Abstract
A major conceptual gap in taste biology is the lack of a general framework for understanding the evolution of different taste modalities among animal species. We turn to two complementary nutritional frameworks, biological stoichiometry theory and nutritional geometry, to develop hypotheses for the evolution of different taste modalities in animals. We describe how the attractive tastes of Na‐, Ca‐, P‐, N‐, and C‐containing compounds are consistent with principles of both frameworks based on their shared focus on nutritional imbalances and consumer homeostasis. Specifically, we suggest that the evolution of multiple nutritive taste modalities can be predicted by identifying individual elements that are typically more concentrated in the tissues of animals than plants. Additionally, we discuss how consumer homeostasis can inform our understanding of why some taste compounds (i.e., Na, Ca, and P salts) can be either attractive or aversive depending on concentration. We also discuss how these complementary frameworks can help to explain the evolutionary history of different taste modalities and improve our understanding of the mechanisms that lead to loss of taste capabilities in some animal lineages. The ideas presented here will stimulate research that bridges the fields of evolutionary biology, sensory biology, and ecology.
Keywords: chemoreception, gustation, homeostasis, nutritional ecology, optimal foraging
This paper describes how the complementary nutritional frameworks, biological stoichiometry theory and nutritional geometry, can inform our understanding of the evolution of multiple taste modalities in animals. We describe how the attractive tastes of Na‐, Ca‐, P‐, N‐, and C‐containing compounds are consistent with principles of both frameworks based on their shared focus on nutritional imbalances and consumer homeostasis.

1. INTRODUCING A GENERAL FRAMEWORK FOR CONSUMPTIVE TASTE EVOLUTION
All organisms are faced with the challenge of procuring the essential elements and biochemicals of life for growth, maintenance, and reproduction, often in proportions that deviate substantially from their environmental availability. For animals, nutrients (i.e., elements and/or biochemicals) are acquired through their diet by consuming foods that may vary considerably in their chemical composition and that frequently provide an inadequate supply of one or more essential nutrients (Raubenheimer et al., 2009). Thus, many animals suffer periodic nutritional imbalances whereby the nutrient content of available foods does not match their nutritional requirements. Such imbalances (i.e., over‐ or undersupply of essential nutrients) can affect consumer metabolism and performance, ultimately resulting in reduced growth and fitness (Simpson & Raubenheimer, 2012; Sterner & Elser, 2002). Because nutritional imbalances between consumers and their foods are both pervasive and consequential, animals have evolved a range of adaptations, from behavioral to physiological and chemosensory, that allow them to modulate nutrient intake, as well as post‐ingestion nutrient processing (i.e., assimilation and allocation), to minimize potential imbalances (Simpson & Raubenheimer, 2012).
Selective foraging is a key behavioral adaptation that allows animals to regulate nutrient intake in order to achieve balanced nutrient supply (Simpson & Raubenheimer, 2012). Choosing exactly which foods to eat, however, requires that animals differentiate among potential food sources of differing chemical composition to select those that are most nutritionally advantageous. One of the primary mechanisms that animals use to assess the nutritional quality of foods is gustation, or taste, which provides animals the ability to evaluate the chemical composition of potential foods prior to ingestion via complex chemosensory systems (Breslin, 2013; Lindemann, 2001; Yarmolinsky et al., 2009). Indeed, there is broad recognition that gustatory systems function primarily as a screening mechanism that drives consumption of nutrient‐ and energy‐dense foods, as well as the rejection of potentially harmful ones based upon the presence, and concentrations, of a variety of different chemicals. These various chemical stimuli interact with specialized receptor cells to produce signals that are transduced and interpreted as unique taste modalities, including the salty, sweet, bitter, sour, and umami tastes familiar to humans (Breslin, 2013; Lindemann, 2001; Roper & Chaudhari, 2017).
The variety of different taste modalities provide organisms with a way to evaluate the quality of food based on multiple aspects of its chemical composition and can be broadly grouped by whether they taste “good,” and therefore promote consumption, or “bad,” and therefore produce an aversive response. Consumptive responses, such as those elicited by sweet and umami tastes, generally promote the ingestion of foods that supply compounds essential for growth and metabolism. Conversely, aversive responses, such as those typically generated by bitter and sour tastes, encourage animals to reject, rather than consume specific foods. Aversive tastes are generally thought to inform consumers that a food may contain toxic or harmful chemicals, such as allelochemicals, though not all chemicals that produce innate aversive responses are necessarily harmful (Breslin, 2013; Yarmolinsky et al., 2009). Additionally, some elements, such as Na, Ca, and P, which are essential but can be toxic when oversupplied, may elicit either consumptive or aversive responses depending on concentration, thereby allowing consumers to regulate intake within a relatively narrow window.
All animals possess gustatory sensing capabilities (Kirino et al., 2013; Simpson & Raubenheimer, 2012), reinforcing the notion that regulation of nutrient intake is highly consequential for consumer fitness. Interestingly, gustatory sensing appears to have evolved largely independently among prominent animal lineages (i.e., mammals and insects) (Yarmolinsky et al., 2009), yet exhibits remarkable convergence around taste capabilities for a small suite of compounds (e.g., amino acids, carbohydrates, Na salts). This suggests that animals have faced similar nutritional constraints throughout their evolutionary history and indicates potential for developing a nutritional framework for understanding the evolution of taste that has broad applicability. Nevertheless, we currently lack a predictive understanding of when and why particular taste modalities might be evolutionarily favored (Simpson & Raubenheimer, 2012), thereby leading to differences in the breadth of gustatory capabilities among species.
In this paper, we turn to two complementary frameworks, biological stoichiometry theory (Elser et al., 1996, 2000; Sterner & Elser, 2002) and nutritional geometry (Raubenheimer & Simpson, 1993; Raubenheimer et al., 2009; Simpson & Raubenheimer, 2012), that have been developed by ecologists to characterize nutritional imbalances (and their consequences) in trophic interactions to draw insights into the evolutionary contexts under which different taste modalities have evolved in animals. In the following sections, we summarize the basic tenets of biological stoichiometry theory and nutritional geometry, including the unifying concept of consumer homeostasis, and draw upon a review of the taste biology literature to show how these complementary frameworks advance our understanding of the evolution of multiple taste modalities in animals. We use the principles of biological stoichiometry to demonstrate that the evolution of multiple nutritive taste modalities can be predicted by identifying individual elements that are typically more concentrated in the tissues of animals than plants. Moreover, we describe how consumer homeostasis can inform our understanding of why some taste compounds (i.e., Na, Ca, and P salts) can be either attractive or aversive depending on concentration. Additionally, we discuss how these frameworks can help to explain the distribution of different taste modalities among diverse animal lineages, including why the taste of Ca (at least at some concentrations) is attractive to some vertebrate species but appears to be strictly aversive to insects. We also discuss how biological stoichiometry and nutritional geometry can improve our understanding of the mechanisms that lead to loss of taste capabilities, as well as the pseudogenization, or loss of function via deleterious mutations, of associated taste receptor genes, in some animal lineages (Antinucci & Risso, 2017; Jiang et al., 2012; Zhao et al., 2010). We also discuss some caveats to our application of these complementary frameworks and, finally, propose several ideas that we hope will stimulate future research, including into the evolutionary history and phylogenetic distribution of different taste modalities, and incorporation of taste into functional trait‐based ecological analyses.
We do not intend for this paper to provide a comprehensive review of taste biology research, as several such papers already exist and remain useful (i.e., Breslin, 2013; Roper & Chaudhari, 2017; Yarmolinsky et al., 2009); nor do we intend this to be a comprehensive discussion and synthesis of biological stoichiometry theory and nutritional geometry (see Sperfeld et al., 2017). Rather, our intent is to highlight linkages between taste and animal nutrition using two prominent nutritional frameworks employed in ecological research. We do, however, draw support for this effort from a comprehensive review of the taste literature, which in some cases (i.e., for P‐ and Ca‐related taste) is limited to a relatively small number of species and model systems, including humans, mice, and livestock. It is important that we acknowledge that animals employ other sensory systems, as well as learned nutritional associations, to assess the nutritional content of their foods and that these systems also contribute to feeding behaviors (Sclafani & Ackroff, 2012; Simpson & Raubenheimer, 2012). Our intent is to focus specifically on the role of nutritional imbalances in driving the evolution of different taste modalities, rather than a broader discussion of mechanisms that regulate feeding preferences and consumption. Finally, we acknowledge that both biological stoichiometry and nutritional geometry may not accurately predict the full suite of consumptive taste capabilities for a species given unique evolutionary histories. Despite these specific limitations, they remain useful for generating hypotheses to further understand broad patterns in taste evolution.
2. NUTRITIONAL FRAMEWORKS IN ECOLOGY
Ecologists have long recognized that the quantity and nutritional quality of foods affect the growth and performance of animals (Raubenheimer et al., 2009). Given that nutritional requirements vary among animal species, shifts in the nutrient composition of available foods can scale beyond individual and population effects to alter community and ecosystem dynamics, including fluxes of energy and nutrients to and from the environment and among trophic levels (Anderson et al., 2020; Sperfeld et al., 2017). Increasing recognition of the role of animal nutrition in driving ecological dynamics, from foraging behavior to consumer‐driven nutrient recycling, has led to the development of several conceptual frameworks that seek to characterize the nature and consequences of nutritional imbalances in trophic interactions (Raubenheimer et al., 2009). Biological stoichiometry theory and nutritional geometry are perhaps the most prominent of these frameworks and have been developed in parallel over the last several decades (Anderson et al., 2020; Sperfeld et al., 2017). Though these frameworks focus on different nutritional currencies (i.e., elements vs. macro‐ and micronutrients), there is substantial conceptual overlap, including an emphasis on homeostasis, that suggest promise in their ability to inform our understanding of how nutritional imbalances have shaped the evolutionary history of gustation in animals (Sperfeld et al., 2017). There are large literatures devoted to the development and application of these frameworks, including several recent papers that compare the two approaches, highlighting their connections and encouraging their synthesis (Anderson et al., 2020; Sperfeld et al., 2017; Wilder & Jeyasingh, 2016). Sperfeld et al. (2017) provide an especially useful summary and comparison of the two frameworks with an emphasis on their conceptual overlap. As such, we provide here only brief summaries of their basic principles to establish their usefulness in describing the ecological contexts under which different taste modalities have evolved.
Biological, or commonly, ecological stoichiometry is the study of the balance of energy and multiple elements in ecological interactions (Elser et al., 1996, 2000; Sterner & Elser, 2002). For the purposes of this paper, we prefer the term “biological stoichiometry,” because its broader scope explicitly integrates cellular and genetic mechanisms (Elser et al., 2000). Similar to the stoichiometry of chemical reactions, biological stoichiometry applies thermodynamic principles to the mass balance of multiple elements in biological interactions (Elser et al., 1996). It is rooted in the observation that individual organisms are composed of elements in relatively fixed ratios (stoichiometric homeostasis) and that available resources frequently supply elements in ratios that deviate from those of the organism. This is particularly true of animals, which tend to be far less plastic in their elemental composition and exhibit far narrower interspecific variation in elemental composition than do plants (and many autotrophic microorganisms; Figure 1), thereby resulting in notable stoichiometric imbalances between plants and animals (Frost et al., 2005; Persson et al., 2010). Core principles of biological stoichiometry include the observations that (1) organisms have limited capacity to alter their elemental composition even when the supply of elements is variable and (2) large imbalances in elemental composition between consumers and their foods (e.g., herbivores and plants; Figure 1) are common (Sterner & Elser, 2002). A central thesis of biological stoichiometry theory is that reconciling these imbalances (i.e., via selective foraging behavior or post‐ingestive regulation) while maintaining elemental homeostasis has consequences for the growth and fitness of animals, as well as the cycling of elements between animals and the environment. Hence, a key strength of biological stoichiometry is the ability to scale and make predictions across different levels of organization (e.g., from consumer taste receptors to ecosystem nutrient supply).
FIGURE 1.
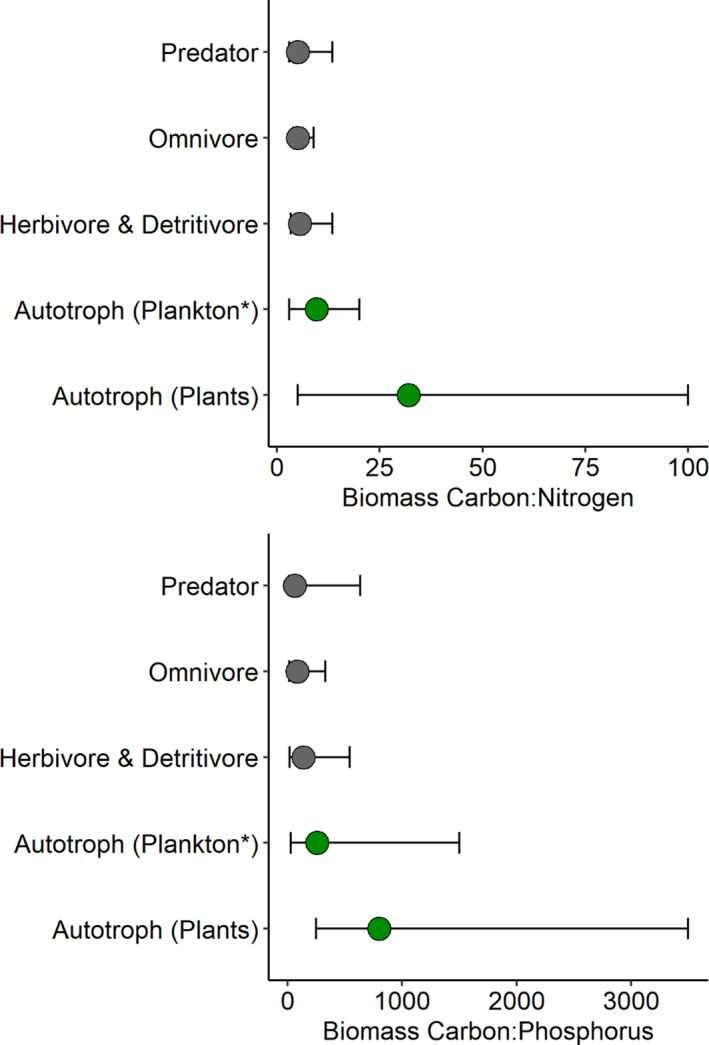
Carbon:nitrogen and carbon:phosphorus ratios of biomass across multiple trophic levels. Circles represent median values, and error bars indicate ranges of C:N and C:P observed for organisms within each trophic level. Autotroph data are from Elser et al. (9), where Autotroph (Plants) includes measurements of foliar chemistry of terrestrial autotrophs (for C:N, n = 406; for C:P, n = 413) and Autotroph (Plankton*) represents nutrient chemistry of seston from freshwater lakes (for C:N, n = 267; for C:P, n = 273). Seston contains some detritus and heterotrophic biomass (i.e., bacteria, protozoa) but is typically dominated by phytoplankton (9). Consumer stoichiometry data are from Vanni et al. (2017) and represent primarily aquatic animals. Data include 190 families from 9 animal phyla (herbivore and detritivore, n = 168; omnivore, n = 77; predator, n = 163)
Biological stoichiometry has historically focused primarily on imbalances between the supply and demand of C, N, and P in trophic interactions. This bias reflects how commonly these elements limit biological productivity across trophic levels in many different ecosystems (Elser et al., 2007; LeBauer & Treseder, 2008) and a tendency to view limiting nutrients through the prism of a single nutrient rooted in Liebig's law of the minimum. However, biological stoichiometry is not constrained to these three elements and incorporation of a wider suite of biologically essential elements into stoichiometric analysis is an emerging frontier (Filipiak et al., 2017; Jeyasingh et al., 2017). Additionally, there is increasing recognition that key molecular and cellular level processes may be colimited by multiple chemical elements (Sperfeld et al., 2012) and that studies of nutrient limitation should consider this possibility and expand the elements considered in stoichiometric analyses (Kaspari & Powers, 2016). A notable weakness of biological stoichiometry theory with respect to its ability to characterize nutritional imbalances is its focus on individual elements (Anderson et al., 2004; Sperfeld et al., 2017). Given that animal nutrition is largely biochemical in nature, focusing solely on the ratios of elements in foods may obscure potential imbalances in the supply of certain essential macro‐ and micronutrients that affect consumer performance and reduce fitness (Anderson et al., 2004).
Similar to biological stoichiometry, nutritional geometry, or the “geometric framework” for animal nutrition, focuses on how the availability of multiple nutrients affect consumer growth and fitness and relies on homeostatic regulation as a conceptual cornerstone (Raubenheimer et al., 2009; Simpson & Raubenheimer, 2011, 2012). However, nutritional geometry differs by focusing on the availability of micro‐ and macronutrients (i.e., amino acids, carbohydrates, lipids), rather than chemical elements, making it a more nutritionally explicit framework (Raubenheimer et al., 2009; Sperfeld et al., 2017) but less easy to scale across levels of biological organization. This framework recognizes that foods are complex mixtures of nutrients and other non‐nutritive compounds and that any single food item is likely to provide an insufficient supply of at least some essential nutrients relative to consumer requirements at a given point in time. Nutritional geometry models relationships among various nutrients (or non‐nutrients, such as allelochemicals in some applications) using a multidimensional state‐space approach to characterize the mechanisms, such as foraging behavior or nutrient assimilation, that animals use to achieve nutritional homeostasis while foraging among foods of varying nutrient composition (Simpson & Raubenheimer, 2011). This geometric approach was designed to provide quantitative predictions regarding how consumers utilize multiple foods that vary in their respective amounts of different nutrients (i.e., proteins and carbohydrates) in the service of homeostatic regulation of nutrient intake (Raubenheimer et al., 2009; Simpson & Raubenheimer, 2011).
Much of the early formulation and application of nutritional geometry focused on how animals forage among various resources to achieve a specific target ratio of two or more nutrients in their diet (Sperfeld et al., 2017). Proponents of nutritional geometry have recognized that taste and other chemosensory systems (i.e., olfaction), as well as learned nutritional associations for various foods, play an important role in nutrient‐based foraging decisions, allowing consumers to achieve balanced supply of multiple nutrients (Simpson & Raubenheimer, 2012). In contrast, most applications of biological stoichiometry have focused on how organisms reconcile imbalances in the supply of essential elements via post‐ingestive mechanisms, such as altering element use efficiencies (i.e., assimilation) and differential excretion of individual elements, rather than through selective foraging. However, the potential for biological stoichiometry theory to inform our understanding of how animals forage among nutritionally heterogenous food resources, particularly through the evolution and employment of chemosensory systems, has been previously suggested (Frost et al., 2005), but remains largely unexplored.
In the following sections, we describe how these two complementary frameworks provide a platform, rooted in the concept of consumer homeostasis, to describe the evolutionary context from which multiple different taste modalities have evolved. A standard assumption of biological stoichiometry is that tissue concentrations of individual elements can often be related to a small number of specific biochemicals. Protein, for example, is the primary N pool in most organisms (Sperfeld et al., 2017; Sterner & Elser, 2002). Thus, biological stoichiometry sometimes treats individual elements (i.e., N) as surrogates for the biochemicals (i.e., amino acids) that stimulate gustatory receptors and produce different taste modalities. Nevertheless, biological stoichiometry and the more biochemically focused nutritional geometry often produce qualitatively similar predictions regarding the ability of animals to taste certain elements or compounds. In cases where simple elemental imbalances are sufficient to predict the evolution of certain taste capabilities, we favor the conceptually simpler biological stoichiometry but acknowledge this does not preclude interpretation from a nutritional geometry perspective. By emphasizing individual elements, biological stoichiometry enjoys broader application and more explicitly links animals to the biogeochemical cycling of elements (Sperfeld et al., 2017; Sterner & Elser, 2002). There are, however, notable cases where we believe nutritional geometry provides greater insight into potential evolutionary drivers of taste, such as for carbohydrate and lipid taste. As such, we rely on both frameworks in our discussion of the evolutionary contexts for different taste modalities, with the common thread being that consumers have relatively specific nutritional requirements, determined largely by their own chemical composition (which is relatively homeostatic), and that potential foods (at least individually) often do not satisfy those requirements.
3. CONSUMER HOMEOSTASIS AND NUTRITIONAL IMBALANCE: AN EVOLUTIONARY CONTEXT FOR NUTRITIVE TASTE
The concept of elemental homeostasis, meaning that organisms have limited capacity to alter their chemical composition even when the supply of chemical elements is variable (Sterner & Elser, 2002), is foundational to both biological stoichiometry theory and nutritional geometry.
A second shared concept is that for many animals, the chemical composition of potential foods can be very different than that of the consumer. These differences result in nutritional imbalances, where certain foods may provide either an under‐ or oversupply of certain nutrients (i.e., elements or biochemicals), that can constrain individual performance and reduce reproductive potential (Simpson & Raubenheimer, 2012; Sterner & Elser, 2002). Because consumers have limited capability to modify their chemical composition to more closely match that of their foods, they must reconcile imbalanced nutrient supply by using pre‐ and/or post‐ingestive mechanisms (Simpson & Raubenheimer, 2012; Sterner & Elser, 2002), such as selective feeding or differential release of individual elements as waste products to achieve nutritional, or maintain tissue, homeostasis. However, post‐ingestion nutrient processing, particularly of nutrients which are oversupplied, may have metabolic costs that affect consumer performance, highlighting the importance of selective foraging to reduce nutritional imbalances at the front end (Boersma & Elser, 2006; Simpson et al., 2004). Gustation is commonly viewed as having evolved to function as a sensory tool that allows animals to assess the chemical composition of their food (Breslin, 2013; Lindemann, 2001; Yarmolinsky et al., 2009). Expanding further, we suggest that the evolution of many individual taste modalities should be explained, at least in part, by common nutritional imbalances in the diets of animals. Moreover, we contend that these common imbalances are the drivers of the remarkable convergence in gustatory evolution among multiple animal lineages (i.e., mammals and insects [Yarmolinsky et al., 2009], birds [Niknafs & Roura, 2018], reptiles [Rowland et al., 2014]). We posit that biological stoichiometry and nutritional geometry provide useful frameworks for identifying and characterizing those imbalances and therefore have great potential to inform our understanding of the evolutionary contexts for many individual taste modalities in animals.
Biological stoichiometry in particular provides a simple, yet useful, framework for characterizing common nutritional imbalances between consumers and their foods by comparing their respective elemental composition (though see carbon, section 4; Elser et al., 2000). We can make some generalizations about consumer nutritional imbalances by identifying elements that are commonly less concentrated in foods than in the tissues of consumers. The common convention in biological stoichiometry is to express the relative concentrations of different elements of consumers and their food as ratios, particularly as C:nutrient (i.e., N or P) ratios. For example, Figure 1 displays biomass C:N and C:P ratios of autotrophs (i.e., plants) and animals across multiple trophic levels. The median C:N and C:P of autotroph biomass is considerably greater than that of animals (at any trophic level; Figure 1), indicating that plant tissue has a greater amount of C per unit N, and C per unit P, than does animal tissue. As such, an animal of typical chemical composition (i.e., around the median) that is feeding on a plant of typical chemical composition will experience undersupply of both N and P, relative to C. We would expect that such elements, here N and P, would have a greater likelihood of limiting consumer growth and metabolism and therefore predict that animals should find them (or the primary chemicals that contain them, as in the case of N and amino acids) pleasing to taste—the pleasing sensation would reward animals for consuming foods that are relatively high in those potentially limiting nutrients.
To illustrate, we compared the whole‐body elemental composition of insects, marine fishes, and mammals with the elemental composition of foliar plant tissue (Figure 2; Table 1) in order to identify which elements are typically less concentrated in plants than in animals. We use plants and animals in this example because of the typically greater difference in the elemental composition of herbivores and plants than carnivores and their animal prey (Figure 1) and with the assumption that this is representative of conditions faced by herbivorous animals (particularly terrestrial) throughout their evolutionary history. Based on principles of biological stoichiometry, we might expect there to be consumptive taste associations for elements that are typically more concentrated in the tissues of animals than in plants, given that these elements are likely to limit performance and reproductive potential. By extension, we would not necessarily expect positive taste associations for elements that are more concentrated in plants than animals. Comparison of animal and plant elemental composition reveals that Na, P, N, and sometimes Ca (i.e., in mammals but not insects) are considerably more concentrated (between ~3.5–40×) in animal than in plant biomass (Figure 2; Table 1). Our comprehensive review of taste research reveals that each of these four elements is indeed associated with at least one chemical, or biochemical, known to have a taste that elicits a consumptive response in some animal species (i.e., N, Na [Yarmolinsky et al., 2009], P [Tordoff, 2017], Ca [Tordoff, 2001]). Conversely, we were unable to find evidence of nutritive taste modalities that were obviously associated with specific elements that are closely balanced between plants and animals (except C, see below), or that are more concentrated in plants than in the tissues of the three animal groups in our analysis.
FIGURE 2.
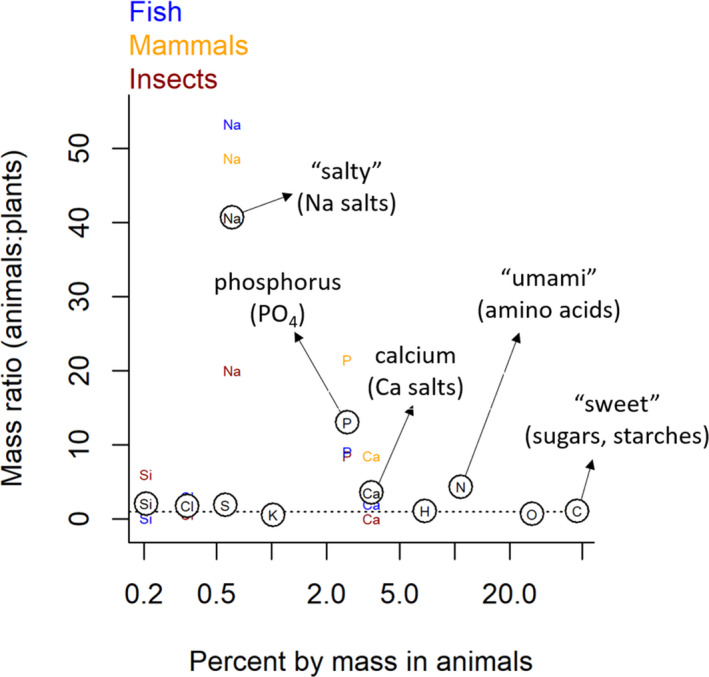
The ratio of biomass concentrations of biologically “essential” elements in animals and plants for all elements which are at least 0.1% of dry mass in animals. Mass ratio is calculated as X A/X P, where X A and X P represent the % of dry mass for element X in animals and plants, respectively. Thus, values >1 indicate elements that are more concentrated in animal than plant tissues, 1 indicates equal concentrations, and values <1 indicate greater concentrations in plant tissue. Circles containing elemental symbols represent the average of data from mammals (orange), insects (black), and fish (blue) compiled by Bowen (Bowen, 1966 and 1979). Data for plants are from Markert (1992) and represent the elemental composition of foliar material
TABLE 1.
The tissue concentrations (as % of dry mass) of essential elements in plants, animals, insects, fish, and mammals
| Element | Atomic mass | Percent of total dry mass | ||||
|---|---|---|---|---|---|---|
| Plants | Animals | Insect | Fish | Mammals | ||
| C | 12.11 | 44.50 | 46.83 | 44.60 | 47.50 | 48.40 |
| O | 15.99 | 42.50 | 26.63 | 32.30 | 29.00 | 18.60 |
| N | 14.01 | 2.50 | 10.80 | 12.30 | 11.40 | 8.70 |
| H | 1.01 | 6.50 | 6.90 | 7.30 | 6.80 | 6.60 |
| Ca | 40.08 | 1.00 | 3.52 | 0.30 | 2.00 | 8.50 |
| P | 30.97 | 0.20 | 2.60 | 1.70 | 1.80 | 4.30 |
| K | 39.09 | 1.90 | 1.02 | 1.10 | 1.20 | 0.75 |
| Na | 22.99 | 0.02 | 0.61 | 0.30 | 0.80 | 0.73 |
| S | 32.97 | 0.30 | 0.56 | 0.44 | 0.70 | 0.54 |
| Cl | 35.45 | 0.20 | 0.35 | 0.12 | 0.60 | 0.32 |
| Si | 28.09 | 0.10 | 0.21 | 0.60 | 7.00E−03 | 1.20E−02 |
| Mg | 24.31 | 0.20 | 9.8E−02 | 0.08 | 0.12 | 0.10 |
| F | 18.99 | 0.0002 | 9.5E−02 | 0.14 | 0.05 | |
| Zn | 65.41 | 0.01 | 2.1E−02 | 0.04 | 8.00E−03 | 1.60E−02 |
| Fe | 55.85 | 0.02 | 1.3E−02 | 0.02 | 3.00E−03 | 1.60E−02 |
| Cu | 63.55 | 1.00E−03 | 2.0E−03 | 5.00E−03 | 8.00E−04 | 2.40E−04 |
| B | 10.81 | 4.00E−05 | 1.1E−03 | 2.00E−03 | 2.00E−04 | |
| Mn | 54.94 | 0.02 | 3.7E−04 | 1.00E−03 | 8.00E−05 | 2.00E−05 |
| Ni | 58.69 | 1.50E−04 | 3.7E−04 | 9.00E−04 | 1.00E−04 | 1.00E−04 |
| Se | 78.96 | 2.00E−06 | 1.7E−04 | 1.70E−04 | ||
| Mo | 95.94 | 5.00E−05 | 8.7E−05 | 6.00E−05 | 1.00E−04 | 1.00E−04 |
| I | 126.9 | 3.00E−04 | 7.8E−05 | 9.00E−05 | 1.00E−04 | 4.30E−05 |
| Co | 58.93 | 2.00E−05 | 5.0E−05 | 7.00E−05 | 5.00E−05 | 3.00E−05 |
| Cr | 51.99 | 1.00E−05 | 2.5E−05 | 3.00E−05 | 2.00E−05 | |
| V | 50.94 | 5.00E−05 | 2.3E−05 | 1.50E−05 | 1.40E−05 | 4.00E−05 |
For many animals, some of these elements (i.e., Ca, Na, P) or associated biochemicals may taste good at some concentrations, but also may produce aversive taste responses when consumed at other, especially high, concentrations. Here, again we can turn to the concept of consumer homeostasis to provide a potential explanation for this pattern. Because oversupply of some essential nutrients, such as Na and Ca, is toxic to consumers and because consumers have limited ability to alter their constitution (homeostasis) consumers may be required to regulate homeostasis by managing nutrient intake within a relatively narrow window. The combination of both consumptive and aversive responses to some essential nutrients, especially those that can have toxic or other negative effects when oversupplied, when supplied at different concentrations can be viewed as helping consumers achieve nutritional homeostasis by regulating nutrient intake (Simpson & Raubenheimer, 2012). Interspecific differences in taste thresholds for certain elements, or chemicals, may reflect variation in chemical composition (which is to say, cellular chemistry) among consumer species or variation in diet that alters imbalances between consumers and their typical foods.
In the following subsections, we discuss taste associations for Na, P, N, and Ca and summarize the evidence that supports these taste capabilities. We provide examples of taste associations, or in some cases preferential feeding behaviors, for these elements within both major vertebrate and invertebrate lineages. Though in some cases, direct links between preferential feeding behaviors and associated taste capabilities have not been explicitly made, we believe this anecdotal evidence is at least suggestive of a role for taste in driving these behaviors and warrants further research. We do, however, acknowledge that such behaviors are not necessarily indicative of taste associations, per se, and may be driven by other characteristics of foods such as texture, or by learned nutritional associations (Simpson & Raubenheimer, 2012).
Nevertheless, consumptive taste associations for each of these four elements have been described for at least some taxa and we argue that the large number of additional species that display preferential feeding behavior suggests that these taste modalities may be more widespread among animals than what is currently known from research on a relatively limited number of organisms. Lastly, there is no general consensus on what criteria must be met to establish a chemosensory stimulus as a basic taste, though the presence of a dedicated gustatory receptor would appear necessary for such a characterization. However, the presence of such receptors, or even seemingly associated consumptive feeding behaviors, does not necessarily indicate neurological taste perception of a particular nutrient. Unfortunately, distinct neurological perception of various taste stimuli has not been assessed in most animals, including many of those from which we draw examples. Therefore, we acknowledge that our definition of taste often overlooks this particular criterion and that we define taste capabilities primarily on the basis of genetic and behavioral evidence.
3.1. Na imbalances and salty taste
Na comprises ~0.3% of the body dry mass of animals but only trace amounts of the mass of terrestrial plants, making it on average ~40× more concentrated in animals than in foliar plant tissue (Figure 1). Na is typically attractive to both vertebrates and at least some invertebrates at particular concentrations (Breslin, 2013; Chen & Dahanukar, 2020; Denton, 1982; Hallem et al., 2006; Richter, 1942; Yarmolinsky et al., 2009). Na salts (i.e., NaCl) are the primary taste molecules associated with gustatory sensing of Na and are responsible for salty taste in humans. The greater Na imbalances between plants and animals than among major animal groups (Figure 2) suggest that selection for Na taste should be stronger for herbivores than predators, and perhaps intermediate for omnivores (Table 1). However, Na taste remains intact and putatively functional in most mammalian species (Antinucci & Risso, 2017; Dethier, 1977; Jiang et al., 2012). Nevertheless, herbivorous mammals appear to have a greater affinity for Na than do carnivorous mammals (Dethier, 1977), and there is considerable evidence of Na‐related feeding behaviors among herbivores (Denton, 1982). For example, many terrestrial herbivores seek out Na‐rich mineral deposits on the landscape. Examples include puddling behavior by insects (Smedley & Eisner, 1995), use of dry or wet licks by vertebrates, drinking of seawater by reindeer, soil eating by mountain gorillas (Blair‐West et al., 1968; Denton, 1982), and preference for Na‐rich aquatic plants over terrestrial plants by moose and other ungulates (Belovsky & Jordan, 1981). Though Na appears to be attractive to most animals at low‐to‐moderate concentrations, aversive responses to high Na concentrations have been reported for both mammals and insects (Breslin, 2013; Hallem et al., 2006; Richter, 1942). Excess levels of dietary Na salts are detrimental to animals and aversive taste responses to high concentrations can be seen as a mechanism to protect against oversupply. Thus, the evolution of both consumptive and aversive taste responses across Na supply gradients is consistent with biological stoichiometry, as it provides animals sensory cues to guide feeding behavior that maintains relatively tight homeostatic regulation of body Na content, a critical component of osmoregulation. Thus, we suggest that dietary Na imbalances (Figure 1) combined with the negative consequences of deviation from strict Na homeostasis have driven selection for both consumptive and aversive Na taste responses.
3.2. P imbalances and P taste
P imbalances are pervasive at lower trophic levels, with P being on average 13× more concentrated in animal than plant biomass (Figure 2). P availability is often rate‐limiting for many organisms, and its relationship to growth has garnered considerable interest from a biological stoichiometry perspective (i.e., growth rate hypothesis, Elser et al., 2003). Though there has historically been substantial interest among ecologists in the role of P as a limiting element in trophic interactions, taste associations for P‐containing compounds have received relatively little attention compared to other basic taste senses (i.e., sweet, salty, and bitter). Nevertheless, P taste has been confirmed in some animal taxa, including cattle (Denton, 1982; Green, 1925; Theiler et al., 1924) and rodents (Tordoff, 2017), and appears to be elicited most strongly by phosphate, which is the primary form of P in many P‐rich biomolecules (i.e., nucleic acids, ATP, phospholipids). Moreover, preferential feeding on P‐rich foods has been observed among numerous invertebrate (i.e., grasshoppers, Ibanez et al., 2017) and vertebrate (i.e., rats, Sweeny et al., 1998) taxa and may be fairly widespread. Because generalist herbivores are more likely to suffer greater dietary P deficiencies than carnivores (Figure 1), and thus stronger selective pressure for P‐associated taste, we propose that P taste should be more common among herbivores (and omnivores) than strict carnivores. Additionally, the higher P requirements imposed by building and maintaining skeletal bone suggest that vertebrates likely experience stronger selection for P taste than do invertebrates. Lastly, excess dietary P supply has been associated with reduced individual growth rates in a variety of organisms (Boersma & Elser, 2006), reflecting the importance of stoichiometrically balanced P supply for consumers. As such, animals may be likely to find P‐associated taste to be aversive at high concentrations, similar to Na and Ca, which may explain why some species reduce consumption rates when reared on high‐P diets (crustaceans [Plath & Boersma, 2001], chickens [Holcombe et al., 1976], and mice [Tordoff, 2017]).
3.3. N imbalances and umami taste
N is, on average, ~4× more concentrated in animal than foliar plant biomass (Figure 2). The frequently limiting role of N makes it a strong candidate for consumptive taste associations. Indeed, such taste associations for various N‐rich amino acids appear to be widespread, having been described in numerous vertebrate and invertebrate taxa. In humans, umami taste is stimulated by the N‐rich amino acids l‐glutamate and l‐aspartate, whereas mice are attracted to many l‐amino acids (Nelson et al., 2001). Insects also make consumptive responses to a variety of amino acids, although the relative sensitivity to different amino acids varies among insect taxa (Chapman, 2003). We posit that selective pressure for N‐associated taste should generally be stronger in herbivores than predators, given the greater N content of animal than plant biomass and thus greater likelihood of N limitation among herbivores (Figure 1). However, retention of umami taste in obligate carnivores may be an indication of the potential for carnivore N limitation. Despite the appearance of stoichiometrically similar prey, carnivores may experience N limitation due to their reliance on amino acids as a source of glucose for cellular respiration and the low carbohydrate content of their food (animal prey, Fagan & Denno, 2004; Kohl et al., 2015).
3.4. Ca imbalances and Ca taste
Ca is, on average, ~3.5× more concentrated in the tissues of animals than in plants, although there is major taxonomic variation in the Ca content of animals due to the production of Ca‐rich skeletal bone in vertebrates (Figure 2). Consumptive taste associations for several Ca salts have been observed among diverse groups of animals including amphibians, birds, and mammals (Gabriel et al., 2009; Niknafs & Roura, 2018; Tordoff, 2001; Tordoff et al., 2008). Calcium taste is likely to be under stronger selection in herbivores than predators, due to the generally lower Ca content of plant biomass (Figure 2; Table 1). Moreover, selection for Ca taste should vary considerably between invertebrates and vertebrates due to large differences in Ca content associated with skeletal bone (and eggshell formation) in the latter. Indeed, Ca appears to be strictly aversive in insects, which are depleted in Ca relative to plants (Figure 1; Chen & Dahanukar, 2020; Lee et al., 2018]), whereas Ca is attractive at low and moderate concentrations to a diverse suite of vertebrates. For example, the Ca content of plant material has been linked to consumptive preferences in rats and mice in laboratory experiments (Tordoff & Sandell, 2009), as well as the feeding behaviors of wild gorilla populations, which use forest clearings to access their preferred Ca‐ and Na‐rich plant species (Magliocca & Gautier‐Hion, 2002). Additionally, dietary Ca deficiencies in herbivorous desert tortoises have been proposed to explain their occasional consumption of numerous Ca‐rich resources such as bones, bone‐rich vulture feces, reptile skin castings, feathers, mammalian hair (Walde et al., 2007), and Ca carbonate stones (Esque & Peters, 1994). Though it remains unclear if these foraging behaviors are informed by gustatory sensing of Ca or some other mechanism, the discovery of Ca taste and associated taste receptors in a variety of species suggests a potential role for gustation (Gabriel et al., 2009; Niknafs & Roura, 2018; Tordoff, 2001; Tordoff et al., 2008). Similar to Na taste, Ca taste is aversive at high concentrations that may result in hypercalcemia (Lee et al., 2018; Tordoff et al., 2012). Thus, the presence of both consumptive and aversive responses to different levels of dietary Ca supply indicates a role for taste in regulating Ca homeostasis in animals and suggests homeostatic maintenance as a likely driver behind the evolution of Ca‐associated taste.
4. CARBON, CARBOHYDRATE, AND LIPID TASTE, A NUTRITIONAL GEOMETRY PERSPECTIVE
We have demonstrated that a relatively simple analysis of elemental imbalances between plants and animals, rooted in foundational concepts of biological stoichiometry, provides a potential explanation of the evolutionary context that gave rise to multiple nutritive taste modalities, including those for Na, N (amino acids), Ca, and P or associated compounds. However, conspicuously absent from this list are nutritive taste associations for certain C‐rich molecules, including the pleasing taste of sugars experienced by humans (sweet taste) and many other organisms. Indeed, biological stoichiometry appears insufficient in providing an explanation for why many animals find certain C‐rich biomolecules, (i.e., sugars) pleasing and others (i.e., structural carbohydrates) not. Carbon is the dominant element by mass in animals and plants and is similarly concentrated in both animal and plant foliar tissues (Figure 2). However, an organism's total demand for C is not determined solely by its body C composition but must also account for additional C for energy metabolism (Frost et al., 2006), given that C compounds are relied upon as energy by nearly all life forms. Thus, focusing strictly on biomass proportions of C in plants and animals suggests that the C concentration of food is unlikely to limit consumer growth and metabolism. As such, dietary C imbalances for consumers may be obscured by the similar C content of animals and plants (Figure 2).
Assessing potential C‐related nutritional imbalances based on bulk C concentration of foods is also difficult given the wide variety of C‐containing biochemicals that vary in their essentiality, digestibility, and ability to be synthesized by consumers. For example, a large proportion of the C in foliar tissue of plants occurs in the form of recalcitrant structural carbohydrates (i.e., cellulose and lignin) that many animals cannot digest, rather than structurally simpler carbohydrates, such as sugars and starches, that are more labile and energy‐dense. Additionally, many essential C‐containing and C‐rich macronutrients, including some lipids and amino acids, cannot be synthesized endogenously by animals and therefore must be acquired through the diet. As such, it would be advantageous for animals to differentiate among foods not by their total C concentration, but by assessing the presence and relative abundances of different C‐containing macronutrients.
Here, we turn to the framework of nutritional geometry to inform our understanding of how the evolution of C‐associated tastes, such as that for sugars, are shaped by the need to regulate nutritional homeostasis (i.e., a target nutrient intake ratio) while foraging among foods that vary in their relative concentrations of multiple essential macronutrients. Nutritional geometry studies have often focused on the relative concentrations of C‐containing macronutrients, including carbohydrates, lipids, and proteins, in multiple foods (Raubenheimer & Simpson, 2018; Simpson & Raubenheimer, 2011; Simpson et al., 2004). Given that some of these nutrients cannot be synthesized by animals and that they are variably concentrated in different foods, one would expect that animals would experience strong selective pressure on mechanisms, including taste, that allow them to detect their presence and assess their concentrations in potential foods (Simpson & Raubenheimer, 2012). In the next several paragraphs, we discuss the evidence for taste associations for carbohydrates and lipids and how nutritional geometry can inform our understanding of their evolutionary history. Although we previously discussed the evolution of amino acid taste in context of dietary N deficiencies, we acknowledge here that nutritional geometry may provide additional insight into the evolution and radiation of amino acid taste in animals. For example, the more nutritionally explicit framework of nutritional geometry may help us understand why different animal species taste different combinations of amino acid molecules by considering the unique nutrient requirements and diets of different species.
Carbon is the primary energy currency of life and it is glucose that fuels the process of cellular respiration in animals. Dietary carbohydrates, such as sugars and starches, are the primary source of glucose for many animals, though it can also be derived from other sources (i.e., lipids, amino acids). Given that foods can vary substantially in their total carbohydrate content and that different carbohydrates (i.e., sugars vs. fiber) vary in their digestibility, animals should experience selective pressure on mechanisms that allow them to detect and differentiate between types of dietary carbohydrates in foods. Indeed, many animals taste simple sugars and find sugar taste attractive, while many mammal species are also able to taste, and enjoy more complex carbohydrates, including starches and starch derivatives (Sclafani, 2004). Animals, however, do not appear to taste complex structural carbohydrates, such as cellulose fiber. Thus, it appears that carbohydrate taste is fine‐tuned for labile, energy‐rich carbohydrates, thereby enabling consumers to regulate their intake of energetically important foods. The link between sugar taste in animals and metabolic C demand that we suggest is supported by multiple lines of evidence. For example, in frugivorous nonhuman primates, sucrose taste threshold increases with decreasing body size (Hladik & Simmen, 1996), indicating that the sweet taste receptors of small‐bodied species are tuned for selecting foods with higher sucrose than those of large‐bodied species. This pattern is consistent with basic allometric scaling rules relating to organismal metabolic rates, where mass‐specific metabolic rates decrease with increasing body size (Brown et al., 2004) and is suggestive of a link between sugar taste thresholds and C demands of at least some primate species. Moreover, bumblebees and honey bees have gustatory receptor neurons that are triggered by especially high sugar concentrations (Miriyala et al., 2018) providing a gustatory mechanism to meet their high metabolic C demand. Likewise, hummingbirds' high metabolic demands for C (Suarez & Welch, 2017) are consistent with the evolution of their sweet taste receptor from their ancestors' umami taste receptor as their diet and habits changed (Baldwin et al., 2014). These patterns are consistent with the principles of nutritional geometry which recognizes animals use sensory systems, like taste, to inform foraging decisions that balance the intake of multiple nutrients that vary in their concentration among different foods.
Lipids, including fatty acids, phospholipids, and steroids, are another important and diverse class of C‐rich macromolecules that are essential in animal nutrition and can be an important source of glucose for cellular respiration. Different types of lipids vary substantially in their nutritional value and some nutritionally important lipids, including various essential fatty acids (i.e., linoleic acid), must be obtained directly from the diet. Animals possess multiple systems to evaluate the lipid content of foods, including taste, olfaction, and gut‐nutrient sensing (Besnard et al., 2015; Mizushige et al., 2007; Sclafani & Ackroff, 2012). However, the extent to which gustation, specifically, is responsible for the palatability of fats remains in question (Besnard et al., 2015; Mizushige et al., 2007; Roper & Chaudhari, 2017). Nevertheless, taste associations for lipid molecules have been demonstrated for a variety of species and range from attractive to aversive depending on the molecule and consumer species (Ahn et al., 2017; Besnard et al., 2015; Roper & Chaudhari, 2017). For example, the attractive taste of lipids experienced by some mammals, including mice, is apparently stimulated by certain long‐chain fatty acid molecules, such as linoleic acid, and not by abundant triglyceride fatty acids, which can produce an aversive response (Besnard et al., 2015; Gaillard et al., 2008; Roper & Chaudhari, 2017). In Drosophila, linoleic and octanoic acids stimulate appetitive gustatory receptor neurons (GRNs) while hexanoic acid activates both attractive and repulsive GRNs (Ahn et al., 2017). The combination of attractive and repulsive tastes induced by various lipids may help consumers achieve nutritional homeostasis with respect to lipid supply by promoting consumption of potentially limiting essential nutrients (i.e., linoleic acid) while protecting against the myriad negative health consequences of overconsumption of fats (Willett, 1994). For example, bumblebees regulate lipid consumption within relatively narrow bounds based on the nutritional composition of their food sources (protein‐to‐lipid ratio), consistent with predictions of nutritional geometry (Raubenheimer et al., 2020; Vaudo et al., 2017). However, it remains unclear the extent to which such behavior is triggered by sensory cues other than taste [i.e., olfaction, texture, post‐ingestive cues (Sclafani & Ackroff, 2012)], highlighting the need to consider how other sensory systems interact to influence foraging behaviors that play a role in regulating the intake of lipids (Simpson & Raubenheimer, 2012).
Nutritional geometry implies that animals that consume a variety of foods of varying nutrient composition will benefit from the ability to detect, whether by taste or another mechanism, the presence of multiple nutrients in their food. Animals should experience particularly strong pressure for nutrient‐sensing systems that detect essential biochemicals that cannot be synthesized and must be consumed. Tastes for certain fatty acids, as discussed above, are examples. Another example is the attractive taste of vitamin C (ascorbic acid) to guinea pigs (Smith & Balagura, 1975) and certain laboratory strains of rats (Yasuo et al., 2019), which are incapable of vitamin C synthesis. Consumptive taste associations for vitamin C, which has a chemical formula of C6H8O6, would not necessarily be predicted from an analysis of stoichiometric imbalances between guinea pigs or rats and their food.
5. LACK AND LOSS OF TASTE SENSES AMONG ANIMAL SPECIES
Understanding why some animal species possess, or lack, certain taste senses has generated considerable interest among taste researchers, particularly in cases where specific taste functions have been lost within some animal lineages (Antinucci & Risso, 2017; Jiang et al., 2012a). The loss of taste receptor function has been linked, in part, to evolutionary shifts in feeding ecology, including dietary specialization, that reduce the selective pressures on taste receptor genes (Feng et al., 2014; Jiang et al., 2012a, 2012b; Liu et al., 2016; Zhao et al., 2010, 2012). For example, sweet taste has been independently lost in multiple lineages of terrestrial carnivores, including the strictly carnivorous felids, cetaceans, vampire bats (Antinucci & Risso, 2017; Jiang et al., 2012a; Zhao et al., 2012), and insectivorous bats (Jiao et al., 2021). This loss of sweet taste has been linked to pseudogenization of the T1R2 (sweet) receptor gene following dietary shifts toward obligate carnivory (Antinucci & Risso, 2017) as the relative lack of carbohydrates in animal tissue is thought to reduce selective pressure for maintaining a functioning sweet taste receptor (Jiang et al., 2012a). Shifts in feeding mode, meaning how an organism feeds (i.e., swallowing vs. chewing prey and fluid feeding), have also been linked to loss of taste functions in some animal lineages. For example, the substantial loss of taste (sweet, umami, bitter, and sour) in cetaceans has been linked to pseudogenization of multiple genes following an ancestral dietary shift from herbivory to carnivory (animals contain fewer carbohydrates and bitter‐tasting compounds than plants), as well as a behavioral shift toward swallowing prey whole, which precludes the release of taste compounds within the oral cavity through mastication (Feng et al., 2014). Additionally, in vampire bats, the shift to blood feeding and use of infrared sensing to locate blood flows have presumably reduced selection for functional carbohydrate and amino acid taste receptors (Zhao et al., 2012) resulting in the loss of sweet and umami tastes, respectively. Despite these notable examples, lineage‐specific pseudogenization events do not always align with shifts in feeding ecology or behavior, suggesting gaps in our understanding of the physiological function of taste receptor genes and the selective pressures that influence their maintenance (Antinucci & Risso, 2017; Feng & Zhao, 2013).
We suggest that biological stoichiometry can provide additional insight into the ecological contexts under which natural selection is likely to favor, or possibly disfavor, the evolution and maintenance of functional taste capabilities across diverse animal lineages. By focusing on differences in the elemental composition of plants and animals, as well as major vertebrate lineages, we can use biological stoichiometry to understand how selective pressure for certain taste capabilities might vary among species on the basis of their trophic position (herbivore, omnivore, or carnivore) and evolutionary history (i.e., mammals and insects). For example, differences in organismal stoichiometry among trophic levels, such as those depicted in Figure 1, suggest that animals are likely to experience differential selective pressure for certain taste capabilities based on their trophic position. The narrower variation in the elemental composition of animals compared with plants (Figure 1) indicates that stoichiometric imbalances for N and P, and potentially Na and Ca (Figure 2), are likely to be greater, and more common, for animals that feed at lower trophic positions, such as generalist herbivores and omnivores, than those that feed at higher trophic levels (i.e., obligate carnivores). Put another way, carnivores are more likely to encounter food that has a chemical composition matching their own, thereby reducing selective pressure on the genes that allow them to differentiate among foods of varying composition. Conversely, generalist herbivores and omnivores consume foods that are much more heterogeneous in their elemental composition and less likely to be in balance with their own chemical composition, meaning they should experience stronger selective pressure on their gustatory systems than strict carnivores.
When taste receptor genes are no longer under strong selective pressure, they should be more likely to accumulate deleterious mutations that result in their becoming broken pseudogenes. To illustrate this concept, we turn to data compiled by Feng and Zhao (2013) who used available genomes of 48 mammals to demonstrate widespread pseudogenization of taste receptor genes, including those for the T1R1 umami receptor. Based on principles of biological stoichiometry, we would expect herbivores to experience much greater dietary N deficiencies than carnivores, because of the higher and more variable C:N ratio of plants than animals (Figure 1), and therefore stronger selective pressure to maintain functioning umami (amino acid) taste receptor genes. Though Feng and Zhao (2013) concluded that there was no common dietary factor that explained the pseudogenization of the T1R1 umami taste receptor across those 48 mammal species, a higher proportion of predatory species (carnivores, insectivores, piscivores) lacked a functioning umami receptor gene (60% defective or absent, n = 10) than omnivores (13.5% defective or absent, n = 22), or herbivores (31% defective or absent, n = 16) in their dataset. Thus, there is some limited evidence that N‐associated umami taste is more common for consumers that experience large dietary imbalances in N supply, suggesting that biological stoichiometry may be useful for understanding general patterns regarding the loss of some taste functions in animals. However, we caution that biological stoichiometry may not accurately predict the presence or absence of specific tastes for individual species as those are undoubtedly shaped by each species unique evolutionary history. For example, the loss of amino acid triggered umami taste in some herbivores, like the giant panda, would not be predicted by biological stoichiometry or nutritional geometry. In the case of the giant panda, loss of umami taste is likely a reflection of reduced selective pressure on taste receptors resulting from the reduced dietary breadth of giant pandas, which almost exclusively consume bamboo, relative to ancestral species (Zhao et al., 2010). As such, the apparently higher rate of pseudogenization among herbivores than omnivores may reflect strict dietary specialization, which can lead to reduced pressure on taste receptor genes, among some herbivores relative to generalist omnivores. Additionally, retention of umami taste in some carnivores, such as felids, may be an indication of predator N limitation, despite the appearance of relatively stoichiometrically balanced prey (Fagan & Denno, 2004; Kohl et al., 2015). In such cases, N limitation may be driven by consumer metabolism, as many predators rely on gluconeogenesis to convert N‐rich amino acids into glucose for cellular respiration due to the lack of carbohydrates in animal prey (Kohl et al., 2015). Finally, some amino acids can be detected by taste receptors other than those involved in umami taste (Chaudhari et al., 2009; Nelson et al., 2002) suggesting that loss of umami taste does not preclude gustatory detection of amino acids.
Though interspecific variation in elemental composition tends to be lower among different animal than plant species, there are several elements that vary considerably among animal species based on broad‐scale phylogenetic relationships. For example, P and Ca tend to be more concentrated in vertebrates than insects, a result of the presence P‐ and Ca‐rich skeletal bones in the former (Figure 1). As such, vertebrates may be more likely to experience, or experience greater magnitude, P and Ca imbalances than insects within any given trophic level, making it more likely they should find P and Ca taste attractive. Indeed, Ca is attractive at some concentrations to a diverse suite of vertebrates (birds, amphibians, mammals), but appears to be strictly aversive in insects, which are depleted in Ca relative to plants (Figure 1; Chen & Dahanukar, 2020; Lee et al., 2018). Thus, we believe that biological stoichiometry can inform our understanding of the phylogenetic distribution of taste by revealing differential elemental imbalances between animals and their food both among major animal lineages and consumer trophic levels, while accepting that the full suite of taste capabilities possessed by any species will ultimately be influenced by that species' evolutionary history.
6. LIMITATIONS OF BIOLOGICAL STOICHIOMETRY AND NUTRITIONAL GEOMETRY
We have previously discussed that dietary specialization has been linked to reductions in diversity of taste capabilities in some species. In such cases, the presence or absence of taste senses may deviate from expectations based solely on principles of biological stoichiometry and nutritional geometry. In cases of extreme dietary specialization, especially those that result in large nutritional imbalances between the consumer and its food, animals may rely on a diverse suite of adaptations ranging from specialized physiology and digestive morphology to mutualisms with endosymbiotic microorganisms to reduce the severity of nutritional deficiencies in their diets. For example, some termites that feed on nutritionally poor wood rely on microbial endosymbionts to breakdown cellulose (Breznak & Brune, 1994), and for microbial N fixation within the gut (Potrikus & Breznak, 1977). Animals that possess such adaptations may circumvent the effects of nutritional limitation on poor quality diets by means other than dietary selectivity informed by gustatory sensing and therefore may experience little selective pressure to maintain functioning gustatory systems.
We have primarily focused our discussion of taste senses on those that promote consumptive, rather than aversive, behavioral responses, though we do discuss aversive responses to high concentrations of compounds that produce consumptive responses under some conditions (i.e., Na, P, and Ca salts). This distinction is important given that some aversive tastes, such as bitter and perhaps to some extent sour, are generally thought to inform consumers that foods may contain toxic compounds, or low pH, that may be harmful if ingested, even in low quantities (Breslin, 2013; Yarmolinsky et al., 2009). Thus, aversive tastes encourage consumers to reject foods and do not necessarily align with our applications of biological stoichiometry theory and nutritional geometry, which we link primarily to consumptive tastes that drive nutrient acquisition. Indeed, aversive tastes may alert consumers to the presence of potentially harmful compounds in foods that may appear nutritious when considered from a strictly biological stoichiometry perspective. Interestingly, animals can, in some cases, learn to tolerate, or even prefer, bitter and sour tastes in their food and drink, particularly when the toxicity of taste compounds is negligible relative to potential nutritional and pharmacological benefits (Capaldi & Privitera, 2008). Though aversive responses to bitter and sour tastes are not necessarily consistent with principles of biological stoichiometry, we have documented several examples where aversive tastes for high concentrations of certain elements, such as Na, P, and Ca, do align. The aversive responses to highly concentrated supply of Na, P, and Ca contrast to the consumptive response that these same elements induce at low and moderate concentrations. These differential responses to low and high levels of dietary Na, P, and Ca can be viewed as adaptations that reduce the potential for physiological consequences associated with deviation from strict homeostatic Na, P, and Ca regulation and thus provide examples of aversive taste responses that are consistent with the core principles of biological stoichiometry theory.
7. FUTURE DIRECTIONS
We have provided numerous hypotheses for understanding the evolution of consumptive taste in animals based on the core principles of biological stoichiometry theory and the complementary framework of nutritional geometry. We believe that our review of the taste biology literature has produced compelling evidence that supports the usefulness of these frameworks for informing our understanding of the evolution of taste. Here, we highlight several observations and questions that have emerged from this analysis that point to exciting avenues for future work that we hope will bridge the fields of taste biology, physiology, biological stoichiometry, ecology, and evolution.
We have previously acknowledged that support for some of the ideas presented in this manuscript come from relatively small bodies of literature. For example, both P‐ and Ca‐associated tastes have been described for a small number of taxa, reflecting the lack of attention for these particular taste capabilities relative to the basic taste modalities recognized in humans (e.g., salty, bitter, sour, sweet, umami). Nevertheless, our analysis suggests that P‐ and Ca‐associated taste may be widespread given the large number of species that display preferential feeding behaviors related to these elements and provides some guidance on where to look for candidate species that might possess these specific taste capabilities (i.e., herbivores and omnivores for P, vertebrates for Ca). Thus, we believe that both P‐ and Ca‐associated tastes are deserving of increased attention in a wider array of taxa, including invertebrates.
In addition to the elements surveyed here (Na, P, N, Ca, and C), several trace elements such as Bo, Fl, Fe, and Zn are present in much greater concentrations in animal tissues than the foliar tissue of plants, suggesting the potential for consumptive taste associations for those elements. For example, it is worth noting that Zn is essential for the proper functioning of taste receptors themselves (Henkin, 1984). Future research may assess the potential for gustatory sensing of those elements in animals, followed by efforts to characterize the genetic and molecular nature of those gustatory systems. Further research is also warranted to better understand potential taste associations for essential biochemicals that might not be predicted by biological stoichiometry. Gustatory sensing of essential biochemicals, particularly those that are not endogenously synthesized by animals, should be highly favored, leading to the evolution of taste associations.
Rapid advances in molecular and genetic techniques have enabled great insight into the chemical and genetic underpinnings of taste in both vertebrate and invertebrate models. We now know the specific receptor genes involved in producing many different taste modalities. This opens the possibility of analyzing the distribution of different taste modalities throughout the animal kingdom and thereby facilitating the extension of taste research to a larger number of species, including a greater survey of invertebrate fauna. This might prove especially useful for ecologists seeking to understand how animals employ behavioral or life‐history strategies to minimize nutritional imbalances in natural settings, thereby influencing interspecific predator–prey interactions, as well as food web and ecosystem dynamics. For example, a species ability to taste a particular nutrient is likely an important trait that influences the role of that species in nutrient cycling. We encourage continued efforts to understand the genetic and molecular bases of different taste capabilities, particularly those exhibited by invertebrates. Identification of specific taste receptors and their associated genes will facilitate broad phylogenetic analyses that reveal the taxonomic distribution and evolutionary history of individual taste capabilities.
CONFLICT OF INTEREST
The authors of this manuscript declare no competing interests between the listed authors, institutions, or any third parties concerning this manuscript.
AUTHOR CONTRIBUTIONS
Lee M. Demi: Conceptualization (lead); data curation (lead); writing‐original draft (lead); writing‐review & editing (lead). Brad W. Taylor: Conceptualization (equal); writing‐original draft (supporting); writing‐review & editing (equal). Benjamin J. Reading: Conceptualization (equal); writing‐original draft (supporting); writing‐review & editing (equal). Michael G. Tordoff: Conceptualization (supporting); writing‐original draft (supporting); writing‐review & editing (equal). Robert R. Dunn: Conceptualization (equal); writing‐original draft (supporting); writing‐review & editing (equal).
ACKNOWLEDGMENTS
This manuscript was improved by comments from P Jiang, JA Balik, R Irwin, and S Jordt. This work was supported by the U.S. National Science Foundation [grant number 1556914] as well as the Department of Applied Ecology and Dr. Jules Silverman at North Carolina State University.
Demi LM, Taylor BW, Reading BJ, Tordoff MG, Dunn RR. Understanding the evolution of nutritive taste in animals: Insights from biological stoichiometry and nutritional geometry. Ecol Evol. 2021;11:8441–8455. 10.1002/ece3.7745
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated, and all data presented (Table 1; Figures 1 and 2) can be accessed from the original sources.
REFERENCES
- Ahn, J. , Chen, Y. , & Amrein, H. (2017). Molecular basis of fatty acid taste in Drosophila. eLife, 6, 1–21. 10.7554/eLife.30115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, T. R. , Boersma, M. , & Raubenheimer, D. (2004). Stoichiometry: Linking elements to biochemicals. Ecology, 85, 1193–1202. 10.1890/02-0252 [DOI] [Google Scholar]
- Anderson, T. R. , Raubenheimer, D. , Hessen, D. O. , Jensen, K. , Gentleman, W. C. , & Mayor, D. J. (2020). Geometric stoichiometry: Unifying concepts of animal nutrition to understand how protein‐rich diets can be “Too much of a good thing”. Frontiers in Ecology and Evolution, 8, 1–12. 10.3389/fevo.2020.00196 [DOI] [Google Scholar]
- Antinucci, M. , & Risso, D. (2017). A matter of taste: Lineage‐specific loss of function of taste receptor genes in vertebrates. Frontiers in Molecular Biosciences, 4, 1–8. 10.3389/fmolb.2017.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin, M. W. , Toda, Y. , Nakagita, T. , O'Connell, M. J. , Klasing, K. C. , Misaka, T. , Edwards, S. V. , & Liberles, S. D. (2014). Evolution of sweet taste perception in hummingbirds by transformation of the ancestral umami receptor. Science, 345, 929–933. 10.1126/science.1255097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belovsky, G. E. , & Jordan, P. A. (1981). Sodium dynamics and adaptations of a moose population. Journal of Mammalogy, 62, 613–621. 10.2307/1380408 [DOI] [Google Scholar]
- Besnard, P. , Passilly‐Degrace, P. , & Khan, N. A. (2015). Taste of fat: A sixth taste modality? Physiological Reviews, 96, 151–176. [DOI] [PubMed] [Google Scholar]
- Blair‐West, J. R. , Coghlan, J. P. , Denton, D. A. , Nelson, J. F. , Orchard, E. , Scoggins, B. A. , Wright, R. D. , Myers, K. , & Junqueira, C. L. (1968). Physiological, morphological and behavioural adaptation to a sodium deficient environment by wild native Australian and introduced species of animals. Nature, 217, 922–928. 10.1038/217922a0 [DOI] [PubMed] [Google Scholar]
- Boersma, M. , & Elser, J. J. (2006). Too much of a good thing: On stoichiometrically balanced diets and maximal growth. Ecology, 87, 1325–1330. 10.1890/0012‐9658(2006)87[1325:TMOAGT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bowen, H. J. M. (1966). Trace elements in biochemistry. Academic Press. [Google Scholar]
- Bowen, H. J. M. (1979). Environmental chemistry of the elements. New York, NY: Academic Press. [Google Scholar]
- Breslin, P. A. S. (2013). An evolutionary perspective on food and human taste. Current Biology, 23, R409–R418. 10.1016/j.cub.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak, J. A. , & Brune, A. (1994). Role of microorganisms in the digestion of lignocellulose by termites. Annual Review of Entomology, 39, 453–487. 10.1146/annurev.en.39.010194.002321 [DOI] [Google Scholar]
- Brown, J. H. , Gillooly, J. F. , Allen, A. P. , Savage, V. M. , & West, G. B. (2004). Toward a metabolic theory of ecology. Ecology, 85, 1771–1789. 10.1890/03-9000 [DOI] [Google Scholar]
- Capaldi, E. D. , & Privitera, G. J. (2008). Decreasing dislike for sour and bitter in children and adults. Appetite, 50, 139–145. 10.1016/j.appet.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Chapman, R. F. (2003). Contact chemoreception in feeding by phytophagous insects. Annual Review of Entomology, 48, 455–484. [DOI] [PubMed] [Google Scholar]
- Chaudhari, N. , Pereira, E. , & Roper, S. D. (2009). Taste receptors for umami: The case for multiple receptors. American Journal of Clinical Nutrition, 90, 738–742. 10.3945/ajcn.2009.27462H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. C. D. , & Dahanukar, A. (2020). Recent advances in the genetic basis of taste detection in Drosophila. Cellular and Molecular Life Sciences, 77, 1087–1101. 10.1007/s00018-019-03320-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton, D. A. (1982). The hunger for salt: An anthropological, physiological and medical analysis. Springer‐Verlag. [Google Scholar]
- Dethier, V. G. (1977). The taste of salt. American Scientist, 65, 744–751. [PubMed] [Google Scholar]
- Elser, J. J. , Acharya, K. , Kyle, M. , Cotner, J. , Makino, W. , Markow, T. , Watts, T. , Hobbie, S. , Fagan, W. , Schade, J. , Hood, J. , & Sterner, R. W. (2003). Growth rate‐stoichiometry couplings in diverse biota. Ecology Letters, 6, 936–943. 10.1046/j.1461-0248.2003.00518.x [DOI] [Google Scholar]
- Elser, J. J. , Bracken, M. E. S. , Cleland, E. E. , Gruner, D. S. , Harpole, W. S. , Hillebrand, H. , Ngai, J. T. , Seabloom, E. W. , Shurin, J. B. , & Smith, J. E. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters, 10, 1135–1142. 10.1111/j.1461-0248.2007.01113.x [DOI] [PubMed] [Google Scholar]
- Elser, J. J. , Dobberfuhl, D. R. , Mackay, N. A. , & Schampel, J. H. (1996). Size, and Life Stoichiomet Toward a unified view of cellular and ecosystem processes. BioScience, 46, 674–684. [Google Scholar]
- Elser, J. J. , Fagan, W. F. , Denno, R. F. , Dobberfuhl, D. R. , Folarin, A. , Huberty, A. , Interlandi, S. , Kilham, S. S. , McCauley, E. , Schulz, K. L. , Siemann, E. H. , & Sterner, R. W. (2000). Nutritional constraints in terrestrial and freshwater food webs. Nature, 408, 578–580. 10.1038/35046058 [DOI] [PubMed] [Google Scholar]
- Esque, T. C. , & Peters, E. L. (1994). Ingestion of bones, stones, and soil by desert tortoises. Fish and Wildlife Resources, 13, 105–112. [Google Scholar]
- Fagan, W. F. , & Denno, R. F. (2004). Stoichiometry of actual vs. potential predator‐prey interactions: Insights into nitrogen limitation for arthropod predators. Ecology Letters, 7, 876–883. 10.1111/j.1461-0248.2004.00641.x [DOI] [Google Scholar]
- Feng, P. , & Zhao, H. (2013). Complex evolutionary history of the vertebrate sweet/umami taste receptor genes. Chinese Science Bulletin, 58, 2198–2204. 10.1007/s11434-013-5811-5 [DOI] [Google Scholar]
- Feng, P. , Zheng, J. , Rossiter, S. J. , Wang, D. , & Zhao, H. (2014). Massive losses of taste receptor genes in toothed and baleen whales. Genome Biology and Evolution, 6, 1254–1265. 10.1093/gbe/evu095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipiak, M. , Kuszewska, K. , Asselman, M. , Denisow, B. , Stawiarz, E. , Woyciechowski, M. , & Weiner, J. (2017). Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS One, 12, 1–31. 10.1371/journal.pone.0183236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, P. C. , Benstead, J. P. , Cross, W. F. , Hillebrand, H. , Larson, J. H. , Xenopoulos, M. A. , & Yoshida, T. (2006). Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecology Letters, 9, 774–779. 10.1111/j.1461-0248.2006.00919.x [DOI] [PubMed] [Google Scholar]
- Frost, P. C. , Evans‐White, M. A. , Finkel, Z. V. , Jensen, T. C. , & Matzek, V. (2005). Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos, 109, 18–28. 10.1111/j.0030-1299.2005.14049.x [DOI] [Google Scholar]
- Gabriel, A. S. , Uneyama, H. , Maekawa, T. , & Torii, K. (2009). The calcium‐sensing receptor in taste tissue. Biochemical and Biophysical Research Communications, 378, 414–418. 10.1016/j.bbrc.2008.11.060 [DOI] [PubMed] [Google Scholar]
- Gaillard, D. , Laugerette, F. , Darcel, N. , El‐Yassimi, A. , Passilly‐Degrace, P. , Hichami, A. , Khan, N. A. , Montmayeur, J. , & Besnard, P. (2008). The gustatory pathway is involved in CD36‐mediated orosensory perception of long‐chain fatty acids in the mouse. The FASEB Journal, 22, 1458–1468. 10.1096/fj.07-8415com [DOI] [PubMed] [Google Scholar]
- Green, H. H. (1925). Perverted appetites. Physiological Reviews, 5, 336–348. 10.1152/physrev.1925.5.3.336 [DOI] [Google Scholar]
- Hallem, E. A. , Dahanukar, A. , & Carlson, J. R. (2006). Insect odor and taste receptors. Annual Review of Entomology, 51, 113–135. 10.1146/annurev.ento.51.051705.113646 [DOI] [PubMed] [Google Scholar]
- Henkin, R. I. (1984). Zinc in taste function. Biological Trace Element Research, 6, 263–280. 10.1007/BF02917511 [DOI] [PubMed] [Google Scholar]
- Hladik, C. M. , & Simmen, B. (1996). Taste perception and feeding behavior in nonhuman primates and human populations. Evolutionary Anthropology, 5, 58–71. [DOI] [Google Scholar]
- Holcombe, D. J. , Roland, D. A. , & Harms, R. H. (1976). The ability of hens to regulate phosphorus intake when offered diets containing different levels of phosphorus. Poultry Science, 55, 308–317. 10.3382/ps.0550308 [DOI] [PubMed] [Google Scholar]
- Ibanez, S. , Millery, A. , D'ottavio, M. , Guilhot, R. , & Vesin, E. (2017). Phosphorus‐rich grasshoppers consume plants high in nitrogen and phosphorus. Ecological Entomology, 42, 610–616. 10.1111/een.12425 [DOI] [Google Scholar]
- Jeyasingh, P. D. , Goos, J. M. , Thompson, S. K. , Godwin, C. M. , & Cotner, J. B. (2017). Ecological stoichiometry beyond redfield: An ionomic perspective on elemental homeostasis. Frontiers in Microbiology, 8, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, P. , Josue, J. , Li, X. , Glaser, D. , Li, W. , Brand, J. G. , Margolskee, R. F. , Reed, D. R. , & Beauchamp, G. K. (2012a). Major taste loss in carnivorous mammals. Proceedings of the National Academy of Sciences of the United States of America, 109, 4956–4961. 10.1073/pnas.1118360109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, P. , Josue, J. , Li, X. , Glaser, D. , Li, W. , Brand, J. G. , Margolskee, R. F. , Reed, D. R. , & Beauchamp, G. K. (2012b). Reply to Zhao and Zhang: Loss of taste receptor function in mammals is directly related to feeding specializations. Proceedings of the National Academy of Sciences of the United States of America, 109, 19104. 10.1073/pnas.1205581109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, H. , Xie, H.‐W. , Zhang, L. , Zhuoma, N. , Jiang, P. , & Zhao, H. (2021). Loss of sweet taste despite the conservation of sweet receptor genes in insectivorous bats. Proceedings of the National Academy of Sciences of the United States of America, 118, 2021516118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspari, M. , & Powers, J. S. (2016). Biogeochemistry and geographical ecology: Embracing all twenty‐five elements required to build organisms. American Naturalist, 188, S62–S73. 10.1086/687576 [DOI] [PubMed] [Google Scholar]
- Kirino, M. , Parnes, J. , Hansen, A. , Kiyohara, S. , & Finger, T. E. (2013). Evolutionary origins of taste buds: Phylogenetic analysis of purinergic neurotransmission in epithelial chemosensors. Open Biology, 3, 1–12. 10.1098/rsob.130015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl, K. D. , Coogan, S. C. P. , & Raubenheimer, D. (2015). Do wild carnivores forage for prey or for nutrients?: Evidence for nutrient‐specific foraging in vertebrate predators. BioEssays, 37, 701–709. 10.1002/bies.201400171 [DOI] [PubMed] [Google Scholar]
- LeBauer, K. , & Treseder, D. (2008). Nitrogen limitation of net primary productivity. Ecology, 89, 371–379. [DOI] [PubMed] [Google Scholar]
- Lee, Y. , Poudel, S. , Kim, Y. , Thakur, D. , & Montell, C. (2018). Calcium taste avoidance in Drosophila. Neuron, 97, 67–74. 10.1016/j.neuron.2017.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann, B. (2001). Receptors and transduction in taste. Nature, 413, 219–225. 10.1038/35093032 [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Liu, G. , Hailer, F. , Orozco‐terWengel, P. , Tan, X. , Tian, J. , Yan, Z. , Zhang, B. , & Li, M. (2016). Dietary specialization drives multiple independent losses and gains in the bitter taste gene repertoire of Laurasiatherian Mammals. Frontiers in Zoology, 13, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliocca, F. , & Gautier‐Hion, A. (2002). Mineral content as a basis for food selection by western lowland gorillas in a forest clearing. American Journal of Primatology, 57, 67–77. 10.1002/ajp.10034 [DOI] [PubMed] [Google Scholar]
- Markert, B. (1992). Establishing of “Reference Plant” for inorganic characterization of different plant species by chemical fingerprinting. Water, Air, and Soil Pollution, 64, 533–538. 10.1007/BF00483363 [DOI] [Google Scholar]
- Miriyala, A. , Kessler, S. , Rind, F. C. , & Wright, G. A. (2018). Burst firing in bee gustatory neurons prevents adaptation. Current Biology, 28, 1585–1594. 10.1016/j.cub.2018.03.070 [DOI] [PubMed] [Google Scholar]
- Mizushige, T. , Noue, K. I. , & Ushiki, T. F. (2007). Why is fat so tasty? Chemical reception of fatty acid on the tongue. Journal of Nutritional Science and Vitaminology, 53, 1–4. 10.3177/jnsv.53.1 [DOI] [PubMed] [Google Scholar]
- Nelson, G. , Chandrashekar, J. , Hoon, M. A. , Feng, L. , Zhao, G. , Ryba, N. J. P. , & Zuker, C. S. (2002). An amino‐acid taste receptor. Nature, 416, 199–202. 10.1038/nature726 [DOI] [PubMed] [Google Scholar]
- Nelson, G. , Hoon, M. A. , Chandrashekar, J. , Zhang, Y. , Ryba, N. J. P. , & Zuker, C. S. (2001). Mammalian sweet taste receptors. Cell, 106, 381–390. 10.1016/S0092-8674(01)00451-2 [DOI] [PubMed] [Google Scholar]
- Niknafs, S. , & Roura, E. (2018). Nutrient sensing, taste and feed intake in avian species. Nutrition Research Reviews, 31, 256–266. 10.1017/S0954422418000100 [DOI] [PubMed] [Google Scholar]
- Persson, J. , Fink, P. , Goto, A. , Hood, J. M. , Jonas, J. , & Kato, S. (2010). To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos, 119, 741–751. 10.1111/j.1600-0706.2009.18545.x [DOI] [Google Scholar]
- Plath, K. , & Boersma, M. (2001). Mineral limitation of zooplankton: Stoichiometric constraints and optimal foraging. Ecology, 82, 1260–1269. 10.1890/0012‐9658(2001)082[1260:MLOZSC]2.0.CO;2 [Google Scholar]
- Potrikus, C. J. , & Breznak, J. A. (1977). Nitrogen‐fixing Enterobacter agglomerans isolated from guts of wood‐eating termites. Applied and Environmental Microbiology, 33(2), 392–399. 10.1128/AEM.33.2.392-399.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer, D. , & Simpson, S. J. (1993). The geometry of compensatory feeding in the locust. Animal Behavior, 45, 953–964. 10.1006/anbe.1993.1114 [DOI] [Google Scholar]
- Raubenheimer, D. , & Simpson, S. J. (2018). Nutritional ecology and foraging theory. Current Opinion in Insect Science, 27, 38–45. 10.1016/j.cois.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Raubenheimer, D. , Simpson, S. J. , & Mayntz, D. (2009). Nutrition, ecology and nutritional ecology: Toward an integrated framework. Functional Ecology, 23, 4–16. 10.1111/j.1365-2435.2009.01522.x [DOI] [Google Scholar]
- Richter, C. P. (1942. –1943). Total self‐regulatory functions in animals and human beings. Harvey Lectures., 38, 63–103. [Google Scholar]
- Roper, S. D. , & Chaudhari, N. (2017). Taste buds: Cells, signals and synapses. Nature Reviews, 18, 485–497. 10.1038/nrn.2017.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland, H. M. , Parker, M. R. , Jiang, P. , Reed, D. R. , & Beauchamp, G. K. (2014). Comparative taste biology with special focus on birds and reptiles. Handbook of olfaction and gustation. John Wiley & Sons Inc. [Google Scholar]
- Ruedenauer, F. A. , Raubenheimer, D. , Kessner‐Beierlein, D. , Grund‐Mueller, N. , Noack, L. , Spaethe, J. , & Leonhardt, S. D. (2020). Best be(e) on low fat: Linking nutrient perception, regulation and fitness. Ecology Letters, 23, 545–554. 10.1111/ele.13454 [DOI] [PubMed] [Google Scholar]
- Sclafani, A. (2004). The sixth taste? Appetite, 43, 1–3. [DOI] [PubMed] [Google Scholar]
- Sclafani, A. , & Ackroff, K. (2012). Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 302, R1119–R1131. 10.1152/ajpregu.00038.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, S. J. , & Raubenheimer, D. (2011). The nature of nutrition: A unifying framework. Australian Journal of Zoology, 59, 350–368. 10.1071/ZO11068 [DOI] [Google Scholar]
- Simpson, S. J. , & Raubenheimer, D. (2012). The nature of nutrition: A unifying framework from animal adaptation to human obesity. Princeton University Press. [Google Scholar]
- Simpson, S. J. , Sibly, R. M. , Lee, K. P. , Behmer, S. T. , & Raubenheimer, D. (2004). Optimal foraging when regulating intake of multiple nutrients. Animal Behavior, 68, 1299–1311. 10.1016/j.anbehav.2004.03.003 [DOI] [Google Scholar]
- Smedley, S. R. , & Eisner, T. (1995). Sodium uptake by puddling in a moth. Science, 270, 1816–1818. 10.1126/science.270.5243.1816 [DOI] [PubMed] [Google Scholar]
- Smith, D. F. , & Balagura, S. (1975). Taste and physiological need in vitamin C intake by guinea pigs. Physiology & Behavior, 14, 545–549. 10.1016/0031-9384(75)90179-1 [DOI] [PubMed] [Google Scholar]
- Sperfeld, E. , Martin‐Creuzburg, D. , & Wacker, A. (2012). Multiple resource limitation theory applied to herbivorous consumers: Liebig's minimum rule vs. interactive co‐limitation. Ecology Letters, 15, 142–150. 10.1111/j.1461-0248.2011.01719.x [DOI] [PubMed] [Google Scholar]
- Sperfeld, E. , Wagner, N. D. , Halvorson, H. M. , Malishev, M. , & Raubenheimer, D. (2017). Bridging Ecological Stoichiometry and Nutritional Geometry with homeostasis concepts and integrative models of organism nutrition. Functional Ecology, 31, 286–296. 10.1111/1365-2435.12707 [DOI] [Google Scholar]
- Sterner, R. W. , & Elser, J. J. (2002). Ecological stoichiometry: The biology of elements from molecules to the biosphere. Princeton University Press. [Google Scholar]
- Suarez, R. K. , & Welch, K. C. (2017). Sugar metabolism in hummingbirds and bats. Nutrients, 9, 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny, J. M. , Seibert, H. E. , Woda, C. , Schulkin, J. , Haramati, A. , & Mulroney, S. E. (1998). Evidence for induction of a phosphate appetite in juvenile rats. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 275, 1358–1365. 10.1152/ajpregu.1998.275.4.R1358 [DOI] [PubMed] [Google Scholar]
- Theiler, K. , Green, H. H. , & Du Toit, P. J. (1924). Phosphorus in the livestock industry. Journal of the Department of Agriculture of South Africa, 8, 460–504. [Google Scholar]
- Tordoff, M. G. (2001). Calcium: Taste, intake, and appetite. Physiological Reviews, 81, 1567–1597. 10.1152/physrev.2001.81.4.1567 [DOI] [PubMed] [Google Scholar]
- Tordoff, M. G. (2017). Phosphorus taste involves T1R2 and T1R3. Chemical Senses, 42, 425–433. 10.1093/chemse/bjx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff, M. G. , Alarcón, L. K. , Valmeki, S. , & Jiang, P. (2012). T1R3: A human calcium taste receptor. Scientific Reports, 2, 3–6. 10.1038/srep00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff, M. G. , & Sandell, M. A. (2009). Vegetable bitterness is related to calcium content. Appetite, 52, 498–504. 10.1016/j.appet.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff, M. G. , Shao, H. , Alarcón, L. K. , Margolskee, R. F. , Mosinger, B. , Bachmanov, A. A. , Reed, D. R. , & McCaughey, S. (2008). Involvement of T1R3 in calcium‐magnesium taste. Physiological Genomics, 34, 338–348. 10.1152/physiolgenomics.90200.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni, M. J. , McIntyre, P. B. , Allen, D. , Arnott, D. L. , Benstead, J. P. , Berg, D. J. , Brabrand, Å. , Brosse, S. , Bukaveckas, P. A. , Caliman, A. , Capps, K. A. , Carneiro, L. S. , Chadwick, N. E. , Christian, A. D. , Clarke, A. , Conroy, J. D. , Cross, W. F. , Culver, D. A. , Dalton, C. M. , … Zimmer, K. D. (2017). A global database of nitrogen and phosphorus excretion rates of aquatic animals. Ecology, 98, 1475. 10.1002/ecy.1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudo, A. D. , Stabler, D. , Patch, H. M. , Tooker, J. F. , Grozinger, C. M. , & Wright, G. A. (2017). Correction: Bumble bees regulate their intake of essential protein and lipid pollen macronutrients. Journal of Experimental Biology, 219, 3962–3970. [DOI] [PubMed] [Google Scholar]
- Walde, A. D. , Delaney, D. K. , Harless, M. L. , & Pater, L. L. (2007). Osteophagy by the desert tortoise (Gopherus agassizii). The Southwestern Naturalist, 52, 147–149. 10.1894/0038‐4909(2007)52[147:OBTDTG]2.0.CO;2 [Google Scholar]
- Wilder, S. M. , & Jeyasingh, P. D. (2016). Merging elemental and macronutrient approaches for a comprehensive study of energy and nutrient flows. Journal of Animal Ecology, 85, 1427–1430. 10.1111/1365-2656.12573 [DOI] [PubMed] [Google Scholar]
- Willett, W. C. (1994). Diet and health: What should we eat? Science, 264, 532–537. 10.1126/science.8160011 [DOI] [PubMed] [Google Scholar]
- Yarmolinsky, D. A. , Zuker, C. S. , & Ryba, N. J. P. (2009). Common sense about taste: From mammals to insects. Cell, 139, 234–244. 10.1016/j.cell.2009.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo, T. , Suwabe, T. , & Sako, N. (2019). Behavioral and neural responses to vitamin C solution in vitamin C‐deficient osteogenic disorder Shionogi/Shi Jcl‐od/od rats. Chemical Senses, 44, 389–397. 10.1093/chemse/bjz028 [DOI] [PubMed] [Google Scholar]
- Zhao, H. , Xu, D. , Zhang, S. , & Zhang, J. (2012). Genomic and genetic evidence for the loss of umami taste in bats. Genome Biology and Evolution, 4, 73–79. 10.1093/gbe/evr126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Yang, J. R. , Xu, H. , & Zhang, J. (2010). Pseudogenization of the umami taste receptor gene Tas1r1 in the giant panda coincided with its dietary switch to bamboo. Molecular Biology and Evolution, 27(12), 2669–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated, and all data presented (Table 1; Figures 1 and 2) can be accessed from the original sources.


