Abstract
The roles of many environmental contaminants in increasing breast cancer risk remain controversial. Arsenic (As) is a major global environmental contaminant and carcinogen. We conducted a systematic review of the role of As and gene-arsenic interactions in susceptibility to breast cancer. Following a systematic literature search using well-defined inclusion/exclusion criteria, a total of 15 epidemiologic studies (two meta-analyses, three systematic reviews, three cohort studies, two case-control studies, and five cross-sectional studies) were reviewed. In addition, several animal, in vitro, in vivo, and in silico (i.e., computer modeling) studies provided mechanistic insights into the association between As and breast cancer. Our review suggests a possible overall main effect of As on breast cancer risk. The evidence for an effect of gene-As interactions on breast cancer risk is strong. Studies that measured levels of As metabolites among participants and/or evaluated interactions between As exposure and genetic or epigenetic factors generally reported positive associations with breast cancer risk. Our analysis of the Comparative Toxicogenomics and the Ingenuity Pathway Analysis Databases provided further evidence for As-gene interactions and their effects on breast cancer-related biologic pathways. Our findings provide potential leads for future epidemiologic studies of As-associated cancer risks and interventions to reduce population exposure.
Keywords: epidemiologic studies, gene-environment interactions, gene-metal interactions, toxicogenomics
Introduction
Globally, breast cancer is the most reported malignancy and the leading cause of cancer death among women. Age-standardized (world) incidence rates of breast cancer range from 25.9 to 94.2 per 100,000 person-years with highest rates in Australia and New Zealand (94.2), Western Europe (92.6), Northern Europe (90.1), and North America (84.8) [1]. In the United States (US), breast cancer is the most reported cancer and the second leading cause of cancer death among women. 1 in 8 women in the US will develop breast cancer in their lifetime and 1 in 35 will die from the disease [2]. There will be an estimated 279,100 new cases of invasive breast cancer and 42,690 deaths due to breast cancer in the US in 2020 [2].
Known modifiable environmental and life-style risk factors for breast cancer include obesity, lack of physical activity, excessive alcohol use and long-term oral contraceptive use [3]. These factors explain relatively small attributable risks of breast cancer. Several environmental contaminants, particularly those that accumulate in human tissue and have carcinogenic and/or estrogenic properties, have also been investigated for their role in susceptibility to breast cancer [4]. Gene-contaminant interactions may also play a role [5]. Given that environmental contaminants constitute modifiable risk factors, identifying contaminants associated with breast cancer and elucidating the mechanisms by which they exert their effects may facilitate primary prevention by reducing exposures, particularly among susceptible subgroups.
Arsenic (As) is a major global environmental contaminant, with population exposure through drinking water, food, and certain occupations [6]. The inorganic form of As (iAs) is found in water and certain grains such as rice [7]. Ingested iAs is eliminated as mostly monomethylarsonic acid (MMA) and dimethylarsenic acid (DMA) along with a small amount as iAs. Exposure to both As and iAs has been linked to risk of several chronic diseases and conditions such as arsenicosis skin lesions, heart disease, diabetes, and cancer [8, 9].
Arsenic and As compounds are designated as Group 1 human carcinogens by IARC [10] and as group A carcinogens by the US Environmental Protection Agency (USEPA) [11]. Exposure to As and iAs has been linked to cancers of the lung, liver, bladder, kidneys, and skin [11, 12]. We conducted a systematic review to examine the association between As and the risk of developing breast cancer. Our interest lies in both the main effect of As as well as the effect of gene-As interactions on breast cancer risk. In our study, interaction is defined as both the modifying effects of genetic factors on As-associated breast cancer risk and the effect of As on biologic (genetic and/or epigenetic) pathways involved in breast cancer.
Methods
The main focus of this systematic review was on the following: 1. Main effect of As on breast cancer risk, 2. Modifying effects of genes and gene-As interactions on breast cancer risk, and 3. Effect of As on genetic and epigenetic mechanisms associated with breast cancer.
A literature search was conducted using PubMed and Medscape interfaces on the Medline database using several keywords and phrases. The following search terms were used along with the term breast cancer: heavy metals, arsenic, gene-arsenic interactions, gene-metal interactions, genetic modifiers and metals, genetic modifiers and arsenic, arsenic toxicogenomics, arsenic and genetic mechanisms, arsenic and epigenetic mechanisms, epidemiologic studies of arsenic, and genetic epidemiologic studies of arsenic. All relevant articles in peer-reviewed journals published between June 1, 2006 and May 31, 2020 were retrieved. All meta-analyses, review articles, cohort studies, case-control studies, cross-sectional studies, case series, and case reports were included. Other types of studies such as animal, in vivo, in vitro, and in silico (i.e., using computer modeling) were scanned and included for context and/or mechanistic insight. Meta-analyses and systematic reviews were given the same weight in our synthesis as original research articles. To ensure every paper was included in our study only once, we excluded all the original research articles that were part of previous review articles and meta-analyses included in our review. Articles only reporting on associations with breast cancer mortality were also excluded. Our search included all publications in English or French; the full text of articles in other languages were also reviewed if their abstracts were in either English or French. Relevant data (such as first author’s name, study design, sample size, effect size, statistical significance, and conclusions) were extracted from each paper systematically and tabulated. Literature reviews and data extractions were conducted in duplicates by various authors.
Results
Our search identified 15 relevant epidemiologic studies (two meta-analyses, three systematic reviews, three cohort, two case-control, and five cross-sectional) on the association between arsenic exposure and breast cancer risk (Table 1). The cohort, case-control and cross-sectional studies were unique in that they were not part of any of the meta-analyses or systematic reviews included in our report. In addition to main effects, two systematic reviews, one case-control, and three cross-sectional studies included discussion of gene-As interactions. Additionally, several relevant animal, in-vitro, in-vivo, and in-silico studies were identified three of which are also included in Table 1.
Table 1:
Studies of arsenic and breast cancer included in our review.
| Author, Year, Place | Study design | Study detail/Population | Collected data | Methods and measuresa | Conclusions |
|---|---|---|---|---|---|
| L. Jouybari et al. (Cancer Management and Research, 2018) [13] | Meta-Analysis | 11 studies from multiple countries | Scalp hair (4), Plasma (1), Toenail (2), and Breast tissue (4) | Standard mean Difference:0.52, 95%CI: −0.12–1.16, p=0.114 | No significant statistical difference in Arsenic status between healthy subjects and breast cancer patients. |
| B. Gamboa-Loira et al. (Environmental Research, 2017) [14] | Meta-Analysis (As and cancer risk) | 1 study on breast cancer from Mexico | Urine | OR for %MMA Q5 vs. Q1=2.63, 95%CI: 1.89–3.66 | Significant positive association between % MMA and breast cancer. |
| N. Khanjani et al. (Reviews on Environmental Health, 2017) [15] | Systematic review | 7 studies from multiple countries | Blood (1), urine (1) urine and Blood (1), Toenail (1), and drinking water (3) | Descriptive | Exposure to As may increase the risk of breast cancer to varying degrees depending on individual/regional exposure patterns and genetic susceptibilities. |
| D.F. Romagnolo et al. (Mol. Nutr. Food Res., 2016) [16] | Systematic review (As and epigenetic alterations) | 4 studies from multiple countries | Blood | Descriptive | Interactions between environmental As exposure and alcohol consumption may interfere with normal folate and B12 metabolism and influence breast cancer risk through hypermethylation of tumor suppressor genes. |
| A.S. Bardach et al. (Science of the Total Environment, 2015) [11] | Systematic review (chronic diseases and Arsenic) | 2 studies on breast cancer from Argentina | Aquifer (1) and groundwater (1) | Descriptive | Higher incidence rate ratio per 100 μg/L increment in iAs concentration for breast cancer. |
| A.J. White et al. (Epidemiology, 2019), USA [17] | Cohort Sister study (2003–2009) | 2,587 incident breast cancer cases among 50,884 breast cancer-free women | The Environmental Protection Agency’s National Air Toxics Assessment database | HR (Q5 vs. Q1)=1.0, 95%CI: 0.9–1.2 | No association between Arsenic exposure and breast cancer. |
| R. Zhang et al. (International Journal of Cancer, 2016), USA [18] | Cohort Nurse’s health study (NHS) (1984–2010) | 8115 incident breast cancer cases among 160,408 women | Validated food frequency questionnaires | Multivariable RRs (≥5 servings/week)=0.95, 95%CI: 0.88–1.03 | Long-term consumption of total rice, white rice, or brown rice was not associated with risk of developing breast cancer. |
| R. Liu et al. (Epidemiology, 2015), USA, California [19] | Cohort California Teacher’s study (1995–1996) | 5361 incident breast cancer cases among 112,379 women free of breast cancer living at a California address | Ambient air | HR (Q5 vs. Q1)=1.7, 95%CI: 1.1–2.5 | Long-term, low-dose exposure to iAs may be a risk factor for breast cancer. |
| K.M. O’Brien et al. (American Journal of Epidemiology, 2019), USA [20] | Case-control Sister (2003–2009) and Two sister (2008–2010) studies | 1,217 disease-discordant sister pairs among 50,884 Participants aged 35–74 years | Toenail | OR (Q4 vs. Q1)=1.07, 95%CI: 0.72–1.57 | Little evidence to support a positive association between young-onset breast cancer and exposure to As. |
| B. Gamboa-Loira et al. (Environ Toxicol Pharmacol, 2017), Mexico [21] | Case-control Population-based (Northern Mexico) | 1016 breast cancer cases and 1028 healthy controls | Urine | Interaction p=0.0002 for MTR c.2756A>G polymorphism and % DMA | MTR c.2756A>G polymorphism may confer protection for breast cancer associated with iAs exposure. |
| G. Michel-Ramirez et al. (Journal of Applied Toxicology, 2020), Mexico [22] | Cross-sectional | 182 women >18 years (77 breast cancer cases and 105 controls) recruited from a clinic (all exposed to As through drinking water) | Blood and urine | Adjusted OR for iAs=1.10, 95%CI: 1.01–1.20, p=0.015 | Urinary levels of iAs were associated with significant increase in risk of breast cancer but no interactions between Yes-associated protein (YAP) gene polymorphisms and As urinary levels were identified. |
| O. Ajayi et al. (Medical Sciences, 2018), Nigeria [23] | Cross-sectional | 79 non-pregnant women 28–80 years of age (52 premenopausal and 27 postmenopausal) recruited from a Clinic | Serum from venous blood | Multiple regression Analysis for As related to TSH in premenopausal ER−: β=−0.305 and PR−: β=−0.304 breast cancer | Arsenic was inversely associated with Thyroid stimulating hormone (TSH) in premenopausal participants with ER− and PR− breast cancer. |
| G. Michel-Ramirez et al. (Journal of Applied Toxicology, 2017), Mexico [24] | Cross-sectional | 120 women (76 newly diagnosed breast cancer cases and 44 controls) from a hospital (all exposed to As from drinking water) | Breast biopsies, urine, and toenails | OR for high cytoplasm YAP expression=0.37, 95%CI: 0.17–0.80, p=0.01 | Significantly lower concentration of cytoplasm Yes-associated protein (YAP) expression in cases suggesting YAP may act as a tumor suppressor. |
| N.S. Joo et al. (Biological Trace Element Research, 2009), Korea [25] | Cross-sectional | 144 women (40 breast cancer cases and 144 body-mass index matched controls) recruited from a hospital | Hair | Comparison of mean As among cases and controls: p<0.001 Association of hair iron level with As: r=−0.537, p<0.001 | Breast cancer patients had higher levels of As in hair compared to healthy controls. Lower hair iron levels among breast cancer cases compared to healthy controls and negative correlation of hair iron with As. |
| K. Schlawicke Engstrom et al. (Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2009), Argentina [26] | Cross-sectional (Northern Argentina) | 104 indigenous women exposed to approximately 200 μg/L of As in drinking water with low %MMA and high % DMA | Urine and blood | Association between urinary metabolite patterns of As and SNPs in three gene groups related to As metabolism | Polymorphisms in AS3MT and in genes involved in one-carbon metabolism and reduction reactions affect As metabolism. |
| D.A. Parodi et al. (Reproductive Toxicology, 2015) [27] | Animal | Pregnant spraguedawley rats on day 7 of gestation | Intra-peritoneal injection of arsenite on days 12 and 17 of gestation and morphological analysis of mammary glands | P<0.05 for all comparisons between case and control rats | In utero exposure to arsenite alters the pre- and post-pubertal development of the mammary gland, and possibly the risk of developing breast cancer. |
| E. Egiebor et al. (International Journal of Environmental Research and Public Health, 2013) [28] | In-vitro | MCF7 breast cancer cell lines exposed to different concentrations of As | Kinetic response of As on MCF7 cells measured by realtime cell electronic sensing (RT-CES) | All concentrations of As induced total cell death, ranging from 5 to 20 h after exposure | As was associated with significant cytotoxicity both in the presence and in the absence of glutathione. |
| L. Smeester et al. (Chemical Research in Toxicology, 2011) [29] | In-vivo/In-silico (Mexico) | 16 individuals (8 with elevated levels of iAs exposure and arsenicosis) | Urine, Blood, and Interactome database | Differences in iAs exposure levels in urine: p<0.001 183 differentially-methylated genes in individuals with high iAs levels (arsenicosis): FDR q value<0.05 | Interactome analysis of 183 genes revealed an interactome of hypermethylated genes enriched for those involved in cancer-associated pathways mediated by p53. Also identified an arsenic-methylated tumor suppressorome made up of 17 known or putative tumor suppressors silenced in human cancers. |
Explanation of statistical measures abbreviations: OR, Odds Ratio; 95% CI, 95% Confidence Interval; HR, Hazard Ratio; Q5, Quintile 5 (Highest); Q1, Quantile 1 (Lowest); RR, Relative Risk.
The meta-analysis by Jouybari et al. (2018) involved 11 case-control studies (one collected plasma, four studied breast tissue, and six studied scalp hair and/or toenails) and did not identify an overall significant difference in As concentrations among breast cancer cases and healthy controls [13]. The meta-analysis by Gamboa-Loira et al. (2017) included 13 studies exploring the risk of cancer in association with As but only one study (Lopez-Carrillo et al. 2014) focusing on breast cancer, which reported a significant positive association between %MMA and significant negative association between %DMA and breast cancer [14].
The systematic review by Khanjani, Jafarnejad, and Tavakkoli (2017) [15] suggested a possible association between As and breast cancer risk based on positive results from four of the seven original studies reviewed. This review also concluded that the increase in risk of breast cancer may vary based on individual genetic susceptibilities [15]. The systematic review by Romagnolo et al. (2016), which focused on the main effect of As along with the modifying effect of genetic and epigenetic factors, reported significantly higher risks (OR=~1.25–1.7) of breast tumorigenesis among female carriers of certain genetic mutations, such as 5382insC, C61G, and 4153delA in BRCA1, in association with higher serum As levels (~4–6 μg/L) [16]. This review also suggested that interaction between environmental As exposure and alcohol use may interfere with normal folate and B12 metabolism and influence breast cancer risk through hypermethylation of tumor suppressor genes[16]. Another systematic review (Bardach et al., 2015 [11]) on the association between high levels of As in surface and drinking water aquifers and breast cancer incidence in Argentina reported (based on findings of two ecological studies) higher incidence rate ratio per 100 μg/L increment in iAs concentration for breast cancer [11].
The results of cohort studies were mixed with 2 of 3 reporting no statistically significant association between As exposure and breast cancer risk (Table 1). White et al. (2018) [17] conducted an investigation involving sister study participants (n=50,884) by recruiting breast cancer-free women with a sister with breast cancer (n=2587) and deducing each participant’s exposure level by matching their residence using the Environmental Protection Agency National Air Toxic Assessment’s census-tract estimates of metal concentrations in air. This study reported no association between As and breast cancer risk [17]. Zhang et al. (2016) [18] examined the association between rice intake and breast cancer risk in the Nurses’ Health Study (NHS) I and II, and found non-significant associations between breast cancer and consumption of rice (reported to contain worrisome levels of arsenic in a 2012 US consumer report) [18]. Liu et al. (2015) [19] examined the association between residential exposure to estrogen-disrupting hazardous air pollutants and breast cancer risk, and found an increase in the risk of hormone receptor-negative breast cancer for exposure to iAs compounds at the highest exposure quintile [19].
The two case-control studies also reported contrasting results (Table 1). The study by O’Brien et al. (2019) [20] assessed recent As exposure in toenail clippings of 1217 disease-discordant sister pairs in the US-based Sister (2003–2009) and Two-Sister (2008–2010) studies and found no association between young-onset breast cancer and toenail concentrations of As [20]. Gamboa-Loira et al. (2017) [21] conducted a population-based case-control study in Northern Mexico involving 1016 cases and 1028 controls. They assessed As exposure through levels of urinary metabolites. They also measured methylation ratios of the metabolites and frequency of polymorphisms in a number of candidate genes. Furthermore, they evaluated interactions between genetic polymorphisms, iAs metabolites, and methylation levels. They found that the MTR c.2756A>G polymorphism may decrease the risk of breast cancer associated with iAs. They also found significant interaction (p=0.002) between MTR c.2756A>G and %DMA on the risk of breast cancer with lower %DMA-associated risk among those with AG+GG genotypes compared to those with AA (i.e., homozygote for the common allele) genotypes [21].
All five cross-sectional studies reported associations and correlations suggestive of an increase in risk of breast cancer due to As exposure (Table 1). Michel-Ramirez et al. (2020) [22] found significant increase in risk of breast cancer in association with high urinary levels of iAs but no interactions with Yes-associated protein (YAP) genetic polymorphisms [22]. Ajayi et al. (2018) [23] studied the difference in metabolite levels of endocrine disruptors and certain hormones such as Thyroid Stimulating Hormone (TSH) among hormone receptor positive and negative breast cancer cases. They found that there were no significant differences in the serum As levels between hormone positive and negative women; however, an inverse relationship was found between TSH and As in premenopausal women with estrogen receptor negative (ER−) and progesterone receptor negative (PR−) breast cancer [23]. Another study by Michel-Ramirez et al. (2017) [24] found a lower concentration of cytoplasm YAP in breast cancer cases suggesting that YAP may act as a tumor suppressor. Joo et al. (2009) [25] found higher levels of As in hair of breast cancer patients compared to healthy controls, and a negative correlation of As levels with iron in hair of breast cancer patients. Schlawicke et al. (2009) [26] studied the association with polymorphisms in selected genes involved in As reduction reactions and one-carbon metabolism. They reported two SNPs in AS3MT and one in CYP17A1 being associated with lower %MMA and higher % DMA, hence possibly conferring protection against breast cancer [26]. They also found that polymorphisms in some genes involved in one-carbon metabolism and reduction reactions (including CYP17A1, MTRR, CDHD, GLRX and PRDX2) affect As metabolism [26]. The results of cross-sectional analyses should be interpreted with caution given the limitations of this study design; however, studies reported here highlight the potential biologic mechanisms involved.
Animal, in vitro, in vivo and in silico studies provided supporting evidence for the postulated mechanisms (Table 1). Based on studies in rats, Parodi et al. (2015) [27] concluded that in utero exposure to arsenite alters pre- and post-pubertal mammary gland development, and possibly the risk of breast cancer. Egiebor et al. (2013) [28] studied exposure of MCF7 cancer cells to As and reported significant cytotoxicity both in the presence and absence of glutathion. Smeester et al. (2011) [29] found that many of the proteins encoded by genes with differentially-methylated CpG islands are known players in As-associated diseases including cancer; they reported an As-methylated complex of 17 potential tumor suppressors known to be silenced in human cancers.
Discussion
Our review of epidemiologic and mechanistic studies published in the last 14 years suggests a possible overall main effect for As on breast cancer risk. The evidence for the effect of gene-As interaction on breast cancer risk is strong with several studies reporting modifying effects of mutations and polymorphisms in some genes on As-associated breast cancer risk as well as an effect for As on the expression of several cancer susceptibility genes and pathways. Our evaluation of epidemiologic evidence to date paints a picture that most of the equivocal findings are from studies that measured As exposure indirectly through As levels in water, air or food (such as rice) and extrapolated to individual-level exposures. Studies that evaluated individual-level exposures directly in serum, hair or nail generally found positive associations between As and breast cancer. Of note, nearly all studies that measured levels of As metabolites among participants and/or evaluated interactions between As exposure and genetic or epigenetic factors reported positive associations with breast cancer risk.
The majority of breast cancer cases in the general population are sporadic (i.e., not due to inherited genetic mutations). Hereditary breast cancers are due to segregation of germline mutations and variations in high-, medium- or low-penetrance susceptibility genes [30–32]. Sporadic breast cancer cases are believed to be due to somatic genetic and epigenetic alterations in the breast tissue. Environmental contaminants may induce somatic mutations and chromosomal abnormalities through a variety of genetic and epigenetic mechanisms. If contaminant-induced mutations occur in the germ cells, they can become hereditary in the next generation. Epigenetic alterations caused by environmental contaminants at the somatic level are hypothetically reversible, hence the importance of studying the carcinogenic potential of trace elements such as As. Understanding interactions between environmental contaminants and genes may help elucidate the disease mechanism and identify susceptible subpopulations.
There has been a wealth of information on the association between As and breast cancer since the earliest epidemiologic review of trace elements and cancer risk by Navarro Silvera et al. (2007) [33], which stated that additional studies were needed before any conclusions could be reached with regards to the association between As and breast cancer. We did not include Navarro Silvera et al. (2007) [33] in our Table 1 in order to avoid duplication since the original epidemiologic studies in that review were included in subsequent reviews that are listed in our Table 1.
With respect to the main effect of As on breast cancer risk, studies (one systematic review and all three cohort studies) that extrapolated individual exposures from geographic/ecologic exposure values produced mixed results. The systematic review by Bardach et al. (2015) [11] only included breast cancer studies that measured As in water; they reported higher incidence rate ratio with higher concentrations of iAs. The cohort studies by White et al. (2019) [17] and Zhang et al. (2016) [18] measured As exposure levels indirectly from census-tract airborne As levels and long-term consumption of rice, respectively, and did not find a significant association with breast cancer. The Liu et al. (2015) [19] cohort study also measured exposure to ambient As and iAs indirectly through census-tract air concentrations at residential addresses but they found borderline association between long-term, low-dose iAs exposure and overall breast cancer risk.
The four studies (one meta-analysis, one case-control study, and two cross-sectional studies) that measured individual As exposure levels directly by analyzing hair, serum, nail, or tissue samples of participants produced mixed but suggestive results. The meta-analysis by Jouybari et al. (2018) reported negative associations. The O’Brien et al. (2019) case-control study also reported negative associations. The cross-sectional study by Ajayi et al. (2018) [23] reported an inverse relationship between TSH and As in premenopausal women with ER− and PR− breast cancer, which may suggest an interference of thyroid hormone metabolism by As and possible link with hormone receptor negative breast cancer. The cross-sectional study by Joo et al. (2009)[25] found higher levels of As in hair of breast cancer patients compared to healthy controls, and a negative correlation of As levels with iron in hair of breast cancer patients. The significance of the negative correlation with iron is not known.
All seven studies that measured As metabolite levels in urine and/or interaction between As and genetic/epigenetic factors reported positive associations. These included the meta-analysis by Gamboa-Loira et al. (2017) [14], the systematic reviews by Khanjani, Jafarnejad, and Tavakkoli (2017) [15] and Romagnolo et al. (2016) [16], the case-control study by Gamboa-Loira et al. (2017) [21], and three cross-sectional studies including two by Michel-Ramirez et al. (2017 and 2020) [22, 24] and one by Schlawicke et al. (2009) [26]. Similar to other contaminants, As is metabolized by a series of reduction and methylation reactions which lead to production of MMA, DMA and iAs as metabolites that are excreted in urine. It is believed that MMA is the more toxic metabolite and people with higher levels of MMA are more susceptible to developing arsenic-related disease. Accordingly, increased risk of breast cancer was found in association with higher levels of MMA by Lopez-Carrillo et al. (2014) [34] (also reviewed in Gamboa-Loira et al. 2017 [14]). This study reported that women in Northern Mexico who had lower capacity to methylate MMA to DMA and/or higher capacity to methylate iAs to MMA had higher risks of breast cancer. Proposed mechanisms of action for MMA include direct genotoxic effect, interference in DNA repair, and histone modification [14].
We have previously shown that polymorphisms in genes that code for carcinogen-metabolizing enzymes, such as N-acetyltransferase (NAT)2, can modify the risk of cancer associated with contaminants such as polycyclic aromatic amines found in tobacco smoke [35, 36]. Therefore, it is plausible that polymorphisms in genes that code for enzymes involved in As metabolism may modify As-associated cancer risk. Consistent with this, Schlawicke et al. (2009) [26] found modifying effects of polymorphisms in AS3MT and genes involved in one carbon metabolism in As-associated breast cancer risk. They reported two SNPs in AS3MT and one in CYP17A1 being associated with lower % MMA and higher %DMA, hence possibly conferring protection against breast cancer. Gamboa-Loira et al. (2017) [21] also assessed As exposure through levels of urinary metabolites and found that the MTR c.2756A>G polymorphism may decrease the risk of breast cancer associated with iAs. They also found a significant interaction between this polymorphism and percent DMA. At least one study has reported an increased risk of breast cancer among BRCA1 mutation carriers exposed to As in Poland (reviewed in Khanjani, Jafarnejad, and Tavakkoli 2017 [15]). Two studies by Michel-Ramirez et al. suggested an increased risk of breast cancer among women with elevated urinary iAs levels, and high levels of YAP expression in tissues of breast cancer cases with chronic exposure to As [22, 24]. YAP is crucial in response to oxidative stress and cytotoxic processes, therefore, this finding may suggest induction of YAP expression in response to As-induced oxidative damage.
Several studies also reported an effect of As on genetic or epigenetic pathways involved in breast cancer. The review by Romagnolo et al. (2016) [16] worked on the hypothesis that endocrine disruptors such as As affect the risk of breast cancer through epigenetic alterations and that foods that target the epigenetic machinery protect against these As-induced alterations. Consistent with their working hypothesis, Romagnolo et al. (2016) [16] found studies that provided evidence of epigenetic alterations associated with As such as inhibition of DNA mismatch repair, cell cycle control, and methylation pathways, and increase of proliferation, inflammation, and angiogenesis pathways. They also found evidence that bioactive foods such as folate and B12 may reduce As-induced mutagenic DNA breaks and tissue damage, whereas alcohol consumption may promote As-induced hypermethylation of tumor suppressor genes [16]. The Bardach et al. (2015) [11] systematic review also included four studies that looked at the effect of As on genetic and epigenetic pathways involved in cancer in general. Those studies suggested As carcinogenicity through epigenetic changes, particularly in DNA methylation, and genotoxic effects through micronucleus induction in certain malignancies such as bladder cancer.
The in vivo, in vitro and in silico studies provided additional evidence and biologic plausibility for the observed associations. The study by Parodi et al. (2015) [27] linking in utero exposure to arsenite with altered mammary gland development and possibly breast cancer, is consistent with As having xenoestrogenic properties [6], and suggests that As may induce carcinogenesis via alterations in cell differentiation and proliferation. Arsenic may also cause oxidative stress through direct production of reactive oxygen species (ROS) or indirect depletion of important antioxidants such as glutathione (GSH). Egiebor et al. (2013) [28] finding that As is associated with significant cytotoxicity in both the presence and absence of GSH suggests that GSH-mediated repair mechanism is either not employed or not sufficient for repairing damage by As.
A growing body of evidence suggests that As carcinogenicity may also result from epigenetic changes, particularly in DNA methylation. The two AS3MT SNPs reported by Schlawicke et al. (2009) [26], rs3740393 and rs11191439, have been consistently shown to be related to arsenic methylation in different populations. Smeester et al. (2011) [29] found that many of the proteins encoded by genes with differentially-methylated CpG islands are known players in As-associated diseases including cancer; this study uncovered an As-methylated tumor suppressorome, a complex of 17 putative tumor suppressors known to be silenced in human cancers. Additional evidence is provided by genome-wide association studies (GWAS), which have identified about 3000 genes with differential DNA methylation at transcriptional start sites following As exposure [12]. As has been shown to be associated with hypermethylation in tumor suppressor genes such as p53 and p16 [12]. This As-associated epigenetic re-programming may induce cancer stem cells (CSC)-like behavior in exposed cells [37], hence increasing the risk of cancer development.
We conducted Gene Ontology (GO) analysis using the Ingenuity Pathway Analysis (IPA) [38] database in order to gain insight into molecular networks affected by As exposure. Additionally, we explored As-gene-disease interaction networks by analyzing the Comparative Toxicogenomics Database (CTD; http://ctdbase.org/). Predominant As-affected pathways in IPA were estrogen receptor signaling, estrogen-dependent breast cancer signaling, inhibition of angiogenesis and p53 signaling (Figure 1). We further explored the protein-protein interaction network of estrogen-dependent breast cancer signaling, which identified EGFR, ER-α, and sp1 as key molecules and proliferation, growth, and estrogen dependent carcinogenesis as key cellular pathways affected by As (Figure 2).
Figure 1:
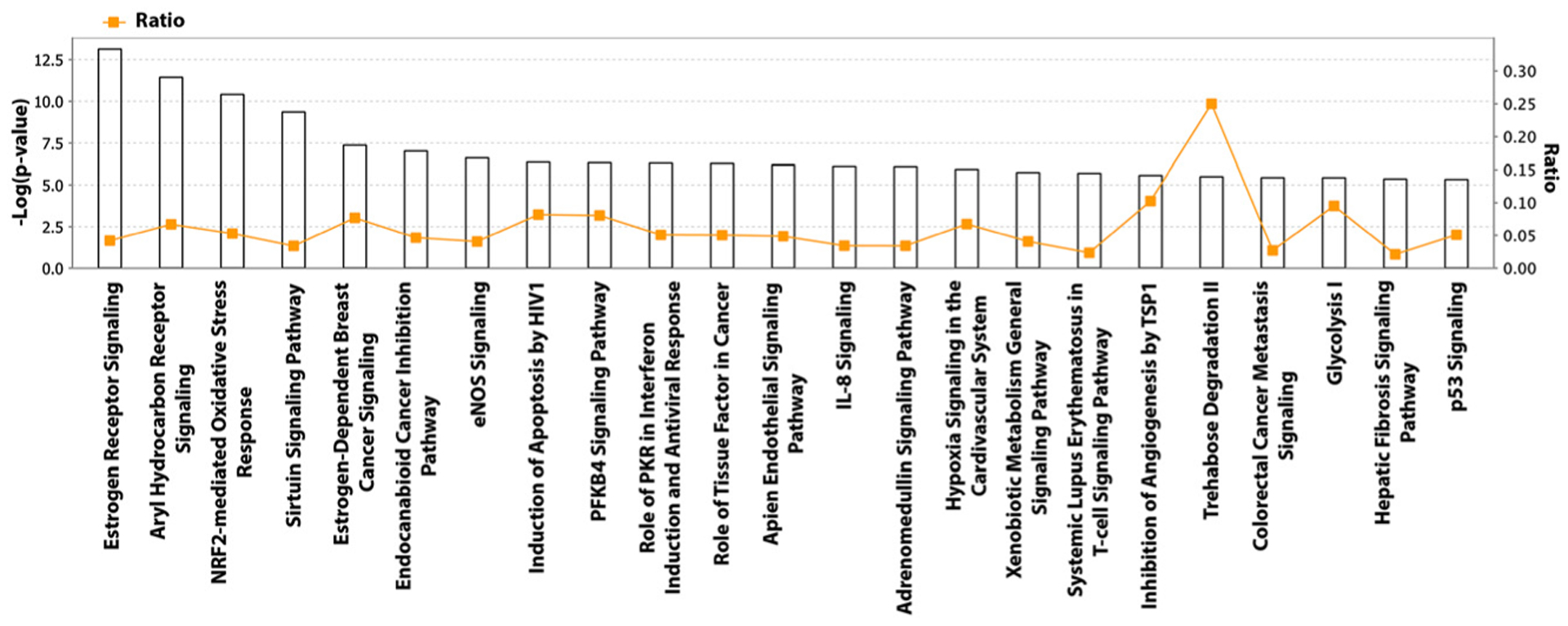
Arsenic-affected pathways in the ingenuity pathway analysis database (IPA).
Figure 2:
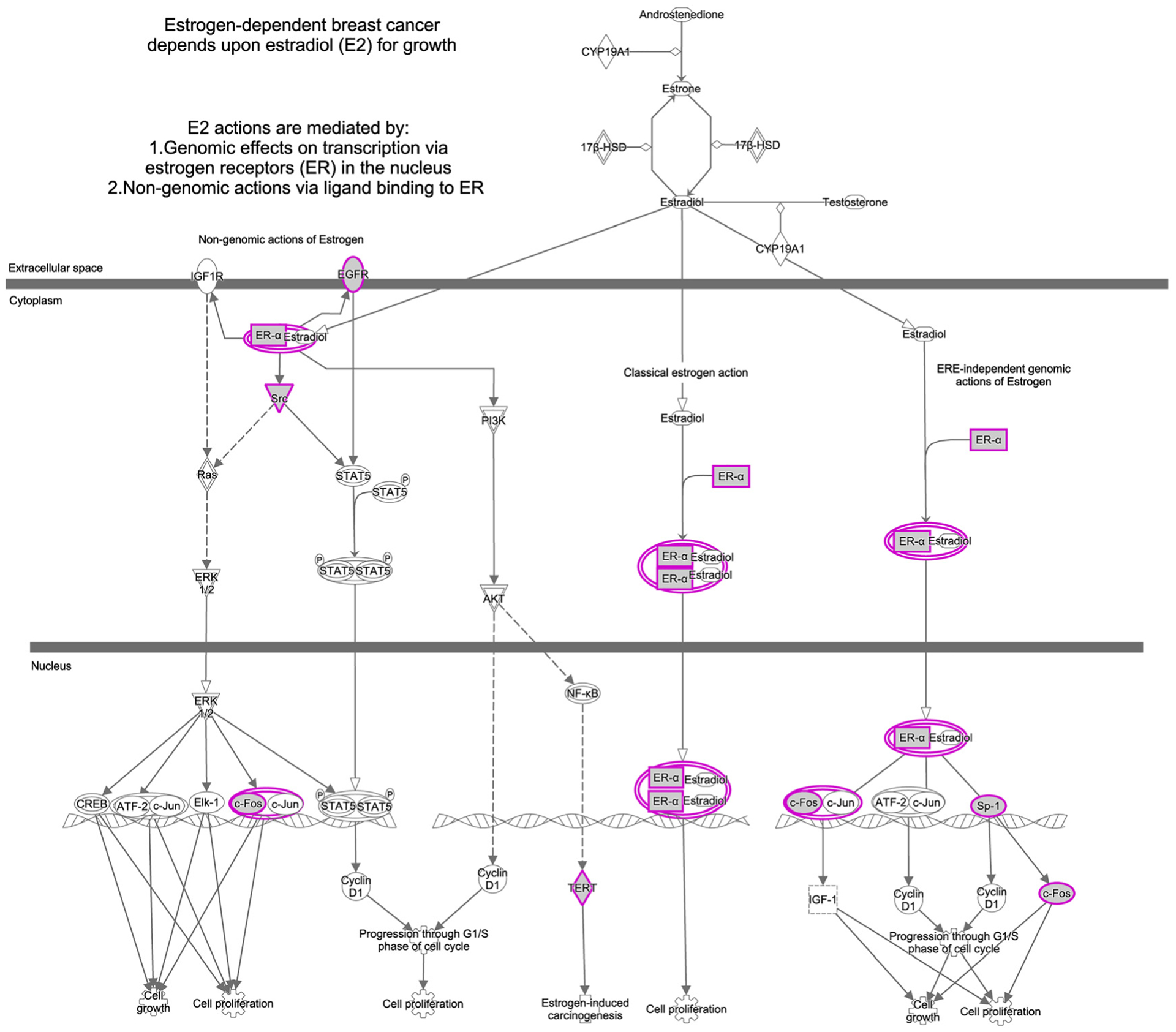
Protein-protein interaction network of estrogen-dependent breast cancer signaling in ingenuity pathway analysis database (IPA).
Our analysis of CTD data revealed several gene networks/biologic pathways involved in breast cancer, which were significantly affected by As. The most significantly-affected network contained genes involved in the regulation of the epithelial-mesenchymal transition. This analysis also highlighted estrogen receptor signaling and estrogen-dependent breast cancer signaling among predominant pathways affected by As (Figure 3A). Figure 3B depicts the As-responsive biologic pathway interactome and confirms the role of As as an endocrine disruptor and xenoestrogen as well as its involvement in breast carcinogenesis. An earlier study utilizing CTD had also reported breast cancer as being associated with the largest number of As-interacting genes [39]. Our overall results confirm the conclusions that As is capable of activating estrogen receptor α, inducing the proliferation of estrogen-dependent breast cancer cells, and increasing the expression of estrogen-regulated genes. Arsenic may induce mutations indirectly by influencing mechanisms that can lead to increased cell proliferation and decreased DNA repair. The gene and pathway lists created by us through analysis of CTD and IPA can be used to select candidate genes for future studies of gene-As interactions.
Figure 3:
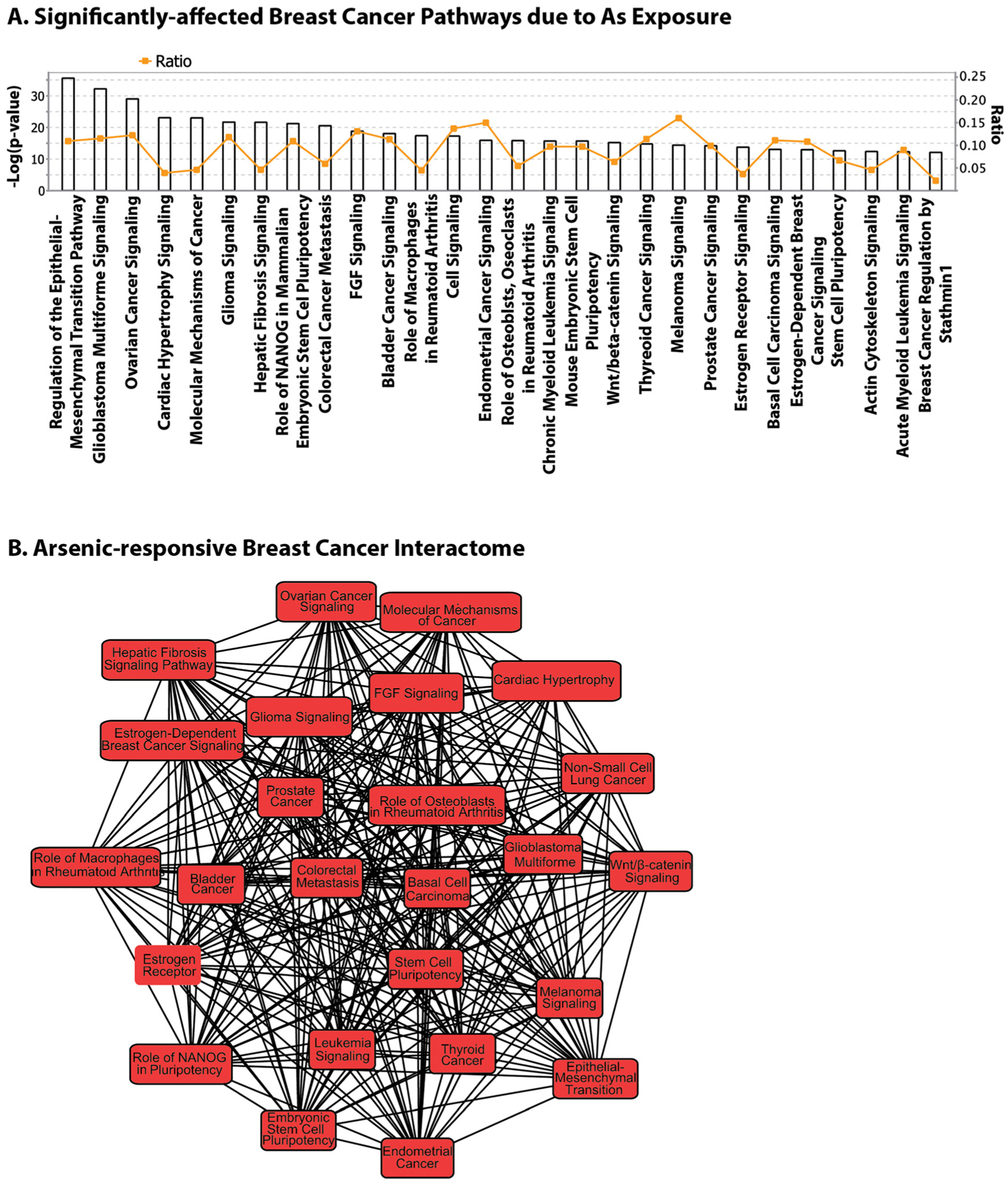
Arsenic-gene-breast cancer interaction in the comparative toxicogenomics database (CTD).
Arsenic is a persistent environmental contaminant and parts of the world including certain regions in the US have naturally high levels of As in the groundwater. Therefore, exposure to As can be chronic and affect a large portion of the population. Studying cancer risks associated with As exposure is of particular interest to public health given the modifiable nature of this carcinogen. A recent paper highlighted the importance of assessing carcinogenic potential of low-dose exposures to combined environmental contaminants, specifically chemical carcinogens, to help determine the triggers and enabling factors during the long latency period of most cancers [40]. This would also apply to metal carcinogens, which besides As include cadmium, lead and nickel, and may help the development of comprehensive preventive strategies.
Conclusions and future directions
Our systematic literature review revealed evidence of a possible main effect of As on breast cancer risk, and strong evidence for an effect of gene-As interactions. Genetic modifiers of As-associated breast cancer risk have been reported and additional modifying loci undoubtedly exist. Several studies have provided evidence for the effect of As on genetic and epigenetic mechanisms involved in breast cancer. Future epidemiologic studies of cancer risk may benefit from measuring individual-level exposures to As and/or As metabolite levels and consider interactions with other metals as well as genetic factors. Knowledge of biologic mechanisms involved can be used to select candidate genes for more targeted genetic epidemiologic studies of As and breast cancer.
Acknowledgments
Research funding: There was no funding involved for this work.
Footnotes
Conflict of interest: Authors declare no conflict of interest.
Informed consent: Informed consent is not applicable.
Ethical approval: The conducted research is not related to either human or animal use.
Contributor Information
Roxana Moslehi, School of Public Health, Albany, USA; Cancer Research Center, University at Albany, State University of New York (SUNY), Albany, NY, 12144, USA.
Cristy Stagnar, School of Public Health, Albany, USA; Drukier Institute for Children’s Health, Weill Cornell Medicine, NY, USA.
Sneha Srinivasan, School of Public Health, Albany, USA.
Pawel Radziszowski, School of Public Health, Albany, USA.
David O. Carpenter, School of Public Health, Albany, USA Institute for Health and the Environment, University at Albany, Albany, NY, USA.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Canc 2019;144:1941–53. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moslehi R, Freedman E, Zeinomar N, Veneroso C, Levine PH. Importance of hereditary and selected environmental risk factors in the etiology of inflammatory breast cancer: a case-comparison study. BMC Canc 2016;16:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiatt RA, Brody JG. Environmental determinants of breast cancer. Annu Rev Publ Health 2018;39:113–33. [DOI] [PubMed] [Google Scholar]
- 5.Peto R, Cancer genes, and the environment. N Engl J Med 2000; 343:1495. discussion 1495–6. [PubMed] [Google Scholar]
- 6.Ronchetti SA, Novack GV, Bianchi MS, Crocco MC, Duvilanski BH, Cabilla JP. In vivo xenoestrogenic actions of cadmium and arsenic in anterior pituitary and uterus. Reproduction 2016;152:1–10. [DOI] [PubMed] [Google Scholar]
- 7.Gundert-Remy U, Damm G, Foth H, Freyberger A, Gebel T, Golka K, et al. High exposure to inorganic arsenic by food: the need for risk reduction. Arch Toxicol 2015;89:2219–27. [DOI] [PubMed] [Google Scholar]
- 8.Palma-Lara I, Martinez-Castillo M, Quintana-Perez JC, Arellano-Mendoza MG, Tamay-Cach F, Valenzuela-Limon OL, et al. Arsenic exposure: a public health problem leading to several cancers. Regul Toxicol Pharmacol 2020;110:104539. [DOI] [PubMed] [Google Scholar]
- 9.Rehman K, Fatima F, Waheed I, Akash MSH. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 2018;119:157–84. [DOI] [PubMed] [Google Scholar]
- 10.Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–Part C: metals, arsenic, dusts, and fibres. Lancet Oncol 2009;10: 453–4. [DOI] [PubMed] [Google Scholar]
- 11.Bardach AE, Ciapponi A, Soto N, Chaparro MR, Calderon M, Briatore A, et al. Epidemiology of chronic disease related to arsenic in Argentina: a systematic review. Sci Total Environ 2015; 538:802–16. [DOI] [PubMed] [Google Scholar]
- 12.Chen QY, DesMarais T, Costa M. Metals and mechanisms of carcinogenesis. Annu Rev Pharmacol Toxicol 2019;59:537–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jouybari L, Saei Ghare Naz M, Sanagoo A, Kiani F, Sayehmiri F, Sayehmiri K, et al. Toxic elements as biomarkers for breast cancer: a meta-analysis study. Canc Manag Res 2018;10:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamboa-Loira B, Cebrian ME, Franco-Marina F, Lopez-Carrillo L. Arsenic metabolism and cancer risk: a meta-analysis. Environ Res 2017;156:551–8. [DOI] [PubMed] [Google Scholar]
- 15.Khanjani N, Jafarnejad AB, Tavakkoli L. Arsenic and breast cancer: a systematic review of epidemiologic studies. Rev Environ Health 2017;32:267–77. [DOI] [PubMed] [Google Scholar]
- 16.Romagnolo DF, Daniels KD, Grunwald JT, Ramos SA, Propper CR, Selmin OI. Epigenetics of breast cancer: modifying role of environmental and bioactive food compounds. Mol Nutr Food Res 2016;60:1310–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White AJ, O’Brien KM, Niehoff NM, Carroll R, Sandler DP. Metallic air pollutants and breast cancer risk in a nationwide cohort study. Epidemiology 2019;30:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Zhang X, Wu K, Wu H, Sun Q, Hu FB, et al. Rice consumption and cancer incidence in US men and women. Int J Canc 2016;138:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu R, Nelson DO, Hurley S, Hertz A, Reynolds P. Residential exposure to estrogen disrupting hazardous air pollutants and breast cancer risk: the California Teachers Study. Epidemiology 2015;26:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien KM, White AJ, Jackson BP, Karagas MR, Sandler DP, Weinberg CR. Toenail-based metal concentrations and young-onset breast cancer. Am J Epidemiol 2019;188:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Gamboa-Loira B, Cebrian ME, Salinas-Rodriguez A, Lopez-Carrillo L. Genetic susceptibility to breast cancer risk associated with inorganic arsenic exposure. Environ Toxicol Pharmacol 2017;56: 106–13. [DOI] [PubMed] [Google Scholar]
- 22.Michel-Ramirez G, Recio-Vega R, Lantz RC, Gandolfi AJ, Olivas-Calderon E, Chau BT, et al. Assessment of YAP gene polymorphisms and arsenic interaction in Mexican women with breast cancer. J Appl Toxicol 2020;40:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajayi O, Charles-Davies M, Anetor J, Ademola A. Pituitary, gonadal, thyroid hormones and endocrine disruptors in pre and postmenopausal Nigerian women with ER-, PR- and HER-2-positive and negative breast cancers. Med Sci 2018;6. 10.3390/medsci6020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michel-Ramirez G, Recio-Vega R, Ocampo-Gomez G, Palacios-Sanchez E, Delgado-Macias M, Delgado-Gaona M, et al. Association between YAP expression in neoplastic and non-neoplastic breast tissue with arsenic urinary levels. J Appl Toxicol 2017;37:1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joo NS, Kim SM, Jung YS, Kim KM. Hair iron and other minerals’ level in breast cancer patients. Biol Trace Elem Res 2009;129: 28–35. [DOI] [PubMed] [Google Scholar]
- 26.Schlawicke Engstrom K, Nermell B, Concha G, Stromberg U, Vahter M, Broberg K. Arsenic metabolism is influenced by polymorphisms in genes involved in one-carbon metabolism and reduction reactions. Mutat Res 2009;667:4–14. [DOI] [PubMed] [Google Scholar]
- 27.Parodi DA, Greenfield M, Evans C, Chichura A, Alpaugh A, Williams J, et al. Alteration of mammary gland development and gene expression by in utero exposure to arsenic. Reprod Toxicol 2015;54:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egiebor E, Tulu A, Abou-Zeid N, Aighewi IT, Ishaque A. The kinetic signature of toxicity of four heavy metals and their mixtures on MCF7 breast cancer cell line. Int J Environ Res Publ Health 2013; 10:5209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeester L, Rager JE, Bailey KA, Guan X, Smith N, Garcia-Vargas G, et al. Epigenetic changes in individuals with arsenicosis. Chem Res Toxicol 2011;24:165–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendt C, Margolin S. Identifying breast cancer susceptibility genes - a review of the genetic background in familial breast cancer. Acta Oncol 2019;58:135–46. [DOI] [PubMed] [Google Scholar]
- 31.Couch FJ, Nathanson KL, Offit K. Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 2014;343:1466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med 2008;359:2143–53. [DOI] [PubMed] [Google Scholar]
- 33.Navarro Silvera SA, Rohan TE. Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control 2007;18:7–27. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Carrillo L, Hernandez-Ramirez RU, Gandolfi AJ, Ornelas-Aguirre JM, Torres-Sanchez L, Cebrian ME. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol 2014;280:53–9. [DOI] [PubMed] [Google Scholar]
- 35.Moslehi R, Chatterjee N, Church TR, Chen J, Yeager M, Weissfeld J, et al. Cigarette smoking, N-acetyltransferase genes and the risk of advanced colorectal adenoma. Pharmacogenomics 2006;7: 819–29. [DOI] [PubMed] [Google Scholar]
- 36.Tan XL, Moslehi R, Han W, Spivack SD. Haplotype-tagging single nucleotide polymorphisms in the GSTP1 gene promoter and susceptibility to lung cancer. Canc Detect Prev 2009;32:403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Yang C. Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: a novel mechanism of metal carcinogenesis. Semin Canc Biol 2019;57: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer A, Green J, Pollard J Jr., Tugendreich S Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014; 30:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis AP, Murphy CG, Rosenstein MC, Wiegers TC, Mattingly CJ. The Comparative Toxicogenomics Database facilitates identification and understanding of chemical-gene-disease associations: arsenic as a case study. BMC Med Genom 2008;1:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodson WH 3rd, Lowe L, Carpenter DO, Gilbertson M, Manaf Ali A, Lopez de Cerain Salsamendi A, et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogenesis 2015;36:S254–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


