, SpO2, albumin level), male sex, and previous use of β-blockers) were associated with ICU admission (all P<0.001). Notably, age was inversely associated with ICU admission, and this was particularly prominent among elderly patients (OR 0.97, 95% confidence interval 0.95–0.99; P=0.0018).
Conclusions
One-third of COVID patients with CVDRF required ICU care during the first phase of the pandemic in Japan. Other than anticipated clinical variables, such as hypoxia and altered mental status, age was inversely associated with the use of the ICU, warranting further investigation.
Key Words: Cardiovascular diseases, COVID-19, ICU admission, ICU length of stay, Intensive care unit (ICU)
In January 2020, the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified.1–3 The virus undergoes human-to-human transmission, resulting in clusters of fatal pneumonia caused by coronavirus disease 2019 (COVID-19). The disease is highly contagious with a certain mortality rate, and the outbreak was declared a “public health emergency of international concern” by the World Health Organization.4 The COVID-19 pandemic has affected 219 countries and territories worldwide; Japan, where the number of reported cases has increased daily, is no exception. To regulate the spread of COVID-19, Japan has taken strict infection control measures, as well as isolated and treated exposed and suspected cases according to international standards.
COVID-19 patients develop critical manifestations, such as acute respiratory distress syndrome (ARDS), septic shock, acute kidney injury, and thromboembolism.5,6 These patients require intensive care unit (ICU) admission and mechanical ventilation, extracorporeal membrane oxygenation, or renal replacement therapy, along with standard therapy;7 however, the number of critical care beds in Japan is relatively lower (7.3 beds/100,000 population) than in other countries.8,9 The number of ICU beds, associated staff, and equipment requirements are limiting but essential medical resources; therefore, understanding and predicting ICU bed demand provides crucial evidence for national medical policy and contingency planning.10,11
In this study, we investigated the current rate and length of ICU admission, the effect of ICU length of stay (LOS) on in-hospital mortality, and clinical variables that affect ICU admission.
Methods
Study Design and Selection of Participants
All participants were informed regarding their participation in the study using the opt-out approach. Written informed consent was not required under Japanese law due to the observational nature of the study, and the study was conducted in accordance with the principles of the Declaration of Helsinki. The study details were first approved by the Clinical Research Committee of Toho University Faculty of Medicine (No. M20253), and subsequently approved by the ethics committee of each participating hospital.
This was a post hoc analysis of CLAVIS-COVID, a nationwide multicenter retrospective observational study. Details regarding the study design have been published elsewhere.12 CLAVIS-COVID included consecutive patients with COVID-19 aged ≥20 years who were hospitalized in 1 of 49 hospitals across Japan between January and May 2020. COVID-19 was diagnosed using a positive nucleic acid amplification test for SARS-CoV-2.13 Detailed inclusion and exclusion criteria, as well as other study information regarding CLAVIS-COVID, are available through the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (ID: UMIN000040598).
In the present study, only patients with cardiovascular diseases or their risk factors (CVDRF) were included for analysis. Cardiovascular diseases were defined as coronary artery disease, myocardial infarction, heart failure, valvular heart disease, arrhythmia, stroke or transient ischemic attack, deep vein thrombosis, pulmonary embolism, peripheral arterial disease, aortic aneurysm, aortic dissection, cardiopulmonary arrest, heart transplantation, left ventricular assist device, cardiac implantable electronic device (artificial cardiac pacemaker, implantable cardioverter defibrillator, and cardiac resynchronization therapy), pericarditis, myocarditis, congenital heart disease, and pulmonary hypertension. Risk factors were defined as diabetes, hypertension, and dyslipidemia.
Data Collection
The main outcome of the study was to clarify the current ICU admission rates and determine the clinical variables that influence ICU admission. Secondary outcomes were to explore the associations between ICU LOS, hospital stay, and in-hospital survival rate. Baseline data, including age, sex, body mass index, smoking status, comorbidities, and laboratory tests, were collected from medical records; in the case of missing data, we specified the number of data analyzed. Hypertension was defined as a history of antihypertensive medication prescriptions, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg. Diabetes was defined as a history of antidiabetic drugs prescriptions, with HbA1c levels ≥6.5%. Dyslipidemia was defined as a history of cholesterol medication, low-density lipoprotein cholesterol ≥130 mg/dL, high-density lipoprotein cholesterol <40 mg/dL for men and <50 mg/dL for women, total cholesterol ≥200 mg/dL, and triglycerides ≥150 mg/dL.
Statistical Analysis
Statistical analyses were performed using R version 3.1.0 (https://www.r-project.org/), JMP version 14 (SAS Institute, Cary, NC, USA), and GraphPad Prism version 7.0 (GraphPad Software, La Jolla, CA, USA). The Shapiro-Wilk test was used to determine whether the data were normally distributed. Results are expressed as mean±SD for normally distributed data and as the median with interquartile range (IQR; 25th–75th percentiles) for non-normally distributed data.
The significance of differences between 2 groups was evaluated using 2-tailed Student’s t-tests; the χ2 test was used to compare categorical variables. To identify the strength and direction of the link between 2 parameters, Spearman’s rank correlation coefficient was calculated. One-way analysis of variance (ANOVA) was used to compare the means of more than 2 groups.
To analyze factors affecting ICU admission, logistic regression analysis was used to examine the association between the incidence of ICU admission and each variable. Factors theoretically related to ICU admission, such as age and sex, as well as factors with P<0.10 in univariate analysis, were simultaneously entered into a multivariate logistic regression model; we also performed analyses between age groups (<65 years old [non-elderly] vs. ≥65 years old [elderly]), as predictive factors for ICU admission may differ with age. The P value for the interaction (Pinteraction) was also calculated between elderly and non-elderly groups. For all tests, P<0.05 was considered significant.
Results
Study Population and Baseline Patient Characteristics
Of the 1,518 patients enrolled in CLAVIS-COVID, 693 (45.7%) with CVDRF were included in the present study (Figure 1). The characteristics of the 693 patients are presented in Table 1. The mean age was 68.3±14.9 years, with 275 patients <65 years of age; 35% were female. The mean body mass index was 24.3±5.1 kg/m2 (n=580; 113 patients with missing data). More than half the patients (56%) were non-smokers (never smoked). The number of patients with hypertension, dyslipidemia, and diabetes was 513 (74%), 269 (38.8%), and 266 (38.4%), respectively. Sixty-nine (10.0%) patients had coexisting hypertension, dyslipidemia, and diabetes. Ninety-three patients (13.4%) experienced cardiopulmonary arrest during hospitalization.
Figure 1.
Study population. CVD, cardiovascular diseases.
Table 1.
Patient Characteristics (n=693)
| Age (years) | 68.3±14.9 |
| Female sex | 244 (35) |
| BMI (kg/m2; n=580) | 24.3±5.1 |
| Smoking status | |
| Never | 385 (56) |
| Current smoker | 87 (13) |
| Ex smoker | 183 (26) |
| Unknown | 38 (5) |
| CVD and/or risk factors | |
| Diabetes | 266 (38.4) |
| Hypertension | 513 (74.0) |
| Dyslipidemia | 269 (38.8) |
| Coronary artery disease/MI | 85 (12.3) |
| Heart failure | 60 (8.7) |
| Valvular heart disease | 19 (2.7) |
| Arrhythmia | 74 (10.7) |
| Atrial fibrillation | 60 (8.7) |
| Ventricular tachycardia | 1 (0.1) |
| Stroke/TIA | 52 (7.5) |
| DVT/PE | 8 (1.2) |
| PAD | 5 (0.7) |
| Aortic aneurysm | 10 (1.4) |
| Aortic dissection | 6 (0.9) |
| Cardiopulmonary arrest | 3 (0.4) |
| Others | 12 (1.7) |
| Laboratory data on admission | |
| White blood cells (/μL; n=678) | 6,453±3,344 |
| Hemoglobin (g/dL; n=679) | 13.2±2.2 |
| Platelets (×104/μL; n=676) | 20.7±9.4 |
| AST (U/L; n=680) | 45.1±47.8 |
| ALT (U/L; n=679) | 35.8±43.9 |
| GGT (U/L; n=629) | 62.6±70.3 |
| LDH (U/L; n=617) | 339.7±169.6 |
| BUN (mg/dL; n=679) | 21.4±15.3 |
| Creatinine (mg/dL; n=679) | 1.2±1.6 |
| Glucose (mg/dL; n=557) | 140.6±61.3 |
| Glycohemoglobin (%; n=389) | 6.7±1.3 |
| LDL-C (mg/dL; n=208) | 97.5±48.3 |
| Triglycerides (mg/dL; n=236) | 135.2±64.9 |
| CRP (mg/dL; n=667) | 7.8± 7.6 |
Data are shown as the mean±SD or n (%). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CRP, C-reactive protein; CVD, cardiovascular disease; DVT, deep venous thrombosis; GGT, γ-glutamyl transpeptidase; LDH, lactate dehydrogenase; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PAD, peripheral arterial disease; PE, pulmonary embolism; TIA, transient ischemic attack.
ICU Rate of Admission and LOS
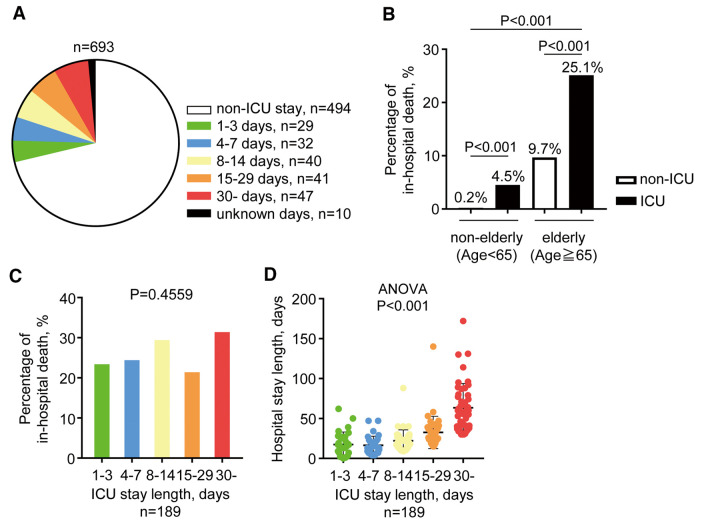
Among the 693 patients, 199 (28.7%) were treated in the ICU (Figure 2A). Ninety-four patients (47.2%) stayed in the ICU for more than 2 weeks, whereas the mean ICU LOS was 19.3±18.5 days (n=189; 10 patients with missing data). Among the 693 patients, 108 (15.6%) died while in hospital; the rate of in-hospital death was significantly higher in the ICU group (n=59; 29.6%) than non-ICU (n=49; 9.9%) group (P<0.001). When divided into 4 groups according to age and ICU admission, in-hospital death rates were significantly (P<0.001) higher for older ICU patients (Figure 2B). In both elderly and non-elderly patients, the rate of in-hospital deaths was significantly higher in the ICU group (1 [0.2%] vs. 9 [4.5%] in the non-elderly non-ICU vs. ICU groups, respectively [P<0.001]; 48 [9.7%] vs. 50 [25.1%] in the elderly non-ICU vs. ICU groups, respectively [P<0.001]; Figure 2B). The percentage of in-hospital deaths did not differ significantly among ICU groups with different LOS (ICU LOS: 24%, 25%, 30%, 22%, and 32% for ICU LOS of 1–3, 4–7, 8–14, 15–29, and 30 days, respectively; P=0.46; Figure 2C).
Figure 2.
Current status of intensive care unit (ICU) admissions and their association with in-hospital deaths. (A) Pie chart showing the proportion of ICU admissions according to length of stay (LOS). (B) Patients were divided into 4 groups according to age and ICU admission and in-hospital deaths were compared between the groups using Chi-squared tests. (C) Comparison of in-hospital deaths according to ICU LOS (Chi-squared test). (D) Association between ICU and hospital LOS (1-way ANOVA). Data are the mean±SD.
Association Between ICU and Hospital LOS
The mean hospital LOS among all patients was 24.2±22.9 days. The hospital LOS was significantly longer in the ICU than non-ICU group (34.2±22.9 vs. 20.2±17.5 days, respectively; P<0.001), whereas patients with an ICU LOS ≥30 days had a significantly longer hospital LOS than those who stayed in the ICU for <30 days (P<0.001). Furthermore, there was a significant correlation between ICU and hospital LOS (n=189; 10 patients with missing data; R=0.72; P<0.001).
Clinical Predictors of ICU Admission
Table 2 presents the results of the logistic regression analysis using data from all 693 patients. In addition to male sex (odds ratio [OR] 2.28, 95% confidence interval [CI] 1.29–4.03, P=0.0047) and the previous use of β-blockers (OR 2.55, 95% CI 1.39–4.70, P=0.0026), several clinical variables that reflect impaired general condition, such as low Glasgow Coma Scale (GCS; OR 0.90, 95% CI 0.81–1.00, P=0.04), low SpO2 (OR 0.92, 95% CI 0.88–0.97, P=0.0021), high C-reactive protein (CRP; OR 1.06, 95% CI 1.02–1.10; P=0.0036), and low albumin (OR 0.27, 95% CI 0.16–0.46, P<0.0001), were associated with ICU admission. An inverse association was found between ICU admission and age (OR 0.97, 95% CI 0.95–0.99, P=0.0018; Table 2).
Table 2.
Predictors of Intensive Care Unit Admission in All Patients
| Variables | Univariate model | Multivariable model | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 0.99 (0.98–1.01) | 0.34 | 0.97 (0.95–0.99) | 0.0018 |
| Male sex | 2.69 (1.82–3.96) | <0.0001 | 2.28 (1.29–4.03) | 0.0047 |
| BMI | 1.03 (0.99–1.06) | 0.10 | ||
| GCS | 0.82 (0.76–0.88) | <0.0001 | 0.90 (0.81–1.00) | 0.04 |
| SpO2 | 0.91 (0.87–0.94) | <0.0001 | 0.92 (0.88–0.97) | 0.0021 |
| Heart rate | 1.01 (1.00–1.02) | 0.01 | 0.99 (0.98–1.01) | 0.26 |
| SBP | 0.99 (0.99–1.00) | 0.08 | 1.01 (1.00–1.02) | 0.13 |
| Temperature | 1.45 (1.18–1.78) | 0.0004 | 1.25 (0.93–1.69) | 0.13 |
| Hypertension | 0.80 (0.55–1.15) | 0.23 | ||
| Diabetes | 1.78 (1.27–2.48) | 0.0008 | 1.13 (0.70–1.84) | 0.63 |
| β-blocker use | 2.17 (1.43–3.31) | <0.0001 | 2.55 (1.39–4.70) | 0.0026 |
| Hemoglobin | 0.94 (0.87–1.01) | 0.0935 | 1.06 (0.93–1.21) | 0.38 |
| CRP | 1.13 (1.10–1.16) | <0.0001 | 1.06 (1.02–1.10) | 0.0036 |
| eGFR | 0.99 (0.99–1.00) | 0.30 | ||
| Albumin | 0.22 (0.16–0.31) | <0.0001 | 0.27 (0.16–0.46) | <0.0001 |
BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; GCS, Glasgow Coma Scale; OR, odds ratio; SBP, systolic blood pressure.
As indicated in Table 3 after adjusting for other confounding factors predicting ICU admission, low SpO2 (OR 0.84, 95% CI 0.75–0.94, P=0.0034), the previous use of β-blockers (OR 4.69, 95% CI 1.42–15.53, P=0.0114), and low albumin (OR 0.15, 95% CI 0.06–0.38, P<0.0001) remained statistically significant in non-elderly patients. Conversely, in elderly patients, low age (OR 0.91, 95% CI 0.88–0.96, P<0.0001), male sex (OR 3.15, 95% CI 1.56–6.37, P=0.0014), low SpO2 (OR 0.95, 95% CI 0.90–1.00, P=0.034), high CRP (OR 1.07, 95% CI 1.02–1.12, P=0.0065), and low albumin (OR 0.41, 95% CI 0.22–0.80, P=0.0066) were significantly associated with ICU admission (Table 3). Different clinical variables were observed between non-elderly and elderly patients; therefore, we calculated the P values for the interactions between each factor. We found that age, SpO2, and albumin showed a significant interaction with age (Supplementary Figure).
Table 3.
Differences Between Predictors for Intensive Care Unit Admission According to Age
| Variables | Non-elderly patients (age <65 years) | Elderly patients (age ≥65 years) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate model | Multivariable model | Univariate model | Multivariable model | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.05 (1.01–1.08) | 0.018 | 1.03 (0.97–1.09) | 0.29 | 0.94 (0.91–0.97) | <0.0001 | 0.91 (0.88–0.96) | <0.0001 |
| Male sex | 2.09 (1.07–4.08) | 0.031 | 0.80 (0.28–2.31) | 0.68 | 3.18 (1.97–5.14) | <0.0001 | 3.15 (1.56–6.37) | 0.0014 |
| BMI | 1.04 (0.99–1.09) | 0.12 | 1.03 (0.98–1.09) | 0.26 | ||||
| GCS | 0.10 (0.01–0.80) | 0.03 | 0.40 (0.02–10.32) | 0.58 | 0.87 (0.81–0.95) | <0.0001 | 0.94 (0.83–1.05) | 0.25 |
| SpO2 | 0.82 (0.75–0.90) | <0.0001 | 0.84 (0.75–0.94) | 0.0034 | 0.94 (0.90–0.97) | 0.0014 | 0.95 (0.90–1.00) | 0.034 |
| Heart rate | 1.01 (1.00–1.03) | 0.093 | 1.01 (0.99–0.104) | 0.32 | 1.01 (1.00–1.02) | 0.055 | 0.99 (0.98–1.01) | 0.37 |
| SBP | 0.99 (0.98–1.00) | 0.18 | 0.99 (0.98–1.00) | 0.23 | ||||
| Temperature | 1.44 (1.02–2.01) | 0.036 | 1.17 (0.64–2.12) | 0.61 | 0.94 (0.90–0.97) | 0.0014 | 1.36 (0.94–1.96) | 0.1 |
| Hypertension | 1.10 (0.63–1.94) | 0.73 | 0.60 (0.37–0.99) | 0.04 | 0.84 (0.41–1.73) | 0.63 | ||
| Diabetes | 2.61 (1.52–4.47) | 0.0005 | 0.71 (0.29–1.78) | 0.47 | 1.39 (0.90–2.14) | 0.04 | 0.97 (0.51–1.85) | 0.94 |
| β-blocker use | 3.25 (1.57–6.71) | 0.0014 | 4.69 (1.42–15.53) | 0.0114 | 1.77 (1.06–2.98) | 0.03 | 2.13 (0.99–4.56) | 0.053 |
| Hemoglobin | 0.76 (0.66–0.88) | 0.00024 | 1.19 (0.92–1.56) | 0.19 | 1.03 (0.93–1.13) | 0.58 | ||
| CRP | 1.15 (1.10–1.20) | <0.0001 | 1.02 (0.96–1.09) | 0.55 | 1.11 (1.07–1.15) | <0.0001 | 1.07 (1.02–1.12) | 0.0065 |
| eGFR | 1.00 (0.99–1.01) | 0.86 | 1.00 (1.00–1.00) | 0.16 | ||||
| Albumin | 0.08 (0.04–0.15) | <0.0001 | 0.15 (0.06–0.38) | <0.0001 | 0.33 (0.22–0.50) | <0.0001 | 0.41 (0.22–0.80) | 0.0066 |
Abbreviations as in Table 2.
Discussion
ICU beds are in special departments that are typically reserved for the most critically ill patients, representing the highest standard level of care available. Some patients with COVID-19 may develop symptoms that require intensive care; thus, as the occupancy of ICU beds and lack of empty ICU beds are directly linked to medical collapse, analyzing the determinants of ICU admission is crucial.
To summarize the main findings of this study, of the COVID-19 patients with CVDRF, 28.7% needed ICU care. The mean ICU LOS was 19.3±18.5 days, and 29.6% of patients died. Finally, male sex, as well as low GCS score, low SpO2, the previous use of β-blockers, high CRP, low albumin, and low age influenced ICU admission; age was found to be significantly prominent in the elderly patient group.
A recent systematic review reported that the median ICU LOS of patients with COVID-19 was 8 days (IQR 5–13 days) within China and 7 days (IQR 4–11 days) outside of China,10 which is shorter than the ICU LOS in the present study (median 13 days; IQR 6–28 days). Considering that our study population included only COVID-19 patients with CVDRF, these patients tended to be in a more severe condition, thus requiring longer care in the ICU. Conversely, the overall in-hospital mortality rate estimated in the present study (15.6%) was less than that reported in previous studies from the US (~20%);14,15 however, we observed that COVID-19 patients with CVDRF who needed ICU care had an approximate 1.5-fold higher risk of in-hospital death than non-ICU patients. Unexpectedly, the in-hospital mortality rate did not worsen with increasing ICU LOS. This may reflect a variety of systemic manifestations of COVID-19, such as pneumonia, ARDS, hypotension, and cardiac, renal, gastrointestinal, and hepatic injury, which differ widely because the expression levels of the ACE2 receptor (the entry receptor for SARS-CoV-2) in each tissue differ among patients.16 Considering that patients who need ICU care have a significantly higher risk of in-hospital death compared with non-ICU patients, management to prevent ICU admission is important to save patients’ lives, and understanding the factors that determine ICU admission is important to prevent patients from being admitted to the ICU.
Unexpectedly, our results showed that low age is a predictive factor for ICU admission; however, this only applied to patients aged ≥65 years when considering the P value for interaction. This may be because older patients chose to withdraw from highly invasive treatment in the ICU at their own will. In addition, it is speculated that social factors, such as religion, family composition, or income, may be involved in this “age paradox”; however, we cannot provide a clear explanation and this aspect should be further evaluated in future studies.
We also found that low albumin and SpO2 levels were independent predictors for ICU admission in all patients. It is well known that albumin is a marker of inflammation and is not solely dependent on nutritional status, and our findings are consistent with those of previous studies demonstrating that inflammatory markers strongly predict patients at risk of respiratory deterioration,17 or that low albumin levels at admission are predictive markers of more severe disease outcomes.18 Interestingly, both factors showed a significant interaction with age, indicating that albumin and SpO2 levels have a strong effect on ICU admission in patients aged <65 compared with ≥65 years.
In summary, assessing these clinical parameters could be helpful for risk stratification and treatment strategies in patients with COVID-19. Further studies are warranted to confirm these results and determine the cut-off value to predict ICU admission for COVID-19 patients with CVDRF.
Conclusions
Our results indicate that approximately one-third of COVID-19 patients with CVDRF require ICU care, whereas approximately one-third of these patients die in hospital. Furthermore, impaired general condition, such as low GSC score, low SpO2, high CRP, and low albumin, along with low age, male sex, and the previous use of β-blockers, were associated with ICU admission. Overcoming COVID-19 infections is an ongoing challenge. We therefore believe that the present study contributes greatly to the treatment of patients with COVID-19, as well as to important decision making designed to prevent collapse of the medical system.
Sources of Funding
This study was supported by the Japanese Circulation Society.
Conflict of Interest
Y.M. is affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi, and has received honoraria from Otsuka Pharmaceutical Co. and Novartis Japan, as well as research funding from Otsuka Pharmaceutical Co. and Pfizer Inc. T. Yonetsu is affiliated with departments endowed by Abbott Vascular Japan, Boston Scientific Japan, Japan Lifeline, WIN International, and Takeyama KK. S. Kohsaka has received unrestricted research grants from the Department of Cardiology, Keio University School of Medicine, Daiichi Sankyo Co., Ltd, and Bristol-Meyers Squibb, as well as lecture fees from AstraZeneca and Bristol-Meyers Squibb.
Disclosures
S. Kohsaka, K.N. are members of Circulation Reports’ Editorial Team.
IRB Information
The study details were first approved by the Clinical Research Committee of Toho University Faculty of Medicine (No. M20253) and subsequently approved by the ethics committee of each participating hospital.
Supplementary Files
Supplementary Figure.
Acknowledgments
The authors acknowledge all the investigators who participated in CLAVIS-COVID, as well as the Japanese Circulation Society.
Data Availability
The deidentified participant data will not be shared.
References
- 1. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al.. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020; 63: 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al.. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis 2020; 20: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 4. Eurosurveillance Editorial Team.. Note from the editors: Novel coronavirus (2019-nCoV). Euro Surveill 2020; 25: 2001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al.. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacLaren G, Fisher D, Brodie D.. Preparing for the most critically ill patients with COVID-19: The potential role of extracorporeal membrane oxygenation. JAMA 2020; 323: 1245–1246. [DOI] [PubMed] [Google Scholar]
- 8. Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EA, et al.. Variation in critical care services across North America and Western Europe. Crit Care Med 2008; 36: 2787–2793. [DOI] [PubMed] [Google Scholar]
- 9. Phua J, Faruq MO, Kulkarni AP, Redjeki IS, Detleuxay K, Mendsaikhan N, et al.. Critical care bed capacity in Asian Countries and regions. Crit Care Med 2020; 48: 654–662. [DOI] [PubMed] [Google Scholar]
- 10. Rees EM, Nightingale ES, Jafari Y, Waterlow NR, Clifford S, Pearson CAB, et al.. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Medicine 2020; 18: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenbaum L.. Facing Covid-19 in Italy: Ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med 2020; 382: 1873–1875. [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto S, Kuroda S, Sano T, Kitai T, Yonetsu T, Kohsaka S, et al.. Clinical and biomarker profiles and prognosis of elderly patients with coronavirus disease 2019 (COVID-19) with cardiovascular diseases and/or risk factors. Circ J 2021; 85: 921–928. [DOI] [PubMed] [Google Scholar]
- 13. Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z.. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol 2021; 19: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S.. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open 2020; 3: e2029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al.. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al.. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26: 1017–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, et al.. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146: 128–136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bedock D, Bel Lassen P, Mathian A, Moreau P, Couffignal J, Ciangura C, et al.. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN 2020; 40: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure.
Data Availability Statement
The deidentified participant data will not be shared.