Abstract
Coexisting species may experience population and range changes alone or jointly in response to environmental change. Here, we used six climate variables and ten modeling algorithms to predict the distribution of two Takydromus species (T. septentrionalis and T. sexlineatus) in China. We identified the sympatric and allopatric areas by comparing projections between the two species based on habitat suitability under present and future climate scenarios. We constructed the hypervolumes of six climate variables for the two species and then evaluated overlaps between hypervolumes. From this study, we know the following. First, minimum temperature of coldest month contributes the most to the prediction of habitat suitability. Second, habitats suitable for the two species will shift northward in response to climate warming. Third, the range of T. sexlineatus will expand across the four future time intervals before 2,100, namely the 2021–2040, 2041–2060, 2061–2080, and 2081–2100 intervals, under both Shared socioeconomic pathway (SSP) 245 and SSP585 scenarios, and the range of T. septentrionalis will also expand in the future except at the 2081–2100 interval under the SSP585 scenario. Fourth, the sympatric areas will contract or expand under the SSP245 scenario and expand across the four future time intervals before 2,100 under the SSP585 scenario. Fifth, the niche hypervolumes of the two species partially overlapped, and the differences in niche centroid show some degree of niche differentiation between the two species. These results allow to conclude that climate warming will not only drive the northward drift of sympatric areas but also increase the size of these areas if nothing is done to limit the emission of greenhouse gases. Given the existence of hybridization and introgression between T. septentrionalis and T. sexlineatus in the field where they coexist, we also conclude that climate warming will increase chances of hybridization and introgression between the two species.
Keywords: allopatry, climatic niche, hybridization and introgression, species distribution models, sympatry, Takydromus lizards
Climate warming will not only drive the northward drift of sympatric areas but also increase the size of these areas if nothing is done to limit the emission of greenhouse gases in the future. Given the existence of hybridization and introgression between two Takydromus lizards in the field where they coexist, climate warming will increase hybridization and introgression between the two species.
1. INTRODUCTION
Speciation is the origin of reproductive isolation and divergence between populations (Mayr, 1963). Isolation by physical and/or ecological barriers is an important driver in allopatric processes that may lead to population differentiation and, ultimately, speciation (Volis et al., 2019). Other processes such as hybridization and introgression between involved species in the secondary contact zones may also have important evolutionary consequences for speciation, especially at the early stages of speciation when reproductive barriers may be weak (Bertrand et al., 2017; Cortés‐Ortiz et al., 2019; Coyner et al., 2015; Sardell & Uy, 2016; Souissi et al., 2018). There has been evidence that gene flow re‐established by hybridization and introgression may lead to genetic re‐admixture between closely related species living in sympatry (Mapel et al., 2021; Martin et al., 2015; Seehausen, 2006). Environmental change as one of the most serious challenging concerns poses multiple threats to organisms, results in shifts in species distribution, and alters interspecific interactions by breaking the physical (geographical) barriers of reproductive isolation and thus the chances of hybridization and introgression (Bellard et al., 2012; Mammola et al., 2019; Parmesan, 2006; Zhang, Mammola, et al., 2020).
Coexisting species may experience population (expansions or declines) and range (expansions or contractions) changes alone or jointly in response to environmental change. Climate warming is one hypothesis often proposed to explain these changes (Liu, White, et al., 2011; Liu, Guo, et al., 2011; Sinervo et al., 2010; Zhang, Capinha, et al., 2020). The earth's temperature has increased by ~0.7°C over the past century, which may substantially alter the entire ecosystem and constitute a serious threat to global biodiversity (McCarthy et al., 2010). In lizards, for example, such a warming trend will lead 39% of global populations and 20% of global species to go extinct by 2080, under the premise of a limited potential for plastic and/or genetic adjustments in response to a warming world (Sinervo et al., 2010). Therefore, it is of great importance to evaluate whether and how climate warming alters the distribution of habitats suitable for coexisting species.
Species distribution models (SDMs) are a powerful tool for delineating habitat suitability in geographic space by statistically exploring the relationships between species occurrence records and environmental variables (Elith & Leathwick, 2009; Guisan & Thuiller, 2005; Guisan et al., 2017; Guisan & Zimmermann, 2000; Phillips et al., 2006). SDMs have been widely used to examine the effects of environmental change in species distribution and their interactions with other species (Austin & Van Niel, 2011; Guisan et al., 2013; Vaclavik & Meentemeyer, 2012), with consensus methods found to be able to cope with prediction variability by combining an ensemble of predictions from different modeling methods (Araújo & New, 2007). The ensemble SDMs simultaneously use several algorithms to predict the present and future maps of habitat suitability, thus avoiding the limitation of occurrence records in data analysis and, compared with single algorithms, improving the accuracy of model predictions (Araújo & New, 2007). Moreover, the projection of ensemble SDMs will help to find the previously undetected distribution of species. Using ensemble SDMs, one may not only determine sympatric and allopatric areas of coexisting species in different periods but also better explain discordance of genome or gene introgression between species in secondary contact zones (Zhang, Capinha, et al., 2020).
Asian grass lizards of the genus Takydromus (Lacertidae) are widely distributed in East and Southeast Asia, southward to northeastern India, Borneo, Sumatra, Bangka, and Java, and northward to the Russian Far East (Arnold, 1997; Arnold et al., 2007). Of the 24 currently recognized species, 15 occur in China, eight only in mainland China, six only in Taiwan, and one (T. kuehnei) on both sides of the Taiwan Strait (Wang et al., 2019). Widespread sympatry between different pairs of Takydromus species occurs in several areas of mainland China, such as T. septentrionalis and T. sexlineatus in southeastern China, T. intermedius and T. septentrionalis in southwestern China, T. kuehnei and T. sexlineatus in southern China, T. septentrionalis and T. wolteri in eastern China, and T. amurensis and T. wolteri in northeastern China (Liu, 1999). Over the past decade, four new Takydromus species have been described in China, two [T. albomaculosus (Wang et al., 2017) and T. yunkaiensis (Wang et al., 2019)] in mainland China, and two (T. luyeanus and T. viridipunctatus; Lue & Lin, 2008) in Taiwan. Interestingly, all these newly described species have a narrow range, inferring that processes other than isolation by physical or ecological barriers might lead to speciation. Here, we use an ensemble modeling approach to predict the contemporary and future sympatric and allopatric areas of T. septentrionalis and T. sexlineatus in the context of global climate warming. More specifically, we aim to (1) identify climate variables that have a key role in determining the distribution of the two species, (2) predict spatiotemporal variation in sympatric and allopatric areas of the two species, and (3) explore the influence of climate warming on hybridization and introgression between the two species. Given the existence of hybridization and introgression between these two Takydromus species in the field where they coexist (Guo, 2019), we predict that these processes of gene exchange will be affected by climate warming if it alters the size of the sympatric areas.
2. MATERIALS AND METHODS
2.1. Study species
The northern grass lizard (T. septentrionalis) and the southern grass lizard (T. sexlineatus) both are small, oviparous, and heliothermic lacertid lizards, with the former endemic to China and more northerly distributed than the latter that is widely distributed in southern China, India (Assam) through Burma and Thailand to Vietnam, south to Sumatra, Java, and Borneo in Indonesia (Liu, 1999). Takydromus septentrionalis is a typical temperate lizard with a distributional range that has a temperate and monsoonal climate with four distinct seasons; T. sexlineatus is basically a warm‐climate lizard with a distributional range that has a subtropical and tropical monsoon climate (Xu & Ji, 2000; Zhang & Ji, 2004). Sympatry between the two species occurs in southeastern China, where they coexist not because they differ so much in the niche axes of diet, space, and time but because they use slightly different microhabitats (Liu, 1999). Female T. septentrionalis can lay up to 9 clutches with 1–5 eggs each per breeding season from April to August (Ji et al., 2007; Luo et al., 2010); female T. sexlineatus can lay up to 5 clutches with 1–4 eggs each per breeding season also from April to August (Xu & Ji, 2000; Zhang & Ji, 2004). Evidence from T. septentrionalis has shown that males are the less choosy sex and can mate at extreme phylogenetic distances, either with conspecific females from distant populations, or even with heterospecific but congeneric females (Guo et al., 2020). Actually, bidirectional heterospecific matings (male T. septentrionalis × female T. sexlineatus, or the opposite direction) happen not frequently but often enough in the field and laboratory (Figure 1).
FIGURE 1.
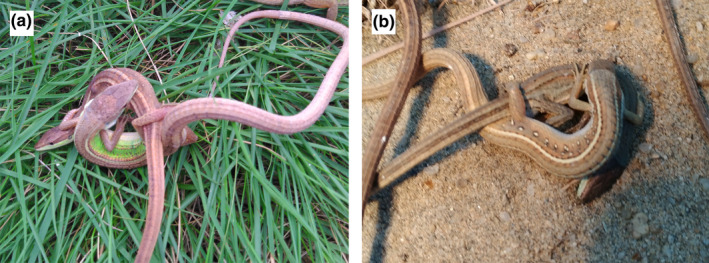
Heterospecific heterosexual mating partners between a male northern grass lizard and a female southern grass lizard (a) and between a female northern grass lizard and a male southern grass lizard (b)
2.2. Species occurrence data and climatic variables
Our study area covers the entire range of the two species in southeastern and central China (~17–38°N, ~97–125°E), including localities occupied only by T. septentrionalis, only by T. sexlineatus, and by both species (Appendix S1). Occurrence records were from our own field investigation, published papers (Du et al., 2005; Xu & Ji, 2000; Yang et al., 2008) and scientific books (Liu, 1999; Zhao & Adler, 1993), and online datasets such as Global Biodiversity Information Facility (http://www.gbif.org), HerpNet/VertNet (http://www.vertnet.org), and National Specimen Information Infrastructure (http://www.nsii.org.cn). We only used one record in a high‐resolution (2.5 × 2.5 arcmin) grid cell, thereby avoiding overrepresentation of climate data in intensively sampled areas. Of the 156 occurrence records, 127 were from T. septentrionalis and 29 from T. sexlineatus (Appendix S1).
As has been reported for other taxa, a number of environmental variables can be used to predict the distribution of reptiles (Franklin et al., 2009; Lin & Wiens, 2017; Falaschi et al., 2019; Lin, Chen et al., 2019). Here, we used six climate variables to predict the distribution of the two Takydromus lizards, including Bio3 (isothermality), Bio5 (maximum temperature of warmest month), Bio6 (minimum temperature of coldest month), Bio15 (precipitation seasonality), Bio17 (precipitation of driest quarter), and Bio18 (precipitation of warmest quarter). We used these six variables for two reasons. First, they are all nonredundant variables with an absolute value of Pearson's correlation coefficient <0.70 (Dormann et al., 2013); all other unused climate variables had an absolute value of Pearson's correlation coefficient ≥0.70 (Figure S1). Second, they have a comparatively more direct role in influencing the life history of lizards (Du et al., 2005; Gao et al., 2014; Lu et al., 2014; Ma et al., 2019). We extracted climate variables for each occurrence point at a spatial resolution of 2.5 × 2.5 arcmin from the WorldClim database (http://www.worldclim.org), using data from 1970 to 2000 as a baseline for the current period. We used the same way to extract climate variables for four future time intervals, namely the 2021–2040, 2041–2060, 2061–2080, and 2081–2100 intervals. The future climate projections were derived from six global circulation models (GCMs): BBC‐CSM2‐MR, CanESM5, CNRM‐CM6‐1, CNRM‐ESM2‐1, MIROC6, and MIROC‐ES2L. Two shared socioeconomic pathways (SSPs), SSP245 (an average scenario) and SSP585 (a pessimistic scenario), were selected to drive climate models for Coupled Model Intercomparison Project Phase 6 (CMIP6). To reduce uncertainties among different GCMs, we averaged projections of the six GCMs as future climates to predict future habitats suitable for the two Takydromus lizards.
2.3. SDM development for habitat suitability
We used 10 modeling algorithms (see Table 1 for names and abbreviations) suitable for species distribution prediction to correlate the distribution of the studying species with the associated climatic data by the biomod2 (Thuiller et al., 2019) package in R 3.5.1. For algorithms requiring species absence records, we generated 10,000 pseudo‐absence records for the species by randomly selecting these pseudo‐absence records from across the study area (Barbet‐Massin et al., 2012; Elith & Leathwick, 2009; Guisan et al., 2017; Thuiller et al., 2019). All modeling procedures were performed under default settings of the biomod2 package (Thuiller et al., 2019).
TABLE 1.
Predictive performance based on the TSS and AUC values of ten modeling algorithms used in this study
| Algorithms | Takydromus septentrionalis | Takydromus sexlineatus | ||
|---|---|---|---|---|
| TSS | AUC | TSS | AUC | |
| Artificial neural network, ANN | 0.716 ± 0.010 | 0.885 ± 0.006 | 0.827 ± 0.032 | 0.922 ± 0.013 |
| Classification tree analysis, CTA | 0.714 ± 0.026 | 0.864 ± 0.012 | 0.851 ± 0.018 | 0.926 ± 0.009 |
| Flexible discriminant analysis, FDA | 0.725 ± 0.018 | 0.893 ± 0.008 | 0.916 ± 0.007 | 0.959 ± 0.004 |
| Generalized additive model, GAM | 0.770 ± 0.017 | 0.900 ± 0.007 | 0.582 ± 0.058 | 0.788 ± 0.030 |
| Generalized boosting model, GBM* | 0.797 ± 0.015 | 0.921 ± 0.007 | 0.822 ± 0.033 | 0.920 ± 0.031 |
| Generalized linear model, GLM* | 0.781 ± 0.015 | 0.915 ± 0.004 | 0.860 ± 0.017 | 0.931 ± 0.005 |
| Maximum entropy, MaxEnt | 0.746 ± 0.020 | 0.894 ± 0.011 | 0.894 ± 0.010 | 0.954 ± 0.003 |
| Multiple adaptive regression splines, MARS | 0.790 ± 0.012 | 0.912 ± 0.005 | NA | NA |
| Random forest, RF | 0.715 ± 0.010 | 0.908 ± 0.004 | 0.840 ± 0.022 | 0.926 ± 0.013 |
| Surface range envelop, SRE | 0.600 ± 0.036 | 0.800 ± 0.018 | 0.584 ± 0.059 | 0.792 ± 0.030 |
Algorithms GBM and GLM marked with asterisks (*) had good predictive performance and were therefore selected to construct the ensemble model. NA (missing data) indicates algorithms that failed to converge. Data are expressed as mean ± standard error.
We performed a fivefold cross‐validation approach with 10 repetitions to evaluate the predictive performance of each algorithm. We randomly divided the occurrence records into five equal groups (four for constructing prediction models and one for testing the prediction models) and repeated this operation 10 times (Thuiller et al., 2019). The model accuracy in each algorithm was assessed using the true skill statistics (TSS; Allouche et al., 2006) and the area under the receiver operating characteristic curve (AUC; Liu, White, et al., 2011; Liu, Guo, et al., 2011). The algorithms with a mean TSS < 0.75 (Nüchel et al., 2018) or a mean AUC < 0.90 (Araújo et al., 2005) were discarded in subsequent analyses. We used the same algorithms to develop the ensemble SDMs for the two Takydromus lizards and compare their habitat suitability under identical conditions (Grenouillet et al., 2011).
We used a randomization methodology in the selected algorithms to assess the relative importance of climate variables used to predict species habitat suitability (Stanton et al., 2012), and then calculated the response curve of the most important variable affecting the polarization distribution of the Takydromus lizards. Both occurrence and pseudo‐absence data were used to predict habitat suitability under the present and future climatic conditions. We got the final result with maps to show habitats suitable for T. septentrionalis and T. sexlineatus by the committee averaging of predictions of the selected model algorithms, and then converted the model outcomes into binary (suitable or unsuitable) maps by a threshold of the maximizing TSS (Liu et al., 2013). We identified the sympatric and allopatric areas by comparing projections between T. septentrionalis and T. sexlineatus based on their habitat suitability under present and future climate change scenarios. Habitats in sympatric areas are suitable for both species, and habitats in allopatric areas are suitable only for one species.
2.4. Comparison of exploited climatic niche
To compare the exploited climatic niche between the two Takydromus lizards (i.e., specialization or generalization), we used the hypervolume package in R 3.5.1 to estimate the climatic niche of each species using a stochastic geometry approach (Blonder et al., 2014, 2018). We constructed the hypervolumes of six climate variables for the two species, which are the definitiveness of habitat suitability and have been used in SDMs. In addition, hypervolumes were randomly resampled to yield 3,000 points across all datasets for each species, thereby adjusting for the influence of sample size on hypervolumes and overlaps. After hypervolume construction, we calculated the Euclidean distance, the Sørensen and Jaccard similarity index, and the unique volume fraction to evaluate overlaps between hypervolumes (Helsen et al., 2020).
3. RESULTS
3.1. Model performance
TSS and AUC values for the 10 modeling algorithms showed high levels of predictive performance in both species; mean TSS values varied from 0.600 to 0.797 in T. septentrionalis and from 0.582 to 0.916 in T. sexlineatus, and mean AUC values varied from 0.800 to 0.921 in T. septentrionalis and from 0.788 to 0.959 in T. sexlineatus (Table 1). Comparatively, GBM and GLM performed better than the other eight algorithms in constructing the ensemble modeling for the two species.
3.2. Variable contribution and response curves
Among the six climate variables, Bio6 contributed the most to the prediction of habitat suitability in both species, with its relative contribution accounting for approximately 89% in T. septentrionalis and 63% in T. sexlineatus (Figure 2). The response curves of the predicted occurrence probability against Bio6 varied with algorithms and species in the projections. Takydromus septentrionalis was predicted to have a high probability of occurrence in regions with Bio6 varying from −1.0 to 6.2°C, and T. sexlineatus in regions with Bio6 greater than 3.9°C (Figure 3).
FIGURE 2.
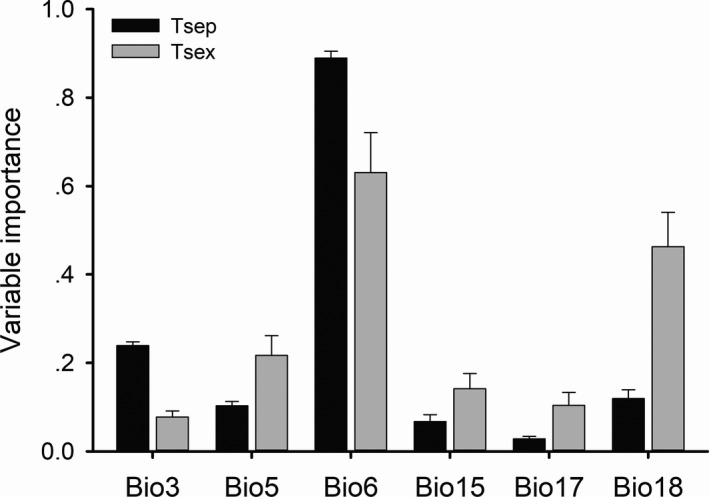
The relative contributions of the six climatic variables used in this study to the species distribution models for T. septentrionalis (Tsep) and T. sexlineatus (Tsex). Data are expressed as mean ± standard error
FIGURE 3.
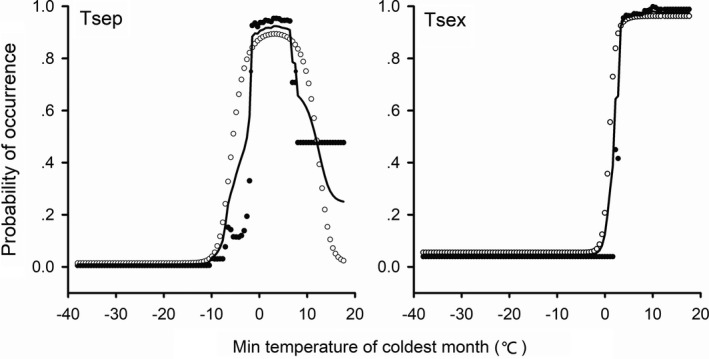
Response curves of the predicted occurrence probability of T. septentrionalis (Tsep) and T. sexlineatus (Tsex) against Bio6 (minimum temperature of coldest month, °C). Solid circles depict response curves of the average predicted occurrence probability in GBM approached with 10 repetitions, hollow circles depict response curves of the average predicted occurrence probability in GLM approached with 10 repetitions, and lines depict response curves of the average predicted occurrence probability in two algorithms
3.3. Present and future projections of habitat suitability
From present SDM projections (Figure 4), we know the following. First, habitats suitable for T. septentrionalis are mainly located in the central and southeastern provinces (Anhui, Fujian, Guangdong, Guangxi, Guizhou, Henan, Hubei, Hunan, Jiangxi, Jiangsu, Shaanxi, Sichuan, Shandong, Yunnan, and Zhejiang) or province‐level municipalities (Chongqing and Shanghai) of China. Second, habitats suitable for T. sexlineatus are mainly located in the southern provinces (Fujian, Guangdong, Guangxi, Guizhou, Hainan, and Yunnan) of China. Third, sympatry between the two species occurs in Fujian, Guangxi, Guangdong, Guizhou, Hunan, and Jiangxi provinces (Figure 4).
FIGURE 4.
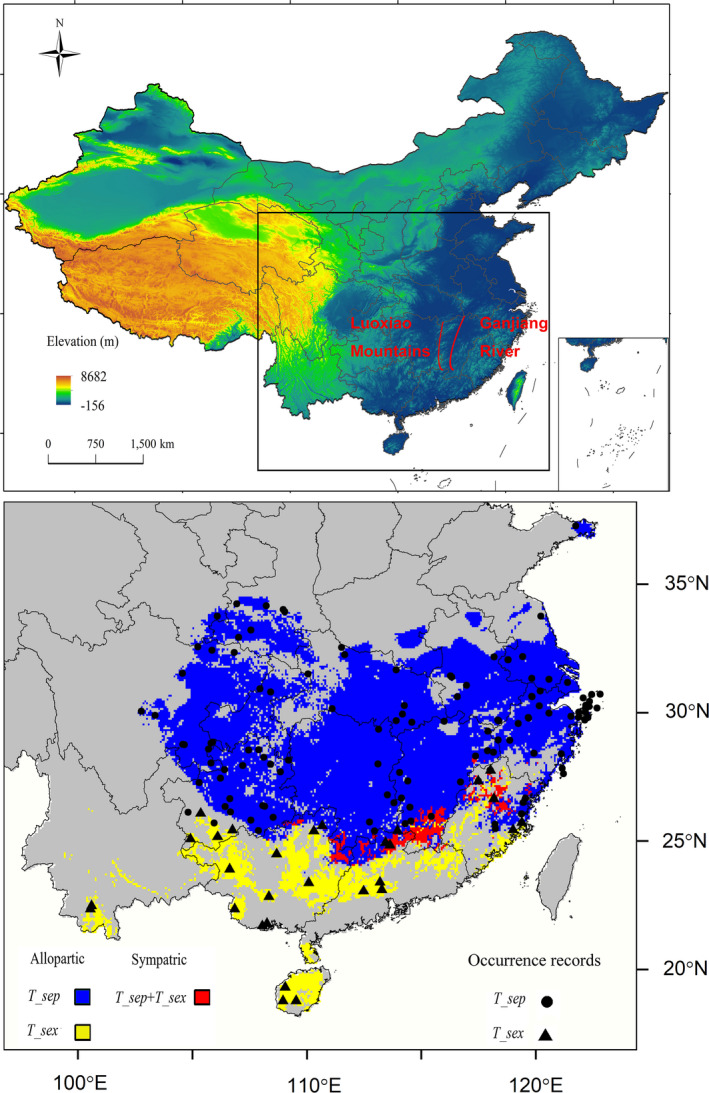
The predicted current sympatric and allopatric areas of T. septentrionalis (Tsep) and T. sexlineatus (Tsex). The available occurrence records of Tsep (black dots) and Tsex (black triangles) were used to build the species distribution models for the two species. The maps show the areas sampled in context with surrounding landmasses
Habitats suitable for the two Takydromus species will shift in the future in response to climate warming, with changes in range size varying with the SSPs using averages of six GCMs as future climatic conditions (Figure 5). The range of T. sexlineatus will expand across the four future time intervals before 2,100 under both SSP245 and SSP585 scenarios (Table 2). The range of T. septentrionalis will also expand in the future except at the 2081–2100 interval under the SSP585 scenario (Table 2). Habitats suitable for the two species will both shift northward in the future, although they respond differently to future climates. Habitats suitable for T. septentrionalis will fade away in Shandong province and decline to some extent in the middle of the species’ range, whereas habitats suitable for T. sexlineatus will expand in Guangxi, Guizhou, and Yunnan provinces (Figure 5). The sympatric areas of the two species will either contract or expand in the future under the SSP245 scenario and expand across the four future time intervals before 2,100 under the SSP585 scenario (Table 2); all of these sympatric areas will be still in Fujian, Guangdong, Guangxi, and Guizhou provinces (Figure 5). More specifically, the sympatric areas will contract at the 2021–2041, 2041–2060, and 2081–2100 intervals but expand at the 2061–2080 interval under the SSP245 scenario, and expand across the four time intervals under the SSP585 scenario (Table 2). Only one sympatric area surrounded by habitats of T. septentrionalis in Wuyi Mountain of Fujian province will be persistent over time (Figure 5).
FIGURE 5.
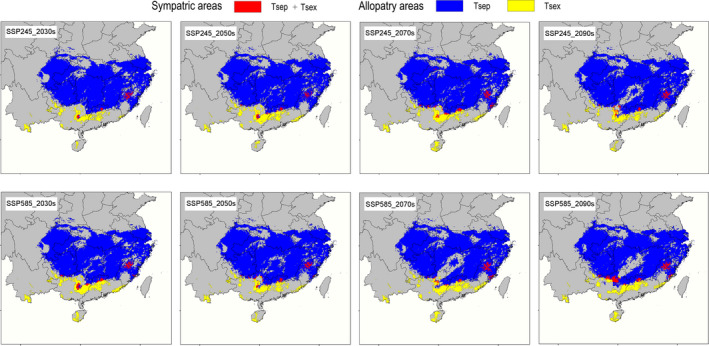
Temporal shifts in the sympatric area of T. septentrionalis (Tsep) and T. sexlineatus (Tsex) under two scenarios at the 2021–2040, 2041–2060, 2061–2080, and 2081–2100 time intervals using averages of six global circulation models as future climates. The maps show the areas sampled in context with surrounding landmasses
TABLE 2.
Percentage changes in sympatric and entire distributional ranges of two Takydromus species under future climate scenarios at the 2021–2040, 2041–2060, 2061–2080, and 2081–2100 time intervals as estimated by the ensemble species distribution model using averages of six global circulation models as future climates
| Time interval | Sympatric distribution | Takydromus septentrionalis | Takydromus sexlineatus | |||
|---|---|---|---|---|---|---|
| SSP245 | SSP585 | SSP245 | SSP585 | SSP245 | SSP585 | |
| 2021–2040 | −40.6 | 6.0 | 17.0 | 18.4 | 24.2 | 3.5 |
| 2041–2060 | −35.9 | 14.4 | 19.6 | 17.1 | 21.7 | 22.3 |
| 2061–2080 | 16.0 | 2.5 | 19.1 | 3.5 | 37.0 | 36.1 |
| 2081–2100 | −13.2 | 57.6 | 13.1 | −15.0 | 19.7 | 16.0 |
3.4. Overlap of exploited climate niche
The niche hypervolumes of the two species estimated by six climate variables partially overlapped (Figure 6). The niche hypervolume was much greater in T. sexlineatus (8.017 × 1010) than in T. septentrionalis (1.754 × 1010), with an overlapping volume of 1.202 × 1010. The Sørensen and Jaccard similarity index of the hypervolume was 0.246 in T. septentrionalis and 0.140 in T. sexlineatus, suggesting a high degree of niche overlap between the two species (Figure 6). The hypervolume centroids of Bio3, Bio5, Bio6, Bio15, Bio17, and Bio18 in order were 26.7, 30.3, 0.9, 62.1, 105.7, and 489.4 for T. septentrionalis, and 34.5, 31.5, 7.9, 72.2, 100.6, and 655.1 for T. sexlineatus. These differences in niche centroid provided evidence for some degree of niche differentiation between the two species (Figure 6).
FIGURE 6.
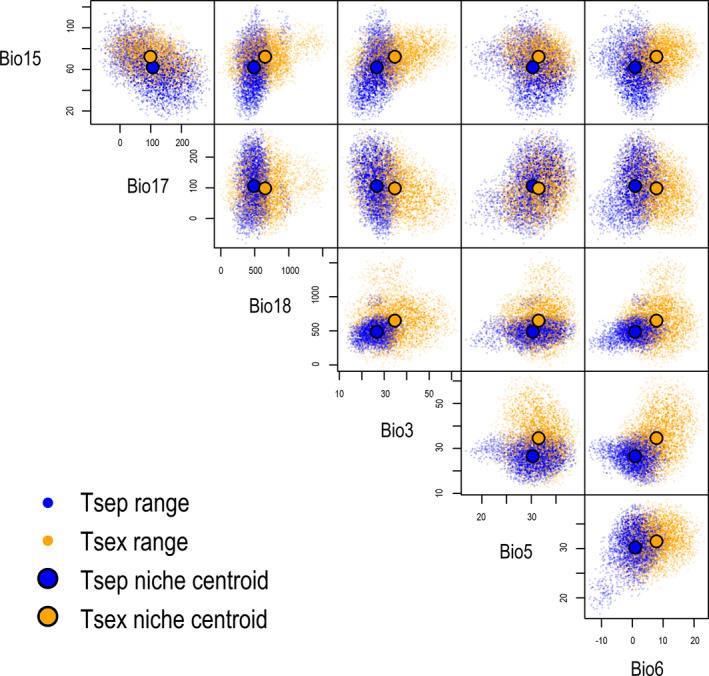
Visualization of the climate niche hypervolumes based on the six climatic variables for the T. septentrionalis (Tsep) and T. sexlineatus (Tsex). Large dots are hypervolume centroids, and small dots are randomized points sampled from the inferred hypervolume to visualize the stochastic description of each hypervolume
4. DISCUSSION
By the end of the 21st century, the global average surface temperature is expected to increase by 3.7°C under the high emission pathway of RCP 8.5 (IPCC Working Group II, 2014). As ambient temperatures rise, many organisms will be subjected to increased selection pressure and even face the risk of local extinction (Sinervo et al., 2010). One way by which organisms may use to cope with the adverse impacts of global warming on their fitness and population persistence is to shift the distributional range and/or the center of distribution (Gienapp et al., 2008; RodríGuez‐Robles et al., 2010). Actually, northward migration under a warming climate has been reported for a diverse array of organism, including plants (Alsos et al., 2012), invertebrates (Bradshaw & Holzapfel, 2006), and vertebrates (Duan et al., 2016; Gienapp et al., 2008; RodríGuez‐Robles et al., 2010). SDM studies generally suggest the distribution of organisms is environmentally dependent (Austin & Van Niel, 2011; Guisan et al., 2013; Vaclavik & Meentemeyer, 2012). However, there is no single algorithm or climate variable that can be used to predict the distribution of all species (Ducci et al., 2015; Qiao et al., 2015). Modeling with a single algorithm may either overestimate or underestimate habitats suitable for a species (Pearson et al., 2006), and using less appropriate climate variables may increase uncertainty in range predictions (Braunisch et al., 2013). Nonetheless, using an ensemble modeling approach and choosing climate variables that directly affect life history may substantially improve the predictive performance of a model and are therefore helpful to obtain a more accurate prediction of habitat suitability for a study species (Thuiller et al., 2009). Here, we used an ensemble of SDMs with high TSS and AUC values to predict habitats suitable for the two Takydromus species under the present climate condition. We found that (1) Bio6 had a key role in determining the distribution of the two species; (2) the 156 occurrence records (127 for T. septentrionalis and 29 for T. sexlineatus) were all in the predicted range of each species; and (3) most records of occurrence were in the range reported for each species (Zhao & Adler, 1993). More interestingly, the model predicted new localities in Wuyi Mountain of Fujian, central Guangxi, and southern Guizhou (Figure 4), where the two species are sympatric, but the occurrence of T. sexlineatus has yet to be verified.
Bidirectional matings between T. septentrionalis and T. sexlineatus have been observed several times in the laboratory (Figure 1), and an earlier study using the STRUCTURE analysis of nuclear and mitochondrial DNA data has shown that hybridization and introgression occur in the areas where the two species coexist and become increasingly pronounced as the geographical distance between heterospecific populations decreases (Guo, 2019). Hybridization and introgression may lead to morphological admixture (Milne et al., 1999; Olave et al., 2018; Wu et al., 2020; Yang et al., 2020), and this seems to be also true in this case as revealed by the fact that adult individuals that are morphologically intermediate between the two Takydromus species have been found in northern Guangdong where they coexist (Yu, 2014). Specifically, these individuals are similar in body size and shape to T. septentrionalis but have a mean number (4.4) of dorsal scales that is much close to that (4) of T. sexlineatus but fewer than that (6) of T. septentrionalis (Yu, 2014). From the exploited climatic niche hypervolumes, we found a great extent of climatic niche overlap between the two species (Figure 6). This suggests that hybridization and introgression between T. septentrionalis and T. sexlineatus are likely to take place in any area where climatic conditions are suitable for both species and allow them to coexist.
The upper limit of thermal tolerance (critical thermal maximum, CTMax) does not differ between T. septentrionalis and T. sexlineatus (~42°C for both species; Qu et al., 2011), whereas the lower limit of thermal tolerance (critical thermal minimum, CTMin) is significantly lower in T. septentrionalis (~4°C) than in T. sexlineatus (~6°C) (Ji et al., 1996; Zhang & Ji, 2004). In lizards, thermal preference (Tp) is positively associated with CTMax but is independent of CTMin (Qu et al., 2011). This explains why T. septentrionalis and T. sexlineatus have a similar Tp level (~32°C for both species; Yang et al., 2008). Therefore, as has been reported for other squamate reptiles (Lin & Wiens, 2017; Lin, Zhu, et al., 2019; Lin, Chen, et al., 2019; Stroud et al., 2020), low winter temperature rather than high summer temperature plays a key role in limiting the distribution of the two species farther north. The lower limit of thermal tolerance (CTMin) generally decreases with the increase in latitude and/or altitude in lizards (Qu et al., 2011). It is therefore not surprising that Bio6 contributes the most to the distribution of the two Takydromus species and that suitable habitats will shift from their currently known ranges to higher latitudinal and/or altitudinal areas in response to a warming world (Figures 4 and 5). Such geographic (latitudinal and/or altitudinal) trends of climate‐driven changes in distribution have been reported for other taxa including plants and animals (Duan et al., 2016; Frenne et al., 2013; Kraft et al., 2011; Smith et al., 2012; Zhang, Mammola, et al., 2020). Specifically, habitats suitable for T. septentrionalis in the south will be lost in the predictable future, whereas those suitable for T. sexlineatus in the area where the two species currently coexist will be preserved or even increase (Figure 5). This may be due to the fact that climate warming adversely affects southern populations of T. septentrionalis by causing the extinction of these populations or forcing them to move northward (Zhu et al., 2010). Heat loads on animals (i.e., operative temperatures, Te; Bakken, 1992) increase as ambient temperature increases. Terrestrial ectotherms in warm environments with high Te are forced to reduce activity time, as activity is constrained when Te > Tp (Deutsch et al., 2008; Huey et al., 2009, 2010; Sinervo et al., 2010). That climate warming increases the risk of population or species extinctions by constraining the activity time allotted for behavioral thermoregulation, foraging, and other activities has been proved to be true by an empirical experiment on agamid lizards (Wang, Gong, et al., 2017; Wang, Ma, et al., 2017). On the contrary, T. sexlineatus is likely to benefit from climate warming at least in its northern limit of distribution, where it is sympatric with T. septentrionalis, but, like other warm‐climate lizards in this area, an even more northerly distribution is constrained by low winter temperature (Du et al., 2011; Ji et al., ,2002, 2006; Qu et al., 2011; Xu & Ji, 2007).
5. CONCLUSIONS
Across the range where the two Takydromus species live in sympatry, there are several physical barriers such as Luoxiao Mountains and the Ganjiang River presumed to limit animal migration (Figure 4; Cai et al., 2012). However, the role of these barriers in limiting the migration of terrestrial reptiles including T. septentrionalis is small or negligible (Cai et al., 2012; Guo et al., 2019; Huang et al., 2007; Lin et al., 2012), thus allowing an inference that introgression and hybridization between T. septentrionalis and T. sexlineatus are less likely to be geospatially nonrandom across the range where they are sympatric. Climate warming will not only drive the northward drift of sympatric areas but also increase the size [by up to 58% under the SSP585 (high greenhouse gas emission) scenario] of these areas if nothing is done to limit the emission of greenhouse gases in the future (Figure 5, Table 2). Given the existence of hybridization and introgression between T. septentrionalis and T. sexlineatus in the field where they coexist, we conclude that climate warming will increase hybridization and introgression between the two species.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Kun Guo: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (equal); Resources (equal); Software (lead); Writing‐original draft (equal); Writing‐review & editing (equal). Jun Zhong: Data curation (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Fan Xie: Investigation (equal); Methodology (equal); Software (equal); Writing‐review & editing (equal). Lin Zhu: Investigation (equal); Methodology (equal); Software (equal); Writing‐review & editing (equal). Yan‐Fu Qu: Data curation (equal); Formal analysis (equal); Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Xiang Ji: Conceptualization (lead); Funding acquisition (lead); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Supervision (equal); Writing‐original draft (lead); Writing‐review & editing (equal).
Supporting information
Appendix S1
Figure S1
ACKNOWLEDGEMENTS
We thank Zhi‐Xin Zhang for help with the data analyses. This work was supported by grants from the National Natural Science Foundation of China (32071493, 31301878, and 31300339) and the Innovation Program of Jiangsu Province for Graduate Students (KYLX16_1285). Our field investigation was permitted by the Provincial Forestry Departments of Anhui, Fujian, Jiangsu, Jiangxi, Shandong, Zhejiang, Hubei, Hunan, Shaanxi, Guangdong, Guangxi, Guizhou, Hainan, and Yunnan.
Guo K, Zhong J, Xie F, Zhu L, Qu Y‐F, Ji X. Climate warming will increase chances of hybridization and introgression between two Takydromus lizards (Lacertidae). Ecol Evol. 2021;11:8573–8584. 10.1002/ece3.7671
Contributor Information
Yan‐Fu Qu, Email: quyanfu@njnu.edu.cn.
Xiang Ji, Email: jixiang@njnu.edu.cn.
DATA AVAILABILITY STATEMENT
The occurrence records used to calibrate and evaluate SDMs are within the article and its supplementary material (Appendix S1).
REFERENCES
- Allouche, O. , Tsoar, A. , & Kadmon, R. (2006). Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). Journal of Applied Ecology, 43, 1223–1232. 10.2307/4123815 [DOI] [Google Scholar]
- Alsos, I. G. , Ehrich, D. , Thuiller, W. , Eidesen, P. B. , Tribsch, A. , Schönswetter, P. , Lagaye, C. , Taberlet, P. , & Brochmann, C. (2012). Genetic consequences of climate change for northern plants. Proceedings of the Royal Society B, 279, 2042–2051. 10.1098/rspb.2011.2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo, M. B. , & New, M. (2007). Ensemble forecasting of species distributions. Trends in Ecology & Evolution, 22, 42–47. 10.1016/j.tree.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Araújo, M. B. , Pearson, R. G. , Thuiller, W. , & Erhard, M. (2005). Validation of species‐climate impact models under climate change. Global Change Biology, 11, 1504–1513. 10.1111/j.1365-2486.2005.01000.x [DOI] [Google Scholar]
- Arnold, E. N. (1997). Interrelationships and evolution of the East Asian grass lizards, Takydromus (Squamata: Lacertidae). Zoological Journal of the Linnean Society, 115, 162–172. 10.1111/j.1096-3642.1997.tb00138.x [DOI] [Google Scholar]
- Arnold, E. N. , Arribas, O. , & Carranza, S. (2007). Systematics of the Palaearctic and Oriental lizard tribe Lacertini (Squamata: Lacertidae: Lacertinae), with descriptions of eight new genera. Zootaxa, 1430, 1–86. 10.11646/zootaxa.1430.1.1 [DOI] [Google Scholar]
- Austin, M. P. , & Van Niel, K. P. (2011). Improving species distribution models for climate change studies: Variable selection and scale. Journal of Biogeography, 38, 1–8. 10.1111/j.1365-2699.2010.02416.x [DOI] [Google Scholar]
- Bakken, G. S. (1992). Measurement and application of operative and standard operative temperatures in ecology. American Zoologist, 32, 194–216. 10.1093/icb/32.2.194 [DOI] [Google Scholar]
- Barbet‐Massin, M. , Jiguet, F. , Albert, C. H. , & Thuiller, W. (2012). Selecting pseudo‐absences for species distribution models: How, where and how many? Methods in Ecology and Evolution, 3, 327–338. 10.1111/j.2041-210X.2011.00172.x [DOI] [Google Scholar]
- Bellard, C. , Bertelsmeier, C. , Leadley, P. , Thuiller, W. , & Courchamp, F. (2012). Impacts of climate change on the future of biodiversity. Ecology Letters, 15, 365–377. 10.1111/j.1461-0248.2011.01736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand, J. A. M. , Borsa, P. , & Chen, W.‐J. (2017). Phylogeography of the sergeants Abudefduf sexfasciatus and A. vaigiensis reveals complex introgression patterns between two widespread and sympatric Indo‐West Pacific reef fishes. Molecular Ecology, 26, 2527–2542. 10.1111/mec.14044 [DOI] [PubMed] [Google Scholar]
- Blonder, B. , Lamanna, C. , Violle, C. , & Enquist, B. J. (2014). The n‐dimensional hypervolume. Global Ecology and Biogeography, 23, 595–609. 10.1111/geb.12146 [DOI] [Google Scholar]
- Blonder, B. , Morrow, C. B. , Maitner, B. , Harris, D. J. , Lamanna, C. , Violle, C. , Enquist, B. J. , & Kerkhoff, A. J. (2018). New approaches for delineating n‐dimensional hypervolumes. Methods in Ecology and Evolution, 9, 305–319. 10.1111/2041-210X.12865 [DOI] [Google Scholar]
- Bradshaw, W. E. , & Holzapfel, C. M. (2006). Evolutionary response to rapid climate change. Science, 312, 1477–1478. 10.1126/science.1127000 [DOI] [PubMed] [Google Scholar]
- Braunisch, V. , Coppes, J. , Arlettaz, R. , Suchant, R. , Schmid, H. , & Bollmann, K. (2013). Selecting from correlated climate variables: A major source of uncertainty for predicting species distributions under climate change. Ecography, 36, 971–983. 10.1111/j.1600-0587.2013.00138.x [DOI] [Google Scholar]
- Cai, Y. , Yan, J. , Xu, X.‐F. , Lin, Z.‐H. , & Ji, X. (2012). Mitochondrial DNA phylogeography reveals a west‐east division of the northern grass lizard (Takydromus septentrionalis) endemic to China. Journal of Zoological Systematics and Evolutionary Research, 50(2), 137–144. 10.1111/j.1439-0469.2012.00655.x [DOI] [Google Scholar]
- Cortés‐Ortiz, L. , Nidiffer, M. D. , Hermida‐Lagunes, J. , Garcia‐Orduna, F. , Rangel‐Negrin, A. , Kitchen, D. M. , Bergman, T. J. , Dias, P. A. D. , & Canales‐Espinosa, D. (2019). Reduced introgression of sex chromosome markers in the Mexican howler monkey (Alouatta palliate × A. pigra) hybrid zone. International Journal of Primatology, 40, 114–131. 10.1007/s10764-018-0056-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyner, B. S. , Murphy, P. J. , & Matocq, M. D. (2015). Hybridization and asymmetric introgression across a narrow zone of contact between Neotoma fuscipes and N. macrotis (Rodentia: Cricetidae). Biological Journal of the Linnean Society, 115, 162–172. 10.1111/bij.12487 [DOI] [Google Scholar]
- Deutsch, C. A. , Tewksbury, J. J. , Huey, R. B. , Sheldon, K. S. , Ghalambor, C. K. , Haak, D. C. , & Martin, P. R. (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of National Academy of Sciences of the United States of America, 105, 6668–6672. 10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carré, G. , Marquéz, J. R. G. , Gruber, B. , Lafourcade, B. , Leitão, P. J. , Münkemüller, T. , McClean, C. , Osborne, P. E. , Reineking, B. , Schröder, B. , Skidmore, A. K. , Zurell, D. , & Lautenbach, S. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36, 27–46. 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- Du, W.‐G. , Ji, X. , Zhang, Y.‐P. , Xu, X.‐F. , & Shine, R. (2005). Identifying sources of variation in reproductive and life‐history traits among five populations of a Chinese lizard (Takydromus septentrionalis, Lacertidae). Biological Journal of the Linnean Society, 85, 443–453. 10.1111/j.1095-8312.2005.00508.x [DOI] [Google Scholar]
- Du, Y. , Lin, C.‐X. , Lin, L.‐H. , Qiu, Q.‐B. , & Ji, X. (2011). Ontogenetic shifts in sexual dimorphism and female reproduction in the Reevese’s butterfly lizard Leiolepis reevesii from China. Journal of Herpetology, 45, 399–405. 10.1670/10-100.1 [DOI] [Google Scholar]
- Duan, R.‐Y. , Kong, X.‐Q. , Huang, M.‐Y. , Varela, S. , & Ji, X. (2016). The potential effects of climate change on amphibian distribution, range fragmentation and turnover in China. PeerJ, 4, e2185. 10.7717/peerj.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci, L. , Agnelli, P. , Di Febbraro, M. , Frate, L. , Russo, D. , Loy, A. , Carranza, M. L. , Santini, G. , & Roscioni, F. (2015). Different bat guilds perceive their habitat in different ways: A multiscale landscape approach for variable selection in species distribution modelling. Landscape Ecology, 30, 2147–2159. 10.1007/s10980-015-0237-x [DOI] [Google Scholar]
- Elith, J. , & Leathwick, J. R. (2009). Species distribution models: Ecological explanation and prediction across space and time. Annual Review of Ecology Evolution Systematics, 40, 677–697. 10.1146/annurev.ecolsys.110308.120159 [DOI] [Google Scholar]
- Falaschi, M. , Manenti, R. , Thuiller, W. , & Ficetola, G. F. (2019). Continental‐scale determinants of population trends in European amphibians and reptiles. Global Change Biology, 25, 3504–3515. 10.1111/gcb.14739 [DOI] [PubMed] [Google Scholar]
- Franklin, J. , Wejnert, K. E. , Hathaway, S. A. , Rochester, C. J. , & Fisher, R. N. (2009). Effect of species rarity on the accuracy of species distribution models for reptiles and amphibians in southern California. Diversity and Distributions, 15, 167–177. 10.1111/j.1472-4642.2008.00536.x [DOI] [Google Scholar]
- Frenne, P. D. , Graae, B. J. , Rodríguez‐Sánchez, F. , Kolb, A. , Chabrerie, O. , Decocq, G. , De Kort, H. , De Schrijver, A. , Diekmann, M. , Eriksson, O. , Gruwez, R. , Hermy, M. , Lenoir, J. , Plue, J. , Coomes, D. A. , & Verheyen, K. (2013). Latitudinal gradients as natural laboratories to infer species’ responses to temperature. Journal of Ecology, 101, 784–795. 10.1111/1365-2745.12074 [DOI] [Google Scholar]
- Gao, J. , Zhang, W. , Dang, W. , Mou, Y. , Gao, Y. , Sun, B.‐J. , & Du, W.‐G. (2014). Heat shock protein expression enhances heat tolerance of reptile embryos. Proceedings of the Royal Society B, 281, 20141135. 10.1098/rspb.2014.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gienapp, P. , Teplitsky, C. , Alho, J. S. , Mills, J. A. , & Merilä, J. (2008). Climate change and evolution: Disentangling environmental and genetic responses. Molecular Ecology, 17, 167–178. 10.1111/j.1365-294X.2007.03413.x [DOI] [PubMed] [Google Scholar]
- Grenouillet, G. , Buisson, L. , Casajus, N. , & Lek, S. (2011). Ensemble modelling of species distribution: The effects of geographical and environmental ranges. Ecography, 34, 9–17. 10.1111/j.1600-0587.2010.06152.x [DOI] [Google Scholar]
- Guisan, A. , & Thuiller, W. (2005). Predicting species distribution: Offering more than simple habitat models. Ecology Letters, 8, 993–1009. 10.1111/j.1461-0248.2005.00792.x [DOI] [PubMed] [Google Scholar]
- Guisan, A. , Thuiller, W. , & Zimmermann, N. E. (2017). Habitat suitability and distribution models: With applications in R. Cambridge University Press. [Google Scholar]
- Guisan, A. , Tingley, R. , Baumgartner, J. B. , Naujokaitis‐Lewis, I. , Sutcliffe, P. R. , Tulloch, A. I. T. , Regan, T. J. , Brotons, L. , McDonald‐Madden, E. , Mantyka‐Pringle, C. , Martin, T. G. , Rhodes, J. R. , Maggini, R. , Setterfield, S. A. , Elith, J. , Schwartz, M. W. , Wintle, B. A. , Broennimann, O. , Austin, M. , … Buckley, Y. M. (2013). Predicting species distributions for conservation decisions. Ecology Letters, 16, 1424–1435. 10.1111/ele.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan, A. , & Zimmermann, N. E. (2000). Predictive habitat distribution models in ecology. Ecological Modelling, 135, 147–186. 10.1016/S0304-3800(00)00354-9 [DOI] [Google Scholar]
- Guo, K. (2019). Climatic correlates of genetics, morphology and distribution and the possibility of hybridization in lizards of the genus Takydromus (Lacertidae). Nanjing, China: PhD Dissertation, Nanjing Normal University.
- Guo, K. , Chen, C. , Liang, X.‐F. , Qu, Y.‐F. , & Ji, X. (2020). Female northern grass lizards judge mates by body shape to reinforce local adaptation. Frontiers in Zoology, 17, 22. 10.1186/s12983-020-00367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, P. , Liu, Q. , Zhu, F. , Zhong, G.‐H. , Che, J. , Wang, P. , Xie, Y.‐L. , Murphy, R. W. , & Malhotra, A. (2019). Multilocus phylogeography of the brown‐spotted pitviper Protobothrops mucrosquamatus (Reptilia: Serpentes: Viperidae) sheds a new light on the diversification pattern in Asia. Molecular Phylogenetics and Evolution, 133, 82–91. 10.1016/j.ympev.2018.12.028 [DOI] [PubMed] [Google Scholar]
- Helsen, K. , Cleemput, E. V. , Bassi, L. , Graae, B. J. , Somers, B. , Blonder, B. , & Honnay, O. (2020). Inter‐and intraspecific trait variation shape multidimensional trait overlap between two plant invaders and the invaded communities. Oikos, 129, 677–688. 10.1111/oik.06919 [DOI] [Google Scholar]
- Huang, S. , He, S.‐P. , Peng, Z. , Zhao, K. , & Zhao, E.‐M. (2007). Molecular phylogeography of endangered sharp‐snouted pitviper (Deinagkistrodon acutus; Reptilia, Viperidae) in mainland China. Molecular Phylogenetics and Evolution, 44, 942–952. 10.1016/j.ympev.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Huey, R. B. , Deutsch, C. A. , Tewksbury, J. J. , Vitt, L. J. , Hertz, P. E. , Pérez, H. J. Á. , & Garland, T. (2009). Why tropical forest lizards are vulnerable to climate warming. Proceedings of the Royal Society B, 276, 1939–1948. 10.1098/rspb.2008.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey, R. B. , Losos, J. B. , & Moritz, C. (2010). Are lizards toast? Science, 328, 832–833. 10.1126/science.1190374 [DOI] [PubMed] [Google Scholar]
- IPCC Working Group II (2014). Climate change 2014: Impacts, adaptation, and vulnerability. Intergovernmental Panel on Climate Change. [Google Scholar]
- Ji, X. , Du, W.‐G. , Lin, Z.‐H. , & Luo, L.‐G. (2007). Measuring temporal variation in reproductive output reveals optimal resource allocation to reproduction in the northern grass lizard, Takydromus septentrionalis . Biological Journal of the Linnean Society, 91, 315–324. 10.1111/j.1095-8312.2007.00791.x [DOI] [Google Scholar]
- Ji, X. , Du, W.‐G. , & Sun, P.‐Y. (1996). Body temperature, thermal tolerance and influence of temperature on sprint speed and food assimilation in adult grass lizards, Takydromus septentrionalis . Journal of Thermal Biology, 21, 155–161. 10.1016/0306-4565(95)00037-2 [DOI] [Google Scholar]
- Ji, X. , Lin, L.‐H. , Lin, C.‐X. , Qiu, Q.‐B. , & Du, Y. (2006). Sexual dimorphism and female reproduction in the many‐lined sun skink (Mabuya multifasciata) from China. Journal of Herpetology, 40, 353–359. [Google Scholar]
- Ji, X. , Qiu, Q.‐B. , & Diong, C.‐H. (2002). Sexual dimorphism and female reproductive characteristics in the oriental garden lizard, Calotes versicolor, from a population in Hainan, southern China. Journal of Herpetology, 36, 1–8. [Google Scholar]
- Kraft, N. J. B. , Comita, L. S. , Chase, J. M. , Sanders, N. J. , Swenson, N. G. , Crist, T. O. , Stegen, J. C. , Vellend, M. , Boyle, B. , Anderson, M. J. , Cornell, H. V. , Davies, K. F. , Freestone, A. L. , Inouye, B. D. , Harrison, S. P. , & Myers, J. A. (2011). Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science, 333, 1755–1758. 10.1126/science.1216393 [DOI] [PubMed] [Google Scholar]
- Lin, L.‐H. , Qu, Y.‐F. , Li, H. , Zhou, K.‐Y. , & Ji, X. (2012). Genetic structure and demographic history should inform conservation: Chinese cobras currently treated as homogenous show population divergence. PLoS One, 7, e36334. 10.1371/journal.pone.0036334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L.‐H. , & Wiens, J. J. (2017). Comparing macroecological patterns across continents: Evolution of climatic niche breadth in varanid lizards. Ecography, 40, 960–970. 10.1111/ecog.02343 [DOI] [Google Scholar]
- Lin, L.‐H. , Zhu, X.‐M. , Du, Y. , Fang, M.‐C. , & Ji, X. (2019). Global, regional and cladistic patterns of variation in climatic niche breadths in terrestrial elapid snakes. Current Zoology, 65, 1–9. 10.1093/cz/zoy026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, T.‐E. , Chen, T.‐Y. , Wei, H.‐L. , Richard, R. , & Huang, S.‐P. (2019). Low cold tolerance of the invasive lizard Eutropis multifasciata constrains its potential elevation distribution in Taiwan. Journal of Thermal Biology, 82, 115–122. 10.1016/j.jtherbio.2019.03.015 [DOI] [PubMed] [Google Scholar]
- Liu, C. , White, M. , & Newell, G. (2011). Measuring and comparing the accuracy of species distribution models with presence‐absence data. Ecography, 34, 232–243. 10.1111/j.1600-0587.2010.06354.x [DOI] [Google Scholar]
- Liu, C. , White, M. , & Newell, G. (2013). Selecting thresholds for the prediction of species occurrence with presence‐only data. Journal of Biogeography, 40, 778–789. 10.1111/jbi.12058 [DOI] [Google Scholar]
- Liu, M.‐Y. (1999). Takydromus Daudin 1802. In Zhao E.‐M., Zhao K.‐T., & Zhou K.‐Y. (Eds.), Fauna Sinica, Reptilia, Vol. 2 (Squamata: Lacertilia) (pp. 257–270). Science Press. [Google Scholar]
- Liu, X. , Guo, Z.‐W. , Ke, Z.‐E. , Wang, S.‐P. , & Li, Y.‐M. (2011). Increasing potential risk of a global aquatic invader in Europe in contrast to other continents under future climate change. PLoS One, 6, e18429. 10.1371/journal.pone.0018429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H.‐L. , Lin, Z.‐H. , Li, H. , & Ji, X. (2014). Geographic variation in hatchling size in an oviparous skink: Effects of maternal investment and incubation thermal environment. Biological Journal of the Linnean Society, 113, 283–296. 10.1111/bij.12322 [DOI] [Google Scholar]
- Lue, K.‐Y. , & Lin, S.‐M. (2008). Two new cryptic species of Takydromus (Squamata: Lacertidae) from Taiwan. Herpetologica, 64, 379–395. 10.1655/07-030.1 [DOI] [Google Scholar]
- Luo, L.‐G. , Ding, G.‐H. , & Ji, X. (2010). Income breeding and temperature‐induced plasticity in reproductive traits in lizards. Journal of Experimental Biology, 213, 2073–2078. 10.1242/jeb.041137 [DOI] [PubMed] [Google Scholar]
- Ma, L. I. , Liu, P. , Su, S. , Luo, L.‐G. , Zhao, W.‐G. , & Ji, X. (2019). Life‐history consequences of local adaptation in lizards: Takydromus wolteri (Lacertidae) as a model organism. Biological Journal of the Linnean Society, 127, 88–99. 10.1093/biolinnean/blz024 [DOI] [Google Scholar]
- Mammola, S. , Piano, E. , Cardoso, P. , Vernon, P. , Dominguez‐Villar, D. , Culver, D. C. , Pipan, T. , & Isaia, M. (2019). Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthropocene Review, 6, 98–116. 10.1177/2053019619851594 [DOI] [Google Scholar]
- Mapel, X. M. , Gyllenhaal, E. F. , Modak, T. H. , DeCicco, L. H. , Naikatini, A. , Utzurrum, R. B. , Seamon, J. O. , Cibois, A. , Thibault, J.‐C. , Sorenson, M. D. , Moyle, R. G. , Barrow, L. N. , & Andersen, M. J. (2021). Inter‐ and intra‐archipelago dynamics of population structure and gene flow in a Polynesian bird. Molecular Phylogenetics and Evolution, 156, 107034. 10.1016/j.ympev.2020.107034 [DOI] [PubMed] [Google Scholar]
- Martin, C. H. , Cutler, J. S. , Friel, J. P. , Touokong, C. D. , Coop, G. , & Wainwright, P. C. (2015). Complex histories of repeated gene flow in Cameroon crater lake cichlids cast doubt on one of the clearest examples of sympatric speciation. Evolution, 69, 1406–1422. 10.1111/evo.12674 [DOI] [PubMed] [Google Scholar]
- Mayr, E. (1963). Animal species and evolution. Harvard University Press. [Google Scholar]
- McCarthy, M. P. , Best, M. J. , & Betts, R. A. (2010). Climate change in cities due to global warming and urban effects. Geophysical Research Letters, 37, L09705. 10.1029/2010GL042845 [DOI] [Google Scholar]
- Milne, R. I. , Abbott, R. J. , Wolff, K. , & Chamberlain, D. F. (1999). Hybridization among sympatric species of Rhododendron (Ericaceae) in Turkey: Morphological and molecular evidence. America Journal of Botany, 86, 1776–1785. 10.2307/2656674 [DOI] [PubMed] [Google Scholar]
- Nüchel, J. , Bøcher, P. K. , Xiao, W. , Zhu, A.‐X. , & Svenning, J. C. (2018). Snub‐nosed monkeys (Rhinopithecus): Potential distribution and its implication for conservation. Biodiversity and Conservation, 27, 1517–1538. 10.1007/s10531-018-1507-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olave, M. , Avila, L. J. , Sites, J. W. Jr , & Morando, M. (2018). Hybridization could be a common phenomenon within the highly diverse lizard genus Liolaemus . Journal of Evolutionary Biology, 31, 893–903. 10.1111/jeb.13273 [DOI] [PubMed] [Google Scholar]
- Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics, 37, 637–669. 10.1146/annurev.ecolsys.37.091305.110100 [DOI] [Google Scholar]
- Pearson, R. G. , Thuiller, W. , Araújo, M. B. , Martinez‐Meyer, E. , Brotons, L. , McClean, C. , Miles, L. , Segurado, P. , Dawson, T. P. , & Lees, D. C. (2006). Model‐based uncertainty in species range prediction. Journal of Biogeography, 33, 1704–1711. 10.1111/j.1365-2699.2006.01460.x [DOI] [Google Scholar]
- Phillips, S. J. , Anderson, R. P. , & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, 231–259. 10.1016/j.ecolmodel.2005.03.026 [DOI] [Google Scholar]
- Qiao, H.‐J. , Soberón, J. , & Peterson, A. T. (2015). No silver bullets in correlative ecological niche modelling: Insights from testing among many potential algorithms for niche estimation. Methods in Ecology and Evolution, 6, 1126–1136. 10.1111/2041-210X.12397 [DOI] [Google Scholar]
- Qu, Y.‐F. , Li, H. , Gao, J.‐F. , Xu, X.‐F. , & Ji, X. (2011). Thermal preference, thermal tolerance and the thermal dependence of digestive performance in two coexisting Phrynocephalus lizards (Agamidae), with a review of species studied. Current Zoology, 57, 684–700. 10.1080/0144929X.2011.553739 [DOI] [Google Scholar]
- RodríGuez‐Robles, J. A. , Jezkova, T. , & Leal, M. (2010). Climatic stability and genetic divergence in the tropical insular lizard Anolis krugi, the Puerto Rican ‘Lagartijo Jardinero de la Montaña’. Molecular Ecology, 19, 1860–1876. 10.1111/j.1365-294X.2010.04616.x [DOI] [PubMed] [Google Scholar]
- Sardell, J. M. , & Uy, J. A. C. (2016). Hybridization following recent secondary contact results in asymmetric genotypic and phenotypic introgression between island species of Myzomela honeyeaters. Evolution, 70, 257–269. 10.1111/evo.12864 [DOI] [PubMed] [Google Scholar]
- Seehausen, O. (2006). Conservation: Losing biodiversity by reverse speciation. Current Biology, 16, R334–R337. 10.1016/j.cub.2006.03.080 [DOI] [PubMed] [Google Scholar]
- Sinervo, B. , Méndez‐de‐la‐Cruz, F. , Miles, D. B. , Heulin, B. , Bastiaans, E. , Cruz, M. V. S. , Lara‐Resendiz, R. , Martínez‐Méndez, N. , Calderón‐Espinosa, M. L. , Meza‐Lázaro, R. N. , Gadsden, H. , Avila, L. J. , Morando, M. , De la Riva, I. J. , Sepulveda, P. V. , Rocha, C. F. D. , Ibargüengoytía, N. , Puntriano, C. A. , Massot, M. , … Sites, J. W. (2010). Erosion of lizard diversity by climate change and altered thermal niches. Science, 328, 894–899. 10.1126/science.1184695 [DOI] [PubMed] [Google Scholar]
- Smith, B. T. , Bryson, R. W. , Houston, D. D. , & Klicka, J. (2012). An asymmetry in niche conservatism contributes to the latitudinal species diversity gradient in New World vertebrates. Ecology Letters, 15, 1318–1325. 10.1111/j.1461-0248.2012.01855.x [DOI] [PubMed] [Google Scholar]
- Souissi, A. , Bonhomme, F. , Manchado, M. , Bahri‐Sfar, L. , & Gagnaire, P. A. (2018). Genomic and geographic footprints of differential introgression between two highly divergent fish species (Solea spp.). Heredity, 121, 579–593. 10.1038/s41437-018-0079-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton, J. C. , Pearson, R. G. , Horning, N. , Ersts, P. , & Akcakaya, H. R. (2012). Combining static and dynamic variables in species distribution models under climate change. Methods in Ecology and Evolution, 3, 349–357. 10.1111/j.2041-210X.2011.00157.x [DOI] [Google Scholar]
- Stroud, J. T. , Mothes, C. C. , Beckles, W. , Heathcote, R. J. P. , Donihue, C. M. , & Losos, J. B. (2020). An extreme cold event leads to community‐wide convergence in lower temperature tolerance in a lizard community. Biology Letters, 16, 20200625. 10.1098/rsbl.2020.0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller, W. , Guéguen, M. , Renaud, J. , Karger, D. N. , & Zimmermann, N. E. (2019). Uncertainty in ensembles of global biodiversity scenarios. Nature Communications, 10, 1446. 10.1038/s41467-019-09519-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller, W. , Lafourcade, B. , Engler, R. , & Araújo, M. B. (2009). BIOMOD‐a platform for ensemble forecasting of species distributions. Ecography, 32, 369–373. 10.1111/j.1600-0587.2008.05742.x [DOI] [Google Scholar]
- Vaclavik, T. , & Meentemeyer, R. K. (2012). Equilibrium or not? modelling potential distribution of invasive species in different stages of invasion. Diversity and Distributions, 18, 73–83. 10.1111/j.1472-4642.2011.00854.x [DOI] [Google Scholar]
- Volis, S. , Zhang, Y.‐H. , Deng, T. , Dorman, M. , Blecher, M. , & Abbott, R. J. (2019). Divergence and reproductive isolation between two closely related allopatric Iris species. Biological Journal of the Linnean Society, 127, 377–389. 10.1093/biolinnean/blz014 [DOI] [Google Scholar]
- Wang, J. , Lyu, Z.‐T. , Yang, C.‐Y. , Li, Y.‐L. , & Wang, Y.‐Y. (2019). A new species of the genus Takydromus (Squamata, Lacertidae) from southwestern Guangdong, China. ZooKeys, 871, 119–139. 10.3897/zookeys.871.35947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.‐Y. , Gong, S.‐P. , Liu, P. , & Wang, X. (2017). A new species of the genus Takydromus (Squamata: Lacertidae) from Tianjingshan forestry station, northern Guangdong, China. Zootaxa, 4338, 441–458. 10.11646/zootaxa.4338.3.2 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Ma, L. , Shao, M. , & Ji, X. (2017). Are viviparous lizards more vulnerable to climate warming because they have evolved reduced body temperature and heat tolerance? Oecologia, 185, 573–582. 10.1007/s00442-017-3979-0 [DOI] [PubMed] [Google Scholar]
- Wu, M.‐Y. , Low, G. W. , Forcina, G. , Van Grouw, H. , Lee, B. P. Y. H. , Oh, R. R. Y. , & Rheindt, F. E. (2020). Historic and modern genomes unveil a domestic introgression gradient in a wild red junglefowl population. Evolutionary Applications, 13, 2300–2315. 10.1111/eva.13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, D.‐D. , & Ji, X. (2007). Sexual dimorphism, female reproduction and egg incubation in the oriental leaf‐toed gecko (Hemidactylus bowringii) from southern China. Zoology, 110, 20–27. 10.1016/j.zool.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Xu, X.‐F. , & Ji, X. (2000). A comparison of egg and hatchling characteristics of two lacertid lizards. Chinese Journal of Zoology, 35(3), 15–19.(In Chinese). [Google Scholar]
- Yang, J. , Sun, Y.‐Y. , An, H. , & Ji, X. (2008). Northern grass lizards (Takydromus septentrionalis) from different populations do not differ in thermal preference and thermal tolerance when acclimated under identical thermal conditions. Journal of Comparative Physiology B, 178, 343–349. 10.1007/s00360-007-0227-7 [DOI] [PubMed] [Google Scholar]
- Yang, W.‐Z. , Feiner, N. , Laakkonen, H. , Sacchi, R. , Zuffi, M. A. L. , Scali, S. , While, G. M. , & Uller, T. (2020). Spatial variation in gene flow across a hybrid zone reveals causes of reproductive isolation and asymmetric introgression in wall lizards. Evolution, 74, 1289–1300. 10.1111/evo.14001 [DOI] [PubMed] [Google Scholar]
- Yu, D.‐N. (2014). The phylogenetic relationship and hybridization of Takydromus lizards in China. Nanjing, China: PhD Dissertation, Nanjing Normal University.
- Zhang, Y.‐P. , & Ji, X. (2004). The thermal dependence of food assimilation and locomotor performance in southern grass lizards, Takydromus sexlineatus (Lacertidae). Journal of Thermal Biology, 29, 45–53. 10.1016/j.jtherbio.2003.10.007 [DOI] [Google Scholar]
- Zhang, Z.‐X. , Capinha, C. , Usio, N. , Weterings, R. , Liu, X. , Li, Y.‐M. , Landeria, J. M. , Zhou, Q. , & Yokota, M. (2020a). Impacts of climate change on the global potential distribution of two notorious invasive crayfishes. Freshwater Biology, 65, 353–365. 10.1111/fwb.13429 [DOI] [Google Scholar]
- Zhang, Z.‐X. , Mammola, S. , Liang, Z.‐Q. , Capinha, C. , Wei, Q.‐W. , Wu, Y.‐A. , Zhou, J. , & Wang, C.‐R. (2020b). Future climate change will severely reduce habitat suitability of the critically endangered Chinese giant salamander. Freshwater Biology, 65, 971–980. 10.1111/fwb.13483 [DOI] [Google Scholar]
- Zhao, E.‐M. , & Adler, K. (1993). Herpetology of China. Published by Society for the Study of Amphibians and Reptiles. [Google Scholar]
- Zhu, L.‐J. , Du, W.‐G. , Sun, B. , & Zhang, Y.‐P. (2010). The effects of warming on female reproduction, egg incubation and hatchling traits in the northern grass lizard, Takydromus septentrionalis . Acta Ecologica Sinica, 30, 4848–4854.(In Chinese with English abstract) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1
Data Availability Statement
The occurrence records used to calibrate and evaluate SDMs are within the article and its supplementary material (Appendix S1).


